Abstract
This retrospective study aimed to assess the impact of subretinal drusenoid deposit (SDD), also known as reticular pseudodrusen, on retinal sensitivity (RS) in patients with intermediate age-related macular degeneration (iAMD) using microperimetry. Eyes meeting the modified criteria of iAMD, including SDD, and total drusen area greater than 0.50 mm2 were included. The presence of SDD, as well as soft drusen (SD), and specific type of SDD (dot or ribbon) were determined through multimodal imaging modalities including fundus photography, fundus autofluorescence, infrared reflectance image and optical coherence tomography. RS was measured using MAIA microperimetry. A total of 302 eyes from 302 patients were analyzed. Patients with SDD were older and predominantly female. Average RS was significantly lower in eyes with SDD than without SDD. (19.75 (5.06) vs. 21.21 (4.53) (average (SD)), P = 0.003). The presence of SD alone did not significantly affect RS. Among eyes with SDD, those with ribbon-type SDD exhibited significantly lower RS (18.02 ± 6.49) than those with dot-type SDD (20.67 ± 4.13; P < 0.001). In multivariable linear regression analysis, older age and the presence of ribbon-type SDD were significantly associated with reduced RS. In conclusion, eyes with iAMD and SDD demonstrate reduced RS, with ribbon-type SDD exerting a greater negative impact on retinal function than dot-type SDD.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is a leading cause of blindness in developed countries. For exudative AMD, intravitreal injection of anti-vascular growth factor (VEGF) are the current standard treatment. While several novel therapeutic agents for advanced AMD are under development, considerable research efforts are also focused on establishing preventive strategies to halt or delay the progression to late-stage AMD1. In this context, intermediate AMD (iAMD) has received increasing attention, as it represents a precursor stage to advanced AMD. One of the key pathologic feature of iAMD is the presence of drusen. Classical soft drusen are deposits located beneath the retinal pigment epithelium (RPE) whereas subretinal drusenoid deposit (SDD) is found between the retina and RPE. SDD is known to increase the risk of developing GA or neovascular AMD2,3.
Central visual acuity is usually not affected in iAMD while patients with advanced AMD typically experience significant visual deterioration. Visual acuity tests primarily assess central visual function. Various visual function tests have been proposed to evaluate the visual function decline in the early state of AMD. For instance, low luminance vision is often impaired in early to intermediate AMD and correlates with the results of a low luminance questionnaire4. Microperimetry has recently emerged as clinical trial end point in several studies5. It enable the detection of subtle defect of retinal sensitivity that may precede measurable loss of visual acuity. A key advantage of microperimetry is its ability to assess retinal sensitivity in specific location based on fundus image. Therefore, microperimetry is particulary useful for evaluating the visual function impairment in iAMD with retinal pathologies such as drusen or SDD. Several studies have employed microperimetry to investigate the retinal sensitivity of iAMD, focusing on the presence of SDD or soft drusen. However, SDD can be further categorized into different types based on its distribution pattern and morphology, such as dot, ribbon and peripheral types. The risk of AMD progression is known to differ according to the pattern of SDD. Therefore, we aimed to examine retinal function impairment in relation to the type of SDD as well as type of drusen, using microperimetry in conjunction with multimodal imaging approaches.
Results
A total of 531 eyes from 302 participants met the inclusion criteria. When both eyes were eligible, the right eye was selected for analysis, resulting in a final dataset of 302 eyes. Representative cases with SDD and SD are shown in Fig. 1, and cases with the SDD alone are shown in Fig. 2. SDD was found more frequently in female patients. (87.3% in SDD vs. 65.9% in non-SDD, P < 0.001, Chi squared test). The mean age of SDD patients was higher compared to that of patients without SDD (75.31 in SDD vs. 72.17 in non-SDD). (Table 1), Patients with eyes having both SDD and SD are older than patients with SD alone. (Tables 1 and 3) Dot type and ribbon type SDD were found in 113 eyes and 69 eyes, respectively. Thirty-eight eyes had SDD but did not fit into either dot or ribbon type (Table 1).
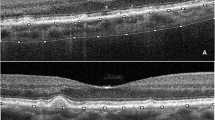
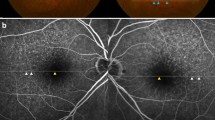
Representative case of soft drusen (SD) alone (A–D) and both SD and subretinal drusenoid deposit (SDD) (E–H). SDD includes both dot and ribbon type SDD. (A) and (E) are fundus photos. (B) and (F) are blue peak fundus autofluorescence (FAF) images. (C) and (G) are infrared reflectance images (IR). (D) and (H) shows pointwise retinal sensitivity and optical coherence tomography (OCT). FAF, IR, and OCT were acquired using the Spectralis OCT device. All images are aligned to display the same retinal region. The MAIA-derived pointwise retinal sensitivity is superimposed on the FAF image. The OCT B scan corresponds to the green arrow in the IR image. Areas outlined in blue indicate SD, while areas within green lines correspond to dot-type SDD. Note that retinal sensitivity is reduced in the area with SDD (H) compared to the region with SD alone (D).
Representative case of dot-type subretinal drusenoid deposit (SDD) (A–D) and ribbon type SDD (E–H). (A) and (E) show color fundus photographs. Magnified insets of the SDD, enclosed in green boxes at the bottom left corner, provide clearer visualization of each SDD type. (B) and (F) are blue peak fundus autofluorescence (FAF) images. C and G are infrared reflectance (IR) images. (D) and (H) show pointwise retinal sensitivity maps and corresponding OCT B-scans. All images are co-registered to display the same macular region. Both dot-type (A–D) and ribbon-type (E–H) SDD involve the macula, defined as more than 10 SDDs within the macula area on an OCT scan. Blue line is indicate the margins of SDD to facilitate cross-modality comparisons of SDD extent and retinal sensitivity. In the dot-type case (A–D), most SDDs are confined within the blue circle, whereas in the ribbon-type case (E–H), SDDs are located outside the blue circle. Retinal sensitivity is markedly reduced in the presence of ribbon-type SDD (H).
The average retinal sensitivity (RS) was significantly lower in eyes having SDD compared to those without SDD. (19.11 (5.36) with SDD vs. 20.87 (4.45) without SDD, P = 0.009), whereas visual acuity (VA) did not differ by the presence of SDD. Eyes with both SDD and SD had lower RS than eyes with SD alone. However, RS and VA were not affected by the presence of SD, when SDD were absent (Tables 2 and 3).
Next, we investigated the influence of location and type of drusen on BCVA and RS. RS was not significantly affected by the location of SD within macula while RS of outer macula region (Ring 1 and 2) was significantly lower when SDD was present. The average RS was not differed by the macular involvement of SDD. Interestingly, only the outermost macula ring (Ring 1) showed a significant reduction in RS with regard to macular involvement of SDD, whereas the sensitivity of the central macula (Ring 2, 3 and S4) remained unaffected. BCVA was not affected by the location of SDD (macular vs. non-macular) (Table 4).
Comparing SDD subtypes, eyes with ribbon-type SDD demonstrated lower average RS than those with dot-type SDD, while no significant difference in BCVA was observed between the two groups (Table 2). This significant difference was observed in all regions of the macula (Table 4).
Univariable linear regression revealed that age and the presence of ribbon-type SDD were significant factors associated with average RS. In the multivariable model, average RS remained independently associated with both age and ribbon-type SDD. RS showed a negative correlation with increasing age and the presence of ribbon-type SDD (Table 5).
Discussion
This study demonstrates the negative impact of SDD on RS in individuals with iAMD. Eyes with SDD exhibited reduced RS, although central vision did not show significant differences. Moreover, the ribbon type SDD had an independent effect on RS, while the presence of dot SDD did not. These findings highlight that both the presence and type of SDD are associated with RS, to the best of our knowledge, this has not been previously reported.
The association between SDD and RS has been investigated in several studies using microperimetry. Comparing eyes with and without SDD, a significant decrease in mean RS was observed in eyes with SDD6. Furthermore, impairment of RS was more pronounced in the presence of SDD compared to SD7,8,9. These findings are consistent with the results of the current investigation. Additionally, longitudinal observation revealed progressive RS decline in retinal areas located above SDD14. This temporal deterioration supports the notion that SDD may contribute to the impairment of retinal function in AMD.
The association between drusen volume and RS in AMD appears to be conflicting10,11. These studies did not specify which types of drusen impact RS. As SDD is relatively smaller than SD and tend to regress, while SD progress and enlarge, it is likely that these volumetric analysis studies predominantly reflected SD. Some studies have also questioned the impact of SDD on RS. Wu et al. reported no association between SDD and microperimetry RS12. Roh et al. demonstrated that neither SDD nor SD were significantly associated with RS reduction after adjusting for confounding variables13. However, these studies included patients across the full AMD spectrum, including advanced stages where SDD may regress and become difficult to detect14. Consequently, the effects of SDD may have been underestimated. In summary, most existing evidence supports a negative effect of SDD on RS, while the role of SD remains less consistent and likely becomes functionally significant only at later stages.
The current study revealed that RS was more reduced in the outer macula (ring 1 and 2) compared to the inner and central macula. (ring 3 and fovea) (Table 4 and Fig. 2) SDD typically originates from the upper mi-dperiphery and spread along the outer macula, often sparing the fovea15,16. This distribution corresponds with areas of high rod photoreceptor density. This trend is also observed in the current work although this is a cross sectional study. Although this is a cross-sectional study, the observed topographic pattern aligns with the known distribution of SDD and explains the greater RS loss in the outer macular regions.
Our multivariable regression analysis revealed that increasing age is associated with decreased RS. This finding is consistent with previous reports evaluating RS in healthy subjects17,18. Age-related RS decline is thought to result from neural degeneration involving photoreceptors, ganglion cells, or visual cortex neurons, as well as from changes in ocular media and pupil size19.
Microperimetry provides a functional assessment of retinal areas beyond the fovea and is thus more comprehensive than visual acuity testing, particularly in diseases like AMD where pathology may not initially affect central vision. In this study, RS of central grids was also not different between drusen types. (Table 4) Moreover, microperimetry generally measures photopic and mesopic retinal function, mainly reflecting cone function. In contrast, rod mediated function can be measured using scotopic microperimetry or dark adaptation test. Scotopic RS has been reported to be decreased in iAMD7,20,21. Comparing areas with SDD to that without visible pathologic alterations, scotopic sensitivity decline was more pronounced than photopic testing in SDD-affected regions21. Sassmannshausen et al. revealed the decrease of both mesopic and scotopic RS in SDD areas and their association with progressive outer retinal degeneration in a longitudinal investigation20. Visual sensitivity recovery time after photo-bleach (Rod intercept time) can be measured using dark adaptometry. Delays of recovery time was associated with the coexistence of SDD22,23,24,25. RS of dark adapted chromatic perimeter was worse in AMD with SDD than without SDD26. These findings are in line with the current study, highlighting the functional impairment associated with SDD across multiple modalities.
The patho-mechanism by which SDD affects retinal function has not been fully elucidated. SDD is considered a risk factor and precursor lesion of atrophic AMD. SDD of iAMD may be associated with outer retinal degeneration processes. Greferath et al. examined the retina of patients with SDD using OCT and histology and demonstrated that SDD was associated with photoreceptor disruption and loss27. In addition, retinal gliosis and RPE abnormalities are frequently observed above SDD. Histological findings also suggest that SDD preferentially affects rod-mediated retinal function, as SDD is predominantly found in the perifoveal region, where rods are highly concentrated, while basal linear deposits are more prevalent in the foveal region, where cones are highly concentrated15. Perifoveal depression of RS, corresponds to outer rings, is also observed in cases with SDD of current study (Table 4). In vivo OCT studies have shown decreased outer retinal and macular thicknesses with progressive decrease in SDD area compared to healthy or areas containing other drusen types20,28,29. SDD is located between RPE and photoreceptor, which may impair the photoreceptor-RPE circulation, so called visual cycle.
SDD types were categorized and compared in the current study. SDD has been reported as a risk factor for both wet AMD and geographic atrophy3,30. Among them, ribbon type SDD has been found to be more strongly associated with advanced AMD31,32,33. It can be inferred that ribbon and dot type SDD represents different stages of lesion evolution rather than a distinct pathology. In the current study, patients with ribbon-type SDD were older on average than those with dot-type, which supports the notion that ribbon-type SDD represents an advanced form. SDD undergo changes in distribution and morphology16. Although ribbon-type SDD has not yet been widely standardized in nomenclature, descriptions of "late-stage SDD" in previous literature likely correspond to this subtype16. The progression of SDD morphology has been studied using OCT34. However, the association between this OCT finding and the SDD subtype by fundus photo has not been investigated yet. The change of SDD subtype should be clarified in further longitudinal study using multimodal image analysis.
This study has several limitations. We did not perform the pointwise-match analysis between drusen and the corresponding RS values. Due to the small size, numerous nature, and blurred margins of SDD, it is challenging to conduct point-by-point analysis or quantitative measurements. Therefore, we compared RS based on the presence of SDD and macular involvement as an alternative approach. In addition, we did not assess pigmentary abnormalities, which may influence RS, because these features were difficult to distinguish from small, amorphous yellow-white SDD35. Another limitation is that axial length, which can influence retinal sensitivity measured by microperimetry in high myopia patients, was not systematically measured in this study. Lastly, study participants were only comprised of Koreans, which calls for further studies to generalize the findings of the present work.
In summary, this study demonstrates an association between SDD and a reduction in RS, in contrast to SD. Analyzing SDD subtypes, ribbon type SDD was independently related to a decrease in average RS. These findings underlie the importance of SDD as a significant pathologic markers in iAMD.
Methods
This study adhered to the tenets of the Declaration of Helsinki, and Institutional Review Board (IRB)/Ethics Committee approval was obtained (Seoul National University Hospital IRB approval no.: 1910-147-1073). Due to the retrospective nature of the study, Seoul National University Hospital IRB waived the need of obtaining informed consent.
Patients were enrolled from the retina clinic of Seoul National University Hospital from 2019 to 2021. Participants were included primarily if at least one eye was diagnosed with iAMD, which was characterized by the presence of drusen larger than 125 ㎛ or medium drusen with pigmentary abnormalities7. In addition, eyes with SDD, even in the absence of medium or large drusen, were also included for the purpose of current study. This extended inclusion criteria was based on emerging evidence indicating that SDD is an important phenotypic feature and risk factor for AMD progression, despite not being included in traditional AMD definitions36,37. To ensure consistency in of drusen burden, only eyes with total drusen area greater than ETDRS circle O-2 (≥ 0.50 mm2) were included. All types of drusen including SDD were incorporated into the drusen area calculation. Eyes with other retinal or optic nerve diseases and glaucoma were excluded. To avoid confounding effects of other ocular pathologies such as media opacity, only participants with best-corrected visual acuity (BCVA) of 20/40 or better were included.
The following imaging techniques were employed: color fundus photography (Kowa Non-Myd 7, Kowa, Tokyo, Japan), fundus autofluorescence (FAF), using an excitation wavelength of 488 nm, infrared reflectance imaging and spectral-domain optical coherence tomography (OCT) (Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany). The OCT scans were obtained using the enhanced-depth imaging (EDI) high-resolution volume protocol centered on the fovea (49 B-scans over a 30° × 25° area, with 30 frames averaged per B-scan using automatic real-time (ART) tracking).
MAIA microperimetry (CentreVue, Padova, Italy) was used to examine the RS. All microperimetry examinations were conducted in a windowless room with all light sources eliminated to ensure scotopic conditions. The Goldmann Ⅲ stimulus size was used, with a background luminance of 4 apostib (1.27 cd/m2). Measurement scale ranged from 0 to 36 dB, and the threshold algorithm followed the standard 4–2 staircase method. The stimulus grid consisted of 37 points covering the central 10°, including one foveal point and concentric rings of 12 points each at radii of 1°, 3°, and 5°. These rings were referred to as Ring 3, 2, and 1, respectively, and the central point as S4. (Supplementary Fig. 1).
The presence of SDD was defined if definite SDD lesions observed on more than one OCT scan and this is also confirmed using one of other imaging modalities (Fundus photo, FAF and IR)38. Subtypes of SDD followed the suggestion proposed by Suzuki et al.39 Ribbon type SDD was defined as interlacing network of drusen-like deposit and dot SDD as whitish dot-like deposit. If ribbon type and dot type SDD coexisted, the dominant type was selected. (Fig. 1). Macular involvement was defined as more than 10 SDD lesions within 6 mm diameter around fovea on either horizontal or vertical OCT scan.
Right eye was selected to analyze if both eyes met the inclusion criteria. Independent T test was performed to compare the BCVA and retinal sensitivity. One-way analysis of variance (ANOVA) was utilized to evaluate the differences among three groups. Heterogeneity was assessed using the chi-squared test. Univariable and multivariable regression analysis were employed to identify significant parameters among multiple variables. Variables with p < 0.1 in univariable analyses were entered into the multivariable model.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Pameijer, E. M. et al. What did we learn in 35 years of research on nutrition and supplements for age-related macular degeneration: A systematic review. Acta Ophthalmol. (Copenh.) 100, e1541 (2022).
Finger, R. P. et al. Reticular pseudodrusen and their association with age-related macular degeneration the melbourne collaborative cohort study. Ophthalmology 123, 599–608 (2016).
Domalpally, A. et al. Prevalence, risk, and genetic association of reticular pseudodrusen in age-related macular degeneration: age-related eye disease study 2 report 21. Ophthalmology 126, 1659–1666 (2019).
Thompson, A. C. et al. Association of low luminance questionnaire with objective functional measures in early and intermediate age-related macular degeneration. Investig. Opthalmol. Vis. Sci. 59, 289 (2018).
Yang, Y. & Dunbar, H. Clinical perspectives and trends: Microperimetry as a trial endpoint in retinal disease. Ophthalmologica 244, 418–450 (2021).
Ooto, S. et al. Reduction of retinal sensitivity in eyes with reticular pseudodrusen. Am. J. Ophthalmol. 156, 1184 (2013).
Corvi, F., Pellegrini, M., Belotti, M., Bianchi, C. & Staurenghi, G. Scotopic and fast mesopic microperimetry in eyes with drusen and reticular pseudodrusen. Retin. Phila. Pa 39, 2378–2383 (2019).
Querques, G. et al. Impact of reticular pseudodrusen on macular function. Retina 34, 321–329 (2014).
Ooto, S., Suzuki, M., Vongkulsiri, S., Sato, T. & Spaide, R. F. Multimodal visual function testing in eyes with nonexudative age-related macular degeneration. Retina 35, 1726–1734 (2015).
Pondorfer, S. G. et al. Association of visual function measures with drusen volume in early stages of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 61, 55 (2020).
Tepelus, T. C., Hariri, A. H., Al-Sheikh, M. & Sadda, S. R. Correlation between mesopic retinal sensitivity and optical coherence tomographic metrics of the outer retina in patients with non-atrophic dry age-related macular degeneration. Ophthalmic Surg. Lasers Imaging Retin. 48, 312–318 (2017).
Wu, Z., Ayton, L. N., Makeyeva, G., Guymer, R. H. & Luu, C. D. Impact of reticular pseudodrusen on microperimetry and multifocal electroretinography in intermediate age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 56, 2100–2106 (2015).
Roh, M. et al. Microperimetry in age-related macular degeneration: Association with macular morphology assessed by optical coherence tomography. Br. J. Ophthalmol. 103, 1769–1776 (2019).
Spaide, R. F. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina 33, 1800–1808 (2013).
Curcio, C. A. et al. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina Phila. Pa 33, 265–276 (2013).
Sarks, J., Arnold, J., Ho, I. V., Sarks, S. & Killingsworth, M. Evolution of reticular pseudodrusen. Br. J. Ophthalmol. 95, 979–985 (2011).
Martín, A. M., Piñero, D. P. & Pérez-Cambrodí, R. J. Normal values for microperimetry with the MAIA microperimeter: Sensitivity and fixation analysis in healthy adults and children. Eur. J. Ophthalmol. 27, 607–613 (2017).
Sabates, F. N., Vincent, R. D., Koulen, P., Sabates, N. R. & Gallimore, G. Normative data set identifying properties of the macula across age groups: Integration of visual function and retinal structure with microperimetry and spectral-domain optical coherence tomography. Retina 31, 1294–1302 (2011).
Hermann, A. et al. Age-dependent normative values for differential luminance sensitivity in automated static perimetry using the Octopus 101. Acta Ophthalmol. (Copenh.) 86, 446–455 (2008).
Sassmannshausen, M. et al. Longitudinal analysis of structural and functional changes in presence of reticular pseudodrusen associated with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 61, 19 (2020).
Steinberg, J. S. et al. Scotopic and photopic microperimetry in patients with reticular drusen and age-related macular degeneration. JAMA Ophthalmol. 133, 690–697 (2015).
Sevilla, M. B. et al. Relating retinal morphology and function in aging and early to intermediate age-related macular degeneration subjects. Am. J. Ophthalmol. 165, 65–77 (2016).
Laíns, I. et al. Structural changes associated with delayed dark adaptation in age-related macular degeneration. Ophthalmology 124, 1340–1352 (2017).
Grewal, M. K. et al. Functional clinical endpoints and their correlations in eyes with AMD with and without subretinal drusenoid deposits-a pilot study. Eye Lond. Engl. 36, 398–406 (2022).
Flamendorf, J. et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology 122, 2053–2062 (2015).
Fraser, R. G. et al. Assessment of retinotopic rod photoreceptor function using a dark-adapted chromatic perimeter in intermediate age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 57, 5436–5442 (2016).
Greferath, U., Guymer, R. H., Vessey, K. A., Brassington, K. & Fletcher, E. L. Correlation of histologic features with in vivo imaging of reticular pseudodrusen. Ophthalmology 123, 1320–1331 (2016).
Chiang, T.T.-K. et al. Macular thickness in intermediate age-related macular degeneration is influenced by disease severity and subretinal drusenoid deposit presence. Invest. Ophthalmol. Vis. Sci. 61, 59 (2020).
Ramon, C., Cardona, G., Biarnés, M., Ferraro, L. L. & Monés, J. Longitudinal changes in outer nuclear layer thickness in soft drusen and reticular pseudodrusen. Clin. Exp. Optom. 102, 601–610 (2019).
Finger, R. P. et al. Reticular pseudodrusen: A risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology 121, 1252–1256 (2014).
Kim, J. H., Chang, Y. S., Kim, J. W., Lee, T. G. & Kim, C. G. Prevalence of subtypes of reticular pseudodrusen in newly diagnosed exudative age-related macular degeneration and polypoidal choroidal vasculopathy in Korean patients. Retina 35, 2604–2612 (2015).
Sakurada, Y. et al. Pseudodrusen pattern and development of late age-related macular degeneration in the fellow eye of the unilateral case. Jpn. J. Ophthalmol. 63, 374–381 (2019).
Shijo, T. et al. Prevalence and characteristics of pseudodrusen subtypes in advanced age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 255, 1125–1131 (2017).
Querques, G. et al. Analysis of progression of reticular pseudodrusen by spectral domain-optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 53, 1264–1270 (2012).
Schneck, M. E. et al. Visual function in eyes with intermediate AMD with and without retinal pigment abnormalities. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 98, 64–72 (2021).
Agrón, E. et al. Reticular pseudodrusen: The third macular risk feature for progression to late age-related macular degeneration: Age-Related Eye disease study 2 report 30. Ophthalmology 129, 1107–1119 (2022).
Agrón, E. et al. An updated simplified severity scale for age-related macular degeneration incorporating reticular pseudodrusen: Age-related eye disease study report number 42. Ophthalmology 131, 1164–1174 (2024).
Wu, Z., Kumar, H., Hodgson, L. A. B. & Guymer, R. H. Reticular pseudodrusen on the risk of progression in intermediate age-related macular degeneration. Am. J. Ophthalmol. 239, 202–211 (2022).
Suzuki, M., Sato, T. & Spaide, R. F. Pseudodrusen subtypes as delineated by multimodal imaging of the fundus. Am. J. Ophthalmol. 157, 1005–1012 (2014).
Funding
Boehringer Ingelheim International GmbH, Biberach. The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
S.L.—design of the work, data collection, data analysis, manuscript writing, T.Y., R.H.—data analysis, E.L., K.B., U.P., K.P., and C.Y.— data interpretation, manuscript review, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lim, S.H., Yamaguchi, T.C.N., Herrmann, R. et al. Influence of subretinal drusenoid deposit on retinal sensitivity in age-related macular degeneration. Sci Rep 15, 34952 (2025). https://doi.org/10.1038/s41598-025-18930-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18930-x