Abstract
This study aimed to investigate the expression and evaluate the function of long non-coding RNA (LncRNA) HOXA11-AS in Esophageal Squamous Cell Carcinoma (ESCC). HOXA11-AS expression was quantified in paired ESCC tumor tissues (n = 50) and adjacent histologically normal tissues (> 5 cm from tumor margin) (n=50) from surgical patients. The relationship between HOXA11-AS expression levels, clinical staging and patient survival was analyzed. Lentiviral transduction was employed to generate stable ESCC cell lines with HOXA11-AS overexpression (lv-HOXA11-AS) or knockdown (sh-HOXA11-AS), accompanied by negative control groups (lv-NC/sh-NC). The impact on malignant phenotype was assessed via CCK-8 proliferation assays, Transwell migration/invasion assays, wound healing assays, and flow cytometry for apoptosis. In vivo tumor growth was evaluated by subcutaneously injecting fluorescently labeled lv-HOXA11-AS or lv-NC cells into BALB/c-nu mice. RNA-sequencing and tissue qRT-PCR confirmed significantly higher HOXA11-AS expression in ESCC versus normal epithelium, and high HOXA11-AS expression was associated with advanced tumor stage and was identified as an independent predictor of poor prognosis. qRT-PCR validated the elevated expression of HOXA11-AS in ESCC cell lines too. HOXA11-AS overexpression promoted proliferation, migration, invasion, and suppressed early apoptosis in ESCC cells, HOXA11-AS knockdown exerted opposing effects. HOXA11-AS overexpression significantly enhanced tumor growth in mouse xenograft models. LncRNA HOXA11-AS is aberrantly overexpressed in ESCC and functions as an oncogene, driving tumor progression by enhancing proliferation, migration, invasion and suppressing apoptosis. Its association with poor prognosis identifies HOXA11-AS as a promising prognostic biomarker and potential therapeutic target for ESCC.
Similar content being viewed by others
Introduction
Esophageal carcinoma (EC) ranks as the tenth most common malignancy globally (3.1% of total cancer incidence) and the sixth leading cause of cancer mortality (5.5% of total cancer deaths)1. This disease demonstrates significant geographic heterogeneity, with the highest incidence observed in East Asia1. Histologically distinct subtypes include adenocarcinoma and small cell carcinoma, yet esophageal squamous cell carcinoma (ESCC) dominates in high-risk regions, constituting over 90% of cases in China2. The aggressive nature of EC—characterized by occult early symptoms, proclivity for metastasis and recurrence, and poorly defined molecular pathogenesis—contributes to its generally poor prognosis3. Given its distinct epidemiological and histopathological profile, ESCC represents a critical public health challenge and serves as the focus of this investigation. Recent multi-omics studies reveal that non-coding RNA, particularly long non-coding RNA (LncRNA), orchestrate ESCC pathogenesis through epigenetic reprogramming and signaling network dysregulation4.
LncRNA, defined as RNA molecules exceeding 200 nucleotides in length that lack protein-coding potential, regulate cellular physiology through multilayered molecular mechanisms5. Epigenetically, LncRNA can affect DNA or mRNA methylation, chromosome structure, and modification status by acting as signals or guiding the recruitment of chromatin repair complexes6. Transcriptionally, LncRNA can regulate the expression of genes in close proximity to the genome through cis-action, and can also regulate the expression of transcriptional activators or repressors targeting distant transcription through trans-action, mainly through transcriptional interference, chromosome silencing, and direct binding to transcription factors7,8. Post-transcriptionally, LncRNA can directly participate in the regulation of biological processes such as mRNA alternative splicing, mRNA editing, protein translation and transport. It can also be used as a competing endogenous RNA (ceRNA) to endogenously compete with the corresponding miRNA to inactivate it and regulate the expression of the corresponding target genes of miRNA9,10,11. LncRNA plays a role in promoting the proliferation, migration and invasion of tumor cells in a variety of cancers, including bladder cancer, colorectal cancer, liver cancer and so on12,13,14.
LncRNA HOXA11-AS is an antisense strand RNA of HOXA1115. Previous studies have found that it can regulate cell proliferation, migration, invasion, and apoptosis of various tumors such oral squamous cell carcinoma, prostate cancer, glioma, skin squamous cell carcinoma and so on through a variety of signaling pathways16,17,18,19. In addition, HOXA11-AS can mediate inflammatory responses by regulating miRNA expression20. However, the mechanism of LncRNA HOXA11-AS in esophageal cancer is still unclear.
In order to explore the role of LncRNA in the occurrence and development of ESCC, this study collected 5 pairs of ESCC cancer tissues and adjacent tissues more than 5 cm from the tumor edge for RNA-seq, and screened out several LncRNA with significantly higher expression levels in ESCC cancer tissues than in adjacent tissues. The LncRNA HOXA11-AS with the highest expression was screened for subsequent study. In addition, 50 pairs of tumor tissues and adjacent tissues of ESCC patients confirmed by pathology were collected to detect whether the expression of HOXA11-AS in ESCC tumor tissues and adjacent tissues was statistically different by qRT-PCR, and further verified in ESCC cell lines. Univariate regression analysis showed that HOXA11-AS could be used as an independent prognostic predictor for survival time, TNM stage and lymph node metastasis. Lentivirus was used to construct HOXA11-AS overexpression, knockdown and negative control models. CCK-8, Transwell, wound healing assays and flow cytometry were used to detect the regulatory effect of HOXA11-AS expression level on the proliferation, migration, invasion and apoptosis of ESCC cells. Immunodeficient mice were used to detect the effect of HOXA11-AS overexpression on tumor growth in vivo.
Method
Clinical data and specimen acquisition
The inclusion criteria were as follows: patients aged over 18 years, pathologically diagnosed with ESCC, without a history of prior surgery, radiotherapy, chemotherapy, esophageal-related diseases, or other tumors. The pathological diagnosis of all patients was confirmed by three pathologists independently. The clinical information collected in this study included age, gender, smoking history, drinking history, imaging examination, serological examination, pathological diagnosis, 9th Tumor node metastasis (TNM) staging, prognosis and other related information. Detailed clinical information about all enrolled patients is summarized in Table 1.
The histological samples collected in this experiment were derived from the surgical samples of ESCC patients treated in Sichuan Provincial People’s Hospital from January 2021 to January 2025. Histologic samples contained cancer tissue and normal esophageal epithelial tissue that was more than 5 cm from the edge of the cancer (hereafter referred to as para-cancer tissue), each measuring 5 mm × 5 mm × 5 mm.
Cytological samples included human normal esophageal epithelial cells (HET-1A) and human ESCC cell lines (including ECA109, KYSE150, KYSE410 and TE-1). Among them, HET-1A, KYSE150 and KYSE410 were frozen in our laboratory. ECA109 and TE-1 were purchased from Shanghai Anwei Cell Co., LTD. This study has been approved by the ethics committee of Sichuan Provincial People’s Hospital (NO.: 2024-537), the relevant studies were conducted in strict accordance with the Declaration of Helsinki. All informed consent was obtained from the patients before all clinical information and specimen collection.
RNA extraction and reverse transcription
The weight of the histological sample was about 100 µg, and the number of cell samples was about 1 × 106 cells. All histological and cell samples were extracted using the Steady Pure Universal RNA Extraction Kit (Accurate Biotechnology Co., Ltd., Changsha, China). After extraction, RNA concentration was measured using Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA) and stored at − 80 °C. All RNA samples were reverse transcribed using Evo M-MLV RT Mix Kit with gDNA Clean for qPCR (Accurate Biotechnology Co., Ltd., Changsha, China). All reverse transcription products were stored at − 20 °C.
Polymerase chain reaction (PCR) experiments and primer design
All primers in this study were downloaded from the National Center for Biotechnology Information (NCBI) and synthesized by Qingke Biological Co., Ltd. (Beijing, China) for amplification of LncRNA HOXA11-AS. GAPDH was used as an internal control for all PCR experiments in this study. The sequences of all primers are listed in Table 2. The SteadyPure Universal RNA Extration Kit (Accurate Biotechnology Co., Ltd., Changsha, China) was used to prepare PCR experiments in this study. The QuantStudio1 Plus real-time PCR instrument (Thermo Fisher Scientific, Waltham, MA, USA) was used for the experiment. The PCR reaction system and reaction conditions are listed in Table 3. The 2−ΔΔT method was used to calculate the relative expression level of HOXA11-AS.
Construction of overexpression and knockdown models and screening of stable transfection strains
The inactivated adenovirus was used to construct overexpression and knockdown models. The overexpression/knockdown plasmids were packaged into lentivirus and stored at − 80 °C for later use. All the lentivirus solutions and infection enhancement solutions (including A and P solutions) used in this experiment were purchased from Shanghai Jikai Gene Biological Co., LTD. Pre-experiments were carried out according to different cell concentrations (1 × 105cells, 5 × 104cells, 1 × 104cells) and lentivirus concentrations (1 × 108 mmol/l, 5 × 108 mmol/l, 1 × 109 mmol/l). The optimal reaction conditions (the lowest lentivirus concentration and cell concentration that could achieve the target fluorescence intensity) were selected for formal experiments. Transfection efficiency was determined by observing GFP fluorescence under fluorescence microscope 72 h after formal infection of lentivirus. The selection of stable transfected strains was completed by puromycin assay. Different drug concentration gradients (0–10 µl/ml) were set, and the lowest drug concentration that could kill all cells was selected for formal test. Drug was added 72 h after lentivirus transfection. After 7 days of co-culture, the cell growth was observed and the overexpression/knockdown efficiency was verified by PCR assay.
Cell proliferation assay
CCK-8 assay was used to verify the effect of HOXA11-AS on ESCC cell proliferation. 1000 cells were seeded in each well, and 90 µl of complete medium and 10 µl of CCK-8 reagent (Biosharp, Anhui, China) were added to 96-well plates at 0, 24, 48, and 72 h after co-culture for 1 h, and the optical density (OD) was measured at 450 nm wavelength.
Migration and invasion assays
Transwell and wound healing assays were used to verify the effect of HOXA11-AS on the migration and invasion of ESCC cells. Serum-free medium was used 24 h before Transwell migration assay, and 5 × 105cells were seeded in the upper chamber. 400 µl serum-free medium was added to the upper chamber, and 600 µl 20% FBS medium was added to the lower chamber for another 24 h. 4% paraformaldehyde (Biosharp, Anhui, China) was used to fix the cells, and 0.1% crystal violet stain (Biosharp, Anhui, China) was used to stain the cells. The cells were observed under a microscope and counted using ImageJ. Wound healing assay was used to verify the migration ability of HOXA11-AS. After the 6-well plate was covered with cells, a 1 ml bullet tip (Biosharp, Anhui, China) was used to scratch vertically, and the cell migration was observed under a microscope and photographed at 0, 12, and 24 h. Transwell assay for invasion was basically the same as the migration assay, except that the cell-like membrane (80 µl Matrigel matrix gel + 640 µl serum-free medium) was prepared before the cells were seeded.
Apoptosis assay
Flow cytometry was used to verify the effect of HOXA11-AS on ESCC cell apoptosis. Annexin V-APC-PI apoptosis kit (Procell Life Science & Technology Co., Lid. Wuhan, China) was used for all flow cytometry experiments in this study. 1 × 106 cells were collected in a flow tube (BD falcon, American) and centrifuged at 300 × g for 5 min. After discarding the supernatant, the cells were resuspended in 1× PBS buffer, centrifuged again and discarding the supernatant. Cells were resuspended in 100 µl of diluted 1× Annexin V Binding Buffer. 2.5 µl of Annexin V-APC staining solution was added 15 min before loading, and PI staining solution was added 5 min before loading, and the full version was incubated in the dark. Before loading, 400 µl of diluted 1× Annexin V Binding Buffer was added and immediately detected in a flow cytometer (JIMBIO iCytal S2, Jiangsu Zhuo Microbial Technology Co., LTD).
Subcutaneous tumor xenograft in nude mice
In this study, T cell-immunodeficient BALB/c-nu mice were used for tumor-bearing experiments. All BALB/c-nu mice were purchased from Hunan Slaike Jingda Experimental Animal Co., LTD. All animal experiments included in this study received ethical approval by the ethics committee of Sichuan Provincial People’s Hospital (NO.: 2024-537), and were performed in accordance with ARRIVE guidelines 2.0. SPF BALB/c-nu mice aged 4–6 weeks and weighing between 16–20 g were selected and kept at 26–28 °C and 50% humidity. No less than 1 × 106cells of the experimental group (overexpression group) and the control group were implanted into the armpit of the bilateral anterior lower limbs of mice, respectively. The tumor diameter was detected by vernier caliper every 3 days, and the tumor volume was measured by in vivo fluorescence microscope every 7 days and photographed. Before measurement, the BALB/c-nu mice were anesthetized with isoflurane inhalation using the anesthesia system included with the in vivo fluorescence imager. Before the tumor volume reached the ethical critical value (tumor diameter less than 2.0 cm), the mice were sacrificed by neck mutilation. Bilateral tumor tissues were removed, and the excess tissue on the surface was removed and weighed and recorded.
RNA-sequencing
Five pairs of ESCC tissues and adjacent tissues were collected and used for LncRNA detection. RNA-Sequencing was used to detect the differentially expressed LncRNA in ESCC tissues and adjacent tissues. Sequencing quality was assessed using FastQC, RNA expression was quantified using HTSeq, and the R was used for volcano mapping.
Statistical analysis
SPSS20.0 statistical software was used for data processing. Independent t test was used to compare the experimental group and the control group. One-Way ANOVA was used for comparison among multiple groups. A two-sided P < 0.05 was considered statistically significant. ImageJ 2.14.0 was used for cell counting and scratch area calculation. GraphPad8.3.0 and R were used for illustration. All the data were strictly verified for the core assumptions of parametric tests (including normality and homogeneity) before undergoing statistical analysis, and were compiled in Supplementary File 1.
Results
HOXA11-AS was screened out and its high expression in ESCC was preliminarily verified
Five pairs of ESCC tissues and para-cancer tissues were collected for RNA-Seq, and LncRNA whose expression levels in cancer tissues were more than 2 times higher than that in adjacent tissues were selected for analysis and visual analysis (Fig. 1A). The results suggested several LncRNA were highly expressed in ESCC tissues. 5 pairs of ESCC tissues and para-cancer tissues were collected for qRT-PCR experiments for further verification. According to the expression level, Blast detection was performed to exclude LncRNA with many alternative splicing isoforms and difficult to design primers with high specificity, and the top five LncRNA with high expression levels were finally selected (Table 4). The LncRNA with the highest relative expression, namely HOXA11-AS, was selected for subsequent experiments.
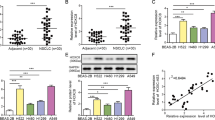
The clinical value of screening HOXA11-AS and the construction of overexpression and knockdown models. (A) The volcano plot screened high and low expression lncRNA from 5 pairs of ESCC cancer tissues and para-cancer tissues; (B) 50 pairs of ESCC tissues and para-cancer tissues were collected to detect the relative expression level of HOXA11-AS; (C) expression levels of HOXA11-AS in ESCC cell lines (ECA109, KYSE150, TE-1, KYSE410, TE-3) and normal esophageal epithelial cell line (HET-1A); ROC curve visualized the diagnostic efficacy of HOXA11-AS in outcome, TNM, age, gender, tumor location, differentiation and lymph node metastasis(D); construction of overexpression models(E) and knockdown models(I) of ECA109, KYSE150 and TE-1 and verification of the relative expression level of HOXA11-AS(F–H, J–L). ns: not significant (P > 0.05); *:P < 0.05; ***: P < 0.001; ****: P < 0.0001.
A total of 50 pairs of ESCC tissues and adjacent tissues were used to detect the expression of HOXA11-AS. qRT-PCR results showed that the relative expression level of HOXA11-AS in ESCC cancer tissues was 2.832 higher than that in para-cancer tissues (P < 0.0001, 95%CI: 2.359–3.305) (Fig. 1B). The ordinary one-way ANOVA revealed a statistically significant difference in HOXA11-AS relative expression levels among the six cell lines (F(5, 12) = 57.23, P < 0.0001, R2 = 0.9598) (Fig. 1C). Post-hoc comparisons demonstrated significantly elevated expression in all five ESCC cell lines(ECA109, KYSE150, TE-1, KYSE410, ECA-109, KYSE150, TE-3) relative to the normal esophageal epithelial cell line (HET-1A): ECA109: 4.230-fold increase (P < 0.0001, 95% CI: 3.323–5.137), KYSE150: 3.727-fold increase (P < 0.0001, 95% CI: 2.820–4.634), TE-1: 2.673-fold increase (P < 0.0001, 95% CI: 1.766–3.580), KYSE410: 1.127-fold increase (P = 0.0144, 95% CI: 0.220–2.034), TE-3: 1.043-fold increase (P = 0.0231, 95% CI: 0.137–1.950).
HOXA11-AS as an independent predictor of ESCC prognosis and TNM staging
To further clarify the effect of HOXA11-AS on TNM staging and prognosis of ESCC patients, relevant information of 50 ESCC patients in 3.1 was collected for univariate analysis. ROC curve (Fig. 1D) showed that the expression level of HOXA11-AS was correlated with the prognosis of ESCC patients (AUC: 0.792; 95%CI: 0.670–0.915) and TNM staging (AUC: 0.816; 95%CI: 0.700–0.816). Suggesting that HOXA11-AS may be an independent predictor of ESCC prognosis and TNM staging.
Construction of HOXA11-AS overexpression and knockdown models
Cell lines with the top three relative expression levels of HOXA11-AS (ECA109, KYSE150, TE-1) were selected for the construction of overexpression (lv-HOXA11-AS), knockdown (sh-HOXA11-AS) models and negative control (lv-NC/sh-NC). GFP fluorescence indicated that the transfection was successful (Fig. 1E, I). The results of PCR experiment showed that the expression of HOXA11-AS in the lv-HOXA11-AS group in ECA109, KYSE150, and TE-1 was 849.3 (P < 0.0001, 95% CI: 742.3–956.3),882.2 (P < 0.0001, 95% CI: 777.1–987.3), and 790.3 (P < 0.0001, 95% CI: 697.6–883.1) times of that in the control group, respectively (Fig. 1F–H), indicating that the overexpression model was successfully constructed. The expression levels of HOXA11-AS in the Sh-HOXA11-AS group were 0.31 (P < 0.001, 95% CI: 0.023–0.4427), 0.41 (P < 0.001, 0.0878–0.5332), and 0.24 (P < 0.001, 95% CI: 0.0034–0.4699) of the sh-NC group, respectively (Fig. 1J–L), suggesting that the knockdown model was successfully constructed. In addition, there was no significant difference in the expression of HOXA11-AS between lv-NC group, sh-NC group and control group in the three cell lines (P > 0.05). The overexpression, knockdown and negative control models constructed in Sect. “Construction of HOXA11-AS overexpression and knockdown models” were used for subsequent functional experiments.
HOXA11-AS up-regulates the proliferation of ESCC cells
The effect of HOXA11-AS on ESCC cell proliferation was detected by CCK-8 method, and the relative cell proliferation rate was calculated by OD change. 24 h after inoculation, the relative proliferation rate of lv-HOXA11-AS in ECA109 was 40.13% higher than that of lv-NC (P = 0.087, 95%CI: − 0.7099 to − 0.0928). The difference increased to 139.8% (P < 0.0001, 95%CI = − 1.706 to − 1.089) at 48 h and 165% (P < 0.0001, 95%CI = − 1.959 to − 1.341) at 72 h (Fig. 2A). The change trend of KYSE150 and TE-1 was the same as that of ECA109 (Fig. 2B, C). AS for knockdown group, the cell proliferation rate of sh-HOXA11-AS was 54.57% lower than that of sh-NC after 48 h (P < 0.01, 95%CI: 0.1348–0.9566), and the difference increased to 125.2% after 72 h (P < 0.0001, 95%CI:0.8413–1.663) and became more evident over time (Fig. 2D). The change trend in KYSE150 and TE-1 was the same as that in ECA109 (Fig. 2E, F). CCK-8 results showed that overexpression of LncRNA HOXA11-AS promoted the proliferation of ESCC cells, while knockdown of LncRNA HOXA11-AS inhibited the proliferation of ESCC cells.
HOXA11-AS regulates ESCC cell proliferation. In ECA109(A), KYSE150(B), TE-1(C) cell lines, the relative proliferation rate of lv-HOXA11-AS group was higher than that of lv-NC group after 24, 48, and 72 h. In ECA109(D), KYSE150(E), TE-1(F) cell lines, the relative proliferation rate of sh-HOXA11-AS group was higher than that of sh-NC group after 24, 48, and 72 h. ns: not significant (P > 0.05); *:P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001.
HOXA11-AS up-regulates the migration and invasion of ESCC cells
Transwell and wound healing assays were used to verify the effect of HOXA11-AS on the migration and invasion of ESCC cells. Transwell assay showed that 24 h after cell inoculation, different numbers of cells in the experimental group and the control group had passed through the chamber membrane (Fig. 3A, D). For migration, the results showed that the number of transmembrane cells in the lv-HOXA11-AS group was significantly higher than that in the lv-NC group, and the number of transmembrane cells in the sh-HOXA11-AS group was significantly lower than that in the sh-NC group in the three cell lines (Fig. 3B, C). For invasion assay, the cells across the class membrane were counted, and the results showed the same trend as the transwell for migration assay (Fig. 3E, F). These results suggest that high expression of HOXA11-AS promotes ESCC cell migration and invasion, while low expression of HOXA11-AS inhibits ESCC cell migration and invasion.
Transwell and wound healing assays were used to verify the regulatory effect of HOXA11-AS on ESCC migration and invasion. Photographs of each group under microscope, including transwell assays for invasion(A) and migration(D) and wound healing assays for ECA109(G), KYSE150(J), and TE-1(M) cell lines; the relative transmembrane number and the relative mobility rate of lv-HOXA11-AS/lv-NC/sh-HOXA11-AS/sh-NC in the transwell for invasion(B, C) and migration(E, F) and the wound healing assay(H, I, K, L, N, O), respectively. ns: not significant (P > 0.05); *:P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001.
Wound healing assays for migration test indicated that the experimental group and the control group had different degrees of cell migration movement 12 h after the start of the experiment (Fig. 3G, J, M). The area of cell migration at different time points was calculated to calculate the relative migration rate. The results suggested that the relative mobility of cells in the lv-HOXA11-AS group in KYSE150 and TE-1 cell lines was significantly higher than that in the lv-NC group after 12 h, and the relative mobility of cells in the sh-HOXA11-AS group in the three cell lines was significantly lower than that in the sh-NC group (Fig. 3H, K, N). The above differences were observed in all three cell lines after 24 h (Fig. 3I, L, O).
These results indicate that overexpression of HOXA11-AS can up-regulate the migration and invasion ability of ESCC cells, while inhibition of HOXA11-AS expression can down-regulate the migration and invasion ability of ESCC cells.
HOXA11-AS inhibited early apoptosis of ESCC cells
Flow cytometry was used to examine the effect of HOXA11-AS on ESCC cell apoptosis (Fig. 4A, C, E). The early apoptosis rate and late apoptosis rate of the experimental group and the control group were calculated, and the visual analysis was performed. For late apoptosis rate, lv-HOXA11-AS was higher than lv-NC 9.123% (P = 0.0002, 95%CI: 5.676–12.57), and sh-HOXA11-AS was lower than sh-NC 4.890% (P = 0.0015, 95%CI: 2.345–7.435) in ECA109(Fig. 4B). In KYSE150, lv-HOXA11-AS was 3.148% higher than lv-NC (P = 0.0040, 95%CI: 1.226–5.070), sh-HOXA11-AS was 11.23% lower than sh-NC (P = 0.0009, 95%CI: 5.877–16.59) (Fig. 4D). In TE-1, lv-HOXA11-AS was 6.863% higher than lv-NC (P < 0.0001, 95%CI: 4.653–9.073), and sh-HOXA11-AS was 12.76% lower than sh-NC (P = 0.0003, 95%CI: 7.568–17.95) (Fig. 4F). However, for early apoptosis, there was no statistically significant difference in each cell line, so we determined that HOXA11-AS had no definite effect on early apoptosis of ESCC (Fig. 4B, D, F). Flow cytometry showed that overexpression of HOXA11-AS inhibited ESCC cells early apoptosis, while knockdown of HOXA11-AS promoted ESCC cells early apoptosis. However, it has no significant effect on the late apoptosis of ESCC cells.
Flow cytometry detected the effect of HOXA11-AS on the apoptosis of ESCC. The apoptosis of lv-NC/lv-HOXA11-AS/sh-NC/sh-HOXA11-AS in ECA109(A), KYSE150(C) and TE-1(E) was measured by flow cytometry. The early and late apoptosis rates (B, D, F) of cells in each group were used to verify the role of HOXA11-AS in ESCC cell apoptosis. ns: not significant (P > 0.05); **: P < 0.01; ***: P < 0.001; ****: P < 0.0001.
HOXA11-AS promotes ESCC tumor proliferation in vivo
After completing the in vitro cell experiments on the functional effects of HOXA11-AS on ESCC, we further implemented in vivo cell experiments. On day 24, the mice were sacrificed and the tumors were removed. The tumor weight (Fig. 5A) and volume (Fig. 5B) were measured and photographed (Fig. 5C). After 24 days, the tumor weight of lv-HOXA11-AS group in ECA109, KYSE150 and TE-1 was 0.1176 g (P = 0.0462, 95%CI: 0.0018–0.2334), 0.2295 g (P = 0.0004, 95%CI: 0.1137–0.3453) and 0.2060 (P = 0.0010, 95%CI: 0.0902–0.3218) higher than that of lv-NC group, respectively. After 24 days, the tumor volumes of the lv-HOXA11-AS group in ECA109, KYSE150, and TE-1 were 264.9mm2 (P = 0.0004, 95%CI: 133.3–396.4), 82.48 mm2 (P = 0.0002, 95%CI: 68.45–96.51) and 381.1mm2 (P < 0.0001, 95%CI: 249.6–512.7) larger than those of the lv-NC group, respectively. In terms of fluorescence intensity, the difference between the lv-HOXA11-AS group and the lv-NC group in ECA109 (P = 0.0041), KYSE150 (P < 0.0001) and TE-1 (P = 0.0384) began to be statistically significant on the 18th day, and the difference became more obvious over time (Fig. 5D–F). These results suggest that overexpression of HOXA11-AS promotes tumor growth in ESCC in vivo, and the promotion effect may have a cumulative effect.
Subcutaneous tumor xenograft in nude mice. Tumor weight (A) and tumor volume (B), and tumor diameter(C) of lv-HOXA11-AS and lv-NC in ECA109, KYSE150, and TE-1 cell lines 24 days after tumor inoculation; changes in tumor fluorescence intensity measured by vivo fluorescence microscopy(D–F). *:P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001.
Discussion
In this study, 5 pairs of ESCC tissues and para-cancer tissues were collected for RNA-Seq, and the LncRNA with the highest expression, HOXA11-AS, was further studied. 50 pairs of ESCC tissues and para-cancer tissues, 5 ESCC cell lines and 1 normal esophageal epithelial cell line to fully verified the high expression of HOXA11-AS in ESCC. The relationships between HOXA11-AS and ESCC patient outcome, TNM stage, age, gender, location, differentiation and lymph node metastasis were analyzed. It was found that HOXA11-AS could be used as an independent predictor of ESCC patient outcome and TNM stage. In addition, in vitro and in vivo experiments were carried out to further explore the role of HOXA11-AS in the tumorigenesis and progression of ESCC. Overexpression(lv-HOXA11-AS), knockdown(sh-HOXA11-AS) and negative control models(lv-NC/sh-NC) were constructed in ECA-109, KYSE150 and TE-1 cell lines, and the related cell phenotypes were verified. The results showed that overexpression of HOXA11-AS promoted the proliferation, migration and invasion of ESCC cells, and inhibited the late apoptosis of ESCC cells, while knockdown of HOXA11-AS reversed the above effects. In vivo experiments were further carried out in BALB/c-nu mice. The experimental results showed that overexpression of HOXA11-AS promoted ESCC tumor proliferation in vivo. This study demonstrated the high expression of HOXA11-AS in ESCC, revealing the strong predictive role of HOXA11-AS as an oncogene in ESCC outcome and TNM staging. Furthermore, HOXA11-AS as a new biomarker and prognostic predictor of ESCC has been demonstrated to promote cell proliferation, migration and invasion, apoptosis in vitro, and tumorigenesis in vivo.
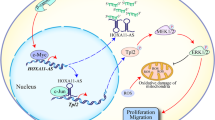
The treatment approach for ESCC has shifted from endoscopic treatment and surgical treatment to the molecular mechanism level21. The in-depth study of LncRNA has unveiled this mystery. As a branch of non-coding RNA, LncRNA plays a role in multiple physiological processes including transcription, epigenetic modification, protein /RNA stability, translation and post-translational modification by interacting with DNA22,23,24. In addition, LncRNA such as LINC00680, SNHG16 can regulate the occurrence and development of ESCC by interacting with downstream proteins, related signaling pathways and miRNAs25,26. HOXA11-AS has regulatory effects on the occurrence and development of various malignant tumors, such as non-small cell lung cancer, osteosarcoma, uveal melanoma, glioma15. Studies have shown that its mechanism of action may include acting as a ceRNA to competitively inhibit miRNA, acting as a nuclear scaffold to recruit transcription factors, forming a signal pathway axis with miRNA and downstream proteins, regulating EMT and other pathways to regulate the malignant progression of tumors17,18,19,27,28,29,30. In addition, HOXA11-AS may regulate the inflammatory response by promoting the secretion of pro-inflammatory factors interleukin-1β, interleukin-6, and tumor necrosis factor-α20. Our research is the first to apply LncRNA HOXA11-AS to study the occurrence and development of ESCC, and it is also the first study of HOXA11-AS in an in vivo xenograft experiment of ESCC. We have confirmed that HOXA11-AS, as a cancer-promoting gene, exerts a promoting effect on various phenotypes of ESCC, including cell proliferation, migration and invasion, and apoptosis. Furthermore, we also found that knocking down HOXA11-AS might result in a slower progression of ESCC compared to ESCC cells that have not undergone any treatment. This suggests that HOXA11-AS may serve as a potential therapeutic target for ESCC. To explain this phenomenon, we reviewed relevant literature and found that LncRNAs usually act as ceRNAs, competitively inhibiting miRNAs and causing downstream functional changes13,25,31. We believe the possible mechanism is that when HOXA11-AS expression is significantly inhibited, its competitive inhibitory effect is relieved, allowing a large number of miRNAs to rapidly release, thereby exerting a significant inhibitory effect on ESCC development. This rapid release of miRNAs may lead to a stronger tumor-suppressing effect than in untreated ESCC cells. The limitation of this study is that the mechanism of HOXA11-AS in ESCC was not thoroughly investigated. By reviewing the relevant literature, this study made the following prospects. HOXA11-AS has been shown to bind to EZH2 in gastric cancer32 and uveal melanoma33, and up-regulate Cyclin expression to regulate cell cycle in human skin cancer19 and Wilms tumor34. Therefore, the role of HOXA11-AS in ESCC cell proliferation may also be explored through EZH2- and Cylin-related pathways in the same gastrointestinal cancer and squamous cell carcinoma. The study of downstream mechanisms can collect fresh tissue samples for cell cycle-related analysis, which may further clarify the specific proliferation regulatory mechanism of HOXA11-AS in ESCC. The therapeutic potential of natural polyphenolic compounds for ESCC mainly operates at the microscopic level of epigenetic regulation, while also coordinating the regulation of signaling pathways and cell cycle progression35. Perhaps by studying the interaction between HOXA11-AS and polyphenolic compounds, new potential therapeutic targets can be identified. For migration and invasion, HOXA11-AS has been confirmed as a ceRNA, which promotes the migration and invasion of tumor cells by regulating the expression of Integrin in patients with melanoma36, prostate cancer37, and gastric cancer38. Therefore, for ESCC, we can also start from the integrin-related pathways to study. Furthermore, LncRNA acts as a ceRNA to remodel the cell cytoskeleton and phosphorylate the movement proteins to upregulate their movement functions25, or promote glycolysis for migration functions, or reduce cell adhesion by promoting EMT39. We believe that the regulation of ESCC’s sugar metabolism reprogramming by LncRNA may be an important research direction. As for cell apoptosis, this study confirmed that HOXA11-AS exerted an inhibitory effect on late apoptosis of ESCC cells, which was closely related to Caspase protein pathway, DNA methylation and ER stress pathways40,41. The methylation correlation analysis of HOXA11-AS may be beneficial to the exploration of downstream mechanisms. We believe that HOXA11-AS may play a central role in ESCC, being both an epigenetic regulatory factor and a ceRNA. It may exert regulatory effects on various downstream proteins through the classic HOXA11-AS-miRNA-protein regulatory network and at the transcriptional level.
Conclusion
This study found that LncRNA HOXA11-AS is highly expressed in ESCC and can be used as an independent predictor of prognosis and TNM staging of ESCC patients. As a tumor promoter, HOXA11-AS promoted ESCC cell proliferation, migration, invasion, apoptosis and tumor growth in vivo. The above effects could be reversed by knocking down its expression. It is suggested that HOXA11-AS may play an important role in the occurrence and development of ESCC as a new biomarker and therapeutic target.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74(3), 229–263. https://doi.org/10.3322/caac.21834 (2024).
Zhou, M. et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 394(10204), 1145–1158. https://doi.org/10.1016/S0140-6736(19)30427-1 (2019).
Ajani, J. A. et al. Esophageal and esophagogastric junction cancers, version 1.2015. J. Nat. Compr. Cancer Netw. 13(2), 194–227. https://doi.org/10.6004/jnccn.2015.0028 (2015).
Cao, W. et al. Multi-faceted epigenetic dysregulation of gene expression promotes esophageal squamous cell carcinoma. Nat. Commun. 11(1), 3675. https://doi.org/10.1038/s41467-020-17227-z (2020).
Tan, Y. et al. LncRNA-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun. 41(2), 109–120. https://doi.org/10.1002/cac2.12108 (2021).
Herman, A. B., Tsitsipatis, D. & Gorospe, M. Integrated LncRNA function upon genomic and epigenomic regulation. Mol. Cell 82(12), 2252–2266. https://doi.org/10.1016/j.molcel.2022.05.027 (2022).
Ponting, C. P., Oliver, P. L. & Reik, W. Evolution and functions of long noncoding RNAs. Cell 136(4), 629–641. https://doi.org/10.1016/j.cell.2009.02.006 (2009).
Liu, J. et al. Long noncoding RNA POU6F2-AS2 is associated with oesophageal squamous cell carcinoma. J. Biochem. 160(4), 195–204. https://doi.org/10.1093/jb/mvw025 (2016).
Tong, Y. S. et al. Association of decreased expression of long non-coding RNA LOC285194 with chemoradiotherapy resistance and poor prognosis in esophageal squamous cell carcinoma. J. Transl. Med. 12(1), 233. https://doi.org/10.1186/s12967-014-0233-y (2014).
Li, Z. et al. Long noncoding RNA MALAT 1 affects the efficacy of radiotherapy for esophageal squamous cell carcinoma by regulating Cks1 expression. J. Oral Pathol. Med. 46(8), 583–590. https://doi.org/10.1111/jop.12538 (2017).
Wang, W. et al. Long noncoding RNA MALAT1 promotes malignant development of esophageal squamous cell carcinoma by targeting β-catenin via Ezh2. Oncotarget 7(18), 25668–25682. https://doi.org/10.18632/oncotarget.8257 (2016).
Cao, H. L., Liu, Z. J., Huang, P. L., Yue, Y. L. & Xi, J. N. LncRNA-RMRP promotes proliferation, migration and invasion of bladder cancer via miR-206. Eur. Rev. Med. Pharmacol. Sci. 23(3), 1012–1021. https://doi.org/10.26355/eurrev_201902_16988 (2019).
Lin, X. et al. LncRNA ITGB8-AS1 functions as a ceRNA to promote colorectal cancer growth and migration through integrin-mediated focal adhesion signaling. Mol. Ther. 30(2), 688–702. https://doi.org/10.1016/j.ymthe.2021.08.011 (2022).
Yuan, K. et al. Long noncoding RNA TLNC1 promotes the growth and metastasis of liver cancer via inhibition of p53 signaling. Mol. Cancer. 21(1), 105. https://doi.org/10.1186/s12943-022-01578-w (2022).
Lu, C. et al. HOXA 11 antisense long noncoding RNA (HOXA 11-AS): A promising lnc RNA in human cancers. Cancer Med. 7(8), 3792–3799. https://doi.org/10.1002/cam4.1571 (2018).
Cheng, Y. et al. LncRNA HOXA11-AS promotes cell growth by sponging miR-24-3p to regulate JPT1 in prostate cancer. Eur. Rev. Med Pharm. Sci. 25(14), 4668–4677. https://doi.org/10.26355/eurrev_202107_26377 (2021).
Wei, C. et al. LncRNA HOXA11-AS promotes glioma malignant phenotypes and reduces its sensitivity to ROS via Tpl2-MEK1/2-ERK1/2 pathway. Cell Death Dis. 13(11), 942. https://doi.org/10.1038/s41419-022-05393-5 (2022).
Niu, X., Yang, B., Liu, F. & Fang, Q. LncRNA HOXA11-AS promotes OSCC progression by sponging miR-98-5p to upregulate YBX2 expression. Biomed. Pharm. 121, 109623. https://doi.org/10.1016/j.biopha.2019.109623 (2020).
Wang, J., Li, X., Li, H. & Li, X. LncRNA HOXA11-AS regulates the proliferation and epithelial to mesenchymal transition of human skin cancer cells. 3 Biotech. 11(1), 12. https://doi.org/10.1007/s13205-020-02557-y (2021).
Li, X. L., Wang, B., Yang, F. B., Chen, L. G. & You, J. HOXA11-AS aggravates microglia-induced neuroinflammation after traumatic brain injury. Neural Regen. Res. 17(5), 1096. https://doi.org/10.4103/1673-5374.322645 (2022).
Niu, C. et al. Endoscopic full-thickness plication for the treatment of gastroesophageal reflux disease: A systematic review and meta-analysis. Endosc. Int. Open 12(02), E199–E210. https://doi.org/10.1055/a-2231-7136 (2024).
Bridges, M. C., Daulagala, A. C. & Kourtidis, A. LNCcation: LncRNA localization and function. J. Cell Biol. 220(2), e202009045. https://doi.org/10.1083/jcb.202009045 (2021).
Schmidt, K. et al. Targeting the oncogenic long non-coding RNA SLNCR1 by blocking Its sequence-specific binding to the androgen receptor. Cell Rep. 30(2), 541-554.e5. https://doi.org/10.1016/j.celrep.2019.12.011 (2020).
Zealy, R. W. et al. Long noncoding RNA complementarity and target transcripts abundance. Biochim. Biophys. Acta BBA Gene Regul. Mech. 1861(3), 224–234. https://doi.org/10.1016/j.bbagrm.2018.02.001 (2018).
Xue, S. T. et al. Long non-coding RNA LINC00680 functions as a ceRNA to promote esophageal squamous cell carcinoma progression through the miR-423-5p/PAK6 axis. Mol. Cancer 21(1), 69. https://doi.org/10.1186/s12943-022-01539-3 (2022).
Ren, L. et al. LncRNA SNHG16 promotes development of oesophageal squamous cell carcinoma by interacting with EIF4A3 and modulating RhoU mRNA stability. Cell. Mol. Biol. Lett. 27(1), 89. https://doi.org/10.1186/s11658-022-00386-w (2022).
Xu, Y., Ren, Z., Wang, X. & Ren, M. The LncRNA HOXA11-AS acts as a tumor promoter in breast cancer through regulation of the miR-125a-5p/TMPRSS4 axis. J. Gene Med. 24(5), e3413. https://doi.org/10.1002/jgm.3413 (2022).
He, Y. & Qiu, X. Suppression of LncRNA HOXA11-AS/miR-124 axis inhibits glioma progression. Cell Biochem. Biophys. 79(4), 815–822. https://doi.org/10.1007/s12013-021-01007-7 (2021).
Guo, T., Yuan, X., Liu, D. F., Peng, S. H. & Xu, A. M. LncRNA HOXA11-AS promotes migration and invasion through modulating miR-148a/WNT1/β-catenin pathway in gastric cancer. Neoplasma https://doi.org/10.4149/neo_2020_190722N653 (2020).
Cheng, Y. et al. LncRNA HOXA11-AS promotes cell growth by sponging miR-24-3p to regulate JPT1 in prostate cancer. Eur. Rev. Med. Pharm. Sci. 25(14), 4668–4677. https://doi.org/10.26355/eurrev_202107_26377 (2021).
Gao, Y. et al. LncRNA PCAT1 activates SOX2 and suppresses radioimmune responses via regulating cGAS/STING signalling in non-small cell lung cancer. Clin. Transl. Med. 12(4), e792. https://doi.org/10.1002/ctm2.792 (2022).
Liu, Z. et al. Over-expressed long noncoding RNA HOXA11-AS promotes cell cycle progression and metastasis in gastric cancer. Mol. Cancer. 16(1), 82. https://doi.org/10.1186/s12943-017-0651-6 (2017).
Lu, Q. et al. LncRNA HOXA11-AS exerts oncogenic functions by repressing p21 and miR-124 in uveal melanoma. DNA Cell Biol. 36(10), 837–844. https://doi.org/10.1089/dna.2017.3808 (2017).
Zhu, S. et al. Long non-coding RNA HOXA11-AS upregulates cyclin D2 to inhibit apoptosis and promote cell cycle progression in nephroblastoma by recruiting forkhead box P2. Am. J. Cancer Res. 10(1), 284–298 (2020).
Niu, C., Zhang, J. & Okolo, P. I. Unlocking the therapeutic potential of natural polyphenols in esophageal cancer. Curr. Treat. Options Oncol. 26(4), 278–290. https://doi.org/10.1007/s11864-025-01308-6 (2025).
Xu, Y., Zhang, J., Zhang, Q., Xu, H. & Liu, L. Long non-coding RNA HOXA11-AS modulates proliferation, apoptosis, metastasis and EMT in cutaneous melanoma cells partly via miR-152-3p/ITGA9 axis. CMAR 13, 925–939. https://doi.org/10.2147/CMAR.S281920 (2021).
Misawa, A., Kondo, Y., Takei, H. & Takizawa, T. Long noncoding RNA HOXA11-AS and transcription factor HOXB13 modulate the expression of bone metastasis-related genes in prostate cancer. Genes 12(2), 182. https://doi.org/10.3390/genes12020182 (2021).
You, L. et al. The long non-coding RNA HOXA11-AS activates ITGB3 expression to promote the migration and invasion of gastric cancer by sponging miR-124-3p. Cancer Cell Int. 21(1), 576. https://doi.org/10.1186/s12935-021-02255-6 (2021).
Ge, X. et al. HOTAIR, a prognostic factor in esophageal squamous cell carcinoma, inhibits WIF-1 expression and activates W nt pathway. Cancer Sci. 104(12), 1675–1682. https://doi.org/10.1111/cas.12296 (2013).
Priestley, P. et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature 575(7781), 210–216. https://doi.org/10.1038/s41586-019-1689-y (2019).
Martínez-Jiménez, F. et al. Pan-cancer whole-genome comparison of primary and metastatic solid tumours. Nature 618(7964), 333–341. https://doi.org/10.1038/s41586-023-06054-z (2023).
Funding
This work was supported by the Natural Science Foundation of Sichuan Province (grant No. 2022NSFSC0780).
Author information
Authors and Affiliations
Contributions
Conceptualization, Wanwen Li and Bin He; methodology, Shenglong Xie; formal analysis, Haiqi Liu; writing—original draft preparation, Wanwen Li; writing—review and editing, Wei Li and Bin He; supervision, Shenglong Xie and Bin He; funding acquisition, Bin He.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All tissue samples, patient-related information and experimental mice collected in this study were approved by the ethics committee (NO.: 2024-537). Informed consent was obtained from the patients for all manipulations of tissue samples and analyses of their clinical information. This study was approved by the ethics committee of Sichuan Provincial People’s Hospital (NO.: 2024-537). All experimental procedures were conducted in accordance with: (a) The ARRIVE Guidelines 2.0; (b) The National Standard GB/T 35892-2018 (China); (c) The 3R Principles (Replacement, Reduction, Refinement).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, W., Xie, S., Liu, H. et al. LncRNA HOXA11-AS promotes the cell proliferation, migration, invasion and inhibits cell apoptosis in esophageal squamous cell carcinoma. Sci Rep 15, 33215 (2025). https://doi.org/10.1038/s41598-025-18534-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18534-5