Abstract
Sweet potato is considered one of the crops enriched with valuable nutritional components, and this plant might possess strongest inhibitory allelochemicals. The present work evaluated the nutritional value and the allelopathic effects of sweet potatoes on seed germination and seedlings performance of Prosopis juliflora. The findings revealed that leaves of sweet potatoes contain more calcium, magnesium, manganese, zinc, copper, protein and flavonoids than the other organs. Food exposure to direct solar positively affected the amounts of phosphorous, magnesium, zinc dry matter, Total Digestible Nutrients (TDN), while greater levels of sodium, potassium, protein, fibres, ash, and flavonoids were recorded indoor. Exposing the samples for 15 and 25 min showed higher values of phosphorous, sodium, fibres, TDN, flavonoids, and fat compared with the non-treated samples. While the values of Total Dissolved Solids (TDS), Electrical conductivity (EC) and salinity were higher in the sweet potatoes shoot water extracts, those of pH were lower. Shoot water extracts exhibited strongest inhibitory effects on the seeds and seedlings responses than the tubers. Electrolyte leakage values were greater in the seedlings treated with shoot water extracts than the tubers. The results of this study demonstrate that growing sweet potatoes in the arid regions significantly improve its nutritional values which could be beneficial for human health. Furthermore, based on the results, proper attention should be given when drying the sweet potatoes to avoid nutrients damage. The tested shoots could be used to control the proliferation and the nuisances of P. juliflora plants in the introduced range.
Similar content being viewed by others
Introduction
World population has grown spectacularly from 1 to 8 billion between 1800 and 2025, and the growth rate is expected to increase significantly in the coming years1. The consequences associated to human population growth could include environmental degradation, resource depletion, pollution increment, biodiversity loss and climate change2. As per this, it seems very important to correlate human population growth with the available natural resources to avoid food shortage and environmental degradation. With this regard, intensive research works have been carried out in the agricultural field but unfortunately, the literature review is less documented regarding the arid regions. Plant growing under arid regions might have important variations in their metabolisms than the other. Therefore, examining the nutritional value of those plants could help improving food production in those extreme geographical zones.
Ipomea batatas (L.) Lam., 1973 commonly called sweet potatoes is an herbaceous perennial vine plant belonging to Convolvulaceae family with more than 400 varieties3. I. batatas (L.) Lam., 1973 is largely cultivated in many countries around the world due to its higher nutritional and medicinal values. With this respect, sweet potatoes plant parts were reported to possess antibacterial, antifungal, anticancer, and anti-inflammatory properties4,5,6. Globally, the production of sweet potatoes is ranked number seventh after rice, maize, wheat, potato, barley and cassava7. Interestingly, this plant species is adapted to grow under various climatic conditions and therefore, the production of this crop might need less care, and less attention compared with other crops8.
Sweet potatoes plant organs are reported to be loaded with greater nutritional components which might vary from variety-to-variety, and from organ-to-organ9. Comparative studies on sweet potatoes cultivars demonstrated that white Triumph sweet potatoes with white skin contained more starch, sugars, proteins, vitamin C, and minerals than the Goldstar and Carmen Ruin cultivars10. Corroborating findings were reported on four varieties of sweet potatoes with the variety Sabe exhibiting higher amounts of water content, vitamin C than the other tested accessions11. Furthermore, sweet potatoes were found containing more fibres, sugar, calcium, vitamin B6 and vitamin A than cassava12.
Plant stage growth, plant age, plant organ-age, and plant organ-types are some of the major factors that determine the quality and quantity of the products resulting from the metabolism13,14. As per this, nutritional components of sweet potatoes can greatly vary from organ-to-organ. Leaves of sweet potatoes were reported to contain significantly higher levels of protein than the stalks, and the stem15. Unfortunately, most of the earlier works on this thematic were not addressed in the arid regions where the weather conditions are the major concerns globally.
Sweet potatoes are enriched with interesting nutritional and medicinal components that are vital for human health. However, some of those nutrients could be degraded due to the cooking and the processing methods. To illustrate this, Anwar and Ghani16 demonstrated that freeze-dried sweet potatoes samples contained significantly lower fat contents than the other treatments, while the amounts of ash were importantly higher under the same treatment. Furthermore17, found that frying method lowering the amounts of vitamin A, C, and E compared with the cooked methods. In the other works18, noticed that applying heat to sweet potatoes significantly reduced the levels of phenolic compounds compared with the other treatments. In addition, sun exposition was seen to reduce the concentrations of total carotenoids, and β-carotene than the hot air19.
To date, plant invasiveness is considered one of the biggest challenges that face environmental authorities in many countries where those plants were introduced. Invasive plants can cause native plants extinctions, biodiversity loss, water table depletion, and considerably alter animals and human health20,21. With regard to agricultural systems, invasive species detrimentally affect crops yield and the total productivity competing with crops for water, nutrients, and light22. Many exotic invasive plants are known to be recalcitrant and to do not have efficient methods to mitigate their proliferation and nuisances.
Among those plants species, exotic Prosopis juliflora commonly called mesquite is ranked in the list of the most worst 100 invasive species with significant negative effects recorded in the introduced range23. P. juliflora is native to Central and South Americas but global assessments on its distribution have showed that this plant has been introduced to around 129 countries24. More recently, the invasiveness of P. juliflora were linked to the processes of climate changes25. This plant species was introduced in those countries with the main goal to combat desertification process and stabilize the soil against natural erosion. Unfortunately, to date, P. juliflora plants have escaped experimental sites and invaded many pastoral lands21,26. Many earlier studies have tried to find out the mitigating methods that could allow to control this invasive plant species but unfortunately, up to date, this plant is still invading many lands in the introduced range27. As per this, prevention and early detection with the main focus on sustainable and community-based approaches could be more efficient to combat the invasiveness of P. juliflora plants than the other methods28.
Sweet potatoes plant organs have been reported to have strongest inhibitory effects on some of the most critical invasive plants29,30,31. Unfortunately, only few previous works have been carried out assessing the allelopathic effects of sweet potatoes on the other plants, and none of the earlier work has attempted to assess the allelopathic effects of this plant considering the exotic invader Prosopis juliflora. P. juliflora plants might have different responses when exposed to sweet potatoes plant organs, and the effects could be detrimental or beneficial. Furthermore, in most of the available published works, plant leaves are addressed to possess strongest allelopathic effects than the other plant part32,33. However, the existing literature review does not present comparative allelopathic works on sweet potatoes including the tubers and other plants parts, which might not allow to elucidate the plant organ with greater effects.
Therefore, in the current study, we attempted to examine the allelopathic effects of sweet potatoes plant parts considering shoot and tubers on the seed germination, and earlier growth of Prosopis juliflora. Plant facing detrimental allelopathic stress could face important physiological damages. Most of the previous works dealing with allelopathic studies had not much interest in addressing these issues. As per this, in our experimental works, we also tried to evaluate electrolyte leakage parameter from the tested P. juliflora seedlings, which is one of the main indicators for the stress magnitude. Furthermore, plants growing in the arid regions might interestingly adjust their metabolisms to cope with the changes occurring in their surrounding habitats. These adjustments in plants metabolism might positively affect the resulting metabolites, which could be therefore beneficial for food production. However, the literature review is relatively poor on the studies considering the nutritional components of sweet potatoes under extreme environments. Besides, nutritional components might considerably vary from plant organ-to-organ and therefore influence the quantity and the quality of each metabolite. As per this exploring nutritive value of sweet potatoes considering plant organs might allow to select the part with higher nutrient contents. Furthermore, methods of cooking and processing might have strong effects on the food quality. Therefore, in the current work, we also tried to demonstrate the impacts of cooking methods (drying and boiling) on the quality of sweet potatoes.
Materials and methods
Samples collection and Preparation
The tested plant organs (flowers, leaves, stem, and tubers) (Fig. 1) of the yellow and the white sweet potatoes cultivars were obtained from the Agricultural Farms of Fujairah Research Centre (25°32’08.4"N 56°21’23.1"E). In this research work, around 1 kg of samples were collected for the different tests.
The collected samples were washed using running tap water and rinsed thrice through distilled water. The current work was conducted in four different parts.
-
In the first experiment, we subjected the flowers, leaves, stem, and tubers to chemical analyses considering the two cultivars to see how chemical elements would vary from organ-to-organ, and between the two sweet potatoes cultivars.
-
In the second study, we tested the impacts of drying conditions (indoor and outdoor) on the chemical analyses of the tubers of the selected sweet potatoes cultivars. The non-treated sweet potatoes were considered as control group.
-
Thirdly, the two sweet potatoes cultivars were cooked at three different time periods including 0, 15, and 25 min with 0 min considered as non-treated group.
-
Fourthly, seed and seedlings of Prosopis juliflora were treated with four levels of shoot and tubers water extracts of the yellow and the white sweet potatoes to see whether these tested extracts could be used to control the proliferation, and the nuisances of P. juliflora plants in the introduced range.
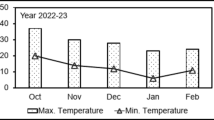
Sun drying method: Sweet potatoes were dried according to34. Briefly, the tested sweet potatoes were peeled manually and cut into small slices of 2 mm thick using the slicer, stainless steel sharp blades. The sliced sweet potatoes were allowed to dry outdoor for three consecutive days on the shelf made from steel until constant dry weight was recorded. Shelf used in this research work was delicately cleaned to avoid contamination prior the drying process. Environmental parameters including atmospheric temperatures and relative humidity were measured using the hygrometer (Anaum, AN1020, Thermo Hygrometer). The readings were performed in three periods of the day (8:00–9:00 am, 12:00–1:00 pm, and 4:00–5:00 pm) (Fig. 3) and then averaged. Indoor drying method: The other sliced sweet potatoes were dried indoor on the bench for one week under 25 °C and 40% for the ambient temperature and humidity respectively. Cooking method: The cooking process was conducted in accordance with the work of35 with slight modifications. Summarizing, the cuts sweet potatoes were cooked at 100 °C in clean water for 15 and 25 min until tender. Thereafter, the boiled samples were allowed to cool at room temperature.
Determination of nutrients and chemical analysis
Dry matter: This was conducted according to the Official Methods of Analysis (AOAC, 922.06).Ash analyses: This parameter was determined by taking 2 g of each sweet potatoes cultivar sample to the silica crucible adjusted at 600° C in muffle Furnace for 2 h (AOAC, 942.05).Fat determination: Analyses on the fat contents were performed using Soxhlet device by taking 2 g of the tested sample, refluxed with petroleum ether for 16 h, and the crude fat was subsequently obtained after evaporating the petroleum (AOAC, 920.39).Crude fibre: This parameter was estimated based on the Official Methods of Analysis (AOAC, 962.09).Tannins: The extraction was performed through titrating the extract with the standard potassium permanganate solution following the work of36.Total digestible nutrients: This element was evaluated according to the work of37.Minerals analyses: Na and K were chemically analysed as per the Association of Official Agricultural Chemists (AOAC, 969.23) whereas, P, Ca, Mg, Mn, Zn, Ni, Cu, and Se were following the Official Agricultural Chemists (AOAC, 2015.01). Heavy metals: Cadmium andlead extraction and determination were performed in accordance with (ICP-AES) (AOAC, 2015.01).Protein determination: This chemical element was obtained following the Official Methods of Analysis International, Edition 2023.
Allelopathy effects of Ipomea batatas plant organs on the seed germination and early growth of Prosopis juliflora.
-
a.
Seeds collection of Prosopis juliflora.
Full mature seeds of Prosopis juliflora were collected around the Emirate of Fujairah. To increase genetic diversification, then the tested seeds were harvested from 10 individuals separated by a distance of around 3 m. The collected seeds were air-dried, manually extracted, and stored in the paper bags for further use.
-
b.
Preparation of the sweet potatoes water extracts.
The collected shoot and tubers were cut into small pieces to facilitate the drying process and then allowed to dry at room temperature for one week. Thereafter, the dried samples were powdered. The stock solution was prepared by taking 200 g of each tested sample to the conical flask containing 1000 ml of distilled water for 24 h. Afterward, the extracts were filtered using Whatman filter paper No 1. The obtained extract was then diluted using distilled water to five concentrations: 0, 5, 10, 15, and 20%, with 0% considered as control group, and stored at 4° C for further use. The values of pH, TDS, EC, and salinity were obtained using the Hygrometer (Anaum, AN1020, Thermo Hygrometer).
-
c.
Assessments of the sweet potatoes water extracts on the seed germination and early growth of Prosopis juliflora.
For the germination test, seeds of Prosopis juliflora were surface sterilized using sodium hypochlorite 10% concentrated for 5 min, washed and rinsed thrice using distilled water. The experiment was arranged with four replicates per group and 25 seeds per dish. Thereafter, five levels of allelochemical including 0, 5, 10, 15, and 20% were applied to each dish, and the seeds were allowed to germinate in the greenhouse (25°32’08.4"N 56°21’23.1"E). To avoid water loss during the experimentations, each dish was wrapped with parafilm. The temperature and humidity were adjusted at 25 ± 2° C and 60%. 14 days post experimentations, the following measurements were performed:
-
Germination percentage (GP) \(\:=\frac{\text{N}\text{u}\text{m}\text{b}\text{e}\text{r}\:\text{o}\text{f}\:\text{g}\text{e}\text{r}\text{m}\text{i}\text{n}\text{a}\text{t}\text{e}\text{d}\:\text{s}\text{e}\text{e}\text{d}\text{s}}{\text{T}\text{o}\text{t}\text{a}\text{l}\:\text{n}\text{u}\text{m}\text{b}\text{e}\text{r}\:\text{o}\text{f}\:\text{s}\text{e}\text{e}\text{d}\text{s}\:}\).
-
Seed vigor index (SVI) = \(\:\frac{\text{S}\text{h}\text{o}\text{o}\text{t}\:\text{l}\text{e}\text{n}\text{g}\text{t}\text{h}\:\text{x}\:\text{g}\text{e}\text{r}\text{m}\text{i}\text{n}\text{a}\text{t}\text{i}\text{o}\text{n}\:\text{p}\text{e}\text{r}\text{c}\text{e}\text{n}\text{t}\text{a}\text{g}\text{e}\text{s}}{100}\).
-
Shoot length: Shoot length was determined using the measuring tape.
-
Root length: This was performed using the measuring tape.
-
Seedlings dry weight: Experimental seedlings were washed, kept in the paper bags, and then dried in the oven adjusted at 70° C until constant dry weight.
-
Electrolyte leakage: This parameter was determined following the work of14.
-
Allelopathy index: Allelopathy index allows to determine the types of responses which could be positive, negative or neutral through the following equations20.
AI = 1 – C/T (T ≥ C) (1) Stimulatory or neutral allelopathic effects.
AI = T/C – 1 (T < C) (2) Inhibitory allelopathic effects.
In the equations, C is the control group, and T is the treatment.
The full dataset associated to the current work is available in the Supplementary Materials Dataset.
Data analysis
In this work, the analyses were performed with three replicates considering nutritional assessments, and electrolyte leakage analyses, while four replicates were emphasized for the allelopathic effects on the seed germination and seedlings performance of Prosopis juliflora. Furthermore, three replicates were also considered to assess the chemical analyses of the tested sweet potatoes plant organs water extracts, and the concentrations. Two-way ANOVA (analyses of variance) were conducted to test the effects of plant organs and sweet potatoes cultivars on the nutritional value Ipomea batatas. Furthermore, two-way ANOVA were also performed to evaluate the impacts of drying methods and sweet potatoes cultivar on the chemical parameters of I. batatas. Moreover, two-way ANOVA were also carried out to estimate the effects of cooking time and sweet potatoes cultivars on the chemical analyses of I. batatas. In addition, two-way ANOVA were also carried out to evaluate the effects of sweet potatoes plant organ extract and concentrations on the chemicals analyses of the tested extracts, seed germination and seedlings attributes, and electrolyte leakage parameter of P. juliflora. Pearson correlation matrix was conducted to estimate the associational effects between the seed germination and the seedlings attributes of P. juliflora and the chemical variables of the tested extracts and the electrolyte leakage. Tukey test (Honestly significant differences, HSD) was performed to identify significant differences between the means, and all the data were statically analysed through SYSTAT 13.0.
Results
Impacts of plant organs (flowers, leaves, stem, and tubes) and sweet potato cultivar (yellow and white) on the mineral composition of Ipomea batatas.
Plant organs and sweet potato cultivars had significant effects on the mineral composition of Ipomea batatas (Fig. 3; Table 1). Overall, phosphorous (76.54 mg/100 g) amounts were greater in the flower of the yellow cultivar than the other organs, and the white sweet potatoes, while sodium contents (78.77 mg/100 g) were significantly higher in the tuber of the white cultivar. The levels of manganese (4.24 mg/100 g), zinc (1.24 mg/100 g), and copper (0.8 mg/100 g) were importantly higher in the leaves of the yellow cultivar than the other organs, and the white sweet potatoes whereas, those of calcium (451.93 mg/100 g) and magnesium (403.07 mg/100 g) were higher the leaves of the white cultivar. In general, the values of potassium (1127.22 mg/100 g) were found to be higher in the stem of the yellow cultivar compared with the other organs, and the white sweet potassium. Furthermore, the tested plant organs of I. batatas were also investigated for nickel and selenium contents but these samples had no detectable levels associated to the analysed chemical elements.
Effects of plant organs (flowers, leaves, stem, and tubes) and sweet potato cultivars (yellow and white) on the proximate and phytochemical analyses of Ipomoea batatas.
Statically, plant organs (flowers, leaves, stem, and tubers) and sweet potatoes cultivars (yellow and white) had significant effects on the proximate and the phytochemical analyses of Ipomea batatas (Fig. 4 Table 2). In sum, flowers samples of the yellow cultivar exhibited higher levels of tannins (4.42%) than the other organs and the white sweet potatoes while, the amounts of protein (4.66%) and total flavonoids (2212.63 mg/kg) were greater in the leaves of the yellow sweet potatoes. Furthermore, stem of the white sweet potatoes showed elevated values of cruder fibre (5.93%) than the other plant organs, and the yellow sweet potatoes whereas, the amounts of dry matter (30.54%), ash (4.11%), and total digestible nutrients (26.4%) were significantly higher in the tubers of the yellow sweet potatoes. The concentrations of fats were lower than 1% for all the tested plant organs.
Impacts of sweet potatoes cultivars (yellow and white) and the drying conditions (out and indoor) mineral composition of Ipomea batatas.
Drying conditions and sweet potatoes cultivars showed significant effects on the minerals analyses of Ipomea batatas (Fig. 5; Table 3). Overall, non-treated tubers of the yellow cultivar exhibited elevated values of calcium (304.53 mg/100 g) and manganese (1.92 mg/100 g) than the treated and the white sweet potatoes while, those of copper (0.6 mg/100 g) were more important in the non-treated tubers of the white cultivar. In general, the amounts of phosphorous (347.14 mg/100 mg) magnesium (213.26 mg/100 g) and zinc (0.74 mg/100 g) were found to be greater in the tubers of the white sweet potatoes. Furthermore, the levels of potassium (1368 mg/100 g) recorded in the tubers of both cultivars were slightly similar (1368 mg/100 g) when dried indoor, but these values were greatly higher than those dried outdoor and the non-treated. Drying both cultivars positively affected the amounts of sodium (437.38 mg/100 g) compared with the non-treated, with higher concentrations recorded in the tubers of the white cultivar.
Effects of drying conditions (out and indoor) and sweet potato cultivars (yellow and white) on the proximate and phytochemical parameters of Ipomea batatas.
Drying conditions and sweet potatoes cultivar showed significant effects on the proximate and phytochemical composition of Ipomea batatas, and the effects were sweet potatoes cultivar dependent (Fig. 6; Table 4). Dry matter contents (92.74%) and total digestible nutrients (88.66%) were higher in the tubers of the yellow cultivar dried outdoor, and these amounts were more important than those of the white and non-treated sweet potatoes. However, the levels of crude protein (5.2%), crude fibre (5.1%) and ash (4.87%) were greater in the tubers of the white cultivar dried indoor, and these values surpassed those of the yellow cultivar and the non-treated group. Overall, tubers of the yellow potatoes dried indoor exhibited significantly higher concentrations of total flavonoid (947.96 mg/kg) than the white cultivar and the non-treated while, the levels of tannins (2.65%) were found to be higher in the tubers of the yellow non-treated sweet potatoes. In general, the amounts of total fats were less than 1% for all the tested sweet potatoes cultivars regardless the treatments.
Impacts of the cooking time (0,15, and 25 min) and sweet potato cultivars (yellow and white) on the mineral composition of Ipomea batatas.
Time cooking and sweet potato cultivars had significant effects on the mineral composition ofIpomea batatas, and the impacts were sweet potatoes dependent (Fig. 7; Table 5). In sum, the values of calcium (304.54 mg/100 g), potassium (570.82 mg/100 g), manganese (1.92 mg/100 g) and zinc (0.52 mg/100 g) recorded in the tubers of the non-treated yellow sweet potatoes were greatly higher than those of the treated regardless the treatment group whereas, the amounts of magnesium (40.94 mg/100 g), and copper (0.6 mg/100 g) were much higher in the tubers of the white cultivar. At contrast, the levels of phosphorous (43.44 mg/100 g) and sodium (96.62 mg/100 g) found in the tubers of the white sweet potatoes exposed to 25 min surpassed those of 15 min, and these amounts were significantly higher compared with those of the yellow cultivars.
Impacts of cooking time (15 and 25 min) and sweet potato types (yellow and white) on the proximate and chemical composition of Ipomea batatas
Cooking time and sweet potatoes cultivars were found to significantly affect the analysed chemical elements, and the effects were sweet potatoes cultivars dependent (Fig. 8; Table 6, and 7). In general, the amounts of dry matter (30.54%), crude protein (3.33%), ash (4.11%) and tannins (2.65%) were found to be greater in the tubers of the non-treated yellow cultivar than the treated samples. The concentrations of total flavonoids (405.28 mg/kg) and crude fat (0.78%) were higher in the tuber of the yellow and white cultivars respectively when exposed to 15 min, and these values were greater than those of 25 min. Overall, the non-treated sweet potatoes cultivars had no detectable contents in fat analyses.
Heavy metals
All the tested sweet potato regardless the treatments were investigated for heavy metals contents that included cadmium and lead but none of the tested potato had any contents in the analysed chemical elements.
Effects of sweet potatoes organs water extracts and concentrations on the chemicals analyses of the tested solutions.
The results in (Fig. 9; Table 8) showed that increasing the concentrations of the different organs of sweet potatoes water extracts in the solutions significantly decreased the values of pH with the control exhibiting greater values (6.46) than the treatments. Furthermore, shoots and tubers of the yellow sweet potatoes showed noticeable highest values of pH than the white cultivar. Contrastingly, 20% of the shoots water extracts of the white cultivar had elevated values of EC (19.74 mS/cm), TDS (9.85 g/L), and salinity (11.76 psu) compared with the yellow, and the non-treated.
Impacts of sweet potatoes cultivars water extracts and concentrations on the seed germination of Prosopis juliflora.
Shoot water extracts of sweet potatoes cultivar had strongest negative effects on the seeds responding of Prosopis juliflora with more effects recorded from the white potatoes (Fig. 10; Table 9). Furthermore, shoot water extracts had significantly higher adverse effects than the tubers water extracts, and exposing the seeds to the concentrations above 10%, completely inhibited the seeds germination of P. juliflora. Non-treated seeds attained 95% of the germination percentages, while seed vigor index values were higher when treated with 5% of the yellow tubers water extract, and these amounts were importantly higher than that of the other levels.
Effects of sweet potatoes cultivars water extracts and concentrations on the early growth of Prosopis juliflora.
Shoot water extracts of sweet potatoes cultivar showed strongest negative effects on the seedlings establishments of Prosopis juliflora than the tubers water extracts (Figs. 11 and 12; Table 10). While strongest inhibitory effects were noticeable from the tested shoot water extracts, tubers water extract of the yellow cultivar exhibited stimulating effects, but these positive effects decreased with the increase of the concentrations of water extracts. Shoot (5 cm) and root lengths (3.5 cm), and seedlings biomass (22.75 mg) were positively affected by 5 and 10% of the tubers water extract of the yellow cultivar, and these values were greater than the non-treated seedlings and the other levels.
Allelopathy index values of the sweet potatoes plant organs water extract on the seed germination and seedlings growth attributes of Prosopis juliflora.
In general, the values of the allelopathy index were more negative for the tested shoot water extracts than the tubers, while positive values were recorded on the seedlings growth of Prosopis juliflora. Reddish colours indicate negative effects while greenish colours highlight the positive effects (Table 11).
Electrolyte leakage values of the seedlings of Prosopis juliflora exposed to sweet potatoes water extracts and concentrations.
Exposing the seedlings of Prosopis juliflora to the sweet potatoes plant organs water extracts had significant effects on the values of the electrolyte leakage (Fig. 13; Table 12). Lower values of electrolyte leakage (36.20%) were obtained when treated the seedlings with 5% of the yellow sweet potatoes tubers water extract, while higher values were recorded from the 10% of the white shoot (76.03%) compared with the other concentrations and the non-treated group.
Pearson correlation matrix between the germination and the growth parameters of Prosopis juliflora, and the tested chemicals elements of the allelochemicals solutions.
In sum, the values of correlation coefficients were negatively associated with seed germination and seedlings attributed considering EC, TDS, salinity and electrolyte leakage variables (Table 13). Blue colours indicate positive effects, while reddish colour indicate negative associational effects.
Discussion
Effects of plant organs (flowers, leaves, stem alone, and tubers) and cultivars on the minerals, nutrients and phytochemical analyses of Ipomea batatas.
Intensive works have been carried out and addressed on the nutritive value of sweet potatoes10,15,38 but unfortunately, almost no published data exist regarding the arid regions. In the present work, plant organ recorded higher significant variations in the nutritional components of sweet potatoes cultivars, and the minerals and the nutrients contents were cultivar dependent. Leaves of the two cultivars were more enriched in minerals and nutrients than the other plant organs. Leaves of the yellow cultivar contained higher values of manganese, zinc, copper, and protein, while the leaves of the white cultivar were loaded with significant levels of calcium, magnesium, and flavonoids. The values of the minerals and nutrients analyses presented in the current study are greatly higher than those of Ranteallo39. Few previous works have tried to conduct comparative analyses on the nutritive components of sweet potatoes emphasizing plant organ effects. In general, plant growing in the extreme environmental conditions face more stress than the other, which can induce significant adjustments at the physiological levels. As per this, significant minerals and nutrients recorded in the leaves of the two cultivars could have helped this plant coping with the surrounding environmental factors.
Macro and micro elements play crucial roles in the plants and animals physiology. For example, in plant body, calcium helps this latter tolerating more abiotic stressful conditions through regulating the movements of stomata, while in the animal organism, this chemical element is crucial for the teeth and bones growth, and it is also involved in the mechanism of muscles contraction13,14. While magnesium, regulates nerve and muscles functioning, hormonal production and bones growth40, manganese strongly impacts bones formation, carbohydrate metabolism and reproductive organs maintenance41. Zinc is the second most abundant micro elements in the human body, and it has higher significance in many physiological functions including cell division, nucleic acid metabolism, and protein synthesis42. With focus on copper, this chemical element might play important function in the electron transfer to help maintaining cellular redox balance43.
Roles play by the flavonoids are indispensable to help plants challenging the harsh conditions, while in the animals body, these phytochemical compounds are reported to improve the immunity and body defence against pathogens and harmful particles such ultraviolet44. The amounts of flavonoids recorded in the tested sweet potatoes cultivars leaves are greatly higher than those of Agbo45. Therefore, leaves of these sweet potatoes cultivars could be used to improve food and medicines production in the arid regions.
Impacts of cultivars (yellow and white) and cooking methods (drying and boiling) on the nutritive components of Ipomea batatas.
Based on the finding results, drying sweet potatoes indoor and under full sunlight had significant effects on the quality of the tested cultivars. While more minerals and nutrients were found to be higher in the white cultivar, phytochemical components including tannins and flavonoids were importantly higher in the yellow cultivar. Greater effects were recorded on the amounts of calcium, manganese, copper and tannins with non-treated sweet potatoes exhibiting elevated concentrations than the treated. Interestingly, the amounts of tannins (2.65%) noticeable in the present work were greatly higher than that of the raw sweet potatoes (0.01%) reported in the literature review46. Environmentally, tannins play crucial role in the mechanisms of survival of many plants species when experiencing dramatical changes occurring in their habitats47.
Traditionally, drying the food under sunlight is cost effective, which help in long-term preservation. The main purpose of drying food is to reduce the moisture content in order to control the activities of the microorganisms that could have adversely altered food quality during the preservation periods. Despite sun-drying method is cost effective, food exposition to direct solar radiation might negatively affect some nutritional components. Food exposure to direct solar radiation could modify the structural configuration of some components and therefore that of some minerals as well. It was observed that exposing the peels of sweet potatoes to direct solar radiation decreased the amounts of calcium, copper, and tannins compared with the other treatments48.
In the present study, it was observed that that exposing the tested sweet potatoes to direct sunlight positively affect the levels of dry matter, phosphorous, magnesium, and zinc compared with the indoor samples and the non-treated. The findings of the present work are in alignment with those addressed in the previous work48. Both dry matter and moisture contents present inverse relationships. Decreasing the food moisture content can importantly increase the concentration of some minerals and therefore strongly influence food quality. In general, when water evaporated from the food, the left solids could be therefore more concentrated in term of percentages, but this could not affect the total amounts of those minerals49. The concentrations of iron and copper were found to significantly increase with the decrease of moisture content in the solution50.
In the current study, drying the selected sweet potatoes indoor significantly increased the concentrations of potassium, sodium, protein, ash, fibres and total flavonoids than the direct solar radiation, and the non-treated sweet potatoes. The levels of protein and ash contents observed in this research work are importantly higher than those reported in the literature review51. Solar radiation coupled with higher temperatures can induce significant changes on the chemistry bonds, which could cause the fragmentation of some specific nutritional components into small fragments52. Therefore, notably changes in chemical structure of those elements would ineluctably affect the overall food quality. Protein, ash, and sodium contents of the sweet potatoes peels have been documented to decrease when expose to solar radiation than air-dried treatment53. Flavonoids play remarkable function in the animal physiology. Flavonoids can help animal body fighting against higher stress and harmful particles that could disrupt many physiological functions. In the present work, the levels of these chemical compounds were greater indoor than outdoor drying methods. In general, arid regions are characterized by higher temperature and long-term radiation than the other regions. Therefore, based on our findings, indoor drying the sweet potatoes could potentially improve the concentrations of some chemical elements in the arid regions depending on the time of the year. The obtained results showed that boiling the tested sweet potatoes at different time periods exhibiting significantly higher minerals and nutrients contents in the non-treated samples than the treated.
In general, calcium, potassium, manganese, zinc, magnesium, copper, dry matter, protein, ash and tannins amounts were importantly greater in the non-treated samples compared with the treated. Exposing sweet potatoes to boiling treatment decreased glucose, fructose, protein, β-carotene, and starch contents than the raw samples54. In general, boiling food can greatly affect the values of pH and electrical conductivity and therefore the solubility of the involved chemicals compounds. Although boiling food can improve the levels of proteins, prolonged exposition to boiling could dramatically affect the food quality55. Boiling might not have direct effects on the peptide bonds, but it might alter weaker bonds related to hydrogen that maintain the protein structure and modify the amounts of some minerals including calcium and magnesium31.
Based on the current results, exposing the selected sweet potatoes to 15 min significantly increased the amounts of flavonoids and fat compared to 25 min and the non-treated samples. Prolonged cooking time might have strong negative effects on the fat quality which could cause fat oxidation and alter the fatty acid structures and generate the formation of harmful compounds56. In the present work, flavonoids concentrations recorded at 25 min were importantly lowered than 15 min. Many flavonoids compounds are heat-sensitive and could be damaged when exposed to high heat during the boiling process. The concentrations of flavonoids compounds of broccoli plant were found to be importantly lowered when exposed to boiling methods than steaming, and microwaved samples57.
Fibres are vital for animal physiology and health. These chemical elements positively affect the digestive system, prevent constipation, and could help managing the body weight58. Interestingly, in our work, exposing the selected samples to 25 min showed higher contents than 15 min and the non-treated. As per this, cooking the tested potatoes for 25 min could constitute an important source of fibres that could be used to enriched food quality in the arid regions.
Allelopathy of sweet potatoes cultivars (yellow and white) and water extracts concentrations on the seed responses and seedlings performance of the exotic invader Prosopis juliflora.
The present study showed that shoot water extracts of the tested sweet potatoes exhibited strongest inhibitory effects on the seed responses of the invader Prosopis juliflora than the tubers water extracts and the non-treated seeds. Furthermore, exposing the tested seeds to the concentrations above 10% of the shoot water extracts completely inhibited their germination. Moreover, germination percentages and seeds vigor index were negatively correlated with EC, TDS, and electrolyte leakage values. In general, strongest negative effects were noticeable in the white cultivar than the yellow and the control. Numerous studies have demonstrated the impacts of aqueous leaves extracts of P. juliflora plant on the seeds germination and seedlings growth of many plants species, but unfortunately there is a lack of scientific evidence examining the detrimental allelopathic effects on the responses of P. juliflora seed germination and seedlings growth. Exploring the allelochemical effects of the other plants on the responses of P. juliflora might allow to identify those with strongest inhibitory effects that could help designing organic herbicide. The results obtained in the allelopathy of the tested potatoes on the seed germination of P. juliflora plants are much strongest than that addressed in the earlier work59 in the auto allelopathy of this invasive plant species. Furthermore, the results of this work showed strongest detrimental effects than those of P. juliflora plant on the other plants responses59,60,61. The obtained results on the detrimental allelopathy could be attributed to the chemical composition of the tested cultivars shoots, as the chemical composition of the shoot of the selected potatoes surpassed that of the tubers in the current experiment.
Allelochemicals are the products of the secondary metabolism synthesised and released into the environment to increase the competitive abilities of the releasing individual or to cope with the negative changes that could potentially damage plant body. In general, seed germination is considered one of the critical stages in plant growth, and this plant stage growth is affected by many factors including intrinsic and extrinsic. Seeds metabolism is strongly correlated with water and oxygen availability which are the major factors that control seeds physiology. With this regard, exposing the seeds to allelochemicals with negative effects could have importantly altered electron transfer chain which is vital for generating energy and building essential components for the emerging seedling62. At the hormonal levels, allelochemicals might importantly alter hormones pathway and induce the synthesis some hormones such abscisic acid which are responsible for seed dormancy in plant detrimentally to the other and therefore alter the germination processes. Therefore, plant shoot of the tested cultivars could be used to manufacture organic compounds that could help controlling the proliferation of Prosopis juliflora plants in the introduced ranges.
In the present work, tubers water extracts showed less negative effects on the seed germination of Prosopis juliflora than the shoots. Justifiably, the levels of toxicity in the tested tubers could be greatly lower compared with the shoots. Toxicological tests on rats exposed to sweet potatoes purple demonstrated positive effects on the physiological and morphological attributes of the experimented rats than the non-treated63. Sweet potatoes water extracts were found to enhance seed germination and seedling growth of Vanda tessellata than the control64. Corroborating trends were reported in the previous work65. Sweet potatoes are documented to contain greater amounts of vitamin A, B, and C which act as strong antioxidant and could help protecting the cell from damage due to the accumulation of free radicals38. As per this, the obtained results could be attributed to the chemical contents of sweet potatoes in vitamins and other chemicals elements.
Based on the current results, seedlings establishment of Prosopis juliflora plant was importantly inhibited when treated with shoot water extracts of the tested sweet potatoes than the tuber water extracts and the non-treated. Comparably to the non-treated, 5 and 10% tubers of the yellow cultivar showed stimulating effects on the seedlings performance of P. juliflora but however, these effects decreased with the increase of the concentrations. Furthermore, shoot and root length, and seedlings dry weight were negatively associated to the EC, TDS, and electrolyte leakage variables. These results could be attributed to the chemical analyses of the tested water extracts. Increasing the concentrations augmented the values of EC, TDS and salinity in the solutions compared with the control, and highest values were recorded in the shoot of the white cultivar. Furthermore, the tested shoots exhibited greater values on the selected chemical elements than the tubers water extracts. Electrolyte leakage is one of the most important indicators of plant facing stress. In the present work, the values of electrolyte leakage increased with the augmentation of the allelochemicals in the solution with shoot showing significantly higher values than the tubers, and the non-treated group. In general EC plays remarkable role in the plant growth and development when the levels are in the acceptance ranges. Adequate EC in the solution might increase nutrients and water availability through increasing cation exchange capacity around the plant root. However, higher concentrations of this chemical element might importantly alter the rates of solubilities of some minerals and nutrients which would obviously expose plant to significant stress conditions. Furthermore, allelochemicals released in the solutions could have induced the synthesis of some hormones such cytokines to disrupt cell division and elongation which could have promoted root growth.
Conclusion
In the current work, considering plant organs effects, the tested leaves revealed greater minerals and nutrients contents than the other selected plant organs. Furthermore, more nutrients were recorded in the yellow cultivar than white. Moreover, flavonoids and tannins were importantly higher in the leaves and flowers of the yellow sweet potatoes respectively compared with the white. With focus on the cooking methods, sodium, potassium, protein, fibres, ash, and total flavonoids were significantly higher when exposed to the indoor drying process than direct solar and non-treated samples. Exposing the tested potatoes to boiling process significantly lowered minerals and nutrients levels than the non-treated samples. The negative effects were strongest at 25 min. With regard to the allelopathic effects, shoots water extract of the tested sweet potatoes exhibited strongest inhibitory effects on the seed germination and seedlings performance of Prosopis juliflora than the tubers water extracts and the non-treated group. Furthermore, seeds responses of P. juliflora plants were completely inhibited when exposed to the allelochemicals concentrations above 10%, and seed germination processes were more adversely affected than the seedlings. Overall, the strongest detrimental effects were recorded with the white cultivar. In addition, the values of electrolyte leakage parameter were lower when treated the seedlings with 5% of the yellow tubers water extracts, and higher with 10% of the white shoot water extract. The values obtained in the chemicals and phytochemical analyses of the present work were greater than some of the earlier work. Furthermore, shoot water extracts of the tested sweet potatoes exhibited strongest inhibitory effects on the seed germination and seedlings performance of P. juliflora, and these results were higher than those documented in the literature review. As per this, shoot of the tested sweet potatoes could be used as supplement to improve food quality in the arid regions. Furthermore, shoot of the selected Ipomea batatas could be used to design organic compounds that could be used to control the proliferation of P. juliflora plants and the other weeds species.
Data availability
All the data supporting the findings of the present work are available within the paper and its Supplementary data file.
References
Zeifman, L., Hertog, S., Kantorova, V. & Wilmoth, J. A world of 8 billion. 1–4 (2022).
Walker, R. J. Population growth and its implications for global security. AJES 75 (4), 980–1004 (2016).
Shamsiev, A., Ubaydullaev, S. & Ostonakulov, T. Selection of the variety of sweet potato and features of their cultivation technology. Bul J. Crop Sci. 57 (3), 1–5 (2020).
Sugata, M., Lin, C. Y. & Shih, Y. C. Anti-inflammatory and anticancer activities of Taiwanese, purple‐fleshed sweet potatoes (Ipomoea batatas L. Lam) extracts. Biomed. Res. Int.768093, 2015(1) (2015). (2015).
Baba, J., Mohammed, S. B., Ya’aba, Y. & Umaru, F. I. Antibacterial activity of sweet orange citrus sinensis on some clinical bacteria species isolated from wounds. JFMCH 5 (4), 1154 (2018).
Silva-Correa, C. R. et al. Potential anticancer activity of bioactive compounds from Ipomoea Batatas. Phcog J. 14 (3), 1–7 (2022).
Truong, V. D., Avula, R. Y., Pecota, K. V. & Yencho, G. C. Sweet potato production, processing, and nutritional quality. Handbook Vegetables Vegetable Processing 811–838 (2018).
Abidin, P. E., Carey, E. E., Mallubhotla, S. & Sones, K. R. Sweet potato cropping guide. Africa Soil. Health Consortium, ISBN 978-1-78639-315-9, 1-62 (2017).
Bovell-Benjamin, A. C. Sweet potato: a review of its past, present, and future role in human nutrition. Adv. Food Nutr. Res. 52, 1–59 (2007).
Krochmal-Marczak, B. et al. Nutrition value of the sweet potato (Ipomoea Batatas (L.) Lam) cultivated in south–eastern Polish conditions. IJAAR 4 (4), 169–178 (2014).
Suparno, A., Prabawardani, S. & Bob, A. The nutritional value of sweet potato tubers [Ipomoea batatas (L.) Lamb.] consumed by infants and children of Dani tribe in Kurulu district, Baliem-Jayawijaya. J. Agri Sci. 8, 1–6 (2016).
Ojone, A. S., Oricha, K. A. & Frank, O. D. n.d.). Planning for food security using roots and tubers as functional foods in nigeria: A review. ECAFS 2, 1–6 (2016).
Tsombou, F. M. et al. Effect of harvest timing and plant parts on the nutritional and chemical profile of five potential fodder plants found in Eastern Coast of united Arab Emirates. Sci. Rep. 14 (1), 11371 (2024).
Tsombou, F. M. et al. Altitudinal influence on survival mechanisms, nutritional composition, and antimicrobial activity of Moringa Peregrina in the summer climate of fujairah, UAE. Sci. Rep. 15 (1), 5635 (2025).
Ishida, H. et al. Nutritive evaluation on chemical components of leaves, stalks and stems of sweet potatoes (Ipomoea Batatas poir). Food Chem. 68 (3), 359–367 (2000).
Anwar, N. Z. R. & Ghani, A. A. Effect of different processing methods on the physicochemical properties and sensory evaluations of sweet potatoes chips. J. Agr Biotech. 10 (2), 51–63 (2019).
Chukwu, O., Nwadike, N. & Nwachukwu, N. G. Effects of cooking and frying on antioxidants present in sweet potatoes (Ipomoea batatas). Acad. Res. Int. 2 (2), 104 (2012).
Kido, M. et al. Effects of cooking methods on caffeoylquinic acids and radical scavenging activity of sweet potato. Foods 13 (7), 1101 (2024).
Bechoff, A. et al. Effect of hot air, solar and sun drying treatments on provitamin A retention in orange-fleshed sweet potato. J. Food Eng. 92 (2), 164–171 (2009).
Huang, Y. et al. Allelopathic effects of aqueous extracts of alternanthera Philoxeroides on the growth of Zoysia Matrella. Pol. J. Environ. Stud. 26 (1), 1–10 (2017).
Slate, M. L. et al. Exotic Prosopis Juliflora suppresses understory diversity and promotes agricultural weeds more than a native congener. Plant. Ecol. 221 (8), 659–669 (2020).
Kubiak, A., Wolna-Maruwka, A., Niewiadomska, A. & Pilarska, A. A. The problem of weed infestation of agricultural plantations vs. the assumptions of the European biodiversity strategy. Agron 12 (8), 1808 (2022).
Pasha, S. V. & Reddy, C. S. Global Spatial distribution of Prosopis juliflora-one of the world’s worst 100 invasive alien species under changing climate using multiple machine learning models. Environ. Monit. Assess. 196 (2), 196 (2024).
Shackleton, R. T., Maitre, L., Pasiecznik, D. C., Richardson, D. M. & N. M., & Prosopis: a global assessment of the biogeography, benefits, impacts and management of one of the world’s worst Woody invasive plant taxa. AoB Plants. 6, 1–18 (2014).
Sintayehu, D. W., Dalle, G. & Bobasa, A. F. Impacts of climate change on current and future invasion of Prosopis Juliflora in ethiopia: environmental and socio-economic implications. Heliyon 6 (8), 1–7 (2020).
El-Keblawy, A. & Al-Rawai, A. Impacts of the invasive exotic Prosopis juliflora (Sw.) DC on the native flora and soils of the UAE. Plant Ecol. 190, 23–35 (2007). (2007).
El-Keblawy, A. et al. Effects of conspecific and congeneric soils and litters on the nodulation and growth of non-native invasive and native prosopis species in arid deserts. J. Arid Environ. 227, 105319 (2025).
Kamiri, H. W., Choge, S. K. & Becker, M. Management strategies of Prosopis Juliflora in Eastern africa: what works where? Diversity 16 (4), 251 (2024).
Shen, S. et al. Allelopathic effects of three sweet potato cultivars (Ipomoea batatas) on the invasive plant Mikania Micrantha. PJBS 21 (1), 8–15 (2018).
Shen, S. et al. Allelochemicals identified from sweet potato (Ipomoea batatas) and their allelopathic effects on invasive alien plants. Front. Plant. Sci. 13, 823947 (2022).
Zhang, S. et al. Allelopathic effects of water extracts of sweet potato (Ipomoea batatas) on seed germination of Ageratum conyzoides. J. Agri Chem. Environ. 12 (2), 124–133 (2023).
Othman, S. H., Lazim, Z. S. & Al-Wakaa, A. H. A. Allelopathic potential of aqueous plant extracts against seed germination and seedling growth of weeds. IOP 1262 (3), 032041 (2023).
El-Rokiek, K. G., Nasr Shehata, A., S. El-Din, S. A. & Tarraf, S. A. An allelopathic evaluation of aqueous Aloe Vera leaf and root extracts on the weed Sonchus oleraceus associated vicia Faba L. Plant. Prot. Res. 64, 11–18 (2024).
Silayo, V. C. K. & Laswai, H. S. Effect of sun-drying on some quality characteristics of sweet potato chips. Afr. J. Food Agri Nut Devel. 3, 1–7 (2007).
Eke-Ejiofor, J. & Onyeso, B. U. Effect of processing methods on the physicochemical, mineral and carotene content of orange fleshed sweet potato (OFSP). J Food Res 8(3), 50-58 (2019).
Khasnabis, J., Rai, C. & Roy, A. Determination of tannin content by titrimetric method from different types of tea. J. Chem. Pharm. Res. 7 (6), 238–241 (2015).
Nakano, M., Matoba, K. & Togamura, Y. An Estimation for total digestible nutrients in fresh herbage from a perennial ryegrass-white clover mixed pasture. JARQ 52 (2), 155–161 (2018).
García-Martínez, R. M., Rodiles-López, J. O. & Martínez-Flores, H. E. Nutritional value and antioxidant capacity of Mexican varieties of sweet potato (Ipomoea Batatas L.) and physicochemical and sensory properties of extrudates. Pol. J. Food Nut Sci. 74 (4), 376–386 (2024a).
Ranteallo, Y., Ahmad, M., Syam, A. & Nilawati, A. Identification and quantification of minerals and vitamins of purple sweet potato (Ipomoea batatas) leave. IOP 1230 (1), 012134 (2023).
Stanojević, M. et al. The impact of chronic magnesium deficiency on excitable tissues—translational aspects. Biol. Trace Elem. Res. 203 (2), 707–728 (2025).
Studer, J. M., Schweer, W. P., Gabler, N. K. & Ross, J. W. Functions of manganese in reproduction. Anim. Reprod. Sci. 238, 106924 (2022).
Liberato, S. C., Singh, G. & Mulholland, K. Zinc supplementation in young children: A review of the literature focusing on diarrhoea prevention and treatment. Clin. Nutr. 34 (2), 181–188 (2015).
Fu, Y. et al. The physiological role of copper: dietary sources, metabolic regulation, and safety concerns. Clin. Nutr. 48, 161–179 (2025).
Zheng, X., Zhang, X. & Zeng, F. Biological functions and health benefits of flavonoids in fruits and vegetables: A contemporary review. Foods 14 (2), 155 (2025).
Agbo, E. A. et al. Antioxidant activities in sweet potatoes leaves steamed with spices. J. Food Res. 9 (4), 1–9 (2020).
Akpe, M. A., Ashishie, P. B. & Akonjor, O. A. Evaluation of some phytochemicals in raw and cooked Ipomea batatas (Lam), (sweet potato), Solanum tuberosum (Irish potato) and Dioscorea cayenensis (yellow yam). J. Appl. Sci. Environ. Manag. 25(9), 1563–1567 (2021).
Muthusamy, M. & Lee, S. I. Abiotic stress-induced secondary metabolite production in Brassica: Opportunities and challenges. Front. Plant Sci. 14, 1323085 (2024). (2024).
Abdullahi, A. I. et al. Proximate composition, mineral content and anti-nutrient of Raw and sun-dried sweet potato Peel. ASUU 8, 34–47 (2021).
Crosby, G. The Key Role of Cooking and Food Preparation in Affecting Nutrient Composition of Foods417–424 (Elsevier, 2024).
Saberi, N. & Vriens, B. The effects of water content on mineralogical and drainage quality dynamics in weathering mine waste rock. Min. Eng. 214, 108791 (2024).
Rezende, L. et al. Study of the drying process of the purple-fleshed sweet potato (Ipomoea Batatas (L.) Lam) in spouted bed. Food Sci. Tech 44, 1-10 (2024).
Kim, Y. J. et al. The effect of irradiation on meat products. Food Sci. Res. 44 (4), 779 (2024).
Agubosi, O. C. P., Imudia, F. D. & Alagbe, J. O. Evaluation of the nutritional value of air dried and sun-dried sweet potato (Ipomoea batatas) peels. EJLSS 14 (22), 43–51 (2022).
Dincer, C. et al. Effects of baking and boiling on the nutritional and antioxidant properties of sweet potato [Ipomoea batatas (L.) Lam.] cultivars. Plant. Foods Hum. Nutr. 66, 341–347 (2011).
Sanusi, M. S. et al. Effects of boiling and ultrasound treatment on proximate composition, minerals and in vitro protein digestibility of Cirina Butyrospermi powder. J. Agri Food Res. 19, 101608 (2025).
Sun, M. et al. Effect of four different cooking methods on the fat digestion characteristics of yellow-feathered chicken. Appl. Food Res. 4 (2), 100465 (2024).
Wu, X. et al. Effects of domestic cooking on flavonoids in broccoli and calculation of retention factors. Heliyon, 5(3), 1-16 (2019).
Anderson, J. W. et al. Health benefits of dietary fiber. Nutr. Rev. 67 (4), 188–205 (2009).
Bibi, S. et al. Allelopathic effects of the invasive Prosopis Juliflora (Sw.) DC. on native plants: perspectives toward aerosystems. Agron 13 (2), 590 (2023).
Shah, R. H. et al. Bioherbicidal assessment of aqueous extracts of mesquite (Prosopis juliflora) on weeds control and growth, yield and quality of wheat. Planta Daninha. 36, e018169995 (2018).
Almoshadak, A. S., Suliman, E. A. H., Aljeddani, G. S. & Kutby, A. M. Impact of mesquite (Prosopis juliflora) on germination and early growth of two Vachellia species. Appl. Ecol. Environ. Res. 22 (4), 3155–3162 (2024).
Ahmad, M., Wolberg, A. & Kahwaji, C. I. Biochemistry, electron transport chain. Boil Chem. 1-8 (2018).
Hisamuddin, A. S. B. et al. Phytochemical component and toxicological evaluation of purple sweet potato leaf extract in male Sprague–Dawley rats. Front. Pharm. 14, 1132087 (2023).
Islam, M. O., Akter, M. & Prodhan, A. Effect of potato extract on in vitro seed germination and seedling growth of local Vanda roxburgii orchid. J. Bang. Agri. Univer. 9(2), 211–215 (2011). (2011).
Shen, S. et al. Allelopathic potential of sweet potato (Ipomoea batatas) germplasm resources of Yunnan Province in Southwest China. Act. Ecol. Sin. 38 (6), 444–449 (2018b).
Acknowledgements
The authors would like to thank Dr. Saher Bano and Eng. Aishah Saeed Sulaiman for the samples collection.
Funding
the current study did not receive any specific funding.
Author information
Authors and Affiliations
Contributions
Authors Contributions Conceptualization, François Mitterand Tsombou and Fouad Lamghari Ridouane; Data curation, François Mitterand Tsombou, Ahmed Mohamed Saeed Ali Alhmoudi and Amani Jumah Ahmed Meleih Aldhanhani; Formal analysis, François Mitterand Tsombou; Investigation, François Mitterand Tsombou, Maryam Ali Saeed Mohamed Alhmoudi and Amani Jumah Ahmed Meleih Aldhanhani; Methodology, François Mitterand Tsombou, Ahmed Mohamed Saeed Ali Alhmoudi, and Amani Jumah Ahmed Meleih Aldhanhani; Project administration, Fouad Lamghari Ridouane and François Mitterand Tsombou; Resources, Maryam Ali Saeed Mohamed Alhmoudi, Ahmed Mohamed Saeed Ali Alhmoudi, and Fouad Lamghari Ridouane; Software, François Mitterand Tsombou; Supervision, François Mitterand Tsombou and Fouad Lamghari Ridouane; Validation, François Mitterand Tsombou, Fouad Lamghari Ridouane and Maryam Ali Saeed Mohamed Alhmoudi; Visualization, François Mitterand Tsombou, Fouad Lamghari Ridouane, Ahmed Mohamed Saeed Ali Alhmoudi, and Amani Jumah Ahmed Meleih Aldhanhani; Writing – original draft, François Mitterand Tsombou and Maryam Ali Saeed Mohamed Alhmoudi; Writing – review & editing, François Mitterand Tsombou and Fouad Lamghari Ridouane.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tsombou, F.M., Alhmoudi, M.A.S.M., Alhmoudi , A.M.S.A. et al. Study on sweet potatoes in relation to nutritional profile against worst invasive plant (Prosopis juliflora) under arid climate of Fujairah. Sci Rep 15, 33564 (2025). https://doi.org/10.1038/s41598-025-18583-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-18583-w