Abstract
Inflammation is a key factor in neurodegenerative diseases (NDs) development, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). Microglial cells maintain immune microenvironment homeostasis in the central nervous system (CNS) and are actively involved in neuroinflammation. Therefore, inhibiting microglia-mediated neuroinflammation may provide therapeutic benefits in treating NDs. Probenecid (Prob), a sulfonamide derivative, has demonstrated neuroprotective and anti-inflammatory properties. This study investigated the anti-inflammatory and antioxidative properties of Prob in a neuroinflammatory model which was established by stimulating BV2 cells with lipopolysaccharide (LPS). The oxidative stress was assessed by measuring the nitric oxide (NO), reactive oxygen species (ROS), superoxide dismutase (SOD), and malondialdehyde (MDA) levels. Tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) were quantified using enzyme-linked immunosorbent assay (ELISA) and RT-qPCR. The wound-healing assay and phagocytosis assay were employed to examine the migration and phagocytosis capacity of BV2 cells, respectively. The mRNA expression of CD16, CD206, iNOS, and Arg-1 was measured using RT-qPCR, while Western blot was used to analyze inflammasome-associated protein levels. The results indicated that Prob suppressed LPS-induced microglial activation and reduced TNF-α and IL-1β production. Moreover, Prob attenuated the migration and phagocytosis capacities of BV2 cells and inhibited oxidative stress. Prob regulated the immune microenvironment, as shown by reduced M1 markers (iNOS and CD16) and increased M2 markers (Arg-1 and CD206). However, the NLRP1 inflammasome activator (muramyl dipeptide, MDP) reversed the effects of Prob. In conclusion, Prob could alleviate LPS-induced inflammation by modulating the NLRP1 inflammasome pathway.
Similar content being viewed by others
Introduction
Neuroinflammation is a key pathological process in DNs, including PD and AD1. Microglia, the intrinsic immune cells of the CNS, maintain immune homeostasis, respond to injury, and influence brain development2.Under normal physiological conditions, microglia remain relatively quiescent. However, microglia polarize into M1 and M2 phenotypes in immune microenvironment homeostasis disruption. These phenotypes play different roles in the progression of inflammatory diseases3. M1-type microglia produce pro-inflammatory cytokines (PICs), such as TNF-α and IL-1β, which trigger inflammatory responses. In contrast, M2-type microglia produce anti-inflammatory cytokines (AICs), including IL-10 and IL-4, that suppress inflammation4. LPS is a potent activator of microglia, stimulating them to secrete various cytokines and promote neuroinflammation5. LPS-induced BV2 microglia are widely used as a neuroinflammation model6. Modulating neuroinflammatory responses represents a potential treatment strategy for neuroinflammatory diseases.
The initiation of the inflammatory response involves the activation of inflammasomes, which are high molecular-weight multiprotein complexes first discovered in 20027. An inflammasome comprises three main components: pro-caspase-1, intracellular pattern recognition receptors (PRRs) such as nucleotide oligomerization domain (NOD)-like receptors (NLRs), and adaptor proteins (ASC)7. Inflammasomes recognize pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) via PRRs, subsequently recruiting and activating caspase-1 through ASC. This activation leads to the maturation of IL-1 family cytokines (IL-18 and IL-1β), triggering an inflammatory cascade7,8. The mechanism of inflammasome activation is well-established and is generally categorized into two pathways9,10 (Fig. 1). On one hand, following CNS injury, DAMPs are activated by PRRs, leading to the NF-kB nuclear translocation, which regulates inflammation-related factor precursors such as pro-IL-1β and pro-IL-18. On the other hand, ATP released from cell death activates the purinergic receptor P2 X4, which opens the pannexin-1 channel, activating the P2 X7 receptor. The activated P2 X7 receptor further stimulates inflammasomes, leading to an inflammatory cascade after CNS injury11,12. The NLRP1 inflammasome, the first to be identified, comprises NLRP1, ASC, and pro-caspase-113, facilitating the maturation and secretion of IL-1β and IL-1814.
Probenecid (Prob), a sulfonamide derivative, is traditionally used to treat gout15. Research has shown that Prob can suppress the pannexin-1 channel and inhibit inflammasome activation16. Studies have also reported its neuroprotective effects in animal models of neurological diseases, such as brain cognitive disorder17 and neuropathic pain18. Our previous research demonstrated Prob’s protective effect in experimental models of spinal cord injury19. These findings indicated that Prob may offer neuroprotection during CNS injury; however, the underlying mechanism remained uncertain. Here, a neuroinflammatory model was created by stimulating BV2 cells with LPS to investigate whether Prob suppresses oxidative stress (OS) and inflammatory reactions via the NLRP1 inflammasome pathway. The findings aim to provide an experimental basis for the potential use of Prob in CNS diseases.
Materials and methods
Main materials
Probenecid (Cat no. 57669) was purchased from Sigma, USA. Lipopolysaccharide (LPS; E. coli O111:B4; HY-D1056A1) was obtained from Med Chem Express, USA. Fetal bovine serum (FBS; FB15015) was sourced from Clark, USA. BV2 cells (CL-0493 A) were acquired from Procell Life Science and Technology Co., Ltd., China, and muramyl dipeptide (MDP; HY-127090) was obtained from Med Chem Express, USA. MEM medium (41500) was supplied by Solarbio Life Sciences, China. The Cell Counting Kit-8 (CCK-8; BS350A) was acquired from Biosharp, China. Fluorescent latex beads (L2778) were purchased from Sigma, USA. FreeZol Reagent (R711-01) was purchased from Vazyme, China.
Cell culture and drug treatment
BV2 cells were cultured in MEM medium containing 10% (v/v) FBS and 1% (v/v) penicillin/streptomycin in a 5% CO₂ incubator at 37 °C. For experiments, cells were plated in culture dishes and incubated for 24 h. After that, the BV2 cells were pretreated with different concentrations of Prob (0, 100, 200 and 400 µM) for 4 h, then LPS (1.6 µg/mL) with or without MDP (10 µg/mL) was added to the medium to cultured for 24 h.
Cell viability experiment
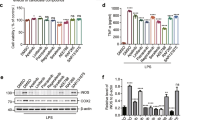
BV2 cells were seeded in 96-well plates (1 × 105 cells/well) and exposed to Prob (0, 50, 100, 200, 400, 800, 1600 and 3200 µM) for 24 h. Then, a 10% CCK-8 solution was added, and cells were incubated at 37 °C for 2 h in the dark. The OD at 450 nm was measured using a spectrophotometer (Thermo Fisher).
Wound-healing assay
BV2 cells were cultured in 6-well plates until they reached 80–90% confluence. A wound was made using a 200 µL pipette tip, and images were captured at 0 h. BV2 cells were then treated with Prob (200 µM) and LPS for 24 h. Control cells were treated with a serum-free culture medium containing 1% DMSO. Images of the cells were captured following the treatment. The migration rate was measured as the percentage reduction in the wound area during closure.
Phagocytosis assay
BV2 cells were seeded in 6-well plates and treated with Prob (200 µM) and LPS for 24 h. Fluorescent latex beads were pre-incubated with FBS at a ratio of 1:5 at 37 °C for 1 h. Following incubation, the mixture was diluted with MEM to achieve the final concentrations of FBS and beads in MEM as 0.05% (v/v) and 0.01% (v/v), respectively. The previous medium was replaced with fluorescent latex bead-containing medium, and the cells were incubated for an additional 2 h at 37 °C. Extracellular fluorescent latex beads were washed off, and the cells were fixed with 4% paraformaldehyde (PFA) at room temperature (RT). The cells were then stained with 4′,6-diamidino-2-phenylindole (DAPI) for 15 min at RT and observed under a Zeiss microscope to evaluate phagocytosis.
ROS assay
Following treatment, cells were harvested and loaded with the DCFH-DA probe provided with the ROS Assay Kit (Cat no. S0033S, Beyotime, China). Briefly, we removed the cell culture medium. Cells were collected and incubated with diluted DCFH-DA (10 µM) then incubated at 37 °C in a cell culture incubator for 20 min. Fluorescence intensity was calculated using a BD FACSVerse flow cytometer (BD Bioscience). Three independent assays were performed according to the manufacturer’s instructions.
Western blot (WB) assay
After the treatment, adherent cells were collected by scraping and centrifuging, and proteins were extracted using RIPA lysis buffer. The protein concentration was quantified with a BCA Protein Assay Kit (CE0014S, CWBIO, China). Proteins were then separated using SDS-PAGE and transferred to PVDF membranes, blocked with 5% skimmed milk, followed by overnight incubation at 4 °C with primary antibodies. Membranes were then incubated with a secondary antibody for 2 h at RT and visualized using a gel imaging system (Bio-Rad). Protein expression levels were quantified using ImageJ software. The specific antibodies used are listed in Table 1.
RT-qPCR
After cell treatment, total RNA was extracted using FreeZol Reagent. cDNA synthesis was performed using the HiScript II 1st Strand cDNA Synthesis Kit (R233-01, Vazyme, China). RT-qPCR analysis was carried out on the Applied Biosystems QuantStudio 3 Real-Time PCR system with ChamQ Universal SYBR qPCR Master Mix (Q711-02, Vazyme). Gene expression levels were quantified using the 2−ΔΔCT method and normalized to β-actin. Primer sequences are detailed in Table 2.
NO assay
Following cell treatment, cell proteins were collected to measure the concentrations of NO. Briefly, collected samples were reacted with reagents (NO: Cat no. A013-2-1, Nanjing Jiancheng, China) at room temperature for 15 min. Then measuring the absorbance at 550 nm wavelengths, and finally calculating the final amount using the calculation formula. Three independent assays were performed according to the manufacturer’s instructions.
SOD assay
The concentrations of SOD were determined using commercia assay kit (SOD: Cat no. RX201865M, Ruixinbio, Quanzhou, China) according to the provided protocols. Following cell treatment, we collected the cells and homogenized them to prepare a suspension then centrifuged and collected the supernatant. The samples were reacted with reagents at 37 °C for 15 min protected from light. Finally, measuring the absorbance at 450 nm.
MDA assay
The concentrations of MDA were determined using commercia assay kit (MDA: Cat no. A003-4-1, Nanjing Jiancheng, China) according to the provided protocols. We collected the cells and homogenize them to prepare a suspension then sequentially added the assay reagents. Heated in a water bath maintained above 95 °C for 40 min. After cooling, centrifuged at 4000 rpm for 10 min. The absorbance was measured at 530 nm. Finally we calculated the final amount using the calculation formula. Three independent assays were performed.
ELISA
BV2 cells were cultured in culture flasks at 37 °C for 24 h and then treated with Prob (0, 100, 200 and 400 µM) and LPS (1.6 µg/mL) for 24 h. Control cells were treated with 1% DMSO-containing serum-free culture medium. Following treatment, TNF-α and IL-1β levels were quantified from cell culture supernatants using ELISA kits (TNF-α: m1002095, Mlbio, China; IL-1β: m1301814, Mlbio, China).
Statistical analyses
Data are presented as mean ± standard deviation. All data were tested for normality with the Shapiro–Wilk test. RT-qPCR data for TNF-α and IL-1β were analyzed using unpaired student’s t-test with Welch’s correction. All other data were analyzed using one-way ANOVA, Tukey’s honest difference was conducted as post hoc analysis. P value < 0.05 was considered statistically significant. Analysis was performed using GraphPad Prism software v.9.0.
Results
Establishment of the inflammation model in LPS-induced BV2 cells
To establish an inflammation model, BV2 cells were stimulated with LPS (1.6 µg/mL). Morphological observation revealed two different phenotypes (Fig. 2A): untreated BV2 cells mainly showed a round shape, while LPS-induced BV2 cells showed a long spindle morphology. To confirm the successful establishment of the inflammation model, the expression levels of PICs were analyzed using RT-qPCR. The results indicated that the levels of TNF-α and IL-1β were significantly elevated in the LPS-treated cells group (LG) compared to the control group (CG) (Fig. 2B). These morphological changes and increased cytokine levels confirmed the successful establishment of the inflammation model in BV2 cells.
Prob attenuates inflammation and oxidative stress in LPS-induced BV2 cells
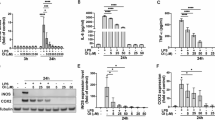
The CCK-8 assay demonstrated that Prob at concentrations of 50, 100, 200 and 400 µM had no significant effect on BV2 cell viability (Fig. 3A). Therefore, 100, 200 and 400 µM concentrations were selected for further experiments. TNF-α, IL-1β, and NO are key pro-inflammatory factors produced in LPS-induced BV2 cells. The Prob’s effect on these pro-inflammatory factors was further investigated. ELISA results demonstrated that TNF-α and IL-1β levels significantly increased in LG, whereas these levels were reduced in the Prob-treated group (Fig. 3C). At the same time, NO levels were measured, revealing a decrease in NO production in the Prob-treated group (Fig. 3C). These findings suggest that Prob effectively inhibits the production of inflammatory factors in LPS-induced BV2 cells.
IL-1β is known to promote OS in microglial cells, leading to tissue and organ damage20. To assess the impact of Prob on OS levels, the concentrations of SOD, MDA, and ROS were measured. The results indicated increased MDA levels post-LPS stimulation and a significant decrease following Prob treatment. Moreover, LPS stimulation resulted in a decrease in SOD levels, while Prob administration (200 µM) restored SOD levels (Fig. 3B). To further evaluate the effect of Prob on ROS levels, flow cytometry (FCM) was employed. The findings showed that Prob treatment reduced ROS production significantly (Fig. 3D). Based on the above results of CCK-8, ROS assay, Antioxidative activity analyses and ELISA, we chose medium Prob administration (200 µM) for the following experiments.
Prob attenuates inflammation and oxidative stress in LPS-induced BV2 cells. A BV2 cells were treated with various concentrations of Prob (0, 50, 100, 200, 400, 800, 1600, and 3200 µM) for 24 h, and cell viability was evaluated with CCK-8. B SOD levels were measured using ELISA and MDA levels were measured using microwell plate method. C Levels of TNF-α and IL-1β in the cell culture supernatant were measured using ELISA, and NO levels were assessed with microwell plate method. D ROS levels were determined using FCM. Data are presented as mean ± SD (n = 3). #P < 0.05, ##P < 0.01 vs. LPS group; *P < 0.05, **P < 0.01 vs. control group.
Prob inhibits the migration and phagocytosis capacity of LPS-induced BV2 cells
The migration ability of microglial cells is closely associated with the inflammatory response21. A wound-healing assay was conducted to assess whether Prob inhibits the migration capacity of LPS-induced BV2 cells. LPS stimulation significantly increased the migration capacity of BV2 cells, whereas Prob treatment effectively attenuated this capacity (Fig. 4A). Additionally, the effect of Prob on the phagocytosis capacity of BV2 cells was evaluated under the same conditions. The phagocytosis capacity of microglial cells was assessed using fluorescent latex microbeads. The results indicated that LPS stimulation increased the number of phagocytic cells, while Prob treatment significantly reduced this number (Fig. 4B).
Prob inhibits the migration and phagocytosis capacity of LPS-induced BV2 cells. A The migration capacity of BV2 cells was assessed using a wound-healing assay. B The phagocytosis capacity of BV2 cells was analyzed using fluorescent latex microbeads. Scale bar: 100 μm. Data are presented as mean ± SD (n = 3). #P < 0.05, ##P < 0.01 vs. LPS group; *P < 0.05, **P < 0.01 vs. control group.
Prob inhibits the expression of inflammasome-associated proteins in LPS-induced BV2 cells
To investigate Prob’s potential anti-inflammatory and antioxidant mechanisms, WB analysis was used to determine whether Prob affects the activation and expression of inflammasome-associated proteins in LPS-induced BV2 cells. The results showed that Prob reduced NLRP1, ASC, and Caspase-1 expression levels in LPS-induced BV2 cells (Fig. 5A, B). These findings suggest that Prob may exert anti-inflammatory and antioxidant effects via the NLRP1 inflammasome pathway.
Prob inhibits the expression of inflammasome-associated proteins in LPS-induced BV2 cells. A NLRP1, ASC, and Caspase-1 expression levels were assessed. B Statistical analyses of the expression results. Data are presented as mean ± SD (n = 3). ##P < 0.01 vs. LPS group; *P < 0.05, **P < 0.01 vs. control group.
Prob inhibits LPS-stimulated inflammation and oxidative stress by modulating the NLRP1 inflammasome pathway
To future prove Prob’s anti-inflammatory and antioxidant effects are mediated through the NLRP1 inflammasome pathway, BV2 cells were treated with Prob (200 µM), LPS (1.6 µg/mL) and MDP (10 µg/mL) for 24 h. The expression levels of inflammasome-associated proteins were assessed by WB, and ROS levels were measured using FCM. WB results showed that Prob significantly inhibited the expression of inflammasome-associated proteins compared to the LG, while MDP treatment notably increased their expression (Fig. 6A). Similarly, ROS levels were significantly elevated after MDP administration (Fig. 6B). These findings suggest that Prob exerts anti-inflammatory and antioxidant effects by modulating the NLRP1 inflammasome pathway.
Prob inhibits LPS-induced inflammation and oxidative stress by modulating the NLRP1 inflammasome pathway. A Protein expression levels of Caspase-1, ASC, NLRP1, and IL-1β. B ROS levels were measured using FCM. Data are presented as mean ± SD (n = 3). $P < 0.05, $$P < 0.01 vs. (LPS + Prob) group; #P < 0.05, ##P < 0.01 vs. LPS group; *P < 0.05, **P < 0.01 vs. control group.
Prob ameliorates the immune microenvironment of LPS-induced BV2 cells by modulating the NLRP1 inflammasome pathway
Given Prob’s ability to reduce inflammation and OS, it was hypothesized that these effects may be due to its modulation of the cellular immune microenvironment. WB and RT-qPCR were employed to detect the expression of markers specific to M1 subtype cells (iNOS and CD16) and M2 subtype cells (Arg-1 and CD206). WB results (Fig. 7A) demonstrated that Prob significantly decreased the expression of M1 subtype markers while increased the expression of M2 subtype markers compared to the LG. However, MDP treatment led to a significant reversal in the expression of these markers. The RT-qPCR results (Fig. 7B) were consistent with the WB findings. These results indicate that Prob ameliorates the immune microenvironment of LPS-induced BV2 cells by modulating the NLRP1 inflammasome pathway.
Prob ameliorates the immune microenvironment by modulating the NLRP1 inflammasome pathway. A Protein expression levels of CD16, iNOS, CD206, and Arg-1 were assessed by WB. B The mRNA expression levels of CD16, iNOS, CD206, and Arg-1 were measured by RT-qPCR. Data are presented as mean ± SD (n = 3). $P < 0.05, $$P < 0.01 vs. (LPS + Prob) group; #P < 0.05, ##P < 0.01 vs. LPS group; **P < 0.01 vs. control group.
Discussion
Neuroinflammation plays an important role in NDs of CNS, including AD, PD, and ischemic stroke (IS)22,23. Microglia, as the primary immune cells in the CNS, are important participants in neuroinflammation24. Microglia-induced neuroinflammation impacts the progression of NDs25,26. Inhibiting microglia-mediated inflammation represents a promising therapeutic strategy for treating NDs.
LPS, a polysaccharide component of gram-negative bacteria27, stimulates microglial activation and promotes neurodegeneration28. LPS is frequently used to establish a model of inflammation. It exerted its effects by interacting with Toll-like receptor 4 (TLR4) on the target host cell membrane. Upon activation by LPS, TLR4 immediately triggered two signaling pathways, myeloid diferentiation primary response protein 88 (MyD88) -dependent/independent pathways, which further leading to the production of pro-inflammatory factors29,30. Whether probenecid affects the TLR4 signaling pathway will be further investigated for our research group.
Excessive release of pro-inflammatory factors is a hallmark of microglial activation and contributes significantly to the development of neuroinflammation. This persistent inflammatory state disrupts neuronal function and ultimately leads to neuronal cell death, which is a key feature in various NDs31. In this study, Prob was demonstrated to suppress the secretion of TNF-α, NO, and IL-1β in LPS-induced BV2 cells. By reducing the levels of these inflammatory mediators, Prob may mitigate the inflammatory cascade that contributes to neurodegeneration. Consistent with our previous research findings19, which further highlights its potential therapeutic effects. OS is another pathological feature frequently observed in NDs32. OS occurs when an imbalance between prooxidants and antioxidants, leading to cellular damage and inflammation33. In the current study, OS was assessed by measuring levels of SOD, a crucial antioxidant enzyme, and MDA, a marker of lipid peroxidation. A significant reduction in SOD levels, along with an increase in MDA levels, was observed in the LG compared to the CG. Prob treatment effectively reversed these changes, which suggested that Prob had antioxidant properties. Furthermore, FCM analysis was conducted to measure the production of ROS, a key indicator of oxidative damage. The results revealed that Prob significantly inhibited ROS production in LPS-induced BV2 cells, further supporting its role in combating OS. These findings collectively indicate that Prob not only suppresses the secretion of neurotoxic PICs but also modulates OS pathways, thereby reducing the levels of neurotoxic mediators during inflammation.
Microglia exhibited significant changes in morphology, migration, and phagocytic activity when activated, playing a vital role in mediating neuroinflammation and immune responses. In this study, BV2 cells exhibited two distinct morphological phenotypes: a round shape and a long spindle shape. Under normal conditions, BV2 cells predominantly displayed a round morphology, indicating a resting or non-activated state. However, upon stimulation with LPS, there was a significant increase in the population of cells adopting the long spindle morphology. This change was consistent with previous studies34, suggesting that LPS promoted microglial activation and transformation into a reactive state. Microglial migration is another key indicator of activation, as these cells move towards sites of injury or inflammation to perform immune functions, such as releasing PICs and clearing debris. The migration ability of microglia, therefore, is closely associated with their role in contributing to the inflammatory response and subsequent neuronal damage35. A wound-healing assay was performed to investigate whether Prob could inhibit microglial migration. The assay results showed that while LPS exposure significantly enhanced the migration of BV2 cells, treatment with Prob attenuated this effect, suggesting its potential anti-inflammatory properties. Furthermore, the phagocytic activity of microglia, which is essential for clearing dead neurons and cellular debris, serves as a critical mechanism for maintaining CNS homeostasis. Properly regulated microglial phagocytosis can help reduce inflammation and promote tissue repair. However, abnormal or excessive activation, such as that induced by LPS, may disrupt this balance, leading to dysregulated phagocytosis and early phagocytic events that can contribute to neuroinflammation and neuronal damage36,37. To assess the impact of Prob on microglial phagocytic activity, BV2 cells were exposed to fluorescent latex microbeads, which allowed for quantification of phagocytosis. The results indicated that Prob reduced the LPS-induced increase in phagocytosis, demonstrating its potential role in modulating neuroinflammatory responses.
Inflammasomes are critical in initiating inflammatory responses and are pivotal in CNS injury mechanisms. Both the NLRP1 and NLRP3 inflammasomes can be activated in LPS-treated microglia38,39, with NLRP3 inflammasome being more frequently activated. In our previous study of spinal cord injury in SD rats19, we examined the expression of NLRP1 inflammasome-associated proteins. The current study primarily focused on the NLRP1 inflammasomes. Activation of inflammasomes promotes the maturation and release of PICs40. The results of the WB experiments in this study showed that Prob significantly reduced the levels of inflammasome-associated proteins, including NLRP1, ASC, and Caspase-1 in LPS-induced BV2 cells. ELISA results further demonstrated that Prob decreased the production of IL-1β and TNF-α. These findings suggest that Prob may reduce PICs by inhibiting the inflammasome pathway in BV2 cells.
MDP, a fragment of peptidoglycan from bacterial cell walls, studies have shown that MDP acts as an activator of NLRP141,42. At the same time, MDP was used as an NLRP1 activator to activate NLRP1 in mouse brain tissue43. To confirm that Prob exerts anti-inflammatory and antioxidant effects by modulating the NLRP1 inflammasome pathway, we treated BV2 cells with MDP. WB and FCM analyses indicated that Prob significantly suppressed inflammasome-related protein levels and ROS levels, while MDP reversed these effects. These data suggest that Prob exerts anti-inflammatory and antioxidant effects by modulating the NLRP1 inflammasome pathway.
Activated microglia differentiate into two phenotypes, M1 and M2, which release PICs and AICs, respectively, influencing the immune microenvironment44,45. Based on the results, we hypothesized that Prob may ameliorate the immune microenvironment. To investigate this, WB and RT-qPCR were used to assess the levels of markers specific to M1 phenotype cells and M2 phenotype cells. The results demonstrated that LPS stimulated the expression of CD16 and iNOS, indicating an increase in M1 phenotype cells, which was consistent with the observation that M1 phenotype cells drive inflammatory responses4. Prob decreased the expression of iNOS and CD16 while increased the Arg-1 and CD206 expression, which was potentially associated with the suppression of inflammasome activation by Prob. However, marker expression patterns reversed following MDP administration. These findings suggest that Prob may modulate the immune microenvironment through the NLRP1 inflammasome pathway.
Some studies have shown that MDP is the ligand for the NOD2 receptor, an intracellular pattern recognition receptor of the NLR family46. LPS is the primary ligand for the TLR4 receptor, a membrane-bound pattern recognition receptor29,30. Complex crosstalk exists between pattern recognition receptor signaling pathways. Studies have reported that NOD2 ligands (such as MDP) and TLR4 ligands (such as LPS) can modulate each other’s activity. It has been shown that the combined effect of MDP and LPS ligands enhances TNF-α level in Bone Marrow stromal cells47. Additionally, LPS and MDP synergistically induce the expression of pro-inflammatory factors in monocytes48. However, it has also been reported that MDP reduced cytokine production when stimulated with TLR ligands in Mouse colonic LP cells49. Based on the specific experimental design and results of our study, we posited that this interaction did not appear to play a significant role.
This study primarily employed the LPS-induced BV2 cells model as an inflammation model to investigate the role of Prob in alleviating LPS-induced neuroinflammation. LPS-induced BV2 microglia are widely used as a neuroinflammation model, however, they may display differences in behavior and response compared to microglial cells in vivo. True neuroinflammation involves various cell types and complex interactions. This limitation suggests that the results of this study may not fully reflect the true conditions in the in vivo environment. Prob can block the Pannexin-1 channel pore to inhibit ATP release, which has inhibitory activities on Pannexin-1 and P2 X7 receptor16,50. Our study focused primarily on molecules downstream of this pathway, however, we neglected to study the mechanism of action of prob on upstream cascades. This will be a focus of our future research, thereby refining our understanding of prob’s mechanisms of action.
Conclusion
The study demonstrates that Prob reduces the activation of BV2 cells and alleviates LPS-induced neuroinflammation. This therapeutic potential is attributed to Prob’s ability to modulate the NLRP1 inflammasome pathway, subsequently improving the immune microenvironment, suppressing inflammation, and attenuating oxidative stress. The findings may contribute to our understanding of neuroinflammatory mechanisms and potential therapeutic interventions for neuroinflammation-associated diseases.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AD:
-
Alzheimer’s disease
- AICs:
-
Anti-inflammatory cytokines
- CNS:
-
Central nervous system
- CCK-8:
-
Cell Counting Kit-8
- CG:
-
Control group
- DAMPS:
-
Damage-associated molecular Patterns
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- ELISA:
-
Enzyme-linked immunosorbent assay
- FBS:
-
Fetal bovine serum
- FCM:
-
Flow cytometry
- IL-1β:
-
Interleukin-1β
- LPS:
-
Lipopolysaccharide
- LG:
-
LPS-treated cells group
- TLR4:
-
Toll-like receptor 4
- MDA:
-
Malondialdehyde
- MDP:
-
Muramyl dipeptide
- NDs:
-
Neurodegenerative diseases
- NO:
-
Nitric oxide
- NLRs:
-
Nucleotide oligomerization domain (NOD)-like receptors
- OS:
-
Oxidative stress
- Prob:
-
Probenecid
- PD:
-
Parkinson’s disease
- PICs:
-
Pro-inflammatory cytokines
- PRRs:
-
Pattern recognition receptors
- PAMPs:
-
Pathogen-associated molecular patterns
- PFA:
-
Paraformaldehyde
- ROS:
-
Reactive oxygen species
- RT:
-
Room temperature
- SOD:
-
Superoxide dismutase
- MyD88:
-
myeloid diferentiation primary response protein 88
- TNF-α:
-
Tumor necrosis factor-α
- WB:
-
Western blot
References
Yang, Q. Q. & Zhou, J. W. Neuroinflammation in the central nervous system: symphony of glial cells. Glia 67 (6), 1017–1035 (2019).
Kwon, H. S. & Koh, S. H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl. Neurodegener. 9 (1), 42 (2020).
Zhang, J. et al. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells. Mol. Immunol. 116, 29–37 (2019).
Gensel, J. C. & Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 1619, 1–11 (2015).
Lykhmus, O. et al. Molecular mechanisms regulating LPS-Induced inflammation in the brain. Front. Mol. Neurosci. 9, 19 (2016).
Park, J. S. et al. Anti-inflammatory mechanisms of isoflavone metabolites in lipopolysaccharide-stimulated microglial cells. J. Pharmacol. Exp. Ther. 320 (3), 1237–1245 (2007).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 10 (2), 417–426 (2002).
Jamilloux, Y. & Martinon, F. Cell-free assay for inflammasome activation. Methods Mol. Biol. 1417, 207–215 (2016).
Crespo Yanguas, S. et al. Pannexin1 as mediator of inflammation and cell death. Biochim. Biophys. Acta Mol. Cell. Res. 1864 (1), 51–61 (2017).
Chen, J. et al. VX-765 reduces neuroinflammation after spinal cord injury in mice. Neural Regen. Res. 16 (9), 1836–1847 (2021).
de Rivero Vaccari, J. P., Dietrich, W. D. & Keane, R. W. Therapeutics targeting the inflammasome after central nervous system injury. Transl. Res. 167 (1), 35–45 (2016).
Kigerl, K. A. et al. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 258, 5–16 (2014).
de Rivero Vaccari, J. P. et al. A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. 28(13), 3404–3414 (2008).
Yap, J. et al. The role of neuronal NLRP1 inflammasome in Alzheimer’s disease: bringing neurons into the neuroinflammation game. Mol. Neurobiol. 56 (11), 7741–7753 (2019).
Robbins, N. et al. The history and future of probenecid. Cardiovasc Toxicol. 12(1), 1–9 (2012).
Silverman, W. R. et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 284 (27), 18143–18151 (2009).
Mawhinney, L. J. et al. Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats. BMC Neurosci. 12, 123 (2011).
Bravo, D. et al. Pannexin 1: a novel participant in neuropathic pain signaling in the rat spinal cord. Pain. 155(10), 2108–2115 (2014).
Qi, Q. et al. Neuroprotective effects of the Pannexin-1 channel inhibitor: probenecid on spinal cord injury in rats. Front. Mol. Neurosci. 15, 848185 (2022).
Huang, M. Y. et al. Corylin inhibits LPS-induced inflammatory response and attenuates the activation of NLRP3 inflammasome in microglia. BMC Complement Altern. Med. 18 (1), 221 (2018).
Zhu, C. et al. Artemisinin attenuates lipopolysaccharide-stimulated proinflammatory responses by inhibiting NF-κB pathway in microglia cells. PLoS One. 7(4), e35125 (2012).
Jin, X. et al. Natural products as a potential modulator of microglial polarization in neurodegenerative diseases. Pharmacol. Res. 145, 104253 (2019).
Stephenson, J. et al. Inflammation in CNS neurodegenerative diseases. Immunology 154 (2), 204–219 (2018).
Calsolaro, V. & Edison, P. Neuroinflammation in alzheimer’s disease: current evidence and future directions. Alzheimers Dement. 12 (6), 719–732 (2016).
Colonna, M. & Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 35, 441–468 (2017).
Yang, L. et al. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol. Dis. 140, 104814 (2020).
Rathinam, V., Zhao, Y. & Shao, F. Innate immunity to intracellular LPS. Nat Immunol. 20(5), 527–533 (2019).
Subedi, L. et al. N,N-disubstituted Azines attenuate LPS-mediated neuroinflammation in microglia and neuronal apoptosis via inhibiting MAPK signaling pathways. BMC Neurosci. 18 (1), 82 (2017).
Ciesielska, A., Matyjek, M. & Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 78 (4), 1233–1261 (2021).
Luo, R. et al. An examination of the LPS-TLR4 immune response through the analysis of molecular structures and protein-protein interactions. Cell Commun. Signal. 23 (1), 142 (2025).
Pajares, M. et al. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells. 9(7), 1687 (2020).
Teleanu, D. M. et al. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 23(11), 5938 (2022).
Cho, C. H. et al. N-Adamantyl-4-methylthiazol-2-amine suppresses amyloid β-induced neuronal oxidative damage in cortical neurons. Free Radic. Res. 50 (6), 678–690 (2016).
Cai, Q., Li, Y. & Pei, G. Polysaccharides from ganoderma lucidum attenuate microglia-mediated neuroinflammation and modulate microglial phagocytosis and behavioural response. J. Neuroinflamm. 14 (1), 63 (2017).
Scheiblich, H. & Bicker, G. Regulation of microglial migration, phagocytosis, and neurite outgrowth by HO-1/CO signaling. Dev. Neurobiol. 75 (8), 854–876 (2015).
Brown, G. C. & Neher, J. J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 15 (4), 209–216 (2014).
Fu, R. et al. Phagocytosis of microglia in the central nervous system diseases. Mol. Neurobiol. 49 (3), 1422–1434 (2014).
Ding, W. et al. Parthenolide ameliorates neurological deficits and neuroinflammation in mice with traumatic brain injury by suppressing STAT3/NF-κB and inflammasome activation. Int. Immunopharmacol. 108, 108913 (2022).
Yan, Y. Q. et al. Parkin regulates microglial NLRP3 and represses neurodegeneration in parkinson’s disease. Aging Cell. 22 (6), e13834 (2023).
Voet, S. et al. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol Med. 11(6), e10248 (2019).
Faustin, B. et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell. 25 (5), 713–724 (2007).
D’Ambrosio, E. A. et al. Differential peptidoglycan recognition assay using varied surface presentations. J. Am. Chem. Soc. 142 (25), 10926–10930 (2020).
Wu, Y. et al. Purinergic neurotransmission receptor P2X4 silencing alleviates intracerebral hemorrhage-induced neuroinflammation by blocking the NLRP1/Caspase-1 pathway. Sci. Rep. 13 (1), 14288 (2023).
Yao, K. & Zu, H.B. Microglial polarization: novel therapeutic mechanism against Alzheimer’s disease. Inflammopharmacology. 28(1), 95–110 (2020).
Xu, A. H. et al. Poly(ADP-ribose) polymerase family member 14 promotes functional recovery after spinal cord injury through regulating microglia M1/M2 polarization via STAT1/6 pathway. Neural Regen. Res. 18 (8), 1809–1817 (2023).
Hsu, L. C. et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and Muramyl dipeptide. Proc. Natl. Acad. Sci. USA. 105 (22), 7803–7808 (2008).
Gorskaya,Y.F. et al. Ligands of NOD2 (Muramyl Dipeptide) and TLR4 (LPS) in 24 h after Combined In Vivo Administration Produce a Synergistic Increase in the Content of Multipotent Stromal Cells in the Bone Marrow and Peritoneal Exudate of CBA Mice. Bull. Exp. Biol. Med. 166(4), 473–476 (2019).
Yang, S. et al. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect. Immunty 69 (4), 2045–2053 (2001).
Watanabe, T. et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J. Clin. Investig. 118 (2), 545–559 (2008).
El-Maadawy, W. H. et al. Probenecid induces the recovery of renal ischemia/reperfusion injury via the blockade of Pannexin 1/P2X7 receptor axis. Life Sci. 308, 120933 (2022).
Acknowledgements
Thanks for the support of the research platform of Second people’s hospital of Bengbu City and Bengbu Medical University and the project sponsor.
Funding
The study was supported by the Research Project of Bengbu Health Commission (No.BBWK2023A103).
Author information
Authors and Affiliations
Contributions
Y.X. Z designed the studies. X.X .W performed the experiments, analyzed the data, and assisted with drafting the manuscript. H.Z.T and J. Z interpreted the data and contributed to drafting the manuscript. J.X and Y.Y. S assisted with manuscript editing, while L.L. Y and S.F. C designed the figures. D. W and Y.X. Z supervised the project, provided guidance in interpreting the results and contributed to the final version of the manuscript. All authors collaborated in writing and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, X., Tian, H., Chen, S. et al. Probenecid relieves LPS-induced neuroinflammation by modulating the NLRP1 inflammasome pathway in BV2 cells. Sci Rep 15, 35192 (2025). https://doi.org/10.1038/s41598-025-19015-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19015-5