Abstract
The identification of cell surface markers specific to pancreatic islet cell subsets is important for both the study of islet biology and for investigating the pathophysiology of diseases in which these cell types are lost or damaged. Analysis of publicly available single-cell RNAseq data showed that LY6H transcripts are highly enriched in the delta cells of the pancreas. This finding was confirmed using immunofluorescence analysis of histological sections of human pancreas, and flow cytometric analysis of human islet preparations. We found that expression of LY6H was robustly associated with pancreatic delta cells in samples derived from both control and diabetic donors. Furthermore, we demonstrate that antibodies against LY6H can be used to substantially enrich for live delta cells from preparations of human islets. This study identified LY6H as a novel cell surface marker of human pancreatic delta cells—a finding that will aid in the identification and characterisation of this important cell type.
Similar content being viewed by others
Introduction
Previous studies have identified cell surface markers that are expressed on pancreatic endocrine cell types—including human pancreatic delta cells1. However, few of these markers are specific for individual endocrine cell types, and to date, no viable strategy has been shown for isolation of viable delta cells of the human pancreas.
Lymphocyte Antigen 6 Family Member H (LY6H) is a member of the LY6 family of proteins that belong to the Ly6/urokinase-type plasminogen activator receptor (uPAR) superfamily. This superfamily is characterised by the presence of a LU domain, a 60–80 amino acid region composed of 6–10 cysteine residues arranged to allow the formation of disulphide bridges which create a three-fingered (3 F) structural motif2. LY6H is believed to act as a modulator of nicotinic acetylcholine receptor (nAChR) activity2, and to play a role in glutamatergic signalling in the brain3. Several previous single-cell transcriptomic studies have identified LY6H transcripts in the delta cell population of human islets and pancreas4,5, however, analysis at the protein level has not been conducted.
In the present study, we show that LY6H is specifically expressed in delta cells of the adult human pancreas, in both control and diabetic subjects. Additionally, we demonstrate that antibodies against LY6H can be used to isolate viable human pancreatic delta cells for further downstream studies.
Methods
Ethical approval
Use of tissue donor material was approved by the St Vincent’s Hospital Human Research Ethics Committee (approval no. SVH HREC-A 011/04). All experiments were performed in accordance with relevant guidelines and regulations. Details of individual donors are provided in Tables 1 and 2.
Islet isolation
Healthy human pancreata were obtained with informed consent from next of kin, for scientific purposes, from heart-beating, brain-dead donors, with research approval from the Human Research Ethics Committee at St Vincent’s Hospital, Melbourne. Human islets were purified by intraductal perfusion and digestion of the pancreases with collagenase AF-1 (SERVA/Nordmark, Germany)6 followed by purification using Ficoll density gradients7. Purified islets were cultured in Miami Media 1 A (Mediatech/Corning 98 − 021, USA) supplemented with 2.5% human serum albumin (Australian Red Cross, Melbourne, VIC, Australia), in a 37 °C, 5% CO2 incubator.
HEK293T culture and transfection
Human embryonic kidney (HEK) 293T cells8 (ATCC CRL-11268) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco; 10564011) supplemented with 10% fetal calf serum (FCS; Gibco; A4766801) and 1% GlutaMAX (Gibco; 35050061) and maintained at 5% CO2 at 37 °C. Cells were passaged upon reaching confluence using TrypLE Select (Life Technologies; 12563029). HEK293T cells were transfected with a pcDNA3.1(+)-P2A-eGFP (Addgene) plasmid containing the LY6H cDNA using Lipofectamine 3000 (Invitrogen; L3000015) as per the manufacturer’s directions. Cells were maintained in 5% CO2 at 37 °C for 48 h prior to immunofluorescence staining.
Immunofluorescence staining
Paraffin sections of donor human pancreas were obtained from the Tom Mandel Islet Isolation Program (St Vincent’s Hospital, Victoria). These donor human pancreata were obtained with informed consent from next of kin, for scientific purposes, from heart-beating, brain-dead donors, with research approval from the Human Research Ethics Committee at St Vincent’s Hospital, Melbourne. Paraffin was removed using xylene, samples were rehydrated, and antigen retrieved using 10 mM citrate buffer. Samples were blocked for 1 h at room temperature in staining buffer (10% foetal calf serum (FCS) (Sigma-Aldrich; 12003 C) in PBS) and 0.1% Triton-X (Sigma-Aldrich; T9284), stained overnight with primary antibodies at 4 °C, stained for 1 h at room temperature with secondary antibodies, and stained with DAPI (Sigma-Aldrich; D9542) for 5 min. Antibody details are provided in Table 3. Samples were mounted using Fluoromount-G (Southern Biotech; 0100-01) and imaged using a LSM780 inverted confocal microscope (Zeiss). Image analysis was performed using ImageJ (version 1.0).
Flow cytometry and sorting
Isolated human islets obtained from the Tom Mandel Islet Isolation Program were digested by resuspension in Accutase (Sigma-Aldrich; A6964) solution for 15 min at 37 °C. Following trituration, cells were washed in PBS and then stained with primary antibody in FACS buffer (2% FCS in PBS) for 30 min on ice. Cells were then washed twice with FACS buffer and stained with the appropriate secondary antibody for 30 min on ice. Antibody details are provided in Table 3. Cells were then washed twice with FACS buffer and then resuspended in 1 µg/ml propidium iodide (Sigma-Aldrich; P4864) to exclude dead cells, prior to cell sorting. Flow sorting was performed on a BD FACSAria Fusion (BD Biosciences). Data was collected and analysed using BD FACSDiva 8.0.1 (BD Biosciences). Isolated cells were resuspended in Trizol reagent (Sigma; 15596026) as a prelude to preparation of RNA.
RNA extraction
Following isolation by flow sorting, cells were resuspended in TRIzol reagent (Thermo-Fisher; 15596026) and RNA extracted as per the manufacturer’s directions.
Bulk RNAseq analysis
RNA extraction of FACS purified populations was performed using TRIzol extraction as directed by the manufacturer (Thermo-Fisher Scientific). RNA samples were processed, quality control performed, and sequenced by the Victorian Clinical Genetics Service, Melbourne (VCGS). Libraries were generated using an either a Truseq Stranded mRNA Library Prep Kit (Illumina) (Sort-1 donor) or a SMARTer Stranded Total RNA-seq Kit v3 - Pico Input Mammalian (Takara) (Sort-2 donor). Sequencing of samples was performed using an NovaSeq 6000 (Illumina) instrument. Between 20 and 30 million 150 bp paired-end reads were obtained per sample. Individual fastq files were aligned to the reference genome (GrCh38 assembly) with the Spliced Transcript Alignment to a Reference (STAR) software (version 2.7.3a)9 using default parameters. Nonuniquely mapping reads and read pairs with unpaired alignments were excluded. Read counts for each gene were determined using featureCounts as part of the Rsubread VERSION library. RNAseq analysis was performed on the raw count table using the limma10 and edgeR11,12 packages within R. Briefly, the counts per million (CPM) value was calculated using the cpm() function in edgeR, and genes expressed at low levels (defined as a CPM value below 0.5 in any sample) were filtered out. The filtered matrix was then used to create a DGEList object in edgeR, and the object TMM normalized using the calcNormFactors() function. For the Sort-2 donor, the count matrix was manually curated to remove sequences that were representative of non-coding RNA sequences. Highly variable genes were identified by estimating the variance of each gene across the different sort fractions from each donor, then sorting genes according to variance value. Unsupervised hierarchical clustering of samples was then performed using the top 1000 variable genes. The top 100 most variable genes for each donor is shown in Supplementary Figs. 1 and 2.
Single cell RNAseq analysis
Processed RNA sequencing data was downloaded from GEO (GSE114412). The count matrix was filtered to remove mitochondrially-encoded genes, genes with less than 1000 UMIFM counts and cells with greater than 25% mitochondrial-DNA content. Variation in the total counts of individual cells was then removed by normalizing the sum of counts for each individual cell to 10,000. The normalized counts were then used for dimensionality reduction and clustering for each dataset, which was performed using the Seurat package within R13. Briefly, highly variable genes were identified using the FindVariableFeatures() function within Seurat. Principle components were then computed and clustering was performed using Louvain community detection in the space of the first 30 principle components. UMAP projections were then computed using the first 30 principle components. Differentially expressed genes within clusters were then computed using the FindAllMarkers() function within Seurat and cluster identity assigned using canonical genes as previously described14.
Results
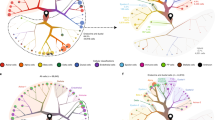
We previously described the re-analysis of a single-cell RNA sequencing dataset (GSM3142001) with the view of identifying novel cell surface markers of specific pancreatic cell subsets14. Briefly, single-cell transcriptional profiling of islets from 4 independent non-diabetic donors were clustered and visualised using uniform manifold approximation and projection (UMAP) (Fig. 1a). Gene expression profiles associated with cell clusters were examined with a view to identifying cell surface markers that would be compatible with live cell analysis and purification techniques, such as flow cytometry. From this re-analysis, we identified LY6H transcripts as being specifically expressed in the delta cell cluster of this dataset (Fig. 1b). In order to examine this in a larger set of donors, a dataset from Elgamal et al.15 was analysed which contained 65 donors (11 autoantibody positive, 10 type 1 diabetic, 17 type 2 diabetic and 27 control). This analysis demonstrates that LY6H expression is found in the delta cell populations (Supplementary Fig. 3a–d). Whilst some cells are seen outside of the delta cell cluster in T1D and T2D donors to express LY6H, upon examination these cells also expressed SOMATOSTATIN (Supplementary Fig. 3e,f).
LY6H is a marker of human pancreatic delta cells. (A) Unsupervised clustering UMAP projection plot for 4 independent donors, with cell types as indicated. (B) Violin plot demonstrating expression of LY6H in the delta cells of the human pancreas as seen in scRNAseq analysis. (C) Immunofluorescence analysis of LY6H expression (green) in pancreatic sections representing control, T1D and T2D tissue donors, co-stained with antibodies recognising SOMATOSTATIN (SST, red) and CHROMOGRANIN A (CHGA, grey). Scale bars for all images are 25 μm.
Consistent with the single-cell transcriptomic data, we found the LY6H expression was restricted to the delta cells within the islets, as identified by SOMATOSTATIN (SST) expression, with all SST-positive cells examined, seen to express LY6H (Fig. 1c). To emphasise this, we have reanalysed the images in Fig. 1 and Supplementary Fig. 4 to quantify the number of SST + cells that co-express LY6H. This analysis across more than 10 islets, demonstrated that all 129 SST-positive cells in these images expressed LY6H. The specificity of the LY6H antibody was validated using a transient expression system (Supplementary Fig. 5a). No co-expression of LY6H was seen within the beta (as marked by INSULIN) or alpha (as marked by GLUCAGON) cells in the islets (Supplementary Fig. 5b).
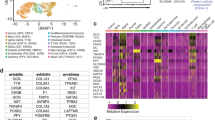
We next tested whether anti-LY6H antibodies could be used to isolate live delta cells from donor islets using flow cytometry. Flow cytometry analysis showed that only a small fraction of cells within the islet preparations expressed LY6H (Fig. 2a, b, Supplementary Fig. 5c), consistent with the relatively low abundance of delta cells within human islets. We isolated RNA from the cell LY6H + population, as well as from cells that did not express LY6H and from unsorted islet cells and subjected these samples to bulk RNAseq analysis (Fig. 2c, Supplementary Fig. 6).
LY6H can be used to isolate human pancreatic delta cells. (A) Flow cytometry plot showing sort gates for human islets using a secondary (IgG-APC) antibody only (Sort-1 donor). (B) Flow cytometry plot showing sort gates for human islets stained an antibody directed against LY6H (Sort-1). (C) Transcript quantification (log2 counts per million (CPM) of LY6H expression in the indicated sorted fractions (US—unsorted). (D) Heatmap showing expression of the top 10 highly expressed genes in LY6H + cells from Sort-1 donor as identified by bulk RNA sequencing mapped against cell clusters from the scRNAseq analysis performed previously6. (E) Transcript quantification (log2 counts per million (CPM)) of INSULIN (INS) and GLUCAGON (GCG) expression in the indicated sorted fractions (US—unsorted). (F) Transcript quantification (log2 counts per million (CPM) of SOMATOSTATIN (SST) in the indicated sorted fractions (US—unsorted).
To determine the identity of LY6H + cells, we mapped the 20 most highly expressed genes in this population to the gene expression profiles associated with specific cell clusters identified by scRNAseq analysis. This association, visualised by means of a heat map (Fig. 2d), demonstrates that LY6H is a specific marker of somatostatin expressing pancreatic delta cells. Additionally, it could be seen that while there was no enrichment in LY6H + cells for either INSULIN or GLUCAGON transcripts (Fig. 2e), it could be seen that there was an enrichment of SOMATOSTATIN transcripts in LY6H + cells (Fig. 2f) as compared to LY6H-negative cells or an unsorted cell fraction. Statistical analysis confirmed that there is a statistically significant increase in SOMATOSTATIN transcripts in the LY6H + cell fraction as compared to the unsorted cell fraction (p = 0.0114, n = 2). From an additional donor (SVI-010-25, Sort-3), we isolated both LY6H + and LY6H- cells and adhered these to poly-l-lysine coated coverslips. Cells were analysed by immunofluorescence for expression of SST. This analysis showed that the vast majority of cells isolated on the basis of LY6H expression also expressed SST, whilst cells that lacked LY6H expression also lacked SST expression (Supplementary Fig. 7).
These analyses confirm the results obtained from the whole pancreas immunofluorescence staining and verifies that LY6H is a novel cell surface marker that can be used to isolate delta cells from the human pancreas for downstream studies.
Discussion
While previous reports have identified cell surface markers facilitating isolation of delta cells from the mouse pancreas16, this is the first report of a marker that enables the purification of a highly enriched population of viable delta cells from the human pancreas. This conclusion was based on flow cytometry experiments, which showed that anti-LY6H antibodies enabled isolation of a population of islet cells that was substantially enriched for somatostatin transcripts, a result consistent with our immunofluorescence analyses of human islets. In addition to enabling identification of delta cells in sections of control human pancreata, we found that LY6H also co-stained SST expressing delta cells in samples derived from both type 1 and type 2 diabetic donors. A limitation of our study is the relatively weak signal strength of the anti-LY6H antibody. This could be either due to the antibody affinity, or to the number of epitopes available for binding per cell. The issue of antibody affinity could be addressed by generating a new antibody with better signal to noise characteristics. Nevertheless, despite the relatively weak signal afforded by currently the available anti-LY6H antibody, our study demonstrates that LY6H can be used as a tool for the isolation of a highly enriched population of delta cells for downstream studies. In the future, further purification of this subset of endocrine cells may be aided by the identification of additional cell surface markers associated with either delta cells or other cell types present within islet cell populations.
LY6H has recently been identified as a modulator of α7 nAcetylcholine Receptor (α7 nAChR) function, where it’s extracellular domain interacts directly with α7 nAChR to inhibit ligand-induced channel activity17. In addition, this work demonstrated that soluble LY6H also possessed nAChR modulatory function, raising the possibility that LY6H activity may not be restricted to the cells which express it. It is well established that endocrine cells within the pancreatic islet are able to regulate each other’s function by both autocrine and paracrine signalling18. Previous studies in the mouse have identified expression of α7 nAChR in both the beta and alpha cells19. As LY6H is a GPI anchored protein, it is plausible that LY6H may be released from the surface of delta cells following the activation of phospholipases20 providing a potential mechanism by which it could affect neighbouring cells. In this scenario, secreted LY6H could modulate α7 nAChR expression on neighbouring alpha and/or beta cells, potentially affecting glucose homeostasis, independent of somatostatin secretion. In this context, it noteworthy that deletion of α7 nAChR in mice leads to a reduced beta cell mass, contributing to a predisposition for later weight gain19. These studies suggest that further investigation into the role of nAChR signalling in the endocrine pancreas function is warranted. In the same vein, it is well established that somatostatin secreted by delta cells modulates insulin and glucagon secretion21. In this light, the ability to purify human delta cells will allow for more detailed study of this cell type and any changes that occur during the diabetogenic process.
Data availability
Bulk RNA-seq data used in this study has been deposited in the Gene Expression Omnibus (GEO) data base and are available under the accessation number GSE264633. All other data supporting findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- T1D:
-
Type 1 diabetes
References
Kumar, U. et al. Subtype-selective expression of the five somatostatin receptors (hSSTR1-5) in human pancreatic islet cells: a quantitative double-label immunohistochemical analysis. Diabetes 48, 77–85 (1999).
Upadhyay, G. Emerging role of lymphocyte antigen-6 family of genes in cancer and immune cells. Front. Immunol. 10, 819 (2019).
Puddifoot, C., Wu, M., Sung, R. J. & Joiner, W. Ly6h regulates trafficking of alpha7 nicotinic acetylcholine receptors and nicotine-induced potentiation of glutamatergic signalling. J. Neurosci. 35, 3420–3430 (2015).
Segerstolpe, A. et al. Single-cell transcriptome profiling of human pancreatic Islets in health and type 2 diabetes. Cell Metabol. 24, 593–607 (2016).
Tritschler, S. et al. A transcriptional cross species map of pancreatic islet cells. Mol. Metabolism. 66, 101595 (2022).
Ricordi, C., Lacy, P., Finke, E. & Olack, B. Scharp, D. Automated method for isolation of human pancreatic Islets. Diabetes 37, 413–420 (1988).
Barbaro, B. et al. Improved human pancreatic islet purification with the refined UIC-UB density gradient. Transplantation 84, 1200–1203 (2007).
Pereira, L. et al. Brachyury and related Tbx proteins interact with the Mixl1 homeodomain protein and negatively regulate Mixl1 transcriptional activity. PLoS ONE. 6, e28394 (2011).
Dobin, A. et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 29, 15–21 (2013).
Ritchie, M. et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Robinson, M., McCarthy, D. & Smyth, G. EdgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
McCarthy, D., Chen, Y. & Smyth, G. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297 (2012).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Schiesser, J., Loudovaris, T., Thomas, H., Elefanty, A. & Stanley, E. Integrin αvβ5 heterodimer is a specific marker of human pancreatic beta cells. Sci. Rep. 11, 8315 (2021).
Elgamal, R. et al. An integrated map of cell type-specific gene expression in pancreatic Islets. BioRxiv 1, 526994 (2023).
Berthault, C., Staels, W. & Scharfmann, R. Purification of pancreatic endocrine subsets reveals increased iron metabolism in beta cells. Mol. Metabolism. 42, 101060. https://doi.org/10.1016/j.molmet.2020.101060 (2020).
Moriwaki, Y. et al. Endogenous neurotoxin-like protein Ly6H inhibits alpha7 nicotinic acetylcholine receptor currents at the plasma membrane. Sci. Rep. 10, 11996. https://doi.org/10.1038/s41598-020-68947-7 (2020).
Drigo, R. A. E. et al. Structural basis for delta cell paracrine regulation in pancreatic Islets. Nat. Commun. 10, 3700 (2019).
Gausseres, B. et al. The constitutive lack of α7 nicotinic receptor leads to metabolic disorders in mouse. Biomolecules 10, 1057. https://doi.org/10.3390/biom10071057 (2020).
Lehto, M. & Sharom, F. PI-specific phospholipase C cleavage of a reconstituted GPI-anchored protein: modulation by the lipid bilayer. Biochemistry 41, 1398–1408 (2002).
Taborsky, G., Smith, P. & Porte, D. Interaction of somatostatin with the A and B cells of the endocrine pancreas. Metabolism 27, 1299–1302 (1978).
Acknowledgements
The authors wish to thank Drs. M. Burton and E. Jones from the flow cytometry and imaging division, Murdoch Children’s Research Institute for their help with flow cytometry and cell sorting. We thank all organ donors and their families for their generosity and for enabling this work. Thanks to the staff of St. Vincent’s Institute involved in the islet isolation program, and DonateLife for obtaining research consent and providing the human pancreata.
Funding
This study was supported by grants from the National Health and Medical Research Council (GNT1186019), Diabetes Australia (Y22G – SCHJ), and the Novo Nordisk Foundation Center for Stem Cell Medicine (reNEW), supported by Novo Nordisk Foundation grant (NNF21CC0073729). Additional infrastructure funding to the Murdoch Children’s Research Institute was provided by the Australian Government National Health and Medical Research Council Independent Research Institute Infrastructure Support Scheme and the Victorian Government’s Operational Infrastructure Support Program.
Author information
Authors and Affiliations
Contributions
JVS, YY and EGS contributed to the acquisition and analysis of data for this work. JVS, TL, HET, AGE and EGS contributed to the conception and design of the experiments or to the analysis or interpretation of the data for this work. JVS and EGS wrote the manuscript and all authors made important contributions to editing and revision of the manuscript. All authors have approved the final version of the manuscript. EGS and JVS are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schiesser, J.V., Yu, Y., Loudovaris, T. et al. LY6H is a marker of human pancreatic delta cells. Sci Rep 15, 33011 (2025). https://doi.org/10.1038/s41598-025-18321-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18321-2