Abstract
Azospirillum brasilense, a plant growth-promoting rhizobacterium that plays a vital role in sustainable wheat production by enhancing nutrient uptake, improving stress tolerance, and reducing reliance on chemical fertilizers. This study aimed to investigate the integrative effects of Azospirillum brasilense inoculation and different mulching practices on the growth, physiology, and soil health of wheat (Triticum aestivum L.) under drought stress, particularly during the critical booting stage. The primary research question focused on identifying whether these combined treatments could mitigate drought-induced damage and enhance plant performance. The experimental was consisted of 9 treatments, including T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch) with randomized complete block design having three replications. Various growth, yield, physiological, and soil nutrient parameters were assessed. Data analysis included ANOVA, cluster heatmap, and principal component analysis (PCA) to evaluate treatment impacts. Drought stress significantly reduced plant height (34.24%), 1000-grain weight (49.05%), and photosynthetic pigments. However, treatments combining A. brasilense with organic mulches (T6: wheat straw and T7: rice husk) substantially improved plant biomass, photosynthetic rate (up to 24.67%), stomatal conductance (7.54%), and soil nutrient uptake. T6 showed the highest increase in chlorophyll a (118.74%) and grain weight (78.78%) compared to drought alone. PCA and heatmap analyses revealed strong clustering of treatments, highlighting T6 as the most effective strategy. The combination of A. brasilense and organic mulching (especially wheat straw) effectively mitigated drought stress in wheat by enhancing physiological resilience, nutrient uptake, and soil health. The demonstrated benefits suggest that incorporating bio-inoculants with locally available mulching materials can be scaled up as a practical intervention for climate-smart agriculture.
Similar content being viewed by others
Introduction
Wheat is a staple food in Pakistan. Approximately 36% of the world’s population consumes wheat as a stable food1. Compared with other cereals, wheat grains constitute the greatest percentage of food calories (20%) and carbohydrates (36%), in addition to various minerals, antioxidants and vitamins2,3. The area of cultivated wheat is increasing gradually around the globe, with increasing demand for wheat. It can be grown under diverse climate conditions4,5. If the population continues to increase at the current growth rate, 60% of the current food production needs to be increased by the middle of the century to meet food security requirements6. However, the current pace of wheat production is not sufficient to meet the increasing global food demand7.
Water is essential for survival. Water deficiency affects plant growth and overall crops in terms of poor grain quality and low production per unit area8. In recent decades, the complex phenomenon of climate change has created challenges for researchers and producers in meeting increasing food demand9,10. Drought is one of the main effects of climate change on crop production11,12. Other factors, such as salinity and heavy metal accumulation in plant tissues, also influence crop yield4,13. In drought situations, water is not available to plants for a prolonged period of time14. A total of 34.9% of the world’s area is under arid and semiarid climate conditions, of which less than 15% has irrigation facilities. The rest of the area is rain-fed14. In the past, due to drought and heat waves, global wheat production was reduced by up to 35%8. This creates an alarming situation in which more food production is needed to feed the increasing population.
Plant species respond to drought in either a deleterious or adaptive way15. Plants with deleterious responses exhibit poor growth, low water-use efficiency, lost turgor, low photosynthesis and transpiration, damaged macromolecules and cell membranes, altered enzyme activities and metabolism, greater reactive oxygen species production and reduced yields16,17. Some plants have the ability of natural drought tolerance. Drought tolerance is the ability of plants to survive and achieve significant production under periodic drought conditions18. To mitigate drought conditions, these plants change their physiology, root structure and production18,19. These plants maintain water uptake by promoting root development, reducing water loss through closing stomata, limiting the shoot growth rate, activating antioxidative defense systems, stabilizing the cell membrane and regulating hormone activity for survival under prolonged drought conditions16,20.
Plant growth-promoting bacteria are beneficial for plant physiological processes under both normal and drought conditions21. These bacteria can also increase the photosynthesis rate and nutrient uptake in plants22. The genus Azospirillum comprises free-living and nitrogen-fixing bacteria that can promote plant growth. The application of A. brasilense is considered a viable economic and eco-friendly technology23. These bacteria promote crop growth under normal and water-stressed conditions21,24,25. These bacteria promote nitrogen fixation and plant growth26, significantly contribute to plant‒soil nitrogen management27, and are beneficial for increasing the zinc concentration in seeds28,29. These bacteria also improve plant drought tolerance through direct or indirect mechanisms21. They improve a plant’s antioxidant defense system24, proline accumulation30 and abscisic acid levels31. Many mulching techniques, including straw mulch, plastic mulch and ridge-furrow mulch, have been proven to be beneficial. Plastic mulch remains in the field and has negative effects on the environment and soil in terms of water and nutrient transport, resulting in low crop yields32. Straw mulching is also a widely adopted technique in arid and semiarid climates where drought is a severe issue because of its ability to retain moisture and hence improve crop production33,34. It maintains moisture in the soil by reducing evaporation losses, reducing soil heat loss to the air and improving the water retention ability of the soil35. Straw mulch provides significant amounts of potassium, nitrogen, phosphorous and other essential nutrients and improves the soil quality of the next crop36. A literature review revealed the combined effects of straw mulch and A. brasilense bacteria on the physiology and yield of wheat crops under drought conditions. Given the water deficit conditions and importance of wheat crops, a study was designed to investigate the combined effects of wheat straw mulch and A. brasilense on wheat plant physiology and crop yield. We hypothesized that the combined application of A. brasilense and mulching practices would more effectively alleviate drought-induced stress in wheat than either treatment alone. Specifically, we anticipated that organic mulches (wheat straw and rice husk), by conserving soil moisture and enhancing nutrient availability, would complement the physiological and soil health benefits provided by A. brasilense. Therefore, the purpose of this study was to evaluate the integrative effects of different mulching practices and A. brasilense inoculation on wheat growth, physiology, and soil health under drought stress.
Materials and methods
Growth conditions and experimental layout
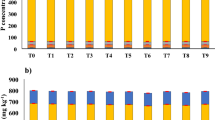
To estimate the combined effects of different types of straw mulch and A. brasilense on wheat crops, this study was conducted at the agricultural research farms of the department of agricultural engineering, khwaja fareed university of engineering and information technology, Rahim Yar Khan, Pakistan (Latitude 28° 25’ 3’’ N, Longitude 70° 18’ 13’’ E). The Cholistan desert surrounds the study area. The weather is hot and dry, with an average temperature of 26.5 °C. The average precipitation in the study area is 101 mm37. The study was designed under a randomized complete block design (RCBD) with three replications. Wheat seeds of an approved wheat variety (Ujala-16) were obtained from the regional agriculture research institute, Bahawalpur, Pakistan, and A. brasilense was obtained from the ayub agriculture research center, Faisalabad, Pakistan. The fields were prepared via standard tillage practices, and seeds were sown via a standard wheat drill. The seed drill was calibrated for a seed rate of 50 kg/acre. The plot size for each treatment was 5 × 7 feet. The recommended NPK fertilizer dose was applied in the field, and an accurate amount for each plot was calculated as 120:80:60 NPK kg/ha1. Sowing of the wheat seeds was done on 15th November 2024 and the harvesting date was 25th April, 2025. All treatments, including control and stress treatments, received the same recommended fertilizer dose to ensure uniform nutrient availability. Three replications for each treatment were selected, and 27 plots were prepared. The experimental setup consisted of 9 treatments, including T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch). For the mulching treatments, a 2-inch thick layer of wheat straw or rice straw was applied uniformly across the field, and a plastic sheet was spread over the field to reduce evaporation losses and measure its effects on crop production. Drought stress was applied after 50 days of sowing at the booting stage, withholding irrigation water, and data were collected as described below. Soil moisture (Fig. 1) and temperature (Fig. 2) are measured at 0–15 cm during the whole crop period with the help of a soil moisture sensor and digital soil thermometer sensor.
Recorded parameters: Plant growth parameters, such as plant height, root length, shoot length, spike length, number of spikelets, leaf area index, ear length, 1000-grain weight and grain weight per plant, were recorded at the time of crop harvesting via standard procedures3,38,39. The crop growth rate (CGR) was calculated via the standard procedure40 using the following formula,
Where W1 is the dry weight of the crop at the time of t1 and W2 is the dry weight of the crop at time t2. T2-t1 is the time interval, and A is the ground area occupied by the crop.
The NPK in plant shoots was determined via the standard procedure reported in previous research41. The MDA content and electrolyte leakage were measured at the anthesis stage by harvesting fresh leaves. The MDA content in wheat leaves is used as an indicator of drought-induced lipid peroxidation. The MDA in wheat leaves was measured following the standard procedure42, whereas electrolyte leakage was measured via the standard method of a previous research43. Free proline contents, photosynthesis rate and carotenoids were measured via the standard techniques described in a an earlier research44,45. We also follow the previous researchers for chlorophylls46 and leaf area calculations16. The photosynthetic rate was measured via a gas analyzer, stomatal conductance was recorded at the anthesis stage, and stomatal conductance was measured via the standard procedure described by a previous researcher1. All physiological and growth related parameters were recorded at the grain filling stage and yield related parameters were recorded after the crop harvesting.
Statistical analysis
An analysis of variance (ANOVA) was conducted on the dataset to explore potential statistically significant differences and dominant patterns among the applied treatments. Pearson’s correlation analysis was used to investigate the linkages and associations between the variables. The data were analyzed via the statistical software package Statistix v. 8.01. The statistical and visualization tool of the R-Studio program was used to conduct principal component analysis, heatmaps and correlation analysis. Heatmap was generated using the heatmap package (v5.5.1) in R “v4.5.1”, https://cran.r-project.org/bin/windows/base/.
Results
Plant growth and yield parameters
The findings of this study, which were based on data analysis, revealed that drought stress affected the growth and yield parameters of wheat plants under in vivo conditions. A linear decrease in all the growth and yield parameters was observed with increasing severity of drought stress. However, the addition of various treatments, viz., mulching and A. brasilense, significantly (p ≤ 0.05) improved the growth and yield characteristics of the wheat plants under drought-stressed conditions (Fig. 3). The positive control (T0) presented a greater ratio of growth and yield parameters than did the other treatments. Compared with normal (positive control) conditions, drought stress (negative control, T1) decreased the plant height (34.24%) (Fig. 1), root length (19.15%) (Fig. 4), shoot length (21.01%) (Fig. 5), spike length (11.62%) (Fig. 6), number of spikelets (24.05%) (Fig. 7), leaf area index (16.18%) (Fig. 8), ear length (23.66%), 1000-grain weight (49.05%), spike per plant (54.49%), CGR (33.06%) (Fig. 9), straw N uptake (52.57%), straw P uptake (67.07%) and straw K uptake (63.76%) (Fig. 10). Compared with the negative control (T1), the combined application of DB + A. brasilense + wheat straw mulch (T6) increased the plant height (46.45%), root length (19.32%), shoot length (24.09%), spike length (11.24%), number of spikelets (29.41%), leaf area index (16.06%), ear length (25.30%), 1000-grain weight (78.78%), spike per plant (103.78%), CGR (46.21%), straw N uptake (91.17%), straw P uptake (181.43%) and straw K uptake (152.49%).
Effects of different mulching practices and Azospirillum brasilense on the plant height of Wheat under drought. T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch). Bars marked with different letters indicate significant differences between treatments at p < 0.05.
Effects of different mulching practices and Azospirillum brasilense on the root length of wheat under drought. T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch). Bars marked with different letters indicate significant differences between treatments at p < 0.05.
Effects of different mulching practices and Azospirillum brasilense on the shoot length of Wheat under drought. T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch). Bars marked with different letters indicate significant differences between treatments at p < 0.05.
Effects of different mulching practices and Azospirillum brasilense on the spike length of wheat under drought. T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch). Bars marked with different letters indicate significant differences between treatments at p < 0.05.
Effects of different mulching practices and Azospirillum brasilense on the no. of spikelets of wheat under drought. T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch). Bars marked with different letters indicate significant differences between treatments at p < 0.05.
Effects of different mulching practices and Azospirillum brasilense on the leaf area index (LAI) of wheat under drought. T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch). Bars marked with different letters indicate significant differences between treatments at p < 0.05.
Effects of different mulching practices and Azospirillum brasilense on the length of ear, 1000-grain weight, grain per spike and crop growth rate (CGR) of Wheat under drought. T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch). Bars marked with different letters indicate significant differences between treatments at p < 0.05.
Effects of different mulching practices and Azospirillum brasilense on straw nitrogen (N), phousporus (P) and potassium (K) uptake wheat under drought. T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch). Bars marked with different letters indicate significant differences between treatments at p < 0.05.
Plant physiological parameters
Malondialdehyde (MDA), electrolyte leakage and proline contents
Compared with the positive control (T0), the negative control (T1) resulted in a greater percentage of MDA (71.97%), electrolyte leakage (126.75%) and proline (187.15%) in wheat. However, the addition of DB + A. brasilense + wheat straw mulch (T6) significantly (p ≤ 0.05) impacted the measured parameters, as the contents of MDA (37.31%), electrolyte leakage (46.39%) and proline (45.26%) were lower than those of the negative control (T1) but greater than those of the positive control (T0) under drought conditions (Fig. 11).
Effects of different mulching practices and Azospirillum brasilense on malondialdehyde (MDA) contents (A), electrolyte leakage (B) and proline content (C) of wheat under drought. T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch). Bars marked with different letters indicate significant differences between treatments at p < 0.05.
Photosynthesis pigments, photosynthesis rate and stomatal conductance parameters
Compared with the negative control treatment, various treatments significantly improved the synthesis of photosynthetic pigments in wheat under drought stress (Fig. 12). Compared with all the other treatments, the drought stress (T1) treatment resulted in the greatest decrease in the accumulation of photosynthetic attributes. However, the application of DB + A. brasilense + wheat straw mulch (T6) had the greatest effect on improving the chlorophyll content and stomatal conductance in wheat leaves. Compared with the negative control (T1), the DB + A. brasilense + wheat straw mulch (T6) treatment increased the chlorophyll a content (118.74%), chlorophyll b content (37.52%), photosynthetic rate (24.67%) and stomatal conductance (7.54%), In contrast, thecarotenoid content was greater in the negative control (T1) (Fig. 13).
Effects of different mulching practices and Azospirillum brasilense on chlorophyll a and b of wheat under drought. T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch). Bars marked with different letters indicate significant differences between treatments at p < 0.05.
Effects of different mulching practices and Azospirillum brasilense on photosynthesis rate, stomatal conductance and carotenoids of Wheat under drought. T0 (control: no mulch, no drought, no soil microbes), T1 (drought stress at the booting stage (DB)), T2 (DB + A. brasilense), T3 (DB + wheat straw mulch), T4 (DB + rice husk mulch), T5 (DB + plastic mulch), T6 (DB + A. brasilense + wheat straw mulch), T7 (DB + A. brasilense + rice husk mulch), and T8 (DB + A. brasilense + plastic mulch). Bars marked with different letters indicate significant differences between treatments at p < 0.05.
Correlation matrix
A clear association was evident among all the growth, yield and physiological variables of the wheat plants under drought stress. The attributes, i.e., proline, MDA, electrolyte leakage and carotenoids, exhibited significant negative correlations with all the measured parameters, in contrast the remaining attributes were positively correlated with each other, as shown in Fig. 14.
Correlation analysis of the measured parameters of wheat plants under various treatments and drought stress in the field trial experiment. PH = Plant height, SL = Shoot length, RL = Root length, Car = Carotenoids, EL = Electrolyte leakage, SKU = Shoot K uptake, SPU = Shoot P uptake, SNU = Shoot N uptake, Chl a = Chlorophyll a, Chl b = Chlorophyll b, PR = Photosynthetic rate, LAI = Leaf area index, YPP = Yield per plant, CGR = Crop growth rate, SC = Stomatal conductance, LE = Length of ear, NS = No. of spikelets, GrS = Grains per spike, ThGW = Thousand grain weight.
Heatmap analysis
The cluster heatmap analysis summarized the responses of the plant growth, yield and physiological characteristics of the wheat plants subjected to various treatments to mitigate drought stress (Fig. 7). The heatmap segmented the tested treatments (1–9) into distinct dendrograms within the framework of trait connection. In the columns, 1 to 2 represent the positive and negative controls, respectively, with respect to the applied treatments. On the other hand, treatments 3–9 depict mulching types and microbes used in combination with drought stress. All the characteristics demonstrated differential relationships fluctuating from positive (red) to negative (blue) limits with respect to the treatments used, as shown in Fig. 15.
Heatmap analysis of measured parameters of wheat plants under various treatments and drought stress conditions in the field trial experiment. PH = Plant height, SL = Shoot length, RL = Root length, Car = Carotenoids, EL = Electrolyte leakage, SKU = Shoot K uptake, SPU = Shoot P uptake, SNU = Shoot N uptake, Chl a = Chlorophyll a, Chl b = Chlorophyll b, PR = Photosynthetic rate, LAI = Leaf area index, YPP = Yield per plant, CGR = Crop growth rate, SC = Stomatal conductance, LE = Length of ear, NS = No. of spikelets, GrS = Grains per spike, ThGW = Thousand grain weight. (Heatmap was generated using the heatmap package in R (v4.5.1, https://cran.r-project.org/bin/windows/base/).
Principal component analysis
All measured parameters (~ 22 variables) are plotted by employing the statistical tool of principal component analysis (PCA) for dimensionality reduction and a method to simplify a large dataset into clusters while maintaining significant patterns (Fig. 16). The total variability was 98.9%, with a PC1 of 97.6% and a PC2 of 1.3%. Two major groups are observed, with one cluster consisting of EL, Car, MDA, proline and others plotted in the other cluster of the PC1 and PC2 quadrants. The groups (PC1 and PC2) are more or less plotted diametrically opposite to each other in the PC quadrants, with loadings scattered across the plot.
Principal component analysis plot showing loadings of measured parameters and contributions of two principal components (PC1 and PC2). PH = Plant height, SL = Shoot length, RL = Root length, Car = Carotenoids, EL = Electrolyte leakage, SKU = Shoot K uptake, SPU = Shoot P uptake, SNU = Shoot N uptake, Chl a = Chlorophyll a, Chl b = Chlorophyll b, PR = Photosynthetic rate, LAI = Leaf area index, YPP = Yield per plant, CGR = Crop growth rate, SC = Stomatal conductance, LE = Length of ear, NS = No. of spikelets, GrS = Grains per spike, ThGW = Thousand grain weight.
Discussion
Many studies have provided evidence for improved crop production through the application of plant growth-promoting bacteria under drought stress. Unlike plants, crops do not possess adaptive features to tolerate drought stress21. Therefore, it is necessary to find agronomic techniques and tools for sustainable application to increase wheat yield under drought stress conditions47,48. The plants without drought stress at the booting stage presented the greatest values for plant height, root length, shoot length, spike length, number of spikelets, leaf area index, 1000-grain weight, grain weight per plant, and crop growth rate49,50,51. The plants subjected to drought stress with no treatment to mitigate drought stress effects presented the lowest plant growth. Similar findings were also reported by previous researchers52,53.
For plants subjected to drought stress at the booting stage, the combined application of A. brasilense and mulch resulted in higher values for crop growth parameters than did the treatments with only the application of A. brasilense or mulch. Previous researchers38 reported that plant growth regulator bacteria improve plant growth by alleviating water stress. In another study52, this was also reported that inoculation with A. brasilense improved the availability of nutrients to plants by providing suitable soil conditions and hence improved plant growth. It also enhances cytokinin production for better cell division and hence improves plant growth1,54. A. brasilense improves plant growth under drought stress and increases the number of spikelets by providing better organic matter, reducing the amount of chelated compounds and increasing nutrient availability3,55. A. brasilense also improves plant growth by adjusting plant water characteristics38. The number of spikelets increases with increasing spike length56. Nutrient availability for plants improves plant growth; hence, the number of spikelets per spike increases through the inoculation of A. brasilense1. Inoculation with A. brasilense improved spike length, and increasing spike length resulted in more spikelets. An increasing number of spikelets results in a greater number of grains and hence a greater grain yield per plant. Similar findings were reported by Zaheer et al.3. A. brasilense produces many plant growth-related hormones, which result in greater grain weight per plant. Similar findings were reported by Raheem et al.57. Iqbal et al.1 reported that increased production of cytokinin by A. brasilense results in increased 1000-grain weight. Greater CK production also results in better root growth, shoot growth and leaf area52. Improved growth parameters resulting from the inoculation of A. brasilense have also been reported by Lu et al.58. Malondialdehyde (MDA) is a product of lipid peroxidation. The results of the present study revealed an increase in MDA under drought stress with no A. brasilense or mulch treatment. Inoculation with A. brasilense and the application of mulch to wheat plants reduced the MDA concentration. Similar results were reported by21. Electrolyte leakage is used as an indicator of membrane damage under drought stress59. Inoculation with A. brasilense and mulching reduced electrolyte leakage in wheat plants. Similar results were reported by60. The proline content reflects the degree of drought stress in a plant. The results of the present study revealed that plants subjected to drought stress presented the greatest accumulation of proline, whereas plants treated with A. brasilense and mulching presented low proline contents. This trend reflected the drought stress level in the wheat plants. Similar results were reported by Zarea et al.21, who reported that the accumulation of proline is the result of environmental stress. Biotic stress, such as heavy metals, low temperature, drought and salinity, causes plants to accumulate proline61. Under drought stress, plants treated with A. brasilense and straw mulch presented greater values of photosynthetic pigments, leaf area, photosynthesis rate and stomatal conductance. In contrast, the plants subjected to drought stress without A. brasilense or mulch presented the lowest values. As discussed earlier, the application of A. brasilense and mulch improved the availability of nutrients and water to wheat plants. Iqbal et al.1 reported that the application of A. brasilense enhances cytokinin production to increase cell division, chlorophyll production and leaf area. Similar findings were also reported by many previous researchers3,52,62.
Mulching also plays a role in conserving water. Hence, it plays a crucial role in mitigating drought stress. Hu et al.32 reported that mulching helps to conserve water in the top 0–80 cm of soil later. In the present study, the availability of water for a longer time resulted in better crop production. Bu et al.63 conducted a study in dry lands and reported that direct solar radiation caused more evaporation losses from the soil. On the other hand, mulching can reduce this evaporation. Xiaoli et al.64 also reported that mulching increases water availability and hence helps improve wheat production. In this study, wheat straw mulch in combination with A. brasilense resulted in better output than did rice straw mulch or plastic mulch alone. Although plastic mulch tends to reduce evaporation losses, better decomposition of straw mulch provides nutrients to wheat plants and results in better crop production. Similar results were reported by Ikram et al.65, where wheat straw presented a relatively high decomposition rate, and a relatively high wheat straw decomposition rate provided nutrients to wheat plants and hence resulted in improved crop growth. Similar results were also reported by Sarwar et al.66, that water preservation resulted in better wheat production.
The observed improvements in photosynthetic rate, stomatal conductance, and carotenoid contents in wheat plants treated with A. brasilense and mulching can be explained by their synergistic effects on plant physiology3,64. Inoculation with A. brasilense enhances nutrient availability, particularly nitrogen, phosphorus, and potassium, which are essential for chlorophyll synthesis and stomatal regulation1,5. Higher nutrient uptake supports greater chlorophyll and carotenoid accumulation, leading to improved light harvesting and photoprotection under drought stress62,67. Moreover, A. brasilense produces phytohormones such as cytokinins, auxins, and gibberellins, which promote better root architecture and water uptake68. This enhanced root system, combined with the moisture conservation capacity of mulches, reduces water stress at the cellular level, maintaining turgor pressure and enabling sustained stomatal opening for efficient gas exchange and photosynthesis69. As a result, these treatments significantly improved plant growth parameters, including plant height, leaf area index, and spike development.
At the metabolic level, the reduction in malondialdehyde (MDA) and electrolyte leakage in treated plants reflects lower oxidative stress and better membrane stability68. A. brasilense inoculation enhances the antioxidant defense system, reducing lipid peroxidation52, while organic mulches further alleviate oxidative damage by maintaining favorable soil temperature and moisture33. Similarly, the decline in proline accumulation in treated plants indicates a lower drought-induced osmotic imbalance, as water availability and nutrient supply were improved through mulch decomposition and bacterial activity52,59. Enhanced nutrient uptake, particularly potassium, plays a vital role in osmotic adjustment and enzyme activation, thereby supporting better grain filling and higher thousand-grain weight36,70. Together, these physiological and metabolic adjustments explain why the combined application of A. brasilense and wheat straw mulch outperformed other treatments, resulting in improved crop growth rate, spikelet formation, and final yield components31,35,52,69. This study has some limitations, as it was conducted at a single site and season, so that results may vary under different climates and soils. Only short-term effects and three mulch types were tested, leaving scope for long-term studies and additional materials. Future work should validate these findings across environments and seasons, explore physiological and molecular mechanisms, and assess economic feasibility to support broader adoption in sustainable agriculture.
Conclusion
The ability of drought stress to mitigate food for increasing populations is becoming a severe issue. To ensure food security, sustainable solutions for crops are needed. A. brasilense is beneficial for crop growth under drought stress. Mulching also provides a sustainable solution to mitigate this situation. Plastic mulch is efficient at conserving water, but straw mulch has additional benefits in terms of nutrient provision through in-field decomposition. In this study, drought stress was applied to wheat plants at the booting stage, and the effects of wheat seed inoculation with A. brasilense and mulching were evaluated. The results revealed that the combined application of A. brasilense with mulching had better effects on plant growth parameters, including plant height, root length, shoot length, spike length, number of spikelets, leaf area index, 1000-grain weight, grain weight per plant, crop growth rate, chlorophyll content, stomatal conductance, and antioxidants. Straw mulch resulted in better results than plastic mulch. Additionally, wheat straw mulch had a greater effect on wheat plants than did rice straw mulch. These findings highlight the potential of combining A. brasilense inoculation with straw mulching as a cost-effective and eco-friendly practice for sustainable wheat production under drought conditions. Such approaches could be integrated into climate-smart agricultural policies to improve crop resilience and ensure food security.
Data availability
Data will be made available upon reasonable request from the corresponding author, Kamran Ikram.
References
Iqbal, R. et al. Maximizing wheat yield through soil quality enhancement: A combined approach with Azospirillum brasilense and bentonite. Plant. Stress. 11, 100321 (2024).
Breiman, A. & Graur, D. Wheat evolution. Isr. J. Plant. Sci. 43, 85–98 (1995).
Zaheer, M. S. et al. Investigating the effect of Azospirillum basilense and Rhizobium pisi on agronomic traits of wheat (Triticum aestivum L). Arch. Agron. Soil. Sci. 65, 1554–1564 (2019).
Din, I. U. et al. Genetic characterization of advance bread wheat lines for yield and stripe rust resistance. ACS Omega. 8, 25988–25998 (2023).
Adnan, M. et al. Comparative efficacy of phosphorous supplements with phosphate solubilizing bacteria for optimizing wheat yield in calcareous soils. Sci. Rep. 12, 11997 (2022).
Liu, L. et al. Individual and combined effects of jointing and booting low-temperature stress on wheat yield. Eur. J. Agron. 113, 125989 (2020).
Hickey, L. T. et al. Breeding crops to feed 10 billion. Nat. Biotechnol. 37, 744–754 (2019).
Gupta, A., Rico-Medina, A. & Caño-Delgado, A. I. The physiology of plant responses to drought. Science 368, 266–269 (2020).
Xiao, L. et al. Estimating spring frost and its impact on yield across winter wheat in China. Agric. For. Meteorol. 260–261, 154–164 (2018).
Tran, T. L. C., Guirguis, A., Jeyachandran, T., Wang, Y. & Cahill, D. M. Mesoporous silica nanoparticle-induced drought tolerance in Arabidopsis thaliana grown under in vitro conditions. Funct. Plant. Biol. 50, 889–900 (2023).
Lankford, B. et al. Irrigation area, efficiency and water storage mediate the drought resilience of irrigated agriculture in a semi-arid catchment. Sci. Total Environ. 859, 160263 (2023).
Guo, J. et al. Parental drought priming enhances tolerance to low temperature in wheat. Funct. Plant. Biol. 49, 946–957 (2022).
Waseem, M., Muhammad Aslam, M. & Kumar Sahu, S. Understanding the mechanistic basis of plant adaptation to salinity and drought. Funct Plant. Biol 51, (2024).
Bhanbhro, N. et al. Revisiting the molecular mechanisms and adaptive strategies associated with drought stress tolerance in common wheat (Triticum aestivum L). Plant. Stress. 11, 100298 (2024).
Ammar, A., Ali, Z., Saddique, M. A. B., Habib-ur-Rahman, M. & Ali, I. Upregulation of TaHSP90A transcripts enhances heat tolerance and increases grain yield in wheat under changing climate conditions. Funct. Plant. Biol 51, (2024).
Kasim, W. A., Osman, M. E. H., Omar, M. N. & Salama, S. Enhancement of drought tolerance in Triticum aestivum L. seedlings using Azospirillum brasilense NO40 and Stenotrophomonas maltophilia B11. Bull. Natl. Res. Cent. 45, 95 (2021).
Bodner, G., Nakhforoosh, A. & Kaul, H. P. Management of crop water under drought: a review. Agron. Sustain. Dev. 35, 401–442 (2015).
Shi, X. et al. Seeking sustainable pathway of crop production by optimizing planting structures and management practices from the perspective of water footprint. Sci. Total Environ. 843, 157091 (2022).
Iqbal, R. et al. Enhancing crop resilience through thiamine: implications for sustainable agriculture in drought-stressed radish. Not Bot. Horti Agrobo. 52, 13472 (2024).
Takahashi, F., Kuromori, T., Urano, K., Yamaguchi-Shinozaki, K. & Shinozaki, K. Drought stress responses and resistance in plants: from cellular responses to long-distance intercellular communication. Front. Plant. Sci. 11, 556972 (2020).
Zarea, M. J. Foliar application of Azospirillum brasilense, salicylic acid and zinc on wheat performance under rain–fed condition. Cereal Res. Commun. https://doi.org/10.1007/s42976-024-00570-y (2024).
Kashif, M. et al. Deciphering the biodesulfurization pathway employing marine mangrove Bacillus aryabhattai strain NM1-A2 according to whole genome sequencing and transcriptome analyses. Genomics 115, 110635 (2023).
Marques, D. M. et al. Does Azospirillum brasilense mitigate water stress and reduce the use of nitrogen fertilizers in maize? South. Afr. J. Bot. 156, 278–285 (2023).
Omer, A. M., Osman, M. S. & Badawy, A. A. Inoculation with Azospirillum brasilense and/or Pseudomonas geniculata reinforces flax (Linum usitatissimum) growth by improving physiological activities under saline soil conditions. Bot. Stud. 63, 15 (2022).
Karimi, N., Goltapeh, E. M., Amini, J., Mehnaz, S. & Zarea, M. J. Effect of Azospirillum zeae and seed priming with zinc, manganese and auxin on growth and yield parameters of wheat, under dryland farming. Agric. Res. 10, 44–55 (2021).
Santos, M. S., Nogueira, M. A. & Hungria, M. Outstanding impact of Azospirillum brasilense strains Ab-V5 and Ab-V6 on the Brazilian agriculture: lessons that farmers are receptive to adopt new microbial inoculants. Revista Brasileira De Ciência Do Solo. 45, e0200128 (2021).
Galindo, F. S. et al. Enhancing agronomic efficiency and maize grain yield with Azospirillum brasilense inoculation under Brazilian savannah conditions. Eur. J. Agron. 134, 126471 (2022).
Silva, V. M., Tavanti, R., Gratão, R. F., Alcock, P. L., Reis, A. R. D. & T. D. & Selenate and selenite affect photosynthetic pigments and ROS scavenging through distinct mechanisms in cowpea (Vigna unguiculata (L.) walp) plants. Ecotoxicol. Environ. Saf. 201, 110777 (2020).
Jalal, A. et al. Nano-zinc and plant growth-promoting bacteria is a sustainable alternative for improving productivity and agronomic biofortification of common bean. Chem. Biol. Technol. Agric. 10, 77 (2023).
Osman, M. S., Badawy, A. A., Osman, A. I. & Abdel Latef, A. A. H. Ameliorative impact of an extract of the halophyte Arthrocnemum macrostachyum on growth and biochemical parameters of soybean under salinity stress. J. Plant. Growth Regul. 40, 1245–1256 (2021).
Cohen, A. C. et al. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 153, 79–90 (2015).
Hu, Y. et al. Exploring optimal soil mulching for the wheat-maize cropping system in sub-humid drought-prone regions in China. Agric. Water Manage. 219, 59–71 (2019).
Yin, W. et al. No-tillage with straw mulching promotes wheat production via regulating soil drying-wetting status and reducing soil-air temperature variation at arid regions. Eur. J. Agron. 145, 126778 (2023).
Wang, J., Ghimire, R., Fu, X., Sainju, U. M. & Liu, W. Straw mulching increases precipitation storage rather than water use efficiency and dryland winter wheat yield. Agric. Water Manage. 206, 95–101 (2018).
Sarkar, S., Paramanick, M. & Goswami, S. B. Soil temperature, water use and yield of yellow sarson (Brassica napus L. var. glauca) in relation to tillage intensity and mulch management under rainfed lowland ecosystem in eastern India. Soil Tillage. Res. 93, 94–101 (2007).
Zhang, J. et al. Effects of dual mulching with wheat straw and plastic film under three irrigation regimes on soil nutrients and growth of edible sunflower. Agric. Water Manage. 288, 108453 (2023).
Punjab Information Technology Board. Climate, District Rahim Yar Khan, Govt. of Punjab. http://rykhan.punjab.gov.pk/climate (2024).
Arzanesh, M. H., Alikhani, H. A., Khavazi, K., Rahimian, H. A. & Miransari, M. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 27, 197–205 (2011).
Galindo, F. S. et al. Wheat yield in the Cerrado as affected by nitrogen fertilization and inoculation with azospirillum Brasilense. Pesq Agropec Bras. 52, 794–805 (2017).
Gardner, F. P., Pearce, R. B. & Mitchell, R. L. Physiology of Crop Plants. (1985).
Chapman, H. D. & Pratt Methods of Analysis for Soils, Plants and Waters. (1961).
Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 125, 189–198 (1968).
Sairam, R. K., Deshmukh, P. S. & Shukla, D. S. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop Sci. 178, 171–178 (1997).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant. Soil. 39, 205–207 (1973).
Arnon, D. & I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in Beta vulgaris. Plant. Physiol. 24, 1–15 (1949).
Horváth, G., Kissimon, J. & Faludi-Dániel, Á. Effect of light intensity on the formation of carotenoids in normal and mutant maize leaves. Phytochemistry 11, 183–187 (1972).
Shan, C., Dong, K., Wen, D., Cui, Z. & Cao, J. A review of m6A modification in plant development and potential quality improvement. Int. J. Biol. Macromol. 308, 142597 (2025).
Jahan, A., Sarkar, M. I. U., Naher, U. A., Biswas, J. C. & Islam, A. Effect of sterile rice spikelets derived Biochar amendment on nutrient leaching and availability in paddy soil under continuous standing water. Geol. Ecol. Landscapes. 8, 574–582 (2024).
Li, R. et al. Hydrological processes in continental valley basins: evidence from water stable isotopes. Catena 259, 109314 (2025).
Lu, S. et al. Optimizing irrigation in arid irrigated farmlands based on soil water movement processes: knowledge from water isotope data. Geoderma 460, 117440 (2025).
Sun, H. et al. A new multiangle method for estimating fractional biocrust coverage from Sentinel-2 data in arid areas. IEEE Trans. Geosci. Remote Sens. 62, 1–15 (2024).
Zaheer, M. S. et al. Effect of rhizobacteria and cytokinins application on wheat growth and yield under normal vs drought conditions. Commun. Soil Sci. Plant Anal. 50, 2521–2533 (2019).
Fakhr, M. A. et al. Investigating the combined effects of β-sitosterol and Biochar on nutritional value and drought tolerance in phaseolus vulgaris under drought stress. Funct. Plant. Biol 51, (2024).
Yi, J. et al. Assessing soil water balance to optimize irrigation schedules of flood-irrigated maize fields with different cultivation histories in the arid region. Agric. Water Manage. 265, 107543 (2022).
Sun, S. et al. Evidence for phosphorus cycling parity in nodulating and non-nodulating N2 ‐fixing pioneer plant species in glacial primary succession. Funct. Ecol. 39, 985–1000 (2025).
Zhou, H., Zhang, C., Tan, S., Dai, Y. & Duan, J. Design of the footprints of uncertainty for a class of typical interval type-2 fuzzy PI and PD controllers. ISA Trans. 108, 1–9 (2021).
Raheem, A., Shaposhnikov, A., Belimov, A. A., Dodd, I. C. & Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil. Sci. 64, 574–587 (2018).
Kazi, N., Deaker, R., Wilson, N., Muhammad, K. & Trethowan, R. The response of wheat genotypes to inoculation with Azospirillum brasilense in the field. Field Crops Res. 196, 368–378 (2016).
Zhou, H. et al. Capability of leaf water content and its threshold values in reflection of soil–plant water status in maize during prolonged drought. Ecol. Ind. 124, 107395 (2021).
De Vega, J. J. et al. Physiological and transcriptional response to drought stress among bioenergy grass miscanthus species. Biotechnol. Biofuels. 14, 60 (2021).
Hussein, H. A. A., Alshammari, S. O., Abd El-Sadek, M. E., Kenawy, S. K. M. & Badawy, A. A. The promotive effect of putrescine on growth, biochemical constituents, and yield of wheat (Triticum aestivum L.) plants under water stress. Agriculture 13, 587 (2023).
AL-Huqail, A. A. et al. Efficacy of priming wheat (Triticum aestivum) seeds with a benzothiazine derivative to improve drought stress tolerance. Funct. Plant. Biol. 50, 915–931 (2023).
Bu, L. et al. The effects of mulching on maize growth, yield and water use in a semi-arid region. Agric. Water Manage. 123, 71–78 (2013).
Xiaoli, C., Pute, W., Xining, Z., Xiaolong, R. & Zhikuan, J. Rainfall harvesting and mulches combination for corn production in the subhumid areas prone to drought of China. J. Agron. Crop Sci. 198, 304–313 (2012).
Ikram, K. et al. Conservation agriculture improves soil and productivity of wheatrice cropping systems in semi-arid region. 55, 51–64 (2021).
Sarwar, M. et al. Silver nanoparticles protect tillering in drought-stressed wheat by improving leaf water relations and physiological functioning. Funct. Plant. Biol. 50, 901–914 (2023).
Xin, W. et al. Genome-Wide association studies identify OsNLP6 as a key regulator of nitrogen use efficiency in rice. Plant Biotechnol. J. pbi.70296 https://doi.org/10.1111/pbi.70296 (2025).
Zaheer, M. S. et al. Inoculation of Azospirillum brasilense and exogenous application of trans-zeatin riboside alleviates arsenic induced physiological damages in wheat (Triticum aestivum). Environ. Sci. Pollut Res. 29, 33909–33919 (2022).
Li, H., Zhao, X., Gao, X., Ren, K. & Wu, P. Effects of water collection and mulching combinations on water infiltration and consumption in a semiarid rainfed orchard. J. Hydrol. 558, 432–441 (2018).
De Vries, J., Evers, J. B., Poelman, E. H. & Anten, N. P. R. Optimal plant defence under competition for light and nutrients: an evolutionary modelling approach. Silico Plants. 2, diaa008 (2020).
Acknowledgements
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R402), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
Open Access funding enabled and organized by Projekt DEAL. Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R402), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
K.I. and A.H. conceived the ideas; M.W.R. designed the methodology; M.Z.M. and M.S.Z., N.A.B. collected and analyzed the data; K.I. and M.R. wrote the manuscript; M.R., K.M.A., R.I., S.L, K.M revised the manuscript; S.M. provided financial support. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding authors
Ethics declarations
Statement of ethical approval in compliance with institutional, national, or international guidelines for using animals
All local, national or international guidelines and legislation were adhered to for the use of plants in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ikram, K., Mansha, M.Z., Mahmood, K. et al. Integrative effects of different mulching practices and Azospirillum brasilense on wheat growth, physiology, and soil health under drought stress. Sci Rep 15, 33435 (2025). https://doi.org/10.1038/s41598-025-19031-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19031-5