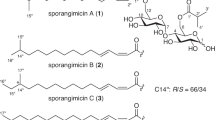
Abstract
Azithromycin (AZT), a persistent macrolide antibiotic, is an emerging environmental contaminant due to its high aqueous stability and role in antimicrobial resistance. In this study, natural serpentinite was chemically exfoliated using dimethyl sulfoxide (DMSO), dimethylformamide (DMF), and urea (U) to enhance surface reactivity and adsorption performance toward AZT. Textural characterization revealed a marked increase in surface accessibility, with BET area rising from 6.1 m²/g (raw) to 23.7 m²/g (DMSO/SP), 18.8 m²/g (DMF/SP), and 15.3 m²/g (U/SP), while pore diameters expanded from 11.1 nm to ~ 16 nm, exposing more reactive hydroxyl groups. Batch adsorption experiments showed pH-dependent uptake, with maximum removal at pH 9. Adsorption kinetics followed the pseudo-first-order model (R² > 0.90), and intra-particle diffusion confirmed a multi-stage adsorption pathway. Statistical physics modeling revealed saturation capacities (Qsat) of 329.7 mg/g for DMSO/SP, 292.3 mg/g for DMF/SP, and 279.9 mg/g for U/SP, confirming the superior adsorption of DMSO-assisted exfoliation. Active site density (Nm) reached 109.5 mg/g for DMSO/SP, compared to 77.5 mg/g for DMF/SP and 84.3 mg/g for U/SP. The number of AZT molecules per site (n) exceeded unity, indicating multi-molecular stacking: DMSO/SP: 3.01–3.44, DMF/SP: 3.77–4.41, U/SP: 3.32–4.40 molecules/site. The mean adsorption energy (ΔE ≈ 5 kJ/mol) confirmed a reversible, exothermic physisorption process dominated by electrostatic attraction. Expanded interlayer spacing facilitated multilayer stacking, while hydroxyl groups promoted hydrogen bonding, creating a synergistic mechanism for enhanced retention. These findings show solvent polarity and molecular geometry governs serpentinite delamination, site density, and adsorption, with DMSO producing the most open, high-capacity adsorbent.
Similar content being viewed by others
Introduction
In the twenty-first century, environmental challenges such as air, soil, and water pollution, along with the accelerating impacts of climate change, have become critical global concerns. Among these, water quality deterioration has gained significant attention due to its effects on ecosystem integrity and public health1,2. Organic and inorganic pollutants contaminate aquatic environments through their toxicity, carcinogenicity, and persistence against natural biodegradation3,4. Pharmaceutical compounds have emerged as major contaminants, increasingly detected in trace amounts and posing serious risks to ecosystems and human health5,6. Among them, antibiotics—widely used in humans, livestock, and aquaculture—are of particular concern1,7. Their rising consumption, driven by population growth and expanded healthcare services, has reached nearly 100,000 tons annually8,9. Unregulated use has led to widespread contamination of water, soils, and food products, fostering antibiotic-resistant bacteria10,11,12 and driving multidrug-resistant infections that threaten ecological balance and undermine conventional treatments worldwide13,14.
Among various antibiotics, azithromycin (AZT) is a second-generation macrolide known for its broad-spectrum antimicrobial activity and enhanced acid stability15,16,17. As a semisynthetic derivative of erythromycin, it was developed to overcome the poor acid resistance and limited bioavailability of its precursor18. AZT is widely used against Gram-positive, Gram-negative, and atypical pathogens, particularly for respiratory and reproductive infections19,20, and gained unprecedented prominence during the COVID-19 pandemic despite limited antiviral evidence21. Due to its limited absorption and minimal metabolism, about 70% of administered AZT is excreted unchanged22, allowing substantial amounts to enter wastewater systems and ultimately reach aquatic environments23. There, AZT disrupts microbial communities and promotes antibiotic-resistant bacteria, posing serious public health risks as infections become harder to treat20. Its stability and persistence enable long-term accumulation in water bodies, intensifying ecological impacts24, leading the U.S. EPA to classify AZT as an emerging pollutant25 and the European Union to list it as a concern for aquatic environments26, and underscoring the urgent need for advanced treatment technologies.
Numerous strategies have been explored for removing azithromycin (AZT) from contaminated water, including adsorption27, membrane filtration28, photocatalytic degradation29, chemical oxidation30, and biological processes31. Among these, adsorption is the most promising due to its simplicity, low energy demand, absence of harmful by-products, broad applicability, and high efficiency in eliminating antibiotic residues32,33,34. Its performance depends on the physicochemical properties of the adsorbent; thus, developing more efficient materials remains a priority. Various adsorbents—activated carbon35, hydrogels36, graphene oxide37, zero-valent iron38, carbon nanotubes39, metal–organic frameworks40, zeolites41, and clay minerals42 —have been used, yet many face challenges such as reduced efficiency under real conditions, secondary pollution risks, high production costs, and limited regeneration, restricting large-scale application43,44,45.
In recent years, considerable research has focused on developing synthetic adsorbents derived from naturally abundant resources, which have shown high efficiency in removing organic contaminants, including antibiotics, from aqueous systems, either as standalone materials or in hybrid forms46,47,48. Among the most promising options, clay-based materials have been widely studied as cost-effective and environmentally sustainable adsorbents for eliminating both organic and inorganic pollutants49,50,51. Clay minerals are naturally occurring layered silicates comprising tetrahedral silicate sheets linked to octahedral hydroxide sheets52,53. Isomorphic substitutions of aluminum (Al³⁺) with magnesium (Mg²⁺) or iron (Fe²⁺/Fe³⁺) generate permanent negative charges, balanced by exchangeable interlayer cations (e.g., Na⁺, Ca²⁺), resulting in a high cation-exchange capacity (CEC) essential for adsorption54,55,56. Structurally, they are classified as 1:1 phyllosilicates (kaolinite, serpentinite) or 2:1 types (montmorillonite, illite), with abundant reactive groups (Si–OH, Al–OH, Mg–OH), natural availability, and chemical stability, making them sustainable and efficient adsorbents for water treatment54,56,57.
To enhance the surface chemistry and optimize the physicochemical properties of clay minerals, various modification strategies have been developed to improve their adsorption performance for both organic and inorganic contaminants48,58,59. These techniques include alkaline treatments, thermal activation, acid leaching, metal-ion pillaring, incorporation of metal oxides, exfoliation, polymer intercalation, structural scrolling, and organic functionalization with surfactants such as cetyltrimethylammonium bromide (CTAB) or natural polymers like starch48,60,61. Such modifications increase specific surface area, enlarge pore volume, alter surface charge, and introduce new reactive functional groups, thereby enhancing the adsorption affinity of clay minerals and enabling efficient removal of a wider range of pollutants from aqueous environments.
The exfoliation modification delaminates layered clay minerals into nanoscale sheets, producing materials with much higher surface area, enhanced adsorption, and improved dispersion properties62,63. This process increases the accessibility of reactive sites, improves ion-exchange capacity, and strengthens chemical interactions with target contaminants64,65. While significant advances have been made in exfoliation and organo-functionalization, most studies focus on aluminosilicate-based clays, such as bentonite and kaolinite, due to their abundance and well-characterized structures48,66. In contrast, magnesium-rich clays like serpentinite remain underexplored despite their distinct magnesium-dominated crystal chemistry, which alters surface reactivity and interlayer behavior, highlighting the need for further functionalization studies to realize their full adsorption potential54,66.
Natural serpentinite (SP, Mg₃Si₂O₅(OH)₄) is a 1:1 layered phyllosilicate mineral formed through the hydrothermal alteration of magnesium-rich silicate minerals such as olivine and pyroxene. It occurs in three polymorphic forms with distinct structural configurations: lizardite, antigorite, and chrysotile54,67,68. Structurally, serpentinite consists of an octahedral Mg–OH sheet bonded to a tetrahedral Si–O sheet, with possible isomorphic substitutions by Fe, Ni, or Al69. Abundant hydroxyl (-OH) groups and surface-bound water molecules contribute to moderate adsorption capacity via hydrogen bonding and electrostatic interactions. However, pristine serpentinite exhibits low specific surface area, limited pore volume, and narrow interlayer spacing, restricting the adsorption and diffusion of molecules48,68. Chemical or physical modification is therefore essential to enhance surface reactivity, increase porosity, and optimize pore size, significantly improving its adsorption potential. Functional, structural, and morphological modification via exfoliation and delamination offers a promising strategy to develop efficient, sustainable, and low-cost adsorbents for antibiotic removal from wastewater48,62.
Common exfoliation techniques for layered clay minerals include high-pressure extrusion, ultrasonic sonication, chemical intercalation, and mechanical grinding62,70. Among these, chemical intercalation is widely recognized as one of the most effective methods for peeling and delaminating of clay minerals65,71. Various intercalating agents, such as N-methylformamide, dimethyl sulfoxide, urea, alkylamines, potassium acetate, formamide, fatty acids, quaternary ammonium salts, and hydrazine hydrate, have been employed71,72. Introducing organic guest molecules into the interlayer spaces expands basal spacing while weakening the hydrogen-bonding network between adjacent clay layers, reducing interfacial adhesion and facilitating exfoliation for improved structural dispersion63,72. Thus, the structure, physicochemical properties, and morphology of exfoliated kaolinite are strongly influenced by the type of intercalating agents used71,72,73. However, there is still a lack of systematic studies linking intercalating agents with the structural evolution and adsorption performance of exfoliated serpentinite, highlighting a key research gap.
To date, and to the best of our knowledge, no systematic investigation has examined the controlled exfoliation and delamination of magnesium-rich serpentinite using multiple chemical intercalating agents. This study addresses this gap by introducing a facile, scalable exfoliation strategy employing three reagents—dimethyl sulfoxide (DMSO), dimethylformamide (DMF), and urea—to tailor the structural, morphological, and physicochemical properties of serpentinite. Unlike conventional approaches, it establishes a direct link between the intercalating agent, the degree of delamination, and the resulting adsorption performance, offering new insights into modifying magnesium-rich clay minerals, which remain underexplored compared to aluminosilicate clays. The exfoliated serpentinite products were developed as sustainable adsorbents for the efficient removal of azithromycin (AZT), an emerging pharmaceutical pollutant of global concern. Their adsorption performance was systematically evaluated under key parameters, and the mechanism was elucidated through steric and energetic analyses using advanced equilibrium models based on statistical physics theory. This study provides the first comparative exfoliation of serpentinite with different organic reagents, revealing how reagent-specific modifications govern adsorption efficiency, and pioneers the integration of magnesium-rich clay mineral engineering with statistical physics modeling for low-cost, eco-friendly adsorbent design.
Experimental work
Materials
The serpentine powder (SP) raw mineral was supplied serpentine quarry, Eastern desert, Egypt. The exfoliation process employed dimethyl sulfoxide (DMSO; CAS No. 67-68-5) with a purity greater than 99.5%, N, N-dimethylformamide (DMF), and urea (U; purity 99%), all of which were obtained from Sigma-Aldrich. Sodium hydroxide (NaOH) pellets and nitric acid (HNO₃) solution were used to adjust the pH during the modification process. Azithromycin (C₃₈H₇₂N₂O₁₂·2 H₂O; molecular weight: 785 g/mol), used in the adsorption as well as photocatalytic experiments, was acquired from Sigma-Aldrich supplier.
Exfoliation of serpentinite
Exfoliation by DMSO (DMSO/SP) and DMF (DMF/SP)
Raw serpentinite powder was first subjected to mechanical activation using a planetary ball mill (Fritsch Pulverisette 7, Germany) for 6 h at 400 rpm with zirconia balls (10 mm diameter, ball-to-powder ratio 10:1). This step reduced the particle size to approximately 20–100 μm. For DMSO-assisted exfoliation, 10 g of the milled powder was dispersed in 100 mL of DMSO/deionized water mixture (8:1 v/v). The suspension was continuously stirred magnetically at 600 rpm for 120 h while being intermittently exposed to ultrasonic irradiation (240 W, 40 kHz; Elmasonic S 120 H, Elma, Germany) for 20 min every 6 h to facilitate interlayer disruption. After this stage, the dispersion was subjected to extended ultrasonication for an additional 24 h to enhance delamination and promote the formation of thin silicate nanosheets. The combined action of shear forces and solvent intercalation weakened the hydrogen bonding between the octahedral and tetrahedral layers, leading to significant exfoliation of the serpentinite structure.
The resulting product (denoted as DMSO/SP) was separated by centrifugation at 6000 rpm for 15 min, repeatedly washed with distilled water until a neutral pH was reached to ensure removal of residual DMSO, and then dried in a convection oven at 65 °C for 12 h. The dried powder was stored in airtight glass vials for further characterization and adsorption studies (Fig. 1).
For DMF-assisted exfoliation, the same procedure was followed, substituting DMSO with DMF while keeping all other processing conditions identical. The obtained material was designated as DMF/SP (Fig. 1).
Exfoliation by urea (U/SP)
The urea-assisted exfoliation of serpentinite was carried out following the procedure adapted from Ref73. , with modifications to optimize reproducibility. In this method, 6 g of finely ground serpentinite powder (20–100 μm) was thoroughly mixed with 3 g of analytical grade urea in an agate mortar for 15 min to ensure uniform dispersion of urea within the mineral particles. The homogeneous mixture was then transferred to a programmable muffle furnace (Nabertherm L5/11, Germany) and subjected to thermal treatment at 95 °C for 48 h under static air atmosphere. This treatment facilitated the intercalation of urea molecules into the interlayer galleries of the silicate framework, weakening the inter-sheet hydrogen bonds.
After thermal treatment, the solid residue was collected by vacuum filtration and sequentially washed with distilled water (3 × 10 min under magnetic stirring) to remove surface-adsorbed urea. A final rinse with ethanol was performed to further eliminate any remaining organic traces. The washed product was then oven-dried at 60 °C for 24 h. To achieve effective exfoliation, the dried intermediate was subjected to post-thermal activation at 120 °C for 1 h, which promoted expansion and partial separation of the silicate layers. The final exfoliated product was designated as U/SP and stored in airtight containers for subsequent characterization and adsorption studies (Fig. 1).
Analytical techniques
A comprehensive suite of analytical techniques was employed to characterize the structural, chemical, morphological, and textural properties of the exfoliated serpentinite-based adsorbents (DMSO/SP, DMF/SP, and U/SP). Crystallinity and phase composition were examined using X-ray diffraction (XRD) on a PANalytical Empyrean diffractometer equipped with Cu Kα radiation (λ = 1.5406 Å) operated at 40 kV and 40 mA. Diffraction patterns were recorded in the 2θ range of 5°–80° with a step size of 0.02° and a scanning rate of 2° min⁻¹, allowing accurate identification of crystalline phases and monitoring of structural ordering following exfoliation. Chemical functionality was investigated by Fourier-transform infrared (FTIR) spectroscopy using a Shimadzu FTIR-8400 S spectrometer. Spectra were collected in the range of 400–4000 cm⁻¹ with a resolution of 4 cm⁻¹ and 32 scans per sample. This analysis provided information on surface functional groups, bonding environments, and possible changes in interlayer interactions induced by the exfoliation agents. Morphological features were visualized by scanning electron microscopy (SEM) using a Zeiss Gemini Ultra 55 instrument. Prior to imaging, samples were sputter-coated with a thin gold layer (~ 5 nm) under vacuum to improve conductivity and image resolution. This enabled detailed observation of the surface morphology and exfoliation-induced structural modifications. Textural properties were determined from nitrogen adsorption–desorption isotherms measured at 77 K using a Beckman Coulter SA 3100 surface area and porosity analyzer. Specific surface areas were calculated by the Brunauer–Emmett–Teller (BET) method, while pore volume and pore size distribution were derived using the Barrett–Joyner–Halenda (BJH) approach from desorption branches. These measurements provided quantitative insights into the porosity development and surface accessibility of the modified serpentinite adsorbents.
Adsorption studies
Batch adsorption studies
The adsorption efficiency of the exfoliated serpentinite-based materials (DMSO/SP, DMF/SP, and U/SP) toward azithromycin (AZT) was evaluated through a systematic series of batch adsorption experiments. The experiments were designed to investigate the influence of key operational variables on adsorption performance, including solution pH, initial AZT concentration, contact time, and temperature. For each run, 100 mL of AZT solution of the desired concentration (50–400 mg/L) was placed in 250 mL Erlenmeyer flasks, with the adsorbent dosage fixed at 0.2 g L⁻¹. The effect of pH was studied in the range of 3.0–9.0, adjusted using 0.1 M HCl or 0.1 M NaOH. Contact time studies were performed over intervals from 30 to 960 min, while thermodynamic experiments were conducted at 303, 313, and 323 K to evaluate temperature dependence.
All adsorption experiments were carried out in a thermostatically controlled orbital shaker (150 rpm) to ensure homogeneity and accurate temperature regulation. At the end of each experiment, the suspensions were filtered through 0.45 μm PTFE membranes, and the residual AZT concentration was quantified by high-performance liquid chromatography (HPLC; Merck/Hitachi L-6200 A system). The equilibrium adsorption capacity (Qe, mg/g) was calculated using the mass balance equation:
where C0 and Ce (mg/L) are the initial and equilibrium AZT concentrations, respectively, V (L) is the solution volume, and m (g) is the mass of the adsorbent.
All adsorption experiments were performed in triplicate (n = 3) using independent aliquots from the same adsorbent batch. Reported values are averages, and error bars represent the standard deviation of these replicates. For theoretical model fittings, smooth curves are presented, while the corresponding experimental data include error bars.
Theoretical traditional and advanced equilibrium studies
To elucidate the adsorption mechanisms and thermodynamic behavior of AZT uptake by the SPNT-based adsorbents, both conventional and advanced equilibrium modeling approaches were employed. Traditional adsorption isotherms, including Langmuir, Freundlich models, were used alongside kinetic models such as pseudo-first-order and pseudo-second-order equations. These were fitted to the experimental data using non-linear regression methods to obtain the best fit parameters. To further deepen the theoretical interpretation, advanced equilibrium models grounded in the principles of statistical physics were also applied. These models provide a molecular-level understanding of the adsorption process, including insights into the adsorption energy, steric parameters, and the nature of the adsorbate–adsorbent interactions.
The qualities of fit and predictive accuracy of the applied models were quantitatively evaluated using several statistical metrics. For classical models, the coefficient of determination (R²) and the Chi-squared (χ²) test were utilized, as defined by Eqs. (2) and (3), respectively. For the advanced models, the root mean square error (RMSE), along with R², was used to assess the reliability of the model predictions, as expressed in Eq. (4). Here, Qe,exp and Qe,cal represent the experimental and calculated adsorption capacities, Q,e,mean is the mean experimental value, m' denotes the number of observations, and p indicates the number of adjustable model parameters:
Results and discussion
Characterization of the adsorbent
XRD analysis
X-ray diffraction (XRD) analysis was employed to characterize the mineralogical composition and crystallinity of raw serpentinite and its exfoliated derivatives. The diffraction pattern of the raw serpentinite (Fig. 2A) revealed the coexistence of two serpentine polymorphs—antigorite and chrysotile—as major constituents48. The presence of antigorite was confirmed by its characteristic basal reflection at 2θ ≈ 12.4°, attributed to the (001) crystallographic plane, accompanied by other distinct peaks at 2θ ≈ 24.5° ((102)), 35.8° ((131)), and 60.2° ((060)) (Fig. 2A). These reflections align well with standard reference data (COD ref. code: 96-900-3104; XRD Cd. No. 00-007-0417), verifying the identity and structural integrity of antigorite. Meanwhile, the peak observed at 2θ ≈ 19.6° corresponds to the (020) plane of chrysotile, as indexed in XRD Cd. No. 00-010-0380 (Fig. 2A). In addition to the serpentine phases, the diffraction pattern displayed peaks corresponding to secondary mineral impurities. These include talc, identified by a basal peak at 2θ ≈ 9.5° ((001)); chlorite at 2θ ≈ 26.7° ((001)); and carbonate phases such as calcite and dolomite, characterized by reflections at 2θ ≈ 29.5° ((104)) and 31.1° ((104)), respectively (Fig. 2A). These accessory minerals are commonly associated with serpentinized ultramafic rocks and are indicative of secondary alteration and carbonation processes that may occur during low-temperature hydrothermal interactions74,75.
Upon chemical modification using intercalating exfoliation agents—namely dimethyl sulfoxide (DMSO), dimethylformamide (DMF), and urea—significant alterations in the XRD patterns were observed (Fig. 2B–D). All modified samples exhibited noticeable changes in the diffraction peak intensities and slight shifts in peak positions, indicative of structural disruption, interlayer expansion, and partial delamination of the serpentine layers48,73. A closer examination of the XRD profiles reveals distinct differences in the degree of structural alteration imparted by each exfoliating agent, confirming the influence of solvent chemistry and polarity on the exfoliation mechanism. In the DMSO-treated sample (DMSO/SP, Fig. 2B), a significant reduction in the intensity of the antigorite peaks is evident—most notably the (001), (102), and (131) reflections. The (001) basal reflection, originally observed at 2θ ≈ 12.4° in the raw sample, is considerably weakened, suggesting a partial collapse or broadening of the basal spacing, likely due to interlayer expansion (Fig. 2B). Furthermore, the overall peak profile appears broader and less defined, indicating decreased crystallinity and enhanced structural disorder. This outcome is consistent with the strong dipolar character and high solvation capacity of DMSO, which allows it to penetrate the interlayer spaces more effectively and disrupt hydrogen bonding between adjacent serpentine layers76,77,78.
The DMF-treated serpentinite (DMF/SP, Fig. 2C) displays intermediate behavior. While the antigorite peaks—especially at 2θ ≈ 24.5° and 35.8°—are notably reduced, they are not as severely diminished as those in the DMSO-treated sample. This suggests a moderate exfoliation effect, attributed to the lower polarity and slightly larger molecular structure of DMF compared to DMSO, which limits its penetration into the tightly stacked layers. Nevertheless, the peak broadening and intensity decline still point to a disruption of long-range crystallographic order, indicating successful partial exfoliation.
The urea-treated sample (U/SP, Fig. 2D) exhibits the least pronounced structural changes among the three modified variants. The main peaks of antigorite and chrysotile are still observable, albeit with reduced intensity, suggesting that urea induces only partial exfoliation or weak intercalation. This behavior may be attributed to urea’s neutral polarity and hydrogen-bonding tendency, which favors surface-level interactions rather than deep intercalation72,79. Nevertheless, the observed attenuation in peak intensity—particularly at 2θ ≈ 24.5° and 35.8°—still indicates a measurable structural modification compared to the raw serpentinite. The minimal shift in peak positions implies that the basal spacing is only slightly affected, potentially due to surface swelling without full delamination.
The pronounced exfoliation achieved by DMSO treatment suggests a substantial increase in surface area, exposure of active hydroxyl groups, and creation of interlayer voids—parameters that directly enhance the adsorption of pollutants, particularly large or complex organic molecules. The moderate exfoliation observed in the DMF variant may be suitable for target-specific adsorption, where a balance between structural integrity and surface accessibility is desired. Conversely, the urea-modified sample, while less disrupted, may still offer sufficient reactive sites for adsorption of smaller ions or act as a support matrix for further functionalization.
FTIR analysis
Fourier Transform Infrared (FTIR) spectroscopy was conducted to examine the molecular structure, functional groups, and surface chemistry of raw and chemically modified serpentinite samples. The technique offers sensitive insight into both the preservation and disruption of structural features during solvent-assisted exfoliation. The FTIR spectra of raw serpentinite (A) and its modified derivatives—DMSO/SP (B), DMF/SP (C), and U/SP (D) are presented in Fig. 3.
The spectrum of the pristine serpentinite (Fig. 3A) displays a series of sharp and well-resolved bands that are characteristic of serpentine group minerals. A dominant absorption band at 3682 cm⁻¹ is assigned to the stretching vibrations of structural hydroxyl groups (Mg–OH) within the octahedral sheets, which are engaged in hydrogen bonding across the interlayer spacing80. This band serves as a diagnostic marker of layered phyllosilicates such as antigorite and chrysotile. In the region between 850 and 1200 cm⁻¹, broad bands are observed corresponding to the Si–O–Si and Si–O–Mg stretching modes, confirming the integrity of the tetrahedral silicate framework48. The lower-frequency region (400–850 cm⁻¹) contains additional diagnostic peaks. Notably, a band near 615 cm⁻¹ is attributed to Si–O–Mg bending vibrations, while the band at 412 cm⁻¹ is associated with Si–O deformation (Fig. 3A). In some reports, these bands have also been linked to Mg–O stretching and Mg–OH bending modes, reflecting interactions between hydroxyl groups and octahedrally coordinated magnesium81,82. These vibrational features are particularly relevant for evaluating the structural units that govern adsorption via ion exchange and surface complexation.
Following exfoliation, all three modified samples exhibited notable alterations in their FTIR spectra, particularly in the hydroxyl stretching and silicate framework regions, indicating effective intercalation and partial disruption of the serpentinite lattice (Fig. 3B-D). Among the modified samples, DMSO/SP (Fig. 3B) showed the most pronounced spectral shifts. The O–H stretching band shifted from 3682 to 3677 cm⁻¹, accompanied by discernible broadening, indicating strong hydrogen bonding interactions between the sulfonyl group (–S = O) of DMSO and the inner Mg–OH units of the silicate layers76,83. This interaction suggests deep intercalation of DMSO molecules, leading to partial delamination of the silicate layers and expansion of the interlayer spacing. Concurrently, the Si–O stretching band shifted from approximately 950 to 940 cm⁻¹, signifying distortion of the tetrahedral silicate sheets. Additionally, the Si–O–Mg vibration at 615 cm⁻¹ was downshifted to 606 cm⁻¹, reflecting layer weakening and partial amorphization. These spectral changes are consistent with significant structural disorder and exfoliation, which are likely to enhance the material’s adsorption performance due to increased surface area, porosity, and exposure of reactive functional groups.
The DMF-exfoliated serpentinite (DMF/SP) (Fig. 3C) showed comparable, though less intense, vibrational changes. The O–H stretching band exhibited a minor redshift to 3680 cm⁻¹, with slight broadening, suggesting moderate hydrogen bonding between the carbonyl group of DMF and the Mg–OH sites84. The Si–O stretching band shifted to 943 cm⁻¹, while the band at 615 cm⁻¹ was shifted to 609 cm⁻¹, indicating partial intercalation and structural rearrangement. Despite these modifications, the retention of relatively sharp bands implies that the overall crystalline framework is partially preserved, with limited chemical disruption compared to the DMSO treatment.
In contrast, the urea-modified sample (U/SP) (Fig. 3D) exhibited the least degree of spectral alteration. The O–H stretching band remained essentially unchanged at 3682 cm⁻¹, with negligible broadening, indicating weak or superficial hydrogen bonding interactions. The Si–O stretching band showed a marginal shift to 947 cm⁻¹, while the Si–O–Mg vibration moved only slightly from 615 to 610 cm⁻¹. These subtle changes suggest that urea primarily interacts with the outer surface hydroxyls rather than penetrating into the interlayer space. This surface-level adsorption without intercalation results in minimal structural distortion or exfoliation, as also corroborated by the XRD findings.
The comparative analysis of the FTIR spectra is in full agreement with the XRD results and provides deeper mechanistic insight into the exfoliation behavior of the three solvents. The extent of structural modification and interlayer interaction appears to depend not only on the polarity of the exfoliating agent but also on its molecular geometry and functional group chemistry. DMSO, with its strong polarity and electron-withdrawing sulfonyl group, demonstrates the most significant structural impact, achieving both intercalation and chemical disruption83,85,86. This process generates disordered silicate layers and exposes numerous active sites—hydroxyl groups—that can serve as adsorption centers for a variety of contaminants. DMF, while capable of moderate intercalation, induces less disruption due to its lower polarity and steric hindrance from its dimethyl amide group. Nevertheless, it still introduces structural disorder and moderate exfoliation, offering a trade-off between activation and retention of lattice stability. In contrast, urea, despite its dual hydrogen bonding capability (via –NH₂ and C = O groups), has limited interaction with the serpentinite structure79. Its strong hydration shell and low interlayer compatibility result in surface-level interaction without meaningful exfoliation or chemical activation.
EDX analysis
The elemental composition of pristine and modified serpentinite samples was examined using energy-dispersive X-ray spectroscopy (EDX) to evaluate the effects of intercalation and surface modification on the material’s chemical structure (Fig. S1). The pristine serpentinite exhibited an elemental profile characteristic of phyllosilicate minerals, with a composition dominated by oxygen (O, 49%), magnesium (Mg, 26.7%), and silicon (Si, 21.3%), reflecting its fundamental magnesium silicate framework. Minor trace elements, including iron (Fe, 1.2%), aluminum (Al, 1.0%), carbon (C, 0.7%), and sulfur (S, 0.1%), were also detected, most likely originating from structural impurities, accessory minerals, or naturally incorporated substitutional elements within the silicate lattice. This composition aligns well with the theoretical stoichiometry of serpentine-group minerals, confirming the integrity and purity of the raw material prior to chemical modification.
Following chemical modification through organic intercalation, substantial changes in the elemental distribution were observed, reflecting successful surface functionalization and molecular incorporation (Fig. S1). In the case of DMSO-intercalated serpentinite (DMSO/SP), there was a notable increase in carbon content, accompanied by a marked decrease in the relative proportions of the major inorganic constituents (O, Si, and Mg) (Fig. S1). This shift can be attributed to the introduction of DMSO molecules into the interlayer region, where their sulfonyl (–S = O) and methyl groups bind strongly to Mg–OH sites, partially displacing structural oxygen and altering the stoichiometric balance.
Similar compositional trends were evident in DMF/SP and U/SP, although the magnitude of the changes varied, reflecting differences in intercalation efficiency and molecular interaction strength (Fig. S1). The increase in carbon content across all modified samples provides direct evidence of organic incorporation, confirming that DMSO, DMF, and urea molecules are effectively immobilized within the serpentinite matrix. The decline in silicon and magnesium signals suggests that the intercalated molecules partly obscure the elemental signals from the silicate lattice, highlighting the formation of a surface-bound organic layer that alters the mineral’s accessible chemical composition.
These compositional modifications have significant implications for the surface chemistry, adsorption capacity, and interfacial properties of the treated serpentinite. The introduction of organic functional groups from DMSO, DMF, and urea enhances the material’s surface heterogeneity and hydrophobicity, which can improve its affinity toward specific pollutants through hydrogen bonding, electrostatic interactions, or π–π stacking mechanisms, depending on the target contaminant. Moreover, the reduction in exposed inorganic sites may alter the mineral’s cation-exchange behavior, thereby influencing its selectivity.
SEM analysis
The scanning electron microscopy (SEM) analysis provides detailed insight into the morphological evolution of serpentinite subjected to chemical modification with DMSO, DMF, and urea, highlighting significant exfoliation, delamination, and in some cases, scrolling effects (Fig. 4). The pristine serpentinite exhibits its intrinsic layered morphology, comprising densely packed platy particles with minimal surface irregularities (Fig. S2). At low magnification, the material appears compact and highly ordered, reflecting the strong interlayer hydrogen bonding and van der Waals forces that stabilize the Mg–OH octahedral and Si–O tetrahedral sheets (Fig. S2.A). At higher magnification, elongated, flakey textures are observed, arranged in tightly bound aggregates, leaving only limited exposure of internal reactive surfaces (Fig. S2. B). This compact and well-crystallized architecture corresponds to the typical structural organization of serpentine-group minerals, where the close packing of the silicate layers restricts porosity and reduces the number of accessible adsorption sites48.
After chemical modification, all treated samples—DMSO/SP, DMF/SP, and U/SP—exhibit considerable morphological changes, with the original compact texture replaced by a more open and porous architecture (Fig. 4). Across all modified samples, the SEM images reveal distinctly separated and intersected nano-rods or needle-like particles, indicating that intercalation of the organic molecules successfully weakened the cohesive forces between adjacent structural units (Fig. 4). This separation demonstrates clear exfoliation and delamination, accompanied by localized rearrangement of the silicate layers. The particles show considerable scrolling or curling, suggesting mechanical relaxation and rolling of delaminated sheets as a result of internal stress release during the intercalation process.
For DMSO/SP, these morphological changes are most pronounced. At lower magnification, the particle network becomes visibly more open with enhanced void formation and reduced aggregation compared to the pristine sample (Fig. 4A). At higher magnifications, the platy aggregates are completely disrupted, revealing well-separated, intersecting nano-rods and partially curled fibers (Fig. 4B and C). The application of DMSO as exfoliated agent leads to extensive exfoliation, delamination, and even considerable scrolling of the serpentinite flaky elongated particles (Fig. 4C). The DMF/SP also exhibits noticeable morphological rearrangements, albeit to a slightly lesser extent (Fig. 4D-F). At lower magnification, the material appears somewhat loosened and less compact than the pristine mineral (Fig. 4D). At higher magnifications, partially separated and intersected nano-rods are observed, with clear edge delamination but more intact compacted layered cores in the interior of the aggregates (Fig. 4E and F). DMF is capable of weakening inter-plates interactions and inducing partial exfoliation but does not generate as extensive scrolling or delamination as DMSO. Even the urea-modified serpentinite demonstrates morphological evolution compared to the raw sample (Fig. 4G, H, and I). The SEM images still reveal discernible separation and intersection of nano-rod and needle-like particles, indicating that surface adsorption of urea is sufficient to disrupt some inter-layered unites bonding. However, the aggregates remain relatively compact, considerable scrolling is observed (Fig. 4G-I).
Collectively, the SEM observations show a clear transition from the dense, tightly bound platy architecture of pristine serpentinite to progressively more exfoliated, delaminated, and intersected nano-rods after chemical modification, with occasional scrolling of the plates. Among the three reagents, DMSO produces the most extensive exfoliation, fiber separation, and visible scrolling. These morphological trends align with the differences in solvent polarity, hydrogen-bonding strength, and steric configuration. From an application perspective, the enhanced morphology seen in all modified samples directly improves adsorption and catalytic performances. Exfoliation and delamination increase the accessible surface area and expose more reactive hydroxyl groups, while scrolling may introduce additional mesoporosity and diffusion channels for adsorbate molecules. As a result, DMSO-modified serpentinite is expected to offer the highest adsorption capacity, followed by DMF-modified samples with intermediate performance, while urea-modified serpentinite exhibits only marginal enhancement unless further activated.
Textural analysis
The nitrogen adsorption–desorption isotherms of the modified serpentinite samples are unequivocally identified as Type IV isotherms according to the IUPAC classification, which is the hallmark of mesoporous solids (Fig. 5). This classification carries important structural and textural implications. Type IV curves reveal a more complex adsorption process that begins with monolayer and multilayer adsorption on the available internal surface at low and intermediate relative pressures, followed by a steep uptake in the higher relative pressure range due to capillary condensation within mesopores87. The presence of such condensation steps confirms that the chemical modification has effectively generated pores in the 2–50 nm mesopore range, which are significantly more accessible than the dense, compact microstructure of pristine serpentinite48. This mesoporous nature is further substantiated by the hysteresis loops superimposed on the Type IV profiles. Notably, all samples exhibit an H3-type hysteresis loop, which emerges at higher relative pressures (P/P₀ > 0.8). This particular loop is strongly associated with non-rigid aggregates of plate-like particles or layered frameworks that form slit-shaped pores rather than well-defined cylindrical channels88. Such a loop type is typical of lamellar materials, confirming that the serpentinite retains its inherent layered morphology even after chemical modification. The H3 loop reflects a heterogeneous pore network dominated by open slit-like voids, suggesting partial exfoliation and delamination without creating a fully ordered mesostructure.
These qualitative interpretations are strongly supported by the quantitative BET analysis, which clearly demonstrates the substantial improvement in textural properties after chemical modification. The pristine serpentinite exhibits a low surface area of only 6.1 m²/g with an average pore diameter of 11.1 nm, reflecting its compact layered structure with limited accessible surface. Upon modification, the surface area increases markedly, reaching 23.7 m²/g for DMSO/SP, 18.8 m²/g for DMF/SP, and 15.3 m²/g for U/SP. The average pore diameters also expand to 16.65 nm (DMSO/SP), 15 nm (DMF/SP), and 15.39 nm (U/SP), confirming that the interlayer spacing and pore network have been enlarged.
The Type IV isotherm with H3 hysteresis loops, therefore, not only confirms the mesoporous nature of the modified serpentinite but also reflects the preservation of its lamellar-derived pore geometry. The combined structural and textural data suggest that these materials possess an interconnected mesoporous network dominated by slit-like voids, which enhances molecular diffusion pathways while maintaining mechanical stability. From a functional standpoint, such controlled mesoporosity has direct implications for adsorption and catalysis. The increased surface area provides a higher density of accessible active sites, while the larger pore diameters facilitate faster diffusion of guest molecules. This makes the modified serpentinite particularly promising for applications such as pollutant adsorption, ion exchange, and catalytic support, where both surface reactivity and transport properties are critical.
Interaction mechanism of exfoliating agents with the serpentinite structure
To elucidate the exfoliation behavior and structural response of serpentinite, a detailed mechanistic understanding of the interactions between the silicate layers and the exfoliating agents—DMSO, DMF, and urea—is essential (Fig. 6). Each reagent exhibits a distinct mode of interaction based on its molecular polarity, hydrogen bonding capability, steric configuration, and affinity toward the hydroxyl-rich layered structure of serpentinite. A schematic 3D illustration of these interactions is presented in Fig. 6.
Dimethyl sulfoxide (DMSO), with a high dielectric constant (ε ≈ 47) and strong dipolar character due to the electronegative sulfonyl (–S = O) group, acts as a highly effective intercalating agent76,83,89,90. Its molecular geometry allows it to penetrate the narrow interlayer galleries of serpentinite, where it forms strong hydrogen bonds with the structural hydroxyl groups (Mg–OH) located within the octahedral sheet (Fig. 6). These interactions destabilize the original hydrogen-bonding network between the tetrahedral and octahedral layers, causing interlayer expansion and partial delamination48,85,91. This mechanism is confirmed by both the redshift of the O–H stretching vibration (3682 → 3677 cm⁻¹) and the broadening of the band, as well as the downshifting of Si–O stretching (950 → 940 cm⁻¹) and Si–O–Mg bending vibrations (615 → 606 cm⁻¹). The simultaneous loss of XRD peak intensity suggests the collapse of long-range order, supporting a scenario of both chemical interaction and structural exfoliation. The outcome is a disordered, high-surface-area structure with exposed active hydroxyls—ideal for adsorption through electrostatic interactions, ligand exchange, and hydrogen bonding.
Dimethylformamide (DMF) possesses moderate polarity (ε ≈ 36.7) and contains a carbonyl (C = O) functional group capable of forming hydrogen bonds with surface hydroxyl groups84,92,93. However, its bulky dimethyl-substituted amide structure imposes steric constraints that limit its penetration into the interlayer region93,94. The result is surface-level intercalation and partial layer separation, with weaker hydrogen bonding interactions compared to DMSO (Fig. 6). This is reflected in the slight shift of the hydroxyl band (3682 → 3680 cm⁻¹) and the Si–O band (950 → 943 cm⁻¹), alongside moderate peak shifts in the fingerprint region (615 → 609 cm⁻¹). These results, combined with moderate XRD peak attenuation, indicate that DMF induces intermediate exfoliation and limited structural deformation. Such partially modified structures retain some crystalline order, offering a balance between reactivity and stability, making them promising for recyclable adsorption systems or composite fabrication.
Urea (CO(NH₂)₂), though rich in hydrogen bonding functionality due to its amine and carbonyl groups, demonstrates the weakest interaction with serpentinite95,96 (Fig. 6). Despite its small molecular size and theoretical capacity for dual hydrogen bonding, urea shows minimal vibrational shifts (O–H remains at 3682 cm⁻¹; Si–O shifts slightly to 947 cm⁻¹) and negligible broadening, suggesting an absence of deep intercalation or structural disruption72,79. This limited reactivity is attributed to its strong hydration shell, which hinder its ability to displace interlayer water or penetrate the hydrophobic silicate sheets (Fig. 6). The preserved sharpness of XRD reflections further confirms the maintenance of the layered structure. Therefore, urea likely.
interacts through surface-level adsorption, forming weak hydrogen bonds or electrostatic interactions with exposed hydroxyl groups, without affecting the inner lattice which results in no significant exfoliation.
Adsorption results
Effect of pH
The pH of the solution is widely recognized as one of the most critical parameters governing pollutant removal in wastewater treatment processes. It directly influences the adsorption capacity by affecting several key factors, including the ionization state of the target contaminant, the surface charge distribution of the adsorbent, the speciation of functional groups on both the adsorbate and adsorbent, and the overall electrostatic and redox interactions within the adsorption system62. Numerous studies have highlighted the strong dependence of antibiotic adsorption on the solution pH, particularly for macrolide antibiotics such as azithromycin (AZT)29. In the present work, the impact of pH on the adsorption of AZT onto chemically modified serpentinite materials—DMSO/SP, DMF/SP, and U/SP—was systematically evaluated over a pH range of 3 to 9 (Fig. 7). The experiments were performed under controlled conditions with an initial AZT concentration of 100 mg/L, an adsorbent dosage of 0.2 g/L, and a fixed contact time of 60 min. The results, depicted in Fig. 7, demonstrate a pronounced enhancement in removal efficiency with increasing pH. At the highly acidic condition of pH 3, the adsorption capacities were relatively low, reaching only 12.7 mg/g for DMSO/SP, 9.6 mg/g for DMF/SP, and 8.7 mg/g for U/SP (Fig. 7). In contrast, at alkaline pH 9, these values markedly increased to 65.7 mg/g, 55.3 mg/g, and 48.2 mg/g, respectively (Fig. 7). Based on these results, pH 9 was identified as the optimal condition for AZT adsorption in subsequent experiments.
The observed pH-dependent adsorption behavior can be rationalized by considering the electrostatic interactions and surface charge properties of both the adsorbent and the antibiotic molecule. AZT is an amphoteric macrolide antibiotic with a dissociation constant (pKa) in the range of approximately 8.6–9.5, indicating that it remains predominantly protonated in strongly acidic media and gradually deprotonates as the solution becomes neutral to alkaline97,98. On the other hand, the surface charge of the modified serpentinite materials is determined by their point of zero charge (pHₚzc), which was measured to be approximately 8.45. Below the pHₚzc, the adsorbent surface remains positively charged due to protonation of surface hydroxyl and oxygen-containing functional groups. Above this value, these groups undergo deprotonation, resulting in a net negative surface charge99.
At low pH values (e.g., pH 3), the AZT molecules are highly protonated, and the adsorbent surface is also positively charged. This charge similarity leads to strong electrostatic repulsion, thereby inhibiting adsorption. Furthermore, the high concentration of H⁺ ions in acidic media competitively occupies the active adsorption sites, reducing the availability of these sites for AZT molecules100. Consequently, the adsorption capacity remains low under acidic conditions. As the pH increases toward neutrality and approaches the pHₚzc, the surface charge of the adsorbents becomes less positive, gradually diminishing electrostatic repulsion. When the pH exceeds the pHₚzc, the adsorbent surface acquires a net negative charge due to deprotonation of surface hydroxyl and carboxyl groups. Simultaneously, AZT molecules begin to lose protons and exist in a partially deprotonated or zwitterionic state. This change in surface and molecular charge polarity significantly enhances electrostatic attraction between AZT and the adsorbent surface. Moreover, the alkaline environment favors the formation of additional hydrogen bonding and dipole–dipole interactions, further contributing to the increased adsorption efficiency.
The substantial improvement in adsorption at pH 9 can therefore be attributed to a synergistic effect of electrostatic attraction and enhanced surface reactivity under alkaline conditions. At this pH, the adsorbents exhibit maximum surface deprotonation, providing more negatively charged active sites, while AZT molecules are less protonated, reducing electrostatic hindrance. This leads to stronger binding affinity and higher adsorption capacities for all modified serpentinite samples. These findings underscore the critical role of pH in modulating the surface charge balance and molecular interactions governing the adsorption process.
Kinetic studies
Effect of contact time
A comprehensive time-dependent adsorption study was conducted to evaluate the adsorption kinetics of azithromycin (AZT) on the chemically modified serpentinite-based materials (DMSO/SP, DMF/SP, and U/SP). The experiments were carried out under controlled conditions, maintaining a constant initial AZT concentration of 100 mg/L, solution volume of 100 mL, pH 9 (the previously determined optimal pH), temperature of 30 °C, and an adsorbent dosage of 0.2 g/L. The contact time was varied over a wide range, from 30 min to 960 min, to capture both the initial adsorption phase and the equilibrium stage.
The results, presented in Fig. 8A, reveal a distinct two-stage adsorption behavior. In the initial phase (0–360 min), a rapid increase in both AZT removal efficiency and adsorption capacity was observed for all three materials. During this period, the equilibrium adsorption capacities approached 166.4 mg/g for DMSO/SP, 148.8 mg/g for DMF/SP, and 133.2 mg/g for U/SP (Fig. 8A). This sharp initial uptake can be primarily attributed to the abundant availability of vacant active sites on the adsorbent surfaces, enabling strong electrostatic interactions and surface complexation with AZT molecules101. Additionally, the concentration gradient between the bulk solution and the adsorbent surface was at its maximum during the early stages, which accelerated mass transfer and facilitated the adsorption process102.
As the contact time progressed beyond 360 min, the adsorption rate markedly declined, indicating the gradual occupation and saturation of high-affinity sites (Fig. 8A). At this stage, only a limited number of lower-energy binding sites remained available, resulting in a much slower uptake rate. Beyond approximately 6 h, the adsorption curves plateaued, signifying that adsorption–desorption equilibrium had been reached and no significant further uptake occurred. The observed trend highlights two important aspects of the adsorption process. First, the rapid initial uptake demonstrates the high accessibility and strong binding affinity of the surface functional groups. Second, the eventual equilibrium plateau confirms that the adsorption process is finite and governed by site saturation, aligning well with previous studies on antibiotic adsorption by mesostructured layered materials103. From a practical perspective, understanding the contact time required to achieve equilibrium is crucial for designing efficient batch or continuous adsorption systems. The results indicate that approximately 360 min (6 h) is sufficient to achieve maximum AZT removal under the investigated conditions, ensuring optimal utilization of the adsorbent while avoiding unnecessary energy or time expenditure in prolonged treatment cycles.
Intra-particle diffusion behavior
To gain deeper insight into the rate-limiting mechanisms governing AZT adsorption onto the modified serpentinite adsorbents (DMSO/SP, DMF/SP, and U/SP), the intra-particle diffusion model was applied. The resulting plots, presented in Fig. 8B, exhibit three distinct linear regions, each characterized by different slopes, clearly indicating that the adsorption of AZT does not proceed through a single, uniform diffusion process. Instead, it involves multiple sequential steps, each dominated by different mass transfer mechanisms along the adsorption pathway104,105.
The first stage, corresponding to the initial linear region, reflects the rapid external adsorption at the outer surface of the adsorbents, commonly referred to as boundary-layer or film diffusion. At this stage, the high driving force generated by the steep concentration gradient between the bulk solution and the adsorbent surface enables an instantaneous transfer of AZT molecules to readily accessible active sites. This phase is primarily controlled by the surface properties of the adsorbents, including the density and distribution of surface-active sites as well as the adsorbent’s surface energy106,107. The observed high initial adsorption rate for all three materials aligns with their surface activation and mesoporosity, particularly for DMSO/SP, which exhibit the most enhanced textural properties.
The second stage, represented by the intermediate linear region, shows a noticeable decrease in adsorption rate. During this stage, the diffusion process transitions from the external surface to the interior pores of the adsorbent particles, indicating that intra-particle diffusion becomes the dominant mass transfer mechanism. The reduced adsorption rate reflects the increasing resistance to diffusion within the porous network, where molecular transport is governed by pore size, connectivity, and the physicochemical interactions between AZT molecules and the internal pore surfaces106,108. This stage highlights the importance of mesoporous structures generated by chemical modification, which facilitate molecular penetration and enhance the accessibility of internal adsorption sites.
The third and final stage is characterized by a plateau region, where the adsorption rate declines significantly and eventually approaches equilibrium. At this point, the majority of high-energy binding sites are already occupied, and the remaining available sites exhibit weaker adsorption affinity. The retention of AZT in this phase is dominated by molecular interactions, such as electrostatic attraction and hydrogen bonding, rather than purely diffusion-controlled processes109. The minimal adsorption observed during this stage confirms the attainment of saturation, where the adsorbents have reached their maximum loading capacity under the experimental conditions. The multi-linear patterns observed in the intra-particle diffusion plots unequivocally confirm that the adsorption of AZT onto DMSO/SP, DMF/SP, and U/SP is a complex, multi-step process rather than a single-rate diffusion mechanism. The sequential involvement of boundary-layer adsorption, pore diffusion, and final equilibrium retention highlights the synergistic role of surface functionalization and mesoporosity in enhancing both the kinetics and capacity of the adsorbents. From a practical standpoint, these findings are highly significant. They demonstrate that while external surface adsorption governs the initial removal efficiency, the development of internal porosity and accessible mesostructures is crucial for sustaining adsorption performance over time.
Time-dependent adsorption behavior of AZT onto DMSO/SP, DMF/SP, and U/SP (A); intra-particle diffusion profiles illustrating the multi-stage adsorption mechanism (B); kinetic fitting of AZT adsorption with pseudo-first-order model (C); and kinetic fitting of AZT adsorption with pseudo-second-order model (D).
Kinetic modeling
The application of adsorption kinetic models is critical for elucidating the time-dependent behavior of the adsorption process and for identifying the nature of the underlying rate-controlling mechanisms. Kinetic modeling not only distinguishes whether adsorption is governed primarily by physical mass transfer phenomena or by chemical surface interactions but also provides valuable insights into the potential practical applicability of the adsorbent in real systems110. In this study, two of the most commonly employed kinetic models—the pseudo-first-order (PFO) (Fig. 8C) and pseudo-second-order (PSO) (Fig. 8D) models—were applied to describe the adsorption kinetics of azithromycin (AZT) onto DMSO/SP, DMF/SP, and U/SP. The PFO model is generally suitable for processes where the adsorption rate is proportional to the number of available unoccupied sites, reflecting predominantly physisorption mechanisms111. In contrast, the PSO model assumes that the adsorption rate is proportional to the square of the number of unoccupied sites, implying that chemisorption involving valence forces (electron sharing or exchange) governs the process111,112.
To rigorously evaluate the kinetics, the experimental time–adsorption data were fitted to the nonlinear forms of the PFO and PSO equations. The goodness of fit was assessed based on the coefficient of determination (R²) and the chi-squared statistic (χ²), with better fitting indicated by higher R² values and lower χ² values. The resulting kinetic parameters are summarized in Table 1, while the nonlinear kinetic plots are presented in Fig. 8C and D. The fitting results reveal that the adsorption of AZT onto all three modified serpentinite adsorbents is better described by the pseudo-first-order model than by the pseudo-second-order model. For DMSO/SP, DMF/SP, and U/SP, the R² values obtained from the PFO model were consistently higher, while the χ² values were correspondingly lower, compared to those from the PSO model. Moreover, the equilibrium adsorption capacities determined experimentally—166.4 mg/g for DMSO/SP, 148.8 mg/g for DMF/SP, and 133.2 mg/g for U/SP—closely matched the theoretical equilibrium capacities predicted by the PFO model (177.14 mg/g, 158.63 mg/g, and 142.8 mg/g, respectively) (Table 1).
This strong correlation strongly suggests that the adsorption kinetics are dominated by physisorption processes, where van der Waals forces, electrostatic attraction, and possibly weak hydrogen bonding drive the uptake of AZT molecules113. While the PSO model also provided a reasonable fit, implying that a minor chemisorption contribution cannot be entirely excluded, the superior agreement of the PFO model highlights that physical interactions are the primary controlling mechanism. This finding is consistent with previous studies, where an initial chemisorbed layer (e.g., involving hydrogen bonding or surface complexation) may serve as a nucleation step that facilitates subsequent multilayer physisorption104,114. In other words, a hybrid adsorption pathway is plausible, where surface modification enhances initial chemical affinity, but the overall adsorption capacity is governed by weaker physical interactions within the porous structure.
From a practical standpoint, the predominance of physisorption suggests favorable reversibility and regeneration potential for the modified serpentinite adsorbents, making them promising candidates for sustainable antibiotic removal in water treatment applications. Moreover, the relatively fast kinetics during the initial phase further supports their suitability for efficient batch or fixed-bed adsorption systems.
Equilibrium studies
Effect of initial concentrations
The influence of the initial azithromycin (AZT) concentration on adsorption performance was systematically investigated to determine the maximum adsorption capacities and equilibrium characteristics of DMSO/SP, DMF/SP, and U/SP. Batch adsorption experiments were conducted over an initial concentration range of 50–400 mg/L, while maintaining constant experimental conditions: solution volume (100 mL), adsorbent dosage (0.2 g/L), and a contact time of 24 h. To evaluate the effect of temperature, the experiments were performed at three different temperatures: 303 K, 313 K, and 323 K. The resulting adsorption capacities and equilibrium profiles are presented in Figs. 9A–C.
The results revealed a positive correlation between initial AZT concentration and the adsorption capacity of all modified serpentinite adsorbents. At low initial concentrations, the driving force for mass transfer was relatively weak, resulting in a slower diffusion rate of AZT molecules toward the adsorbent surface. As the initial concentration increased, the concentration gradient between the solution bulk and the adsorbent surface became steeper, thereby enhancing the mass transfer rate and increasing the likelihood of AZT molecules interacting with available active sites115. This led to a progressive increase in adsorption capacity with increasing concentration. However, beyond a certain threshold, further increases in AZT concentration had a diminishing effect on adsorption performance, indicating that the adsorbent surfaces were approaching saturation and the available binding sites had become largely occupied. This behavior is typical of adsorption systems where the number of active sites is finite, and equilibrium is achieved when the rates of adsorption and desorption become equal116.
At equilibrium, the maximum adsorption capacities of the adsorbents followed a consistent trend across the tested temperatures. For DMSO/SP, the equilibrium capacities were 325.3 mg/g at 303 K, 287.3 mg/g at 313 K, and 247.4 mg/g at 323 K (Fig. 9A). DMF/SP exhibited slightly lower capacities of 290.6 mg/g at 303 K, 267.1 mg/g at 313 K, and 246.3 mg/g at 323 K (Fig. 9B), while U/SP demonstrated the lowest capacities of 279.3 mg/g at 303 K, 255.8 mg/g at 313 K, and 237.8 mg/g at 323 K (Fig. 9C). The superior adsorption performance of DMSO/SP can be attributed to their enhanced surface reactivity resulting from the DMSO-assisted exfoliation process, which introduces sulfate groups and increases the density of oxygen-containing functional moieties48,117. These modifications provide additional binding sites capable of electrostatic interactions and hydrogen bonding with AZT molecules, thereby improving adsorption affinity. DMF/SP and U/SP, although modified, possess relatively fewer reactive sites compared to DMSO/SP, leading to lower adsorption capacities.
An important observation from this study is the temperature dependence of the adsorption process. All adsorbents exhibited a gradual decline in adsorption capacity with increasing temperature, indicating that the adsorption of AZT is exothermic in nature. This behavior is consistent with the general thermodynamic trend for antibiotic adsorption onto layered or mesostructured adsorbents, where elevated temperatures reduce the extent of molecular interactions between the adsorbate and the adsorbent surface53. In other words, higher thermal energy promotes desorption, thereby lowering the equilibrium uptake at elevated temperatures.
Classic isotherm models
Classical adsorption isotherm models are widely used to describe the equilibrium distribution of solute molecules between the liquid phase and the adsorbent surface, offering valuable insights into the adsorption mechanism, the affinity of the adsorbate toward the surface binding sites, and the theoretical maximum adsorption capacity115. In this study, the equilibrium adsorption of azithromycin (AZT) onto DMSO/SP, DMF/SP, and U/SP was analyzed using three established models: Langmuir, Freundlich, and Dubinin–Radushkevich (D–R). Each model was fitted using its nonlinear expression as summarized in Table 2, and the accuracy of the fits was evaluated based on the coefficient of determination (R²) and the chi-squared statistic (χ²), ensuring a reliable comparison of model performance.
The Langmuir isotherm model, presented in Figs. 9D–F, assumes that adsorption occurs as a monolayer on a homogeneous surface containing a finite number of identical active sites, with no interaction between the adsorbed molecules65,118. From the Langmuir analysis, the theoretical maximum adsorption capacities (Qmax) were determined as 329.82 mg/g at 303 K, 285.70 mg/g at 313 K, and 247.12 mg/g at 323 K for DMSO/SP. For DMF/SP, the capacities were 292.74 mg/g at 303 K, 265.58 mg/g at 313 K, and 243.26 mg/g at 323 K, whereas U/SP exhibited slightly lower Qmax values of 280.67 mg/g, 253.13 mg/g, and 233.78 mg/g across the same temperature range (Table 2). Furthermore, the calculated dimensionless separation factor (RL) values were all below 1, indicating that AZT adsorption onto the modified serpentinite materials was thermodynamically favorable across the tested concentration ranges (Table 2)107,109. The Freundlich isotherm model, illustrated in Figs. 9G–I, provides a more generalized description of adsorption on heterogeneous surfaces, where the adsorption sites possess varying affinities and energies119. Based on the R² and χ² values, the Freundlich model offered the best fit for the adsorption data of all three adsorbents, outperforming the Langmuir model. This superior fit suggests that the modified serpentinite surfaces are energetically heterogeneous, leading to multilayer adsorption rather than ideal monolayer coverage. The Freundlich heterogeneity constant (1/n) fell within the favorable range of 0–1, confirming that AZT exhibited high affinity for the surface binding sites of the modified serpentinite adsorbents (Table 2)120,121.
To further elucidate the adsorption energy profile, the Dubinin–Radushkevich (D–R) model was applied (Figs. 9J–L). The D–R model does not assume a uniform surface and is particularly useful for estimating the mean free energy of adsorption (E), which distinguishes between physical and chemical adsorption mechanisms115. Generally, E < 8 kJ/mol indicates physisorption dominated by van der Waals forces or electrostatic interactions, 8–16 kJ/mol suggests a mixed physisorption–chemisorption mechanism, while E > 16 kJ/mol reflects strong chemisorption121,122. In this study, the calculated E values for AZT adsorption onto DMSO/SP, DMF/SP, and U/SP were consistently below 8 kJ/mol, demonstrating that the adsorption process is primarily governed by physical interactions rather than chemical bonding (Table 2).
In summary, the equilibrium modeling of AZT adsorption onto DMSO/SP, DMF/SP, and U/SP reveals a heterogeneous multilayer adsorption mechanism predominantly driven by physical interactions, with DMSO/SP exhibiting the highest adsorption potential due to their enhanced surface functionality and mesostructural properties. These findings hold significant practical implications. The heterogeneous multilayer adsorption suggested by the Freundlich model indicates that the modified serpentinite adsorbents are suitable for treating real wastewater streams with variable pollutant concentrations, as they can accommodate diverse binding energies and multiple adsorption layers. Moreover, the predominance of physical interactions implies that the adsorbents can be easily regenerated and reused, enhancing their cost-effectiveness and sustainability in long-term applications.
Effect of initial AZT concentration on the adsorption capacity of DMSO/SP (A), DMF/SP (B), and U/SP (C). Nonlinear equilibrium fitting of AZT adsorption using the Langmuir model for DMSO/SP (D), DMF/SP (E), and U/SP (F); Freundlich model for DMSO/SP (G), DMF/SP (H), and U/SP (I); and Dubinin–Radushkevich (D–R) model for DMSO/SP (J), DMF/SP (K), and U/SP (L).
Advanced isotherm modeling
Traditional equilibrium models such as the Langmuir and Freundlich isotherms, while widely applied, provide only a limited understanding of adsorption mechanisms as well as the underlying physicochemical phenomena. These simplified models fail to capture the complexity of real adsorption systems, where the process is influenced by a combination of steric effects, surface heterogeneity, and energetics arising from adsorbate–adsorbent interactions66,113. To address these limitations, advanced isotherm models based on statistical physics principles have been increasingly utilized to provide a more realistic and physically meaningful description of adsorption behavior. Unlike traditional models, statistical physics-based isotherms simulate the equilibrium process by incorporating steric and energetic parameters that reflect the molecular arrangement of adsorbates on the surface as well as the energy distribution of the adsorption sites. Such models enable a deeper interpretation of the adsorption mechanism by connecting the experimental macroscopic data with molecular-level processes.
In this study, statistical physics-based isotherm modeling was applied to explore the adsorption of azithromycin (AZT) onto DMSO/SP, DMF/SP, and U/SP. This approach allowed for the simultaneous evaluation of steric parameters, including the number of AZT molecules adsorbed per receptor site (n), the total number of receptor sites available per gram of adsorbent (Nm), and the maximum adsorption capacity at complete site saturation (Qsat). At the same time, several energetic parameters were obtained, such as the internal energy of the adsorption system (Eint), adsorption entropy (Sa), the mean adsorption energy (E), and the enthalpy (G), which collectively provide insights into the spontaneity, favorability, and driving forces of the adsorption process. To ensure accuracy and reliability in parameter estimation, the equilibrium data were analyzed using non-linear regression techniques, and the Levenberg–Marquardt optimization algorithm was employed to achieve robust convergence and minimize residual errors. By optimizing multiple parameters simultaneously, this method improved the interpretability of the model outputs and provided a better mechanistic understanding of the adsorption system. Among the tested statistical physics-based models, the monolayer adsorption model of one energetic site was applied to represent the adsorption properties of the three adsorbents. The optimized steric and energetic parameters, along with the corresponding fitting indices, are summarized in Table 2 and are visually represented in Figs. 10A–C.
Steric properties
Number of adsorbed AZT (n) per each site
The steric parameter n provides critical insight into the adsorption configuration and molecular orientation of azithromycin (AZT) on the surfaces of DMSO/SP, DMF/SP, and U/SP. In the context of statistical physics-based isotherm modeling, n represents the average number of AZT molecules that can be accommodated at a single receptor site. When n is less than one, the adsorption is generally interpreted as a single-molecule docking event in which the molecule lies horizontally, occupying a single site in a flat configuration. Conversely, n values greater than one imply a vertical or non-parallel molecular arrangement, where multiple AZT molecules interact simultaneously with the same adsorption site, suggesting multi-docking or multi-anchoring adsorption phenomena109,123.
The results obtained in this study clearly indicate that n consistently exceeds unity for all three adsorbents, highlighting the prevalence of multi-molecular adsorption mechanisms48. Specifically, for DMSO/SP, the n values ranged from 3.01 to 3.44 across the studied temperature range (Fig. 10D), suggesting that up to four AZT molecules can simultaneously attach to a single receptor site. For DMF/SP, n values were slightly higher, spanning 3.77 to 4.41 (Fig. 10E), indicating that as many as five AZT molecules may bind per site. Similarly, U/SP exhibited n values between 3.32 and 4.40 (Fig. 10F), again implying that up to five AZT molecules can occupy the same site. The fact that n values are consistently above one for all the exfolaited samples confirms that multi-docking adsorption dominates the equilibrium configuration65, meaning that each active site does not simply bind a single AZT molecule but rather accommodates multiple molecules, forming stacked or aggregated adsorption layers. This multi-layer, vertical stacking is indicative of the significant structural and surface chemical changes induced by exfoliation and functionalization, which create high-affinity adsorption regions with increased spatial availability and enhanced molecular interactions. Such behavior is typically associated with the presence of newly generated functional groups, expanded interlayer spacing, and improved surface heterogeneity, which collectively facilitate the accommodation of multiple adsorbate molecules at a single site. An important observation is the temperature dependence of the n parameter, which showed a gradual increase with rising temperature (from 303 K to 323 K) for all three adsorbents. This trend suggests that higher temperatures promote molecular aggregation of AZT in the vicinity of the adsorbent surface, thereby enhancing multi-molecule docking62. These findings have several significant implications. First, the multi-molecular adsorption observed here reflects the high density of adsorption sites generated by chemical exfoliation. Second, the ability of a single site to accommodate several molecules suggests a strong synergistic effect between steric packing and surface energetics, allowing for enhanced pollutant removal even at elevated concentrations.
Occupied active sites density (Nm)
The parameter Nm represents the density of occupied active sites on the adsorbent surface, offering a direct indication of the total number of reactive adsorption sites available for azithromycin (AZT) molecules during the adsorption process. In the framework of statistical physics-based isotherm modeling, Nm provides valuable information on how surface modification affects the structural accessibility and reactivity of the adsorbent. The obtained values for Nm on DMSO/SP, DMF/SP, and U/SP are shown in Figs. 10G–I and summarized in Table 2.
For DMSO/SP, Nm values reached 109.54 mg/g at 303 K, decreasing to 87.30 mg/g at 313 K and further to 71.72 mg/g at 323 K (Fig. 10G; Table 2). DMF/SP exhibited lower site densities, with values of 77.54 mg/g at 303 K, 67.51 mg/g at 313 K, and 55.07 mg/g at 323 K (Fig. 10H; Table 2). U/SP showed an intermediate performance, with corresponding Nm values of 84.31 mg/g, 64.32 mg/g, and 53.02 mg/g across the same temperature range (Fig. 10I; Table 2). These results clearly demonstrate that DMSO-assisted exfoliation substantially enhances the total number of available adsorption sites compared to DMF- and urea-assisted modifications. The superior performance of DMSO/SP can be attributed to several synergistic factors. First, the DMSO-mediated exfoliation process generates a larger specific surface area, exposing previously inaccessible interlayer regions and creating new adsorption domains. Second, this modification introduces a higher density of surface functional groups, such as hydroxyl and sulfate species, which act as high-affinity binding sites for AZT molecules. Third, the improved porosity and surface heterogeneity in DMSO/SP enhance molecular accessibility and facilitate stronger adsorbate–adsorbent interactions. Collectively, these structural and chemical modifications result in a higher Nm value, confirming that DMSO/SP possess a denser and more reactive adsorption surface than their DMF- and urea-treated counterparts.
Another important observation is the temperature-dependent decline in Nm for all three adsorbents. As the temperature increased from 303 K to 323 K, a progressive reduction in site density was observed. This behavior can be explained by the fact that higher temperatures promote AZT molecular aggregation in solution, reducing the mobility and diffusion efficiency of individual molecules and thereby limiting their ability to access distinct adsorption sites. Furthermore, elevated temperatures increase the kinetic energy of the adsorbed molecules, which can disrupt weaker physical interactions such as van der Waals forces and electrostatic attraction, resulting in fewer stably occupied sites124,125.
The differences in Nm among the three adsorbents have significant implications for adsorption design and performance. The markedly higher site density in DMSO/SP confirms that this modification strategy not only increases the number of physically accessible sites but also improves their reactivity, enabling greater pollutant capture even at higher concentrations. In contrast, DMF/SP and U/SP, while still improved compared to raw serpentinite, exhibit fewer reactive sites due to less extensive exfoliation and functionalization. This finding aligns with the observed trends in adsorption capacity from classical isotherm modeling, reinforcing the conclusion that surface modification strongly governs the steric properties of adsorption and ultimately determines the removal efficiency of AZT.
Evaluation of AZT adsorption behavior on DMSO/SP, DMF/SP, and U/SP based on the monolayer adsorption model with a single energy site: (A–C) model fitting curves for AZT uptake on DMSO/SP (A), DMF/SP (B), and U/SP (C); (D–F) variation in the number of AZT molecules adsorbed per site for DMSO/SP (D), DMF/SP (E), and U/SP (F); (G–I) changes in uptake site density for DMSO/SP (G), DMF/SP (H), and U/SP (I); and (J–L) corresponding changes in the saturation uptake capacities for DMSO/SP (J), DMF/SP (K), and U/SP (L).
Adsorption capacity at the saturation state of (Qsat)
The saturation adsorption capacity (Qsat) represents the theoretical maximum quantity of azithromycin (AZT) that can be adsorbed by the modified serpentinite materials under ideal equilibrium conditions. This parameter reflects the ultimate adsorption potential of the adsorbent and is governed primarily by two steric factors: the density of available active binding sites (Nm) and the number of AZT molecules that can dock at each site (n). Thus, Qsat provides an integrated measure of the surface accessibility, structural functionality, and molecular packing behavior of the adsorbent material.
Among the three adsorbents examined, DMSO/SP exhibited the highest Qsat values, reaching 329.71 mg/g at 303 K, which decreased to 285.66 mg/g at 313 K and further to 246.71 mg/g at 323 K (Fig. 10J; Table 2). DMF/SP showed slightly lower saturation capacities of 292.32 mg/g, 256.31 mg/g, and 242.85 mg/g across the same temperature range (Fig. 10K; Table 2). Urea-modified serpentinite (U/SP) demonstrated the lowest Qsat values of 279.90 mg/g at 303 K, 252.77 mg/g at 313 K, and 233.28 mg/g at 323 K (Fig. 10L; Table 2). The superior adsorption potential of DMSO/SP can be directly attributed to the extensive surface exfoliation and functionalization achieved by DMSO treatment. This modification significantly increases the density of high-affinity adsorption sites and enhances the structural openness of the adsorbent, thereby allowing more AZT molecules to occupy the available receptor sites. DMF-assisted exfoliation produces fewer surface defects and functional groups compared to DMSO, leading to moderately lower Nm values and, consequently, a reduced Qsat. Similarly, urea-assisted modification generates the least pronounced structural changes, resulting in fewer accessible binding sites and the lowest saturation capacity among the three materials.
A consistent decline in Qsat was observed for all adsorbents with increasing temperature, underscoring the exothermic nature of the adsorption process. At elevated temperatures, the thermal energy of AZT molecules increases, which can disrupt the weak physical interactions—that primarily govern their binding to the adsorbent surface. Additionally, higher temperatures can induce molecular desorption, thereby reducing the total number of occupied sites at equilibrium115. This temperature-induced decline in Qsat correlates more strongly with reductions in Nm than with changes in n, suggesting that adsorption capacity is more sensitive to the total number of available binding sites than to the molecular configuration or stacking behavior at each site123.
Adsorption energy and mechanism
The adsorption energy (ΔE) is a critical parameter for elucidating the underlying nature of the interaction between azithromycin (AZT) molecules and the adsorbent surface. Understanding these energy dynamics helps distinguish between physical adsorption (physisorption) and chemical adsorption (chemisorption), thereby guiding the rational design and optimization of adsorption systems for improved efficiency. Typically, adsorption is classified as physical when ΔE values are below 40 kJ/mol, while values exceeding 80 kJ/mol are indicative of chemisorption, which involves stronger valence forces and bond formation. Within the domain of physisorption, several weak interactions contribute to adsorbate retention, including hydrogen bonding (ΔE < 30 kJ/mol), dipole–dipole interactions (ΔE = 2–29 kJ/mol), van der Waals forces (ΔE = 4–10 kJ/mol), electrostatic attractions (ΔE = 2–50 kJ/mol), and hydrophobic effects (ΔE ≈ 5 kJ/mol)126,127. These benchmark values serve as reference ranges for identifying the dominant forces involved in adsorption mechanisms.
In this study, the ΔE values were determined using Eq. (6), which relates the energy of adsorption to the solubility of AZT (S), the universal gas constant (R = 0.008314 kJ/mol·K), the equilibrium concentration at half-saturation for each adsorbent, and the absolute system temperature (T):
The calculated adsorption energy values for AZT uptake onto DMSO/SP, DMF/SP, and U/SP ranged from − 5.04, − 4.99, and − 4.69 kJ/mol to − 5.31, − 5.20, and − 5.05 kJ/mol, respectively (Table 2). These values fall well within the characteristic energy range of physisorption, confirming that the adsorption process is dominated by weak, non-covalent interactions rather than chemical bonding. Specifically, the relatively low magnitude of ΔE suggests the involvement of dipole–dipole interactions, van der Waals forces, electrostatic attractions, and hydrogen bonding, which collectively stabilize the AZT molecules on the exfoliated serpentinite surfaces.
On a molecular level, AZT is a large macrolide antibiotic with multiple functional groups, including amine moieties, hydroxyl groups, ether linkages, and tertiary amino sugar residues, which can engage in various weak interactions (Fig. 11)128. On the adsorbent side, the chemical modifications introduced by DMSO, DMF, and urea exfoliation expose and enhance surface hydroxyl groups, Lewis acid/base sites, Si–OH groups, and in the case of DMSO modification, additional sulfate functional groups. These functional moieties provide multiple adsorption sites that interact with AZT through a combination of electrostatic attraction, hydrogen bonding, dipole–dipole forces, and van der Waals interactions (Fig. 11). The electrostatic interactions are particularly important under the optimized pH (9), where AZT exists predominantly in a partially deprotonated zwitterionic or neutral form, while the adsorbent surfaces are negatively charged due to deprotonation of surface hydroxyl groups (pH > pHpzc ≈ 8.45). This charge difference facilitates long-range electrostatic attraction between the protonated regions of AZT and negatively charged hydroxyl or sulfate groups on DMSO/SP (Fig. 11). This mechanism is likely more pronounced for DMSO/SP because DMSO-assisted exfoliation introduces additional polar sulfate groups that can act as high-affinity electrostatic sites, explaining their slightly more favorable ΔE values.
In parallel, hydrogen bonding plays a stabilizing role. The hydroxyl and ether functionalities of AZT can donate or accept hydrogen bonds with surface –OH groups, or coordinated water molecules on the adsorbent surface129. The modified serpentinite structures, particularly those treated with DMSO, have a higher density of surface hydroxyl groups, which enhances their capacity to form hydrogen-bonding networks. These bonds, while individually weak (ΔE < 30 kJ/mol), collectively contribute significantly to the stabilization of AZT molecules on the adsorbent. Dipole–dipole interactions and van der Waals forces further complement the adsorption mechanism. The macrolide structure of AZT contains several polarizable bonds and dipolar functional groups, which can align with the surface dipoles of the modified serpentinite layers, reinforcing adsorption through short-range physical forces48. The relatively low ΔE values (approximately − 5 kJ/mol) suggest that these interactions are moderate in strength but sufficient to drive spontaneous adsorption without requiring external energy input.
The negative ΔE values not only confirm the exothermic nature of the process but also indicate that the adsorption is spontaneous and energetically favorable, aligning with the observed decline in adsorption capacity at elevated temperatures62. When temperature increases, the added thermal energy disrupts these weak physical forces, promoting partial desorption and thereby reducing the overall adsorption capacity (Qsat), as observed in the steric property analysis. An important aspect revealed by the energy analysis is that the adsorption mechanism remains predominantly reversible, which is highly beneficial for environmental applications. Physisorption-dominated processes allow for easy regeneration and reuse of adsorbents, reducing operational costs and enhancing sustainability in large-scale water treatment systems.
Taken together, the adsorption energy analysis highlights a multi-mechanistic physisorption process involving electrostatic attraction between charged AZT groups and deprotonated surface sites, hydrogen bonding with hydroxyl groups, dipole–dipole alignment between polar AZT moieties and surface dipoles, and van der Waals stabilization arising from molecular proximity (Fig. 11). These interactions act synergistically to achieve stable adsorption while maintaining low energy barriers for desorption, ensuring both efficiency and regenerability of the adsorbents126,127.
Thermodynamic functions
Entropy
The adsorption entropy (Sa) provides crucial information about the molecular-level ordering and configurational dynamics that accompany azithromycin (AZT) binding onto exfoliated serpentinite surfaces, namely DMSO/SP, DMF/SP, and U/SP. As a thermodynamic descriptor, Sa captures changes in the degree of disorder within the adsorbent–adsorbate system and reflects the extent to which surface sites are organized or reorganized during the adsorption process65,130. The entropy values were calculated using Eq. (7), which integrates key steric parameters such as the site density (Nm), the number of AZT molecules adsorbed per site (n), and the half-saturation equilibrium concentration (C₁/₂) specific to each adsorption system131
This equation enables an accurate assessment of how AZT molecules interact with the adsorbent surface under varying concentration and temperature conditions, offering a window into both thermodynamic favorability and structural reorganization.
The entropy profiles derived from this model exhibit a clear concentration-dependent decline in Sa across all three exfoliated serpentinite systems (Figs. 12A–C). Specifically, as AZT loading increases, entropy progressively decreases, particularly at higher equilibrium concentrations. This trend reflects a gradual loss of configurational freedom and surface disorder as more AZT molecules occupy reactive adsorption sites, eventually leading to a state of structural saturation62. Despite the reduction in entropy at high concentrations, the initial rise in Sa at lower concentrations suggests favorable docking of AZT molecules into available surface cavities, supporting the hypothesis that the adsorption mechanism is driven by enthalpically and entropically balanced interactions during the early stages of uptake131.
For DMSO/SP, the maximum entropy values were recorded at 67.6 mg/L (303 K), 68.4 mg/L (313 K), and 70.82 mg/L (323 K) (Fig. 12A). DMF/SP exhibited peak Sa values at 69.8 mg/L, 70.94 mg/L, and 72.13 mg/L at the same respective temperatures (Fig. 12B), while U/SP displayed slightly higher maxima at 78.9 mg/L (303 K), 77.11 mg/L (313 K), and 76.7 mg/L (323 K) (Fig. 12C). Notably, these concentration points are closely aligned with the calculated half-saturation concentrations (C₁/₂) for each system, suggesting that entropy is maximized when half the available adsorption sites are occupied. At this critical point, the system exhibits a balance between molecular mobility and localized binding, allowing for optimal surface reorganization and binding site utilization. Beyond the half-saturation threshold, further AZT uptake is limited by reduced accessibility of reactive sites, a phenomenon that correlates with decreased entropy and increased spatial hindrance48. This entropy decline also implies that molecular mobility of AZT decreases as the surface becomes more crowded, leading to constrained diffusion pathways and a more rigid adsorbed phase. The magnitude of this entropy drop is also influenced by the surface chemistry of the adsorbents. For instance, the stronger surface reactivity and structural openness of DMSO/SP facilitate earlier ordering and site occupation, whereas the more moderate structural modification of DMF/SP and U/SP allows for slightly higher entropy retention at similar AZT concentrations.
From a practical standpoint, this thermodynamic behavior holds several important implications. First, the entropy profile supports the idea of a two-phase adsorption mechanism: an initial phase where entropy increases due to favorable orientation and surface interaction of AZT molecules, followed by a second phase characterized by entropy reduction as saturation is approached. Second, the alignment of entropy maxima with C₁/₂ values provides a reliable predictive metric for identifying the optimal operational concentration range for each adsorbent. Lastly, the entropy trends reinforce the steric analysis findings, confirming that adsorption-induced ordering plays a significant role in controlling the overall uptake efficiency and equilibrium dynamics132. In summary, the entropy function (Sa) offers a powerful thermodynamic indicator of surface dynamics during AZT adsorption. The observed concentration-dependent entropy decline—particularly around and beyond the half-saturation threshold—highlights the progressive ordering, reduced diffusion, and structural rigidity that emerge during site saturation. These insights further affirm the physisorption-dominated, yet structurally adaptive nature of AZT uptake on chemically based exfoliated serpentinite materials.
Internal energy and free enthalpy
A detailed thermodynamic assessment was conducted to elucidate the energetic behavior governing the adsorption of azithromycin (AZT) onto exfoliated serpentinite surfaces, specifically DMSO/SP, DMF/SP, and U/SP. Two essential parameters were examined: the internal energy (Eint), which reflects the heat exchange associated with molecular immobilization, and the free enthalpy (G), which represents the configurational driving force and spontaneity of adsorption115,133. Both were calculated using the estimated parameters form the advanced equilibrium model, such as the number of AZT molecules per site (n), the adsorption site density (Nm), and the half-saturation concentration (C₁/₂) in addition to the translational partition function (Zv). The internal energy was determined using Eq. 8:
Similarly, the free enthalpy (G) was derived from Eq. 9 where kB is the Boltzmann constant and T is the absolute temperature.
The calculated Eint values were consistently negative for all three exfoliated serpentinite systems across the investigated temperature range (303–323 K) (Fig. 12D-F). This strongly indicates that AZT adsorption is exothermic, releasing energy as molecules transfer from the disordered aqueous phase to a more stabilized adsorbed state115,133. A slight decrease in the magnitude of Eint was observed with increasing temperature, suggesting that the adsorption interactions become progressively weaker at higher thermal energy. Such behavior is characteristic of physisorption, where the stabilizing forces are dominated by electrostatic attraction, hydrogen bonding, dipole–dipole interactions, and van der Waals forces, all of which are reversible and sensitive to thermal agitation. Among the three adsorbents, DMSO/SP exhibited the most negative Eint values, reflecting their higher surface reactivity and the presence of additional sulfate and sulfoxide groups introduced during DMSO exfoliation. These modifications generate more energetically favorable adsorption domains, enhancing the stabilization of AZT compared to DMF/SP and U/SP, which have fewer reactive sites and lower structural heterogeneity.
The free enthalpy G also remained negative across all equilibrium concentrations and temperatures, confirming that the adsorption process is spontaneous and thermodynamically favorable for each material (Fig. 12G-I)48,131. Similar to the trend observed for Eint, the magnitude of G decreased slightly with increasing temperature, indicating a small reduction in spontaneity under elevated thermal conditions (Fig. 12G-I). Nevertheless, the negative G values clearly demonstrate that no external energy is required to initiate or sustain AZT uptake. The differences in G among the materials are consistent with their degree of exfoliation and functionalization: DMSO/SP displayed the most favorable (more negative) G values due to their higher density of reactive hydroxyl and sulfate groups, while DMF/SP and U/SP showed moderately less favorable energetics.
Together, the combined negative values of internal energy and free enthalpy provide a coherent thermodynamic interpretation of the adsorption process. The negative internal energy confirms that AZT binding is exothermic and energetically stabilized by weak yet cooperative interactions between the antibiotic functional groups and the exfoliated serpentinite surface. The negative free enthalpy further confirms the spontaneous and favorable configurational rearrangement that accompanies adsorption under ambient conditions. The slight temperature dependence of both parameters highlights the reversible nature of adsorption, which is typical of physisorption, where thermal input can partially disrupt surface–molecule interactions without altering the structural integrity of the adsorbent.
From an application standpoint, these findings indicate that AZT adsorption onto modified serpentinite surfaces is energy efficient, as no external heat is required to drive the process. The reversible nature of the interactions facilitates easy adsorbent regeneration without the need for harsh treatments, which is advantageous for sustainable large-scale operations. Furthermore, the clear correlation between temperature, internal energy, and free enthalpy allows for accurate prediction and optimization of adsorption performance under varying operational conditions. Among the three adsorbents studied, DMSO/SPNTs exhibited the most favorable thermodynamic behavior, validating the importance of extensive exfoliation and functionalization in enhancing adsorption capacity and energetic stabilization.
Recyclability
The long-term applicability of an adsorbent depends not only on its initial performance but also on its stability and regeneration potential, which are decisive for industrial and environmental deployment. To evaluate these aspects, the recyclability of the exfoliated serpentinite-based adsorbents (DMSO/SP, DMF/SP, and U/SP) was systematically assessed through repeated adsorption–desorption cycles for azithromycin (AZT). Prior to reuse, the spent materials were thoroughly rinsed with distilled water for 10 min (repeated several times) and subsequently dried at 60 °C for 8 h to ensure complete removal of residual contaminants and restoration of active sites. Batch adsorption experiments were conducted under controlled conditions (initial AZT concentration: 50–400 mg/L; solution volume: 100 mL; adsorbent dosage: 0.2 g/L; contact time: 24 h; temperature: 303 K). The performance of the adsorbents over five successive cycles is summarized in Fig. 11. All three materials retained a significant fraction of their initial adsorption capacity after repeated use, demonstrating satisfactory reusability and structural robustness.
Specifically, DMSO/SP exhibited the highest recyclability, with adsorption capacities of 325.3, 311.7, 290.4, 268.2, and 256.8 mg/g over cycles 1–5, respectively. DMF/SP also maintained considerable efficiency, recording 290.6, 277.6, 261.6, 243.9, and 231.6 mg/g across the five runs. U/SP displayed slightly lower but still promising reusability, with values of 279.3, 270.3, 254.8, 225.9, and 208.5 mg/g. The observed decline in capacity with successive cycles can be attributed to the progressive occupation of strongly binding sites and partial loss of surface functionality during regeneration. Nevertheless, the preservation of more than 70–80% of the initial adsorption efficiency after five cycles highlights the durability of the exfoliated serpentinite frameworks. This stability is particularly noteworthy given the relatively harsh regeneration process and supports their potential for practical water purification applications.
From a mechanistic standpoint, the superior performance of DMSO/SP may be related to its enhanced exfoliation degree and the higher density of accessible functional groups, which facilitate more effective reactivation of adsorption sites after desorption. Comparatively, the moderate decline in DMF/SP and U/SP suggests differences in structural reordering or weaker site regeneration, yet both still exhibit performance levels consistent with reusable adsorbents reported in recent literature. Overall, these results confirm that the developed serpentinite-based materials combine high adsorption efficiency with satisfactory recyclability, positioning them as sustainable and cost-effective candidates for real-world applications in antibiotic removal from aqueous systems.
Comparative study
A comparative assessment of the prepared exfoliated serpentinite adsorbents (DMSO/SP, DMF/SP, and U/SP) with other reported materials for azithromycin (AZT) removal highlights their outstanding performance and practical potential (Table. S2). Among the tested materials, DMSO/SP exhibited the highest adsorption capacity (329.7 mg/ g), outperforming not only DMF/SP (292.3 mg/g) and U/SP (279.9 mg/g), but also a wide range of conventional and engineered adsorbents such as activated carbon, biochar, natural zeolite, graphene oxide, and several functionalized clays and MOF composites reported in the literature. This superior efficiency can be attributed to the enhanced surface area, increased porosity, and higher density of accessible hydroxyl groups generated during DMSO-assisted exfoliation.
In addition to efficiency, the cost-effectiveness of serpentinite-derived adsorbents represents a major advantage. Serpentinite is a naturally abundant ultramafic rock available at very low cost compared with advanced synthetic materials such as MOFs, graphene derivatives, or engineered nanocomposites. The exfoliating agents employed (DMSO, DMF, and urea) are widely available and inexpensive, making the overall preparation process scalable and economically viable for industrial applications.
Safety is another critical parameter for real-world implementation. The serpentinite framework is composed of stable silicate structures that exhibit high resistance to leaching under neutral adsorption conditions. Moreover, the preparation procedure included extensive washing steps to remove residual exfoliating agents, thereby eliminating risks of secondary contamination. Compared with some MOFs or metal oxide-based nanomaterials that may release toxic ions during use, serpentinite-based adsorbents provide a safer and more environmentally friendly alternative. Finally, the wide geological availability of serpentinite across different regions of the world further supports its large-scale applicability in water treatment. Unlike advanced nanomaterials that suffer from supply limitations and complex synthesis procedures, serpentinite represents a low-cost and sustainable raw material that can be readily valorized into high-performance adsorbents.
Taken together, these findings establish exfoliated serpentinite materials—particularly DMSO/SP—as highly efficient, safe, low-cost, and widely available adsorbents. Their superior performance compared with both natural and synthetic alternatives underscores their potential as a sustainable solution for mitigating pharmaceutical contaminants such as AZT in aquatic systems.
Safety considerations of the obtained adsorbents
The modified serpentinite adsorbents (DMSO/SP, DMF/SP, and U/SP) show excellent potential for removing pharmaceutical pollutants such as azithromycin (AZT), while meeting stringent requirements for environmental, human health, and industrial safety. Serpentinite, a naturally abundant silicate mineral with negligible ecotoxicity, serves as the stable base material. The exfoliation process using dimethyl sulfoxide (DMSO), dimethylformamide (DMF), and urea was optimized to avoid persistent, bioaccumulative, or toxic residues. Post-synthesis washing and thermal stabilization ensured compliance with permissible leaching limits under US-EPA Method 1311 and the EU Water Framework Directive (2000/60/EC). The final adsorbents exhibited chemical stability across a broad operational pH (3–9) without releasing secondary contaminants, aligning with ISO 14001:2015 environmental management standards.
From a human health perspective, the materials are safe to handle under standard occupational practices. OSHA and NIOSH guidelines set strict exposure limits for DMF, but residual levels in the final adsorbents are far below the permissible exposure limit of 10 ppm. DMSO is recognized as low-toxicity, while urea is benign, and the surface modifications introduce no bioactive or cytotoxic properties. These adsorbents comply with ECHA REACH regulations for industrial materials and follow NIOSH-recommended protocols for nanoparticle safety. Industrial scalability is supported by their thermal stability, non-flammability, and non-reactivity, meeting ANSI/NSF Standard 61 for water treatment materials and ISO 22,241 safety requirements for chemical handling. Regeneration can be achieved via mild chemical washing or low-temperature desorption, adhering to Green Chemistry Principles and minimizing resource consumption.
Environmentally, the adsorbents play a crucial role in mitigating antimicrobial resistance (AMR). WHO and UNEP have identified residual antibiotics in wastewater as critical contaminants driving resistant bacterial strains. By achieving high removal efficiency at environmentally relevant concentrations, these materials support WHO AMR guidelines while protecting aquatic ecosystems and human health. Spent adsorbents pose no hazardous waste risks under the Basel Convention and comply with US-EPA and EU effluent discharge standards, without releasing heavy metals, carcinogens, or endocrine disruptors.
Sustainability further enhances their value. Serpentinite is inexpensive, widely available, and environmentally benign, while the synthesis process uses low-cost reagents and minimizes carbon emissions, aligning with ISO 14,064 for greenhouse gas reductions and UN Sustainable Development Goals (SDG 6 and SDG 12). Their physisorption-driven uptake mechanism allows for multiple regeneration cycles, supporting circular economy principles and reducing material demand.
In summary, DMSO/SP, DMF/SP, and U/SP are environmentally safe, non-toxic, industrially viable, and fully compliant with major regulatory frameworks (US-EPA, WHO, ECHA-REACH, OSHA, ISO). By combining high adsorption efficiency with safety, sustainability, and regulatory adherence, these adsorbents offer a robust, scalable solution for removing pharmaceutical contaminants and mitigating AMR risks in water systems worldwide.
Conclusions
This study demonstrated that solvent-assisted exfoliation is a highly effective strategy to convert natural serpentinite into advanced adsorbents for azithromycin (AZT) removal, with clear differences in performance depending on the exfoliating agent. Among the tested modifications, DMSO produced the most delaminated structure with the highest surface accessibility, yielding superior adsorption capacity (Qsat 329.7 mg g⁻¹) and active site density, compared to DMF (292.3 mg g⁻¹) and urea (279.9 mg g⁻¹). Statistical physics modeling confirmed a multilayer physisorption mechanism (ΔE ≈ 5 kJ mol⁻¹) dominated by electrostatic attraction and stabilized by hydrogen bonding, while the steric parameter (n > 1) revealed multi-molecular stacking at active sites. These findings highlight the decisive role of solvent polarity and molecular geometry in governing serpentinite exfoliation, surface heterogeneity, and pollutant uptake efficiency. Importantly, the comparative results establish DMSO-exfoliated serpentinite as the most durable and efficient option, offering a scalable and cost-effective route for sustainable water treatment applications.
Recommendation
Future work will focus on post-adsorption characterization to validate the interaction pathways of AZT on DMSO/SP, DMF/SP, and U/SP.
Data availability
The data will be available up on request to corresponding author.
References
Imanipoor, J., Mohammadi, M. & Dinari, M. Evaluating the performance of L-methionine modified montmorillonite K10 and 3-aminopropyltriethoxysilane functionalized magnesium phyllosilicate organoclays for adsorptive removal of Azithromycin from water. Sep. Purif. Technol. 275, 119256 (2021).
Prasad, G., Jayadev, A. & Zolezzi, G. Profiling contaminants of emerging concern on the risk posed to an Indian community. Water Air Soil. Pollut. 236, 1–17 (2025).
Basheer, A. A. Chemical chiral pollution: impact on the society and science and need of the regulations in the 21st century, Chirality. 30 402–406. (2018).
Barik, D. et al. Environmental pollutants such as endocrine disruptors/pesticides/reactive dyes and inorganic toxic compounds metals, radionuclides, and metalloids and their impact on the ecosystem, in Biotechnology for Environmental Sustainability. Springer 391–442. (2025).
de Andrade, J. R., Oliveira, M. F., da Silva, M. G. & Vieira, M. G. Adsorption of pharmaceuticals from water and wastewater using nonconventional low-cost materials: a review. Ind. Eng. Chem. Res. 57, 3103–3127 (2018).
Zaidi, N. et al. Pharmaceuticals and personal care products as emerging contaminants: environmental fate, detection, and mitigation strategies. Int. J. Environ. Anal. Chem. 1–29. (2025). https://doi.org/10.1080/03067319.2025.2484456
Xie, Q. et al. Assessment of the environmental health and human exposure risk of emerging contaminants in groundwater of a typical agricultural irrigation area in the North China plain. J. Hazard. Mater., 495, 138985 (2025).
Terefinko, D. et al. Removal of clinically significant antibiotics from aqueous solutions by applying unique high-throughput continuous-flow plasma pencil and plasma brush systems. Chem. Eng. J. 452, 139415 (2023).
Khumalo, S. M., Makhathini, T. P., Bwapwa, J. K., Bakare, B. F. & Rathilal, S. The occurrence and fate of antibiotics and nonsteroidal anti-inflammatory drugs in water treatment processes: A review. J. Hazard. Mater. Adv. 10, 100330 (2023).
Ajala, A. O. et al. Adsorptive removal of antibiotic pollutants from wastewater using biomass/biochar-based adsorbents. RSC Adv. 13, 4678–4712 (2023).
Zhong, S. F. et al. Hydrolytic transformation mechanism of Tetracycline antibiotics: reaction kinetics, products identification and determination in WWTPs. Ecotoxicol. Environ. Saf. 229, 113063 (2022).
Hjazi, A. et al. Optimization of removal of sulfonamide antibiotics by magnetic nanocomposite from water samples using central composite design. Water Resour. Ind. 30, 100229 (2023).
Werkneh, A. A. & Islam, M. A. Post-treatment disinfection technologies for sustainable removal of antibiotic residues and antimicrobial resistance bacteria from hospital wastewater. Heliyon 9, e15360 (2023).
Gajic, I. et al. The emergence of multi-drug-resistant bacteria causing healthcare-associated infections in COVID-19 patients: a retrospective multi-centre study. J. Hosp. Infect. 137, 1–7 (2023).
Saadi, Z., Fazaeli, R., Vafajoo, L., Naser, I. & Mohammadi, G. Promotion of clinoptilolite adsorption for Azithromycin antibiotic by tween 80 and triton X-100 surface modifiers under batch and fixed-bed processes. Chem. Eng. Commun. 208, 328–348 (2021).
Li, D. et al. Role of adsorption during nanofiltration of sulfamethoxazole and Azithromycin solution. Sep. Sci. Technol. 56, 1996–2010 (2021).
Ali, S. A., Abid, M., Ahmed, A., Wadood, A. & Ahmed, S. Nitazoxanide and Azithromycin for the early treatment of COVID-19: A multi‐spectroscopic, biochemical and computational drug–drug interaction study. J. Chin. Chem. Soc. 70, 1568–1579 (2023).
Arif, M. et al. Impregnation of Biochar with montmorillonite and its activation for the removal of Azithromycin from aqueous media. Environ. Sci. Pollut Res. 30, 78279–78293 (2023).
Xiang, J. J., Xi, C. D. Z., Liao, Q. G., Yuan, L. J. & Zhang, D. W. Detection of Azithromycin residue in broiler feathers by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. 1152, 122225 (2020).
Cano, P. A., Jaramillo-Baquero, M., Zúñiga-Benítez, H., Londoño, Y. A. & Peñuela, G. A. Use of simulated sunlight radiation and hydrogen peroxide in Azithromycin removal from aqueous solutions: optimization & mineralization analysis. Emerg. Contam. 6, 53–61 (2020).
da Silva, C. F. et al. Impact of the pandemic on antimicrobial consumption patterns, infect. Control Hosp. Epidemiol. 42, 1170–1172 (2021).
Davoodi, S., Dahrazma, B., Goudarzi, N. & Gorji, H. G. Adsorptive removal of Azithromycin from aqueous solutions using Raw and saponin-modified nano diatomite. Water Sci. Technol. 80, 939–949 (2019).
Hanamoto, S. & Ogawa, F. Predicting the sorption of Azithromycin and Levofloxacin to sediments from mineral and organic components. Environ. Pollut. 255, 113180 (2019).
Sobhan Ardakani, S., Cheraghi, M., Jafari, A. & Zandipak, R. PECVD synthesis of zno/si thin film as a novel adsorbent for removal of Azithromycin from water samples. Int. J. Environ. Anal. Chem. 102, 5229–5246 (2022).
Maier, M. L. V. & Tjeerdema, R. S. Azithromycin sorption and biodegradation in a simulated California river system, Chemosphere. 190 471–480. (2018).
Senta, I., Kostanjevecki, P., Krizman-Matasic, I., Terzic, S. & Ahel, M. Occurrence and behavior of macrolide antibiotics in municipal wastewater treatment: possible importance of metabolites, synthesis byproducts, and transformation products. Environ. Sci. Technol. 53, 7463–7472 (2019).
Ma, Y. et al. Hydrothermal synthesis of magnetic sludge Biochar for Tetracycline and Ciprofloxacin adsorptive removal. Bioresour Technol. 319, 124199 (2021).
Goyat, R. et al. Synthesis and characterization of nanocomposite based polymeric membrane (PES/PVP/GO-TiO2) and performance evaluation for the removal of various antibiotics (amoxicillin, Azithromycin & ciprofloxacin) from aqueous solution. Chemosphere 353, 141542 (2024).
Mehrdoost, A., Yengejeh, R. J., Mohammadi, M. K., Haghighatzadeh, A. & Babaei, A. A. Adsorption removal and photocatalytic degradation of Azithromycin from aqueous solution using pac/fe/ag/zn nanocomposite. Environ. Sci. Pollut Res. 29, 33514–33527 (2022).
Ibrahim, S. M., Saad, N. & Ahmed, M. M. Abd El–Aal, Novel synthesis of antibacterial pyrone derivatives using kinetics and mechanism of oxidation of Azithromycin by alkaline permanganate. Bioorg. Chem. 119, 105553 (2022).
Al-Hakkani, M. F., Gouda, G. A., Hassan, S. H., Mohamed, M. M. & Nagiub, A. M. Environmentally Azithromycin pharmaceutical wastewater management and synergetic biocompatible approaches of loaded Azithromycin@ hematite nanoparticles. Sci. Rep. 12, 10970 (2022).
Arif, M. et al. Synthesis, characteristics and mechanistic insight into the clays and clay minerals-biochar surface interactions for contaminants removal-A review. J. Clean. Prod. 310, 127548 (2021).
Awad, A. M. et al. Adsorption of organic pollutants by natural and modified clays: a comprehensive review. Sep. Purif. Technol. 228, 115719 (2019).
Ali, I. et al. Removal of copper (II) and zinc (II) ions in water on a newly synthesized polyhydroquinone/graphene nanocomposite material: kinetics, thermodynamics and mechanism. Chemistry Select. 4, 12708–12718. (2019).
Ameen, F. et al. RETRACTED: Modeling of adsorptive removal of azithromycin from aquatic media by CoFe2O4/NiO anchored microalgae-derived nitrogen-doped porous activated carbon adsorbent and colorimetric quantifying of azithromycin in pharmaceutical products. Elsevier. (2023).
Verma, A. et al. A. García-Penas, Graphene oxide/chitosan hydrogels for removal of antibiotics. Environ. Technol., 77, 1–31. (2025).
Zou, S. J. et al. A hybrid sorbent of α-iron oxide/reduced graphene oxide: studies for adsorptive removal of Tetracycline antibiotics. J. Alloys Compd. 863, 158475 (2021).
Ali, I. et al. Green Preparation of activated carbon from pomegranate Peel coated with zero-valent iron nanoparticles (nZVI) and isotherm and kinetic studies of amoxicillin removal in water. Environ. Sci. Pollut Res. 27, 36732–36743 (2020).
Mousavi, S. A. & Janjani, H. Antibiotics adsorption from aqueous solutions using carbon nanotubes: a systematic review. Toxin Rev., 24 3, 24–234(2020).
Zhang, J. et al. Recent advances in performance improvement of Metal-organic frameworks to remove antibiotics: mechanism and evaluation, sci. Total Environ. 811, 152351 (2022).
Al-Jubouri, S. M., Al-Jendeel, H. A., Rashid, S. A. & Al-Batty, S. Antibiotics adsorption from contaminated water by composites of ZSM-5 zeolite nanocrystals coated carbon. J. Water Process. Eng. 47, 102745 (2022).
Hacıosmanoğlu, G. G. et al. Antibiotic adsorption by natural and modified clay minerals as designer adsorbents for wastewater treatment: A comprehensive review. J. Environ. Manage. 317, 115397 (2022).
Ali, I. et al. Fast removal of samarium ions in water on highly efficient nanocomposite based graphene oxide modified with polyhydroquinone: isotherms, kinetics, thermodynamics and desorption. J. Mol. Liq. 329, 115584 (2021).
Ali, I. et al. Preparation and characterization of nano-structured modified montmorillonite for dioxidine antibacterial drug removal in water. J. Mol. Liq. 331, 115770 (2021).
Ali, I. et al. Preparation and characterization of oxidized graphene for actinides and rare Earth elements removal in nitric acid solutions from nuclear wastes. J. Mol. Liq. 335, 116260 (2021).
Ahrouch, M., Gatica, J., Draoui, K. & Vidal, H. Adding value to natural clays as low-cost adsorbents of methylene blue in polluted water through honeycomb monoliths manufacture. SN Appl. Sci. 1, 1595 (2019).
Rammal, J. et al. Comparative analysis of natural and synthetic materials for wastewater treatment: plant powders, activated carbon, biochar, zeolite, and nanomaterials. J. Chem. Rev. 7, 566–590 (2025).
Shemy, M. H. et al. Engineering high-performance CTAB-functionalized magnesium silicate nano-adsorbent for efficient removal of Cd2+, Co2+, and Cu2 + from single-metal aqueous solutions. Front. Chem. 13, 1583305 (2025).
Ahrouch, M., Gatica, J. M., Draoui, K., Bellido-Milla, D. & Vidal, H. Clay honeycomb monoliths for the simultaneous retention of lead and cadmium in water. Environ. Technol. Innov. 27, 102765 (2022).
Salam, M. A., Abukhadra, M. R. & Mostafa, M. Effective decontamination of as (V), hg (II), and U (VI) toxic ions from water using novel muscovite/zeolite aluminosilicate composite: adsorption behavior and mechanism. Environ. Sci. Pollut Res. 27, 13247–13260 (2020).
Alouani, M. E. et al. A mini-review on Natural and Modified Clays for Removal of Organic and Inorganic Pollutants from Wastewater and their Other Applications15–41 (Advanced Systems for Environmental Monitoring, IoT and the application of Artificial Intelligence, 2024).
Değermenci, G. D., Değermenci, N., Emin, N. & Aşıkuzun, E. Characterization of Mg-rich natural serpentine clay mineral and removal of reactive blue 19 from aqueous solutions. EQA Int. J. Environ. Qual. 47, 40–55 (2022).
Perelomov, L. et al. Adsorption Antibiot. Nat. Clay Minerals Minerals 15 733. (2025).
Wang, Z. et al. Facet-dependent adsorption of heavy metal ions on Janus clay nanosheets. J. Hazard. Mater. 461, 132548 (2024).
Mejri, C. & Oueslati, W. A Critical Review of Clay Minerals for Groundwater Protection and Treatment, Groundw (Front. Tech. Chall., 2024).
Kumari, N. & Mohan, C. Basics of clay minerals and their characteristic properties. Clay Clay Min. 24, 1–29 (2021).
Elmi, C. Physical-chemical properties of nano-sized phyllosilicates: recent environmental and industrial advancements. Encyclopedia 3, 1439–1460 (2023).
Alomari, A. D. A. Chemically modified clay for adsorption of contaminants: trends, advantages and limitations-a concise review. Int. J. Environ. Anal. Chem., 1–24. (2024). https://doi.org/10.1080/03067319.2024.2311296
Heryanto, H., Tahir, D., Akmal, A. & Setiawan, V. Advancements in clay mineral adsorbents of montmorillonite and kaolinite for effective water contamination remediation. Int. J. Environ. Sci. Technol. (Tehran), 1–26. (2025). https://doi.org/10.1007/s13762-025-06630-y
Barakan, S. & Aghazadeh, V. The advantages of clay mineral modification methods for enhancing adsorption efficiency in wastewater treatment: a review. Environ. Sci. Pollut Res. 28, 2572–2599 (2021).
Zhu, Y., Kottarath, S., Iroh, J. O. & Vaia, R. A. Progressive intercalation and exfoliation of clay in polyaniline–montmorillonite clay nanocomposites and implication to nanocomposite impedance. Energies 15, 5366 (2022).
Ali, S. M., Mohamed, R. A., Abdel-Khalek, A. A., Ahmed, A. M. & Abukhadra, M. Physicochemical, steric, and energetic characterization of kaolinite based silicate nano-sheets as potential adsorbents for Safranin basic dye: effect of exfoliation reagent and techniques. Front. Chem. 12, 1455838 (2024).
Abdo, S. M., El-Hout, S. I., Shawky, A., Rashed, M. N. & El-Sheikh, S. M. Visible-light-driven photodegradation of organic pollutants by simply exfoliated kaolinite nanolayers with enhanced activity and recyclability. Environ. Res. 214, 113960 (2022).
Das, S. et al. A review on clay exfoliation methods and modifications for CO2 capture application. Mater. Today Sustain. 23, 100427 (2023).
Allah, A. F. et al. Effective remediation of toxic metal ions (Cd (II), Pb (II), hg (II), and Ba (II)) using mesoporous glauconite-based iron silicate nanorods: experimental and theoretical studies. Microporous Mesoporous Mater. 382, 113390 (2025).
Gaber, W. et al. Facile exfoliation of natural Talc into separated mesoporous magnesium silicate nano-sheets for effective sequestration of phosphate and nitrate ions: characterization and advanced modeling. Front. Chem. 13, 1571723 (2025).
Rooney, J. S., Tarling, M. S., Smith, S. A. & Gordon, K. C. Submicron Raman spectroscopy mapping of serpentinite fault rocks. J. Raman Spectrosc. 49, 279–286 (2018).
Chen, H. et al. Activated calcium-rich serpentine tailings and their superefficient removal for arsenite, arsenate and cadmium. Environ. Technol. Innov. 35, 103726 (2024).
Wenk, H. R., Kattemalavadi, A., Zhang, Y., Kennedy, E. R. & Borkiewicz, O. Exploring microstructures and anisotropies of serpentinites. Contrib. Mineral. Petrol. 180, 23 (2025).
Zheng, W. & Lee, L. Y. S. Beyond sonication: advanced exfoliation methods for scalable production of 2D materials. Matter 5, 515–545 (2022).
Zuo, X., Wang, D., Zhang, S., Liu, Q. & Yang, H. Effect Intercalation Agents Morphology Exfoliated Kaolinite Minerals 7 249. (2017).
Makó, É., Kovács, A. & Kristóf, T. Influencing parameters of direct homogenization intercalation of kaolinite with urea, dimethyl sulfoxide, formamide, and N-methylformamide, appl. Clay Sci. 182, 105287 (2019).
Shawky, A., El-Sheikh, S. M., Rashed, M. N. & Abdo, S. M. El-Dosoqy, Exfoliated kaolinite nanolayers as an alternative photocatalyst with Superb activity. J. Environ. Chem. Eng. 7, 103174 (2019).
Amarbayar, N. et al. Progressive carbonation and Ca-metasomatism of serpentinized ultramafic rocks: insights from natural occurrences and hydrothermal experiments. Contrib. Mineral. Petrol. 178, 38 (2023).
Abdel-Rahman, A. et al. Ultramafic rocks and their alteration products from Northwestern Allaqi province, southeastern desert, egypt: petrology, mineralogy, and geochemistry. Front. Earth Sci. 10, 894582 (2022).
Zhang, S. et al. Mechanism responsible for intercalation of dimethyl sulfoxide in kaolinite: molecular dynamics simulations. Appl. Clay Sci. 151, 46–53 (2018).
Singh, P., Veneeta, K. & Kaur Thermodynamic insight of DMSO-DMA and DMSO-DMF binary mixtures across varying temperatures. J. Mol. Liq. 415, 126286 (2024).
Würfel, H. & Heinze, T. Acidic dimethyl sulfoxide: A solvent system for the fast dissolution of pectin derivatives suitable for subsequent modification. Carbohydr. Polym. 348, 122872 (2025).
Zhang, S. et al. Mechanism associated with kaolinite intercalation with urea: combination of infrared spectroscopy and molecular dynamics simulation studies. J. Phys. Chem. C. 121, 402–409 (2017).
Altowyan, A. S. et al. Design and characterization of zeolite/serpentine nanocomposite photocatalyst for solar hydrogen generation. Materials 15, 6325 (2022).
Valouma, A., Verganelaki, A., Tetoros, I., Maravelaki-Kalaitzaki, P. & Gidarakos, E. Magnesium oxide production from Chrysotile asbestos detoxification with oxalic acid treatment. J. Hazard. Mater. 336, 93–100 (2017).
Yu, C. et al. The formation of fungus-serpentine aggregation and its immobilization of lead (II) under acidic conditions. Appl. Microbiol. Biotechnol. 105, 2157–2169 (2021).
Wang, J., Fu, L., Yang, H., Zuo, X. & Wu, D. Energetics, interlayer molecular structures, and hydration mechanisms of dimethyl sulfoxide (DMSO)–kaolinite nanoclay guest–host interactions. J. Phys. Chem. Lett. 12, 9973–9981 (2021).
Okamura, T. & Nakagawa, J. Contribution of intramolecular NH··· O hydrogen bonds to magnesium–carboxylate bonds. Inorg. Chem. 52, 10812–10824 (2013).
Wu, S., Lee, W. C., Thenuwara, H. N. & Wu, P. Quantitative criteria for solvent selection in Liquid-Phase exfoliation: balancing exfoliation and stabilization efficiency. Nanomaterials 15, 370 (2025).
Tatang, H. B. et al. Kaolinites structural defects related to Urea and dimethyl sulfoxide intercalation. Appl. Clay Sci. 255, 107415 (2024).
Thommes, M. et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical Report). Pure Appl. Chem. 87, 1051–1069 (2015).
Danchova, N. et al. Porous silica gels doped with gold nanoparticles: preparation, microstructure, optical and textural properties. Gels 11, 454 (2025).
ZOGO MFEGUE, B., MBEY, J. A., COULIBALY, S. L., ONANA, V. L. & NDJIGUI, P. D. DMSO deintercalation in Kaolinite–DMSO intercalate: influence of solution Polarity on removal. J. Compos. Sci. 5, 97 (2021).
Gamal, R., Sheha, E. & Kholy, M. E. Dimethyl sulfoxide as a function additive on halogen-free electrolyte for magnesium battery application. RSC Adv. 13, 11959–11966 (2023).
Leal, P. V. B., Pereira, D. H., Papini, R. M. & Magriotis, Z. M. Effect of dimethyl sulfoxide intercalation into kaolinite on Etheramine adsorption: experimental and theoretical investigation. J. Environ. Chem. Eng. 9, 105503 (2021).
Zhao, M., Casiraghi, C. & Parvez, K. Electrochemical exfoliation of 2D materials beyond graphene. Chem. Soc. Rev. 53, 3036–3064 (2024).
Meziane, O., Bensedira, A., Guessoum, M. & Haddaoui, N. Preparation and characterization of intercalated kaolinite with urea, dimethyl formamide and an alkylammonium salt using guest displacement reaction. J. Mater. Environ. Sci. 8, 3625–3635 (2017).
Xie, X. & Hayashi, S. NMR study of kaolinite intercalation compounds with formamide and its derivatives. 1. Structure and orientation of guest molecules. J. Phys. Chem. B. 103, 5949–5955 (1999).
Nieuwland, C., van Dam, A. N., Bickelhaupt, F. M. & Guerra, C. F. Urea hydrogen-bond donor strengths: bigger is not always better. Phys. Chem. Chem. Phys. 27, 4099–4108 (2025).
Paladino, A., Vitagliano, L. & Graziano, G. The action of chemical denaturants: from globular to intrinsically disordered proteins. Biology 12, 754 (2023).
Cela-Dablanca, R. et al. A. Núñez-Delgado, Azithromycin adsorption onto different soils, Processes. 10 2565. (2022).
Guimarães, M., Somville, P., Vertzoni, M. & Fotaki, N. Investigating the critical variables of Azithromycin oral absorption using in vitro tests and PBPK modeling. J. Pharm. Sci. 110, 3874–3888 (2021).
Talaiekhozani, A. et al. Comparison of Azithromycin removal from water using UV radiation, Fe (VI) oxidation process and ZnO nanoparticles. Int. J. Env Res. Public. Health. 17, 1758 (2020).
Balarak, D., Mahvi, A. H., Shahbaksh, S., Wahab, M. A. & Abdala, A. Adsorptive removal of Azithromycin antibiotic from aqueous solution by Azolla filiculoides-based activated porous carbon. Nanomaterials 11, 3281 (2021).
El-Sherbeeny, A. M. et al. Effective retention of radioactive Cs + and Ba2 + ions using β-cyclodextrin functionalized diatomite (β-CD/D) as environmental adsorbent; characterization, application, and safety. Surf. Interfaces. 26, 101434 (2021).
Duan, Y. et al. Adsorption of Sb(V) in wastewater by fe/mn binary oxides loaded sludge derived activated carbon: performance, mechanisms and applicability. Environ. Technol. Innov. 36, 103881 (2024).
Alhalili, Z. & Abdelrahman, E. A. Facile synthesis and characterization of manganese ferrite nanoparticles for the successful removal of Safranine T dye from aqueous solutions. Inorganics 12, 30 (2024).
Abdel Salam, M. et al. Synthesis and characterization of green zno@ polynaniline/bentonite tripartite structure (G. Zn@ PN/BE) as adsorbent for as (V) ions: integration, steric, and energetic properties. Polymers 14, 2329 (2022).
Qada, E. E. Kinetic behavior of the adsorption of malachite green using Jordanian diatomite as adsorbent. Jordan J. Eng. Chem. Ind. 3, 1–10 (2020).
Lin, X. et al. Facile Preparation of dual La-Zr modified magnetite adsorbents for efficient and selective phosphorus recovery. Chem. Eng. J. 413, 127530 (2021).
Albukhari, S. M., Salam, M. A. & Abukhadra, M. R. Effective retention of inorganic selenium ions (Se (VI) and se (IV)) using novel sodalite structures from muscovite; characterization and mechanism. J. Taiwan. Inst. Chem. Eng. 120, 116–126 (2021).
Abukhadra, M. R. et al. Insight into the adsorption properties of chitosan/zeolite-A hybrid structure for effective decontamination of toxic cd (II) and as (V) ions from the aqueous environments. J. Polym. Environ. 30, 295–307 (2022).
Wang, M., Yan, R., Shan, M., Liu, S. & Tang, H. Fabrication of crown ether-containing copolymer porous membrane and their enhanced adsorption performance for cationic dyes: experimental and DFT investigations. Chemosphere 352, 141363 (2024).
Wang, J. & Guo, X. Adsorption kinetic models: physical meanings, applications, and solving methods. J. Hazard. Mater. 390, 122156 (2020).
Alsharif, M. A. Understanding Adsorption: Theories, Techniques, Adsorption-Fundamental Mechanisms and Applications: Fundamental Mechanisms and Applications, 1. (2025).
Hu, Q., Pang, S. & Wang, D. In-depth insights into mathematical characteristics, selection criteria and common mistakes of adsorption kinetic models: A critical review. Sep. Purif. Rev. 51, 281–299 (2022).
Hassan, M. et al. Magnetic Biochar for removal of perfluorooctane sulphonate (PFOS): interfacial interaction and adsorption mechanism. Environ. Technol. Innov. 28, 102593 (2022).
Jasper, E. E., Ajibola, V. O. & Onwuka, J. C. Nonlinear regression analysis of the sorption of crystal Violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 10, 1–11 (2020).
Farhan, A. M. et al. Tailoring the synergistic effect of integrated polypyrrole hydrogel on the adsorption activity of rice husk-based activated carbon (polypyrrole/activated carbon composite) for bisphenol-A and 4-chlorophenol: experimental and theoretical analysis. Front. Bioeng. Biotechnol. 13, 1556887 (2025).
Ashrafi, G., Nasrollahzadeh, M., Jaleh, B., Sajjadi, M. & Ghafuri, H. Biowaste-and nature-derived (nano) materials: biosynthesis, stability and environmental applications. Adv. Colloid Interface Sci. 301, 102599 (2022).
Mbey, J. A. et al. DMSO intercalation in selected kaolinites: influence of the crystallinity. Chemengineering 4, 66 (2020).
Kola, D. Y. & Edebali, S. Facile synthesis of zeolitic-imidazole framework‐67 (ZIF‐67) for the adsorption of Indigo Carmine dye. Can. J. Chem. Eng. 103, 2373–2385 (2025).
Zhang, X., Zhai, Z., Feng, X., Hou, H. & Zhang, Y. Recent advances of metal–organic framework for heavy metal ions adsorption. Langmuir 40, 17868–17888 (2024).
Sherlala, A. I. A., Raman, A. A. A., Bello, M. M. & Buthiyappan, A. Adsorption of arsenic using Chitosan magnetic graphene oxide nanocomposite. J. Environ. Manage. 246, 547–556 (2019).
Huang, Y. et al. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 194, 462–469 (2018).
Shaban, M., Abukhadra, M. R., Shahien, M. G. & Khan, A. A. P. Upgraded modified forms of bituminous coal for the removal of safranin-T dye from aqueous solution. Environ. Sci. Pollut Res. 24, 18135–18151 (2017).
Mobarak, M., Ali, R. A. M. & Seliem, M. K. Chitosan/activated coal composite as an effective adsorbent for Mn(VII): modeling and interpretation of physicochemical parameters. Int. J. Biol. Macromol. 186, 750–758 (2021).
Yang, X. et al. Insight into the adsorption and oxidation activity of a zno/piezoelectric quartz core-shell for enhanced decontamination of ibuprofen: steric, energetic, and oxidation studies. Chem. Eng. J. 431, 134312 (2022).
Sellaoui, L., Ali, J., Badawi, M., Bonilla-Petriciolet, A. & Chen, Z. Understanding the adsorption mechanism of Ag + and Hg2 + on functionalized layered double hydroxide via statistical physics modeling. Appl. Clay Sci. 198, 105828 (2020).
Ashraf, M. T. et al. Synthesis of cellulose fibers/Zeolite-A nanocomposite as an environmental adsorbent for organic and inorganic selenium ions; characterization and advanced equilibrium studies. J. Mol. Liq. 360, 119573 (2022).
Ali, R. A. M. et al. New insights into the surface oxidation role in enhancing congo red dye uptake by Egyptian Ilmenite ore: experiments and physicochemical interpretations. Surf. Interfaces. 26, 101316 (2021).
Pradhan, B. L., Lodhi, L., Dey, K. K. & Ghosh, M. Analyzing atomic scale structural details and nuclear spin dynamics of four macrolide antibiotics: erythromycin, clarithromycin, azithromycin, and roxithromycin. RSC Adv. 14, 17733–17770 (2024).
Castro-Jiménez, C. C., Saldarriaga-Molina, J. C., García, E. F., Torres-Palma, R. A. & Acelas, N. Azithromycin removal from water via adsorption on drinking water sludge-derived materials: kinetics and isotherms studies. PLoS One. 20, e0316487 (2025).
Shikuku, V. O. et al. Single and binary adsorption of sulfonamide antibiotics onto iron-modified clay: linear and nonlinear isotherms, kinetics, thermodynamics, and mechanistic studies. Appl. Water Sci. 8, 175 (2018).
Dhaouadi, F. et al. Statistical physics interpretation of the adsorption mechanism of Pb2+, Cd2 + and Ni2 + on chicken feathers. J. Mol. Liq. 319, 114168 (2020).
Sellaoui, L. et al. Experimental and theoretical studies of adsorption of ibuprofen on Raw and two chemically modified activated carbons: new physicochemical interpretations. RSC Adv. 6, 12363–12373 (2016).
Dhaouadi, F. et al. Adsorption mechanism of Zn2+, Ni2+, Cd2+, and Cu2 + ions by carbon-based adsorbents: interpretation of the adsorption isotherms via physical modelling. Environ. Sci. Pollut Res. 28, 30943–30954 (2021).
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Author information
Authors and Affiliations
Contributions
1. Amira S. Diab: Methodology, Visualization, validation, Data curation, Formal analysis, Writing -original draft, Writing – review & editing, 2. Hassan A. Rudayni: Formal analysis, Funding, Data curation, methodology, software, validation, Writing - original draft, Writing – review & editing. 3. Reham A. Mohamed: Supervision, Validation, Writing - original draft, Writing – review & editing. 4. Ahmed A. Allam: Methodology, Data curation, Formal analysis, Writing -original draft. 5. Mostafa R. Abukhadra: Conceptualization, Formal analysis, supervision, Resources, Data curation, Visualization, methodology, validation, Writing - original draft, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Diab, A.S., Rudayni, H.A., Mohamed, R.A. et al. Green clay engineering via dimethyl sulfoxide, dimethylformamide, and urea-assisted exfoliation of serpentinite for efficient azithromycin adsorption as emerging pharmaceutical pollutant. Sci Rep 15, 35279 (2025). https://doi.org/10.1038/s41598-025-18893-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18893-z