Abstract
Ischemic stroke remains a leading cause of death and disability worldwide, yet effective neuroprotective therapies are lacking. Neuroinflammation and pyroptosis, a lytic and inflammatory form of programmed cell death, exacerbate secondary brain injury post-ischemia. This study investigated the neuroprotective effects of T817MA, a neurotrophic agent, in a rat model of transient middle cerebral artery occlusion (MCAO), focusing on its anti-pyroptotic mechanisms via Sirtuin1 (Sirt1) signaling. Animals were pretreated orally with T817MA at 30 mg/kg for 20 days, and brain edema was evaluated by brain water content. Neurological dysfunction was determined by modified neurological severity score (mNSS) and rotating pole test, and neuroinflammatory markers were assayed by immunostaining. Pyroptosis and potential molecular mechanisms were detected by measuring related proteins using western blot. T817MA treated rats exhibited reduced brain edema, improved neurological recovery, and attenuated motor-coordination deficits post-MCAO. T817MA suppressed neuroinflammation, evidenced by decreased activation of microglia (ionized calcium binding adapter molecule 1, Iba-1) and astrocytes (glial fibrillary acidic protein, GFAP). Mechanistically, T817MA inhibited caspase-1 cleavage, IL-1β release, and Gasdermin D (GSDMD)-mediated pyroptosis in neurons. Notably, T817MA prolonged Sirt1 activation up to 48 h post-ischemia, while Sirt1 inhibition with sirtinol reversed its protective effects on brain edema, caspase-1 activation, and IL-1β levels. These findings demonstrate that T817MA mitigates ischemic brain injury by suppressing neuroinflammation and pyroptosis through Sirt1-dependent pathways, highlighting its potential as a therapeutic candidate for ischemic stroke.
Similar content being viewed by others
Introduction
Stroke remains one of the leading causes of long term disability and ranks fifth among the causes of death, with over 795,000 cases occurring annually in the United States1. Ischemic stroke is the most prevalent type of stroke, accounting for approximately 87% of the total stroke cases. The current standard treatment for ischemic stroke is thrombolysis with tissue plasminogen activator (TPA) or thrombectomy with endovascular devices, but no neuroprotective drugs have been proven to be effective in treating neurological dysfunction in patients2.
Neuroinflammation following ischemic stroke aims to clear debris, repair damaged tissues, and restore homeostasis. However, a prolonged inflammatory response can exacerbate neuronal damage, disrupt blood-brain barrier (BBB) integrity, and promote secondary injury mechanisms3. Pyroptosis is a recently discovered form of programmed cell death characterized by necroptotic and inflammatory features. It can be activated by the canonical pathway mediated by the caspase-1 dependent cleavage of Gasdermin D (GSDMD) and release of IL-1β, as well as the noncanonical pathway mediated by the inflammatory caspase, caspase-114. Targeting pyroptosis related cascades might be beneficial for the treatment of ischemic stroke, and many pyroptotic inhibitors, such as small-molecule compounds and traditional natural products, have been shown to be effective in experimental models5,6.
T817MA, also known as edonerpic maleate, is a recently synthesized compound with neurotrophic potential to the central nervous system7. It was shown to promote experience-dependent synaptic α-amino-3-hydroxy-5-methyl-4-isoxazole -propionic-acid receptor (APMAR) trafficking in the cortex, which is closely involved in neural plasticity and cortical reorganization8. In primary cultured cortical neurons, T817MA attenuated the oxidative stress-induced cytotoxicity by mitigating mitochondrial dysfunction and ROS production through a newly synthesized protein-mediated mechanism9. More recently, T817MA was shown to protect against traumatic brain injury (TBI) by regulating ionotropic glutamate receptors (iGluRs) signaling, reducing oxidative stress, and modulating collapsing response mediator protein 2 (CRMP2) and Arc expression10. However, the effect of T817MA on neuronal pyroptosis following ischemic stroke remains undetermined.
Sirt1, encoded by the SIRT1 gene located on chromosome 10 in humans, functions to remove acetyl groups from proteins, thereby regulating the activity of various transcription factors and proteins11. It is widely expressed in the central nervous system (CNS), including neurons, astrocytes, microglia, and brain microvascular endothelial cells (BMECs). Numerous studies have demonstrated the neuroprotective role of Sirt1 in ischemic stroke. Activation of Sirt1 can alleviate ischemia-induced neuronal damage through multiple mechanisms, such as promotion of mitochondrial biogenesis, inhibition of p53-mediated neuronal apoptosis, suppression of the nuclear factor -kappa B (NF-κB)-dependent neuroinflammation, and remove of damaged organelles and misfolded proteins via autophagy12. Given the multiple beneficial effects of Sirt1 in ischemic stroke, targeting Sirt1 has emerged as a potential therapeutic strategy. Activation of Sirt1 can be achieved through various means, such as the use of small molecule activators, caloric restriction, and exercise13,14,15.
In the present study, we investigated the effect of T817MA on brain damage caused by transient middle cerebral artery occlusion (MCAO), as well as the potential underlying mechanisms with focus on Sirt1-mediated inhibition of pyroptosis.
Materials and methods
Ethics and animals
Male Sprague-Dawley (SD) rats (weight: 300–350 g) were housed in a regular 12 h light/dark cycle with free access to food and water at a relatively constant temperature (approximately 22 ℃). The study was performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and reported in compliance with the ARRIVE guidelines. All experimental procedures used in this study were approved by the Animal Ethics Committee of Shaanxi Provincial People’s Hospital (Xi’an, China, No. 2022-038).
Middle cerebral artery occlusion (MCAO)
Brain ischemia was induced in rats using the intraluminal filament technique as previously described with modifications. Briefly, animals were anesthetized with 5% isoflurane in oxygen and maintained at 2-2.5% during surgery. Following midline neck incision, the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were carefully exposed. A 4 − 0 silicone-coated nylon monofilament was introduced into the ECA lumen and advanced 18–20 mm distal to the carotid bifurcation to occlude the middle cerebral artery origin. Cerebral blood flow reduction (> 70% baseline) was confirmed using laser Doppler flowmetry. After 60 min of occlusion, reperfusion was initiated by filament withdrawal under anesthesia. Sham-operated controls received identical procedures without filament insertion. To test the effects of T817MA on brain ischemia, animals were orally treated with 30 mg/kg T817MA for 20 days before MCAO. The maximum concentration of T817MA was observed in the plasma (Fig. S1A) and the brain (Fig. S1B) in animals within 1 h after the oral administration.
Experimental design
Experiment 1. The animals were divided into three groups: sham, MCAO and MCAO + T817MA. The sham group rats were subjected to surgical procedures without artery occlusion. The MCAO group rats were subjected to artery occlusion. The rats in MCAO + T817MA group were treated with chronic administration of T817MA and subjected to MCAO. Brain water content, expression of glial fibrillary acidic protein, (GFAP), ionized calcium binding adapter molecule 1 (Iba-1), Cleaved-Caspase-1, IL-1β and GSDMD were detected at 24 h after MCAO. Neurological dysfunction measurements were performed at 0, 1, 3 and 7 days after MCAO. Western blot was performed at 0, 3, 6, 12, 24 48 and 72 h following MCAO.
Experiment 2. The animals were divided into four groups: sham, MCAO, MCAO + T817MA and MCAO + T817MA + Sirtinol. The sham group rats were subjected to surgical procedures without artery occlusion. The MCAO group rats were subjected to artery occlusion. The rats in MCAO + T817MA group were treated with chronic administration of T817MA and subjected to MCAO. The rats in MCAO + T817MA + Sirtinol group were treated with chronic administration of T817MA, treated with Sirtinol and subjected to MCAO. Brain water content, expression of IL-1β and Cleaved-Caspase-1 were detected at 24 h after MCAO.
Measurement of brain edema
Brain water content was quantified using the gravimetric method to determine brain edema. Rats were euthanized under deep anesthesia (5% isoflurane) at predetermined timepoints post-injury. Brains were rapidly extracted (< 2 min) and divided into ipsilateral and contralateral hemispheres using a rodent brain matrix. Tissue samples were immediately weighed on pre-cooled aluminum foil to obtain wet weight (WW) using an analytical balance. Samples were then dehydrated in an oven at 105 °C for 72 h until constant weight was achieved, confirmed by three consecutive stable measurements at 24 h intervals. Dry weight (DW) was recorded, and brain water content (%) was calculated using the formula: [(WW - DW)/WW] × 100.
Modified neurological severity score (mNSS)
Neurological function was systematically evaluated using the mNSS scores at 0, 1, 3, and 7 days after MCAO. The mNSS encompasses motor, sensory, reflex, and balance assessments. For motor function, rats were observed for spontaneous movement and limb symmetry during walking. One point was deducted for each abnormal limb movement or dragging, with a maximum of 3 points. Sensory function was tested by gently touching the vibrissae on the contralateral side of the ischemic hemisphere; failure to turn towards the stimulus was scored as 1 point. Reflex integrity was examined through the forelimb and hindlimb withdrawal reflexes, where loss of reflex elicited 1 point per limb. Balance was evaluated by placing rats on a 12-mm-diameter wooden rod; inability to maintain posture for 30 s resulted in a 1-point deduction, with a maximum of 2 points. Total mNSS scores range from 0 (normal function) to 18 (severe neurological deficit), and all assessments were conducted by trained researchers blinded to the experimental groups to minimize bias.
Rotating pole test
Sensorimotor coordination was evaluated using a rotating pole apparatus according to established protocols with modifications16. Animals were required to traverse a 150-cm-long horizontal metallic rod (diameter 3 cm) rotating at predetermined angular velocities (3 or 6 revolutions per minute). Following three consecutive training trials (1 rpm, non-recorded), each subject underwent two timed test trials at increasing speeds with 15-min inter-trial intervals.
Immunohistochemistry
Coronal Sect. (20 μm) from paraformaldehyde-perfused brains were collected using a cryostat (Leica CM1950) at − 20 °C. After antigen retrieval in citrate buffer (pH 6.0, 95 °C, 20 min), sections were blocked with 10% normal goat serum in 0.3% Triton X-100/PBS for 1 h at room temperature (RT). Primary antibody incubation with anti-Iba-1 (1:100, ab153696), anti-GFAP (1:100, ab7260), anti-NeuN (1:200, ab177487), or anti-GSDMD (1:100, sc393581) was performed overnight at 4 °C in a humidified chamber. Following three PBS washes (5 min each), sections were incubated with Alexa Fluor 594- or 488-conjugated IgG (1:1000, Invitrogen, A-11005) for 2 h at RT. Nuclei were counterstained with DAPI (5 µg/mL, Sigma, 5 min), and slides were mounted with anti-fade medium. Fluorescence images were captured using a confocal microscope (Zeiss LSM 900) with identical exposure settings.
Measurement of IL-1β content
IL-1β levels in brain homogenates were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Rat IL-1β Quantikine ELISA, R&D Systems, #RLB00) following the manufacturer’s protocol.
Western blot
Equal amounts of protein (30 µg/lane) were separated on 10% SDS-PAGE gels and transferred to 0.45 μm PVDF membranes at 300 mA for 90 min. Membranes were blocked with 5% non-fat milk in TBST (Tris-buffered saline with 0.1% Tween-20) for 1 h at RT, then incubated overnight at 4 °C with following primary antibodies: anti-Cleaved-Caspase-1 (1:800, CST#89332), anti-Sirt1 (1:1000, ab110304) and anti-β-actin (1:5000, Sigma, A5441). After three TBST washes (10 min each), membranes were probed with HRP-conjugated secondary antibodies for 1 h at RT. Protein bands were visualized using chemiluminescent detection system.
Statistical analysis
Statistical analysis was performed using SPSS 16.0. Statistical evaluation of the data was performed by one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparisons.
Results
T817MA protects against ischemic brain injury
To investigate the effects of T817MA on brain ischemia, animals were orally treated with 30 mg/kg T817MA for 20 days before MCAO. Brain water content was measured to detect brain edema, and we found that the increased brain water content in cerebrum induced by MCAO was attenuated by T817MA (P = 0.0114, Fig. 1A). The results of mNSS scores showed that MCAO-induced neurological dysfunction was reduced by T817MA at 7 d (P = 0.0038), but not 1 or 3 d post ischemia (Fig. 1B). In addition, rotating pole test was used to evaluate the effects of T817MA on functional neurological recovery following brain ischemia. MCAO induced an increased time needed for traversing the rotating pole in both 3 rpm (Fig. 1C) and 6 rpm (Fig. 1D) tests. These motor-coordination deficits were attenuated by T817MA at 3 d in 3 rpm test (P = 0.0261, Fig. 1C) but not in 6 rpm test (Fig. 1D).
T817MA protects against ischemic brain injury. (A) T817MA reduces brain water content after brain ischemia. (B) T817MA reduces mNSS scores after brain ischemia. (C,D) T817MA promotes post-ischemia motor-coordination recovery in the rotating pole test at 3 rpm (C) and 6 rpm (D). n = 6 in each group. Data are shown as mean ± SD. #p < 0.05 vs. Sham group. *p < 0.05 vs. MCAO group.
T817MA attenuates neuroinflammation after brain ischemia
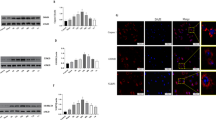
To investigate the effect of T817MA on microglia activation after MCAO, we performed immunostaining using Iba-1 antibody (Fig. 2A). The results showed that the increased number of Iba-1 positive cells induced by MCAO was reduced by T817MA (P = 0.0113, Fig. 2B). We also performed immunostaining with GFAP antibody to detect activation of astrocytes (Fig. 2C), and the number of GFAP positive cells in MCAO + T817MA group was lower than that in MCAO group (P = 0.002, Fig. 2D).
T817MA attenuates neuroinflammation after brain ischemia. (A and B) Immunostaining (A) and quantitative analysis (B) show that T817MA decreases the number of Iba-1 positive cells after brain ischemia. (C,D) Immunostaining (C) and quantitative analysis (D) show that T817MA decreases the number of GFAP positive cells after brain ischemia. Scale bar = 50 μm. n = 3 in each group. Data are shown as mean ± SD. #p < 0.05 vs. Sham group. *p < 0.05 vs. MCAO group.
T817MA inhibits neuronal pyroptosis after brain ischemia
The western blot analysis was performed to detect caspase-1 activation, and the results showed that the increased expression of cleaved-caspase-1 was markedly attenuated by T817MA (P = 0.007, Fig. 3A). As shown in Fig. 3B (P = 0.018), the increased level of IL-1β after MCAO was also reduced by T817MA treatment. In addition, we performed immunostaining using GSDMD antibody to detect pyroptosis (Fig. 3C), and the results showed that the increased number of GSDMD positive neurons induced by MCAO was significantly decreased by T817MA (P = 0.003, Fig. 3D).
T817MA inhibits neuronal pyroptosis after brain ischemia. (A) T817MA inhibits cleaved-caspase-3 expression after brain ischemia. (B) T817MA decreases the level of IL-1β after brain ischemia. (C,D) Immunostaining (C) and quantitative analysis (D) show that T817MA reduces the number of GSDMD positive neurons after brain ischemia. Scale bar = 50 μm. n = 3 (A,C,D) n = 6 (B) in each group. Data are shown as mean ± SD. #p < 0.05 vs. Sham group. *p < 0.05 vs. MCAO group.
T817MA prolongs Sirt1 activation after brain ischemia
To determine the potential involvement of Sirt1 in T817MA-induced protection, we detected the expression of Sirt1 using western blot (Fig. 4). In MCAO-treated group, the expression of Sirt1 increased from 3 h to 24 h post injury, and peaked at 6 h. However, T817MA treatment prolonged the Sirt1 activation to 48 h after MCAO, and the Sirt1 expression at 12 (P = 0.005), 24 (P = 0.006) and 48 h (P = 0.023) in T817MA group were higher than that in MCAO group.
Effects of Sirt1 Inhibition on T817MA-induced protection
To confirm the involvement of Sirt1 in our study, we repeated the above experiments after pretreated with the Sirt1 inhibitor sirtinol. The results of brain water content showed that sirtinol increased brain edema after MCAO and T817MA treatment (P = 0.005, Fig. 5A). The results of western blot showed that the T817MA-induced inhibition on caspase-1 cleavage was partially prevented by sirtinol (P = 0.029, Fig. 5B). As shown in Fig. 5C (P = 0.012), a similar result on IL-1β expression was also observed.
Effects of Sirt1 inhibition on T817MA-induced protection. (A) The reduced brain water content induced by T817MA was prevented by sirtinol. (B) The decreased expression of cleaved-caspase-1 induced by T817MA was prevented by sirtinol. (C) The decreased level of IL-1β induced by T817MA was prevented by sirtinol. N = 6 (A,C) and n = 3 (B) in each group. Data are shown as mean ± SD. *p < 0.05 vs. MCAO + T817MA group.
Discussion
The treatments for ischemic stroke have been far from satisfactory due to the complexity of the types of neuronal death, among which the newly characterized programmed cell death pyroptosis plays a key role6. In the present study, we identified T817MA as a neuroprotective agent against MCAO-induced pyroptosis in rats. We found that (a) T817MA attenuates brain edema and preserves neurological function following brain ischemia; (b) T817MA inhibits the MCAO-induced activation of microglia and astrocytes; (c) T817MA alleviates neuronal pyroptosis after brain ischemia; (d) T817MA prolongs Sirt1 activation to 48 h after brain ischemia; and (e) the sirt1 inhibitor sirtinol prevented the T817MA-induced effects on brain edema and neuronal pyroptosis.
As an inflammatory and lytic form of programmed cell death, pyroptosis plays dual regulatory roles in both exacerbating tissue damage and shaping post-stroke immune responses following brain ischemia17. In the acute stage of both ischemia and reperfusion, neurons in the core area of damage mainly suffered necrotic death, including necrosis, necroptosis and pyroptosis18. These necrotic neurons are characterized by ruptured cell membranes, which cause the release of cytokines and damage-associated molecular patterns (DAMPs), such as purine, high mobility group box 1 (HMGB1), heat shock protein, and peroxidase family proteins, leading to severe neuroinflammatory responses19. These inflammatory responses, as evidenced by activation of microglia, astrocytes and increased levels of IL-1β following MCAO, were all attenuated by T817MA, indicating its anti-inflammatory activity. Pyroptosis is primarily mediated by inflammasomes, such as NOD-like receptor family pyrin domain containing 3 (NLRP3), NLR family CARD domain containing 4 (NLRC4), and absent in melanoma 2 (AIM2), which activate caspase-1 or caspase-4/5/11, leading to cleavage of GSDMD20. A previous study showed that increased levels of caspase-1 mRNA and protein, as well as the cleaved caspase-1 protein, were found in the core area of ischemia in both neurons and glial cells21. As the executioner of pyroptosis, the activation of GSDMD aggravated brain damage and neurological dysfunction, and knockdown of GSDMD markedly inhibited cerebral infarction22. In patients, elevated levels of pyroptosis-related proteins, including GSDMD and caspase-1, in serum and cerebrospinal fluid (CSF) correlate with stroke severity and poor prognosis23. In the present study, MCAO-induced cleavage of caspase-1 was shown by western blot analysis, and the increased level of GSDMD was confirmed by immunostaining. These changes in pyroptosis related factors and neuroinflammatory responses following brain ischemia were reduced after T817MA treatment, suggesting that anti-pyroptotic activity might be involved in the T817MA-induced protection against experimental brain ischemia.
Sirt1, a NAD+-dependent deacetylase, has emerged as a critical regulator of mitochondrial function and cellular survival in ischemic stroke24,25. Recent studies highlight its neuroprotective effects through multiple mitochondrial pathways, though gaps remain in understanding its precise mechanisms and translational potential12. In a rat model of transient brain ischemia, Sirt1 overexpression mitigated mitochondrial dysfunction by upregulating PGC-1α, which in turn suppressed dynamin-related protein 1 (DRP1)-mediated excessive mitochondrial fission and preserved mitochondrial membrane potential26. As a neuroprotective agent, T817MA has been demonstrated to promote Sirt1 activation and regulate mitochondrial dynamics following subarachnoid hemorrhage in rats27. The dynamic changes in Sirt1 expression during different phases of ischemia (acute vs. chronic) and its cell-type-specific roles (neurons, astrocytes, microglia) require further exploration. In the present study, increased Sirt1 expression from 3 to 24 h after MCAO was observed, which was further prolonged by T817MA treatment. Mitochondrial dysfunction can promote pyroptosis through disturbance of tricarboxylic acid circulation, inhibition of oxidative phosphorylation, dysregulation of electron transport chain, as well as the abnormal calcium steady-state imbalance28. A recent study showed that sevoflurane alleviated cognitive dysfunction and neuronal pyroptosis in the hippocampus via activation of Sirt1 in a septic encephalopathy model29. In a rat model of rotenone-induced parkinsonism, linagliptin promoted the activation of Sirt1-Nrf2 pathway, and suppressed the NLRP3 inflammasome-dependent pyroptosis30. Similarly, our results showed that the Sirt1 inhibitor sirtinol partially prevented the protective effects of T817MA against brain ischemia, as well as its regulation of pyroptosis, as evidenced by expression of cleaved-caspase-1 and IL-1β. These data strongly suggest that T817MA-induced protection is dependent on Sirt1-mediated inhibition of pyroptosis.
There are some limitations to the present study. First, animals were orally pre-treated with T817MA for 20 days before MCAO. Although pretreatment with neuroprotective agents has demonstrated efficacy in experimental models of cerebral ischemia, its clinical translation faces significant limitations. A primary challenge lies in the unpredictable nature of stroke onset, which restricts the feasibility of administering drugs prophylactically in at-risk populations31. Also, chronic pretreatment may introduce systemic toxicity or off-target effects, as evidenced by the dose-dependent hepatotoxicity observed in prolonged use of NMDA receptor antagonists32,33. In addition, the Sirt1 inhibitor Sirtinol was used to investigate the involvement of Sirt1 in T817MA-induced protection. While inhibitors offer the advantage of pharmacological modulation and potential reversibility, they are not devoid of constraints. One significant limitation lies in the specificity and selectivity of these inhibitors, which may cross-react with other proteins, thereby complicating the interpretation of experimental results34. More experiments using siRNA-mediated knockdown of Sirt1 might be helpful for further understanding the role of Sirt1 in T817MA-induced protection.
Conclusion
In summary, our present study demonstrated that the protective agent T817MA protects against the MCAO-induced brain damage and inflammation in rats. These protective effects were associated with the Sirt1-mediated inhibition of neuronal pyroptosis.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Martin, S. S. et al. 2025 heart disease and stroke statistics: A report of US and global data from the American heart association. Circulation https://doi.org/10.1161/CIR.0000000000001303 (2025).
Peng, T. et al. Anti-inflammatory effects of traditional Chinese medicines on preclinical in vivo models of brain Ischemia-Reperfusion-Injury: prospects for neuroprotective drug discovery and therapy. Front. Pharmacol. 10, 204. https://doi.org/10.3389/fphar.2019.00204 (2019).
Cheng, W., Zhao, Q., Li, C. & Xu, Y. Neuroinflammation and brain-peripheral interaction in ischemic stroke: A narrative review. Front. Immunol. 13, 1080737. https://doi.org/10.3389/fimmu.2022.1080737 (2022).
Pan, Y. et al. Pyroptosis in development, inflammation and disease. Front. Immunol. 13, 991044. https://doi.org/10.3389/fimmu.2022.991044 (2022).
Wei, S., Feng, M. & Zhang, S. Molecular characteristics of cell pyroptosis and its inhibitors: A review of activation, regulation, and inhibitors. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms232416115 (2022).
Zheng, Y., Xu, X., Chi, F. & Cong, N. Pyroptosis: A newly discovered therapeutic target for Ischemia-Reperfusion injury. Biomolecules 12 https://doi.org/10.3390/biom12111625 (2022).
Hirata, K. et al. A novel neurotrophic agent, T-817MA [1-3-[2-(1-benzothiophen-5-yl) ethoxy] propyl-3-azetidinol maleate], attenuates amyloid-beta-induced neurotoxicity and promotes neurite outgrowth in rat cultured central nervous system neurons. J. Pharmacol. Exp. Ther. 314, 252–259. https://doi.org/10.1124/jpet.105.083543 (2005).
Abe, H. et al. CRMP2-binding compound, Edonerpic maleate, accelerates motor function recovery from brain damage. Science 360, 50–57. https://doi.org/10.1126/science.aao2300 (2018).
Fukushima, T., Koide, M., Ago, Y., Baba, A. & Matsuda, T. T-817MA, a novel neurotrophic agent, improves sodium nitroprusside-induced mitochondrial dysfunction in cortical neurons. Neurochem Int. 48, 124–130. https://doi.org/10.1016/j.neuint.2005.08.012 (2006).
Chen, T. et al. Edonerpic maleate regulates glutamate receptors through CRMP2- and Arc-mediated mechanisms in response to brain trauma. Cell. Death Discov. 8, 95. https://doi.org/10.1038/s41420-022-00901-0 (2022).
Manjula, R., Anuja, K. & Alcain, F. J. SIRT1 and SIRT2 activity control in neurodegenerative diseases. Front. Pharmacol. 11, 585821. https://doi.org/10.3389/fphar.2020.585821 (2020).
Tang, H. et al. New insights into Sirt1: potential therapeutic targets for the treatment of cerebral ischemic stroke. Front. Cell. Neurosci. 17, 1228761. https://doi.org/10.3389/fncel.2023.1228761 (2023).
Chang, N. et al. Emerging roles of SIRT1 activator, SRT2104, in disease treatment. Sci. Rep. 14, 5521. https://doi.org/10.1038/s41598-024-55923-8 (2024).
Verdin, E. AROuSing SIRT1: identification of a novel endogenous SIRT1 activator. Mol. Cell. 28, 354–356. https://doi.org/10.1016/j.molcel.2007.10.013 (2007).
Baur, J. A. Biochemical effects of SIRT1 activators. Biochim. Biophys. Acta. 1804, 1626–1634. https://doi.org/10.1016/j.bbapap.2009.10.025 (2010).
Dumbrava, D. A. et al. Mesenchymal stromal cell-derived small extracellular vesicles promote neurological recovery and brain remodeling after distal middle cerebral artery occlusion in aged rats. Geroscience 44, 293–310. https://doi.org/10.1007/s11357-021-00483-2 (2022).
Long, J. et al. Targeting pyroptosis as a preventive and therapeutic approach for stroke. Cell. Death Discov. 9 https://doi.org/10.1038/s41420-023-01440-y (2023).
Li, L. et al. Targeting pyroptosis to treat ischemic stroke: from molecular pathways to treatment strategy. Int. Immunopharmacol. 133, 112168. https://doi.org/10.1016/j.intimp.2024.112168 (2024).
Gou, X. et al. Pyroptosis in stroke-new insights into disease mechanisms and therapeutic strategies. J. Physiol. Biochem. 77, 511–529. https://doi.org/10.1007/s13105-021-00817-w (2021).
Ye, A. et al. Targeting pyroptosis to regulate ischemic stroke injury: molecular mechanisms and preclinical evidences. Brain Res. Bull. 165, 146–160. https://doi.org/10.1016/j.brainresbull.2020.10.009 (2020).
Li, Q., Dai, Z., Cao, Y. & Wang, L. Caspase-1 Inhibition mediates neuroprotection in experimental stroke by polarizing M2 microglia/macrophage and suppressing NF-kappaB activation. Biochem. Biophys. Res. Commun. 513, 479–485. https://doi.org/10.1016/j.bbrc.2019.03.202 (2019).
Zhang, D. et al. Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro. J. Neurosci. Res. 97, 645–660. https://doi.org/10.1002/jnr.24385 (2019).
Liu, L., Cai, Y. & Deng, C. Identification of ANXA3 as a biomarker associated with pyroptosis in ischemic stroke. Eur. J. Med. Res. 28, 596. https://doi.org/10.1186/s40001-023-01564-y (2023).
Petegnief, V. & Planas, A. M. SIRT1 regulation modulates stroke outcome. Transl Stroke Res. 4, 663–671. https://doi.org/10.1007/s12975-013-0277-y (2013).
Zhang, J. F., Zhang, Y. L. & Wu, Y. C. The role of Sirt1 in ischemic stroke: pathogenesis and therapeutic strategies. Front. Neurosci. 12, 833. https://doi.org/10.3389/fnins.2018.00833 (2018).
Xu, H. et al. SIRT1 regulates mitochondrial fission to alleviate high altitude hypoxia inducedcardiac dysfunction in rats via the PGC-1alpha-DRP1/FIS1/MFF pathway. Apoptosis 29, 1663–1678. https://doi.org/10.1007/s10495-024-01954-5 (2024).
Chen, W. W. et al. T817MA regulates mitochondrial dynamics via Sirt1 and Arc following subarachnoid hemorrhage. Neuroscience 531, 1–11. https://doi.org/10.1016/j.neuroscience.2023.06.020 (2023).
Yang, L. et al. Research progress of mitochondrial dysfunction induced pyroptosis in acute lung injury. Respir Res. 25, 398. https://doi.org/10.1186/s12931-024-03028-1 (2024).
Chen, H., Peng, Y., Wang, L. & Wang, X. Sevoflurane attenuates cognitive dysfunction and NLRP3-dependent caspase-1/11-GSDMD pathway-mediated pyroptosis in the hippocampus via upregulation of SIRT1 in a sepsis model. Arch. Physiol. Biochem. 128, 1413–1420. https://doi.org/10.1080/13813455.2020.1773860 (2022).
Ghaith, W. Z., Wadie, W. & El-Yamany, M. F. Crosstalk between SIRT1/Nrf2 signaling and NLRP3 inflammasome/pyroptosis as a mechanistic approach for the neuroprotective effect of linagliptin in parkinson’s disease. Int. Immunopharmacol. 145, 113716. https://doi.org/10.1016/j.intimp.2024.113716 (2025).
Dirnagl, U., Iadecola, C. & Moskowitz, M. A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 22, 391–397. https://doi.org/10.1016/s0166-2236(99)01401-0 (1999).
Espadinha, M. et al. Optimization of bicyclic lactam derivatives as NMDA receptor antagonists. ChemMedChem 12, 537–545. https://doi.org/10.1002/cmdc.201700037 (2017).
Matousova, M. et al. Pregn-5-en-3beta-ol and androst-5-en-3beta-ol Dicarboxylic acid esters as potential therapeutics for NMDA hypofunction: in vitro safety assessment and plasma stability. Steroids 147, 4–9. https://doi.org/10.1016/j.steroids.2018.09.012 (2019).
Martin, K. J. & Arthur, J. S. Selective kinase inhibitors as tools for neuroscience research. Neuropharmacology 63, 1227–1237. https://doi.org/10.1016/j.neuropharm.2012.07.024 (2012).
Funding
This study has been funded by the Shaanxi Province Natural Science Foundation Research Program (No.2021JM-554 and No.2022JM-587).
Author information
Authors and Affiliations
Contributions
Conceptualization was done by T. W. and J. M. L.; investigation was done by T. W., Y. J. P., X. W., and M. M. Z.; data collection and organization were conducted by W. L.; original draft was written by T. W.; review and editing were performed by J. M. L. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, T., Pan, YJ., Zhang, MM. et al. T817MA protects against inflammation and pyroptosis in response to brain ischemia via activating Sirt1 signaling. Sci Rep 15, 33600 (2025). https://doi.org/10.1038/s41598-025-18871-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18871-5