Abstract
The Cardiometabolic Index (CMI), which combines abdominal obesity and lipid levels, has been shown to be associated with non-alcoholic fatty liver disease (NAFLD). NAFLD, through various mechanisms, can lead to cardiometabolic multimorbidity (CMM). Therefore, the aim of this study was to investigate the relationship between CMI and the occurrence of CMM in individuals with NAFLD. This cross-sectional study included 5,993 individuals with NAFLD from the National Health and Nutrition Examination Survey (NHANES) cycles between 1999 and 2018. Weighted multivariable analysis was used to assess the association between CMI and CMM, with stratified and Restricted Cubic Spline (RCS) analyses. The findings revealed a statistically significant positive correlation between CMI levels and CMM risk in NAFLD patients across all three models. Furthermore, stratified analysis indicated that this relationship was more pronounced in females. RCS analysis revealed a nonlinear relationship between CMI and CMM. Our analysis demonstrates a clear positive association between CMI and CMM in U.S. adults with NAFLD, particularly pronounced in females. These findings suggest that CMI may serve as a valuable indicator for assessing the risk of CMM in U.S. adults with NAFLD, providing critical insights for the development of more effective intervention strategies.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a common and rapidly growing chronic condition, with a global prevalence of 32.4% and the highest incidence observed in industrialized nations, particularly the United States1. Characterized by lipid accumulation, insulin resistance, and liver damage induced by metabolic stress, NAFLD is closely associated with metabolic disturbances, including hyperglycemia, dyslipidemia, inflammation, coagulation abnormalities, and hypertension2,3,4. Consequently, NAFLD exerts a persistent disruption on metabolic homeostasis and profoundly impacts target organs, including the heart, brain, kidneys, and peripheral vasculature, ultimately leading to adverse health outcomes, notably an increased risk of cardiovascular mortality5,6,7,8. As the hepatic manifestation of metabolic syndrome, NAFLD is strongly linked to a spectrum of metabolic disorders, most notably type 2 diabetes(T2DM) and cardiovascular diseases (CVDs)9. Conditions including diabetes, hypertension, coronary artery disease, and stroke, commonly referred to as cardiometabolic diseases (CMDs), share overlapping etiologies and are strongly associated with NAFLD. These diseases are major contributors to global health burdens and mortality rates10,11. Furthermore, the coexistence of two or more CMDs, termed cardiometabolic multimorbidity (CMM), represents one of the most severe forms of multimorbidity. CMM is associated with a 3.7-6.9-fold higher risk of all-cause mortality and a 12-15-year reduction in life expectancy at age 60 compared to individuals without CMDs12.Given the high prevalence of NAFLD and its complex relationship with CMM, despite significant efforts in the prevention and treatment of NAFLD, the prognosis for NAFLD patients remains challenging. Therefore, identifying the risk of CMM in NAFLD patients is critical for reducing the incidence and mortality associated with CMM.
The Cardiometabolic Index (CMI) was proposed by Japanese scholar Wakabayashi13 in 2015, it is calculated based on triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and waist-to-height ratio (WHtR). WHtR is primarily used to assess obesity, while TG/HDL-C is employed to evaluate lipid levels. Both WHtR and TG/HDL-C are biomarkers of insulin resistance (IR) and are significantly associated with IR14,15. During IR, the reduced sensitivity of adipose tissue to insulin enhances lipolysis, which releases free fatty acids into the bloodstream. This excess of free fatty acids subsequently drives increased triglyceride synthesis in the liver16. Consequently, IR promotes hepatic fat accumulation, contributing to the onset and progression of NAFLD, as well as the development of atherosclerosis, hypertension, heart failure, and other CVDs17,18. Several studies have demonstrated a relationship between CMI and NAFLD, with the prevalence of NAFLD increasing as CMI rises19,20. Subsequent studies have also highlighted a strong correlation between CMI and diabetes, hypertension, stroke, CVDs, and kidney disease20suggesting that CMI is a novel indicator of visceral fat distribution and metabolic dysfunction with potential value as a marker of metabolic diseases21. However, the relationship between CMI and the risk of CMM in individuals with NAFLD remains unclear. Therefore, using data from the National Health and Nutrition Examination Survey (NHANES), which represents the general U.S. population, this study aims to investigate the association between CMI and the risk of CMM in NAFLD patients aged 18 and older.
Materials and methods
Study design and subjects
The NHANES, initiated by the U.S. Centers for Disease Control and Prevention (CDC) in the 1960s, is a large-scale, nationally representative survey designed to assess the health and nutritional status of the U.S. population. Using a complex, multistage stratified probability sampling approach, NHANES collects extensive cross-sectional data, including information on demographics, diet, physical activity, clinical measures, and laboratory results. Participants give informed consent, ensuring that ethical guidelines are followed and data privacy is maintained. As a crucial resource for public health research, NHANES data play a key role in monitoring health trends, assessing disease prevalence, identifying risk factors, and shaping health policies. The dataset is an invaluable tool for researchers in fields such as epidemiology, nutrition, and environmental health. The data is available to the public at [https://www.cdc.gov/nchs/nhanes].
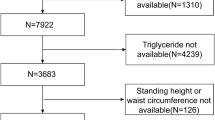
The data for this study were sourced from the NHANES dataset, spanning from 1999 to 2018, with an initial cohort of 101,316 participants. Given the lack of abdominal ultrasound data in NHANES, the diagnosis of NAFLD was determined using the United States Fatty Liver Index (US FLI), where NAFLD was defined as a US FLI value greater than 3022,23. To ensure the robustness of the study, several exclusion criteria were applied: (1) participants under 18 years of age (N = 42,112); (2) individuals with factors such as excessive alcohol consumption (defined as > 3 drinks per day for men and > 2 for women), a positive hepatitis B or C status, missing components needed to calculate the US FLI, or a US FLI ≤ 30 (N = 52,753)22,23; and (3) those missing CMI data (N = 97) or CMM data (N = 361). After applying these exclusions, the final study population consisted of 5,993 individuals with NAFLD. A detailed flowchart outlining the participant selection process is provided in Fig. 1.
Definition of CMI, CMD and CMM
In this study, CMI was treated as the exposure variable, calculated as the product of the TG/HDL-C ratio and the waist-to-height ratio (waist circumference/height), in accordance with prior studies13. The CMDs considered in this analysis included hypertension, diabetes, coronary heart disease (CHD), and stroke24,25.Diabetes was identified through any of the following criteria: (1) self-reported physician diagnosis of diabetes; (2) current use of antidiabetic medications; (3) fasting plasma glucose ≥ 126 mg/dL or glycated hemoglobin ≥ 6.5%26. Hypertension was defined by any of the following: (1) self-reported history of hypertension; (2) use of antihypertensive medications; (3) systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg27. Stroke and CHD diagnoses were based on participants’ self-reported medical history. CMM was defined as the co-occurrence of two or more CMDs24,25.
Definition of covariates
In our analysis, we included several potential confounders as covariates, such as age, sex (female, male), race (non-Hispanic white, non-Hispanic black, Mexican American, and others), Body Mass Index(BMI) categories (< 25, 25–30, ≥ 30 kg/m²)28smoking status (non-smokers, former smokers, and current smokers), alcohol consumption (non-drinkers, light, moderate, and heavy drinkers)29physical activity levels (low, moderate, high, or very high, based on metabolic equivalents [MET-minutes/week])30and the use of medications for hypertension (yes, no), lipid-lowering agents (yes, no), and diabetes (yes, no). Hyperlipidemia was defined as meeting any of the following criteria: (1) triglycerides (TG) ≥ 150 mg/dL, (2) total cholesterol (TC) ≥ 200 mg/dL, (3) low-density lipoprotein cholesterol (LDL-C) ≥ 130 mg/dL, (4) high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL for males or < 50 mg/dL for females, or (5) use of lipid-lowering medications31. The poverty-to-income ratio (PIR) was divided into three categories: low-income (< 1.3), moderate-income (1.3–3.5), and high-income (> 3.5)32.To assess NAFLD, we used the modified US FLI and Fibrosis-4(FIB4) score. The US FLI has been validated as a predictor of hepatic steatosis, with a score greater than 30 suggesting the presence of NAFLD33. The FIB4 score was utilized to evaluate the risk of advanced liver fibrosis34.
The formula is as follows35:
The FIB-4 score formula is as follows36:
Statistical analysis
This study followed the NHANES guidelines and utilized a stratified, non-random sampling approach. We applied the appropriate NHANES sampling weights: the wtmec4 year weight for survey cycles from 1999 to 2002 and the wtmec2 year weight for cycles from 2003 to 2018. These weights were consistently used in all statistical analyses, including descriptive statistics and regression models, to ensure the accuracy and generalizability of our findings. Continuous variables were expressed as weighted means ± standard error (SE) and analyzed using weighted linear regression, while categorical variables were presented as weighted percentages ± weighted proportions and examined with weighted Rao-Scott chi-square tests. To evaluate the independent relationship between CMI levels and the risk of CMM/CMD in individuals with NAFLD, multivariable logistic regression models were used, adjusting for relevant clinical confounders. The clinical covariates were incorporated into the adjusted regression models if they met any of the following criteria: (1) a P-value < 0.10 in univariate analysis for the association between the covariate and the outcome variable, (2) inclusion of the covariate resulted in a greater than 10% change in the regression coefficients, or (3) the covariate was identified as a potential confounder based on previous studies37,38. As shown in Table S1, Table S2, Table S3 and Table S4 in Supporting Information Appendix A, the first two criteria were applied to select variables. Three adjustment models were applied: Model 1 (unadjusted), Model 2 (adjusted for age, sex, and race), and Model 3 (further adjusted for PIR, BMI, smoking and alcohol consumption, physical activity, and the use of lipid-lowering medications). Additional stratified analyses and interaction tests were conducted based on age, sex, race, and BMI to explore potential effect modifications. Restricted Cubic Spline (RCS) models were used to examine potential non-linear associations between CMI levels and the odds of CMM in NAFLD patients. We constructed RCS models using different knot numbers and selected the optimal model based on the lowest Akaike Information Criterion (AIC), which balances model fit and complexity. A significance level of P ≤ 0.05 was considered, and all statistical analyses were performed using R software (version 4.2.2) and Empower (R) (version 2.0) software.
Results
Basic characteristic of study participants
Table S5 in Supporting Information Appendix A shows the baseline characteristics of those patients who were excluded due to missing partial information of CMI and CMM. The study cohort consisted of a large sample of 5,993 participants. Based on the quartiles of CMI, participants were divided into four groups, with their baseline characteristics summarized in Table 1. Significant differences were observed across the groups. Compared to the Q1 group, individuals in higher CMI quartiles demonstrated a greater prevalence of CMM, higher BMI, elevated FLI, and an increased incidence of diabetes and dyslipidemia, and a higher proportion of middle-aged adults (40–59 years old). Furthermore, they had higher rates of smoking, a greater proportion of males, and a higher percentage of non-Hispanic White individuals. In terms of laboratory measures, most parameters, including fasting blood glucose, glycated hemoglobin, ALT, AST, GGT, serum cholesterol, triglycerides (TG), LDL-C, and uric acid, exhibited an increasing trend with higher CMI levels. In contrast, HDL-C levels showed a declining trend (p < 0.001). Figure 2 presents the overlap of the prevalence rates of the four major CMDs.
Association between CMI and CMM in NAFLD
We investigated the association between CMI levels and the risk of CMM in patients with NAFLD, presenting the results using three different multivariable logistic regression models in Table 2. The findings revealed a statistically significant positive correlation between CMI levels and CMM risk in NAFLD patients across all three models.
We further explored the association between CMI and the presence of one, two, or three CMDs in the population. The results, presented in Table 3 using three different multivariable logistic regression models, revealed that CMI was significantly associated with the presence of two or three CMDs across all three models.
Dose-response relationships between CMI and CMM in NAFLD
We used RCS to explore the potential non-linear relationship between CMI and the risk of CMM in patients with NAFLD (Fig. 3). The model with four knots placed at the 5th, 35th, 65th, and 95th percentiles produced the lowest AIC value (Table S6), indicating the best model fit. The resulting dose–response curve visually depicted the association between CMI and CMM risk. The RCS analysis revealed a statistically significant nonlinear relationship (P for nonlinearity < 0.001). Specifically, CMM risk rose rapidly with increasing CMI levels until around 2.3, after which the increase became more gradual.
Multivariable odds ratio (or) for CMM based on CMI stratified by sex, age, race, BMI. Each stratification adjusted for all the factors (age, sex, race, PIR, BMI, smoking behavior, drinking behavior, physical activity, and lipid-lowering therapy) except the stratification factor itself. CMM cardiometabolic multimorbidity, CMI cardiometabolic index, PIR the poverty-to-income ratio, BMI body mass index.
Subgroup analysis
To further elucidate the complex relationship between CMI and the risk of CMM in patients with NAFLD, we conducted subgroup analyses and interaction tests within predefined subgroups (Fig. 4). Among these subgroups, the association between CMI and CMM prevalence was notably stronger in female NAFLD patients (P for interaction = 0.011). In contrast, interaction tests in other subgroups did not show significant results (P for interaction > 0.05).
Dose-response relationship between CMI and CMM. Values represent difference in predicted response in reference to CMI of mean. Red solid lines represent restricted cubic spline models. The black vertical dashed line indicates the CMI median, and the red vertical dashed line indicates the changepoint. Adjusted for age, sex, race, PIR, BMI, smoking behavior, drinking behavior, physical activity, and lipid-lowering therapy. CMM cardiometabolic multimorbidity, CMI cardiometabolic index, PIR the poverty-to-income ratio, BMI body mass index.
Discussion
This study investigated the potential relationship between CMI and the risk of CMM in the general adult NAFLD population in the United States. This association remained statistically significant after adjusting for all confounding variables (adjusted OR: 1.196, 95% CI: 1.077–1.328). RCS analysis demonstrated that the levels of CMI displayed a nonlinear relationship with CMM. Furthermore, Subgroup analysis revealed that the association between CMI and CMM prevalence was significantly stronger in female NAFLD patients. Finally, further investigation revealed that CMI remained positively associated with the presence of two or three CMDs. To our knowledge, this is the largest sample size study to date examining the relationship between CMI and CMM in the adult NAFLD population in the United States.
CMDs are a leading cause of death worldwide, and with the aging population, the incidence of CMD is rising significantly. This trend is unsurprising, as many CMDs share overlapping risk factors and etiologies, with complex bidirectional interactions between these diseases 10. The coexistence of multiple CMDs (CMM) significantly increases the risk of mortality 12, yet the precise pathophysiological mechanisms remain under investigation. It is currently believed that the development of CMM results from the combined effects of genetic, environmental, and lifestyle factors, which promote the onset and interrelationship of individual CMDs. Common pathophysiological pathways include chronic low-grade inflammation, IR, and dyslipidemia39. Furthermore, studies have shown that individuals with one CMD or CMM are 1.41 and 1.89 times more likely, respectively, to experience higher levels of psychological stress compared to those without CMDs40. Thus, CMM has a more profound negative impact on health compared to individual CMDs41. Previous studies have demonstrated a significant association between the CMI and increased CMDs risk in the general population13,42, and CMI is also considered a potential predictor of NAFLD. However, research regarding whether CMI can predict the risk of CMM in NAFLD patients remains limited. Therefore, this study aims to investigate the relationship between CMI and the risk of CMM in NAFLD patients. Our results reveal that elevated CMI levels are significantly associated with an increased risk of CMM in NAFLD patients.Meanwhile, we found that they exhibit a significant nonlinear relationship, consistent with previous studies in hypertensive populations43.This association remains statistically significant even after adjusting for various confounders. This study addresses a significant gap in the existing literature and provides valuable insights for early risk assessment and intervention in the clinical management of NAFLD patients.
Additionally, our study found that the relationship between CMI and CMM was more pronounced in female NAFLD patients, which is consistent with previous research. Previous studies have shown that females exhibit more significant gender differences in CMI, such as stronger associations with hyperglycemia and diabetes, Furthermore, the relationships between IR, TG, and the TG/HDL-C ratio are also more pronounced in women. Other studies have suggested that CMI has a stronger predictive ability for NAFLD in women, younger individuals, and non-obese subjects13,44,45. Similarly, gender differences in CMI regarding cerebrovascular diseases and hypertension have been confirmed, with female CMI showing stronger correlations with stroke and hypertension than in males46,47.One possible explanation is that women are more prone to the breakdown of visceral fat, producing non-esterified fatty acids (FFA), which, when transported to the liver, have a more significant impact on lipid metabolism. In addition, sex hormones may further influence the relationship between CMI and NAFLD by regulating fat distribution48,49.Therefore, gender-specific assessment is crucial in clinical management. Future studies should further investigate the interaction mechanisms between sex hormones and lipid metabolism to optimize prevention and treatment strategies.
Although the association between NAFLD and CMD has been extensively studied, the specific mechanisms underlying the role of CMI in the risk of CMM in NAFLD patients remain incompletely understood. Research has indicated that NAFLD is an important predictor of adverse cardiovascular outcomes and is closely associated with an increased risk of CMD, independent of traditional risk factors50,51,52. Dyslipidemia may be one of the key mechanisms, manifested by elevated TG, reduced HDL-C, and excessive circulating very-low-density lipoprotein cholesterol (VLDL-C), VLDL-C is considered the central factor in the dysregulation of TG and HDL-C, and can further lead to other lipid abnormalities, such as elevated LDL-C, which accelerate atherosclerotic changes53,54. Therefore, the dysregulation of triglyceride-rich lipoproteins is considered a key factor in the cardiovascular risk of NAFLD patients55. In this context, various lipid parameters and lipid ratios have been widely explored, with CMI appearing as a better predictor of NAFLD, potentially related to IR56,57. Adipose tissue IR promotes lipolysis, releasing large amounts of FFAs that are taken up by the liver and converted into LDL-C, thereby accelerating atherosclerosis progression58,59. The inflammatory response and the generation of reactive oxygen species can exacerbate IR, creating a vicious cycle that triggers systemic inflammation60. Notably, IR plays a significant role in the excessive production of VLDL-C in NAFLD patients. Abnormal expression of key molecules in the insulin signaling pathway, such as phosphatases and protein kinases, leads to hepatic IR, which in turn causes the overexpression of key proteins involved in lipid metabolism. This stimulates the liver to produce large amounts of VLDL-C, subsequently inducing dyslipidemia61. Moreover, IR impairs the liver’s ability to clear VLDL-C, leading to excessive accumulation of triglyceride-rich lipoprotein remnants62. IR may also be linked to an imbalance in adipokines and cytokines, characterized by reduced levels of anti-inflammatory adipokines and increased levels of pro-inflammatory cytokines (including interleukins IL-8, IL-6, IL-1b, interferon(IFN)-γ, etc.). The proliferation and dysfunction of adipose tissue, particularly visceral adipose tissue, downregulate the synthesis of anti-inflammatory adipokines while upregulating pro-inflammatory cytokines. This inflammatory environment in NAFLD promotes the formation of atherosclerosis63,64,65. In conclusion, the interplay between IR dyslipidemia, and inflammation may contribute to the association between CMI and CMM in NAFLD. However, further research is needed to elucidate these mechanisms and provide a stronger foundation for targeted interventions.
The main strength of this study lies in its novel use of large-scale cross-sectional NHANES data to investigate the relationship between CMI levels and CMM risk in NAFLD patients. However, several limitations should be acknowledged. First, the cross-sectional design of the study precludes the establishment of a causal relationship between CMI levels and CMM risk, and further validation through longitudinal studies or randomized controlled trials is needed. Second, the diagnosis of NAFLD was based on the USFLI score rather than the gold standard in clinical practice-liver biopsy. Third, laboratory results were based on a single measurement, which did not account for potential long-term variations in these biomarkers. Fourth, although numerous confounding variables were controlled for, the possibility of residual confounding cannot be fully excluded. Fifth, the diagnosis of CMM was partially based on baseline questionnaires and self-reports, which may introduce information bias or underreporting. Finally, the study cohort consisted only of adult NAFLD patients from the United States, limiting the generalizability of the findings. Future multi-center, prospective studies are needed to further elucidate these relationships.
Conclusions
Our cross-sectional study found a nonlinear relationship between CMI and CMM in adult NAFLD patients. These findings indicate a potential association between CMI and cardiometabolic burden in this population, highlighting the relevance of CMI in metabolic health assessment. This underscores the importance of monitoring CMI levels in NAFLD patients and exploring early strategies to mitigate CMM risk.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
Abbreviations
- CMM:
-
Cardiometabolic multimorbidity
- NAFLD:
-
Non-alcoholic fatty liver disease
- CMI:
-
Cardiometabolic Index
- NHANES:
-
National Health and Nutrition Examination Survey
- RCS:
-
Restricted cubic spline
- U.S:
-
United States
- T2DM:
-
Type 2 diabetes
- CVD:
-
Cardiovascular disease
- TG:
-
Triglyceride
- WHtR:
-
Waist-to-height ratio
- IR:
-
Insulin resistance
- CMD:
-
Cardiometabolic disease
- HDL-C:
-
High-density lipoprotein cholesterol
- CHD:
-
Coronary heart disease
- LDL-C:
-
Low-density lipoprotein cholesterol
- BMI:
-
Body Mass Index
- MET:
-
Metabolic equivalents
- PIR:
-
The poverty-to-income ratio
- TC:
-
Total cholesterol
- FIB-4:
-
Fibrosis-4
- SE:
-
Standard error
- HbA1c:
-
Glycated hemoglobin
- FFA:
-
Non-esterified fatty acids
- VLDL-C:
-
Very-low-density lipoprotein cholesterol
- IL:
-
Interleukins
- IFN:
-
Interferon
- CDC:
-
Centers for disease control and prevention
- USFLI:
-
Ultrasound fatty liver index
References
Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 7, 851–861. https://doi.org/10.1016/s2468-1253(22)00165-0 (2022).
Santos, R. D., Valenti, L. & Romeo, S. Does nonalcoholic fatty liver disease cause cardiovascular disease? Current knowledge and gaps. Atherosclerosis 282, 110–120. https://doi.org/10.1016/j.atherosclerosis.2019.01.029 (2019).
Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908–922. https://doi.org/10.1038/s41591-018-0104-9 (2018).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol. (Baltimore Md). 64, 73–84. https://doi.org/10.1002/hep.28431 (2016).
Alon, L. et al. Risk of cardiovascular events in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur. J. Prev. Cardiol. 29, 938–946. https://doi.org/10.1093/eurjpc/zwab212 (2022).
Byrne, C. D. & Targher, G. NAFLD: a multisystem disease. J. Hepatol. 62, 47–64. https://doi.org/10.1016/j.jhep.2014.12.012 (2015).
Long, M. T. et al. Nonalcoholic fatty liver disease and vascular function: cross-sectional analysis in the Framingham heart study. Arterioscler. Thromb. Vasc. Biol. 35, 1284–1291. https://doi.org/10.1161/atvbaha.114.305200 (2015).
McPherson, S. et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J. Hepatol. 62, 1148–1155. https://doi.org/10.1016/j.jhep.2014.11.034 (2015).
Adams, L. A., Anstee, Q. M., Tilg, H. & Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 66, 1138–1153. https://doi.org/10.1136/gutjnl-2017-313884 (2017).
Mendis, S., Davis, S. & Norrving, B. Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 46, e121–122. https://doi.org/10.1161/strokeaha.115.008097 (2015).
Han, E. & Lee, Y. H. Non-Alcoholic fatty liver disease: the emerging burden in cardiometabolic and renal diseases. Diabetes Metabolism J. 41, 430–437. https://doi.org/10.4093/dmj.2017.41.6.430 (2017).
Di Angelantonio, E. et al. Association of cardiometabolic Multimorbidity with mortality. Jama 314, 52–60. https://doi.org/10.1001/jama.2015.7008 (2015).
Wakabayashi, I. & Daimon, T. The cardiometabolic index as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin. Chim. Acta. 438, 274–278. https://doi.org/10.1016/j.cca.2014.08.042 (2015).
Lechner, K., Lechner, B., Crispin, A., Schwarz, P. E. H. & von Bibra, H. Waist-to-height ratio and metabolic phenotype compared to the Matsuda index for the prediction of insulin resistance. Sci. Rep. 11, 8224. https://doi.org/10.1038/s41598-021-87266-z (2021).
Yeh, W. C. et al. Elevated triglyceride-to-HDL cholesterol ratio is an indicator for insulin resistance in middle-aged and elderly Taiwanese population: a cross-sectional study. Lipids Health Dis. 18, 176. https://doi.org/10.1186/s12944-019-1123-3 (2019).
Huang, W., Wang, H., Shen, Z., Wang, X. & Yu, X. Association between TyG index and risk of carotid atherosclerosis in NAFLD patients: a retrospective cohort study. Front. Endocrinol. 15, 1448359. https://doi.org/10.3389/fendo.2024.1448359 (2024).
Smith, G. I. et al. Insulin resistance drives hepatic de Novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 130, 1453–1460. https://doi.org/10.1172/jci134165 (2020).
Cui, H., Liu, Q., Wu, Y. & Cao, L. Cumulative triglyceride-glucose index is a risk for CVD: a prospective cohort study. Cardiovasc. Diabetol. 21, 22. https://doi.org/10.1186/s12933-022-01456-1 (2022).
Xi, W. F. & Yang, A. M. Association between cardiometabolic index and controlled Attenuation parameter in U.S. Adults with NAFLD: findings from NHANES (2017–2020). Lipids Health Dis. 23, 40. https://doi.org/10.1186/s12944-024-02027-x (2024).
Zou, J. et al. Association between the cardiometabolic index and non-alcoholic fatty liver disease: insights from a general population. BMC Gastroenterol. 22 https://doi.org/10.1186/s12876-022-02099-y (2022).
Wang, H. et al. Body adiposity index, lipid accumulation product, and cardiometabolic index reveal the contribution of adiposity phenotypes in the risk of hyperuricemia among Chinese rural population. Clin. Rheumatol. 37, 2221–2231. https://doi.org/10.1007/s10067-018-4143-x (2018).
Mazidi, M., Huybrechts, I. & Kengne, A. P. Associations between serum lipophilic antioxidants levels and non-alcoholic fatty liver disease are moderated by adiposity. Eur. J. Clin. Nutr. 73, 1088–1090. https://doi.org/10.1038/s41430-019-0413-1 (2019).
Zhang, K. et al. The association between serum vitamin A and NAFLD among US adults varied in different BMI groups: a cross-sectional study. Food Funct. 14, 836–844. https://doi.org/10.1039/d2fo02204d (2023).
Zhang, A. et al. Associations of serum lead, cadmium, and mercury concentrations with all-cause and cause-specific mortality among individuals with cardiometabolic Multimorbidity. Ecotoxicol. Environ. Saf. 280, 116556. https://doi.org/10.1016/j.ecoenv.2024.116556 (2024).
Jiang, Z. et al. Long-term influence of air pollutants on morbidity and all-cause mortality of cardiometabolic multi-morbidity: A cohort analysis of the UK biobank participants. Environ. Res. 237, 116873. https://doi.org/10.1016/j.envres.2023.116873 (2023).
Wang, L. et al. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999–2018. Jama 326, 1–13. https://doi.org/10.1001/jama.2021.9883 (2021).
Xu, J. P. et al. Systemic inflammation markers and the prevalence of hypertension: A NHANES cross-sectional study. Hypertens. Re. Offi. J. Jpn. Soc. Hypertens. 46, 1009–1019. https://doi.org/10.1038/s41440-023-01195-0 (2023).
Garvey, W. T. et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. 22 (Suppl 3), 1–203. https://doi.org/10.4158/ep161365.gl (2016).
Rattan, P. et al. Inverse association of telomere length with liver disease and mortality in the US population. Hepatol. Commun. 6, 399–410. https://doi.org/10.1002/hep4.1803 (2022).
Kim, D., Konyn, P., Cholankeril, G. & Ahmed, A. Physical activity is associated with nonalcoholic fatty liver disease and significant fibrosis measured by fibroscan. Clin. Gastroenterol. Hepatology: Official Clin. Pract. J. Am. Gastroenterological Association. 20, e1438–e1455. https://doi.org/10.1016/j.cgh.2021.06.029 (2022).
Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III). Jama 285, 2486–2497. https://doi.org/10.1001/jama.285.19.2486 (2001).
Johnson, C. L. et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat. 2, 1–24 (2013).
Ruhl, C. E. & Everhart, J. E. Fatty liver indices in the multiethnic united States National health and nutrition examination survey. Aliment. Pharmacol. Ther. 41, 65–76. https://doi.org/10.1111/apt.13012 (2015).
Henry, A. et al. Vigorous physical activity provides protection against all-cause deaths among adults patients with nonalcoholic fatty liver disease (NAFLD). Aliment. Pharmacol. Ther. 57, 709–722. https://doi.org/10.1111/apt.17308 (2023).
Chen, C. et al. Copper exposure association with prevalence of non-alcoholic fatty liver disease and insulin resistance among US adults (NHANES 2011–2014). Ecotoxicol. Environ. Saf. 218, 112295. https://doi.org/10.1016/j.ecoenv.2021.112295 (2021).
Tsou, P., Wu, C. J. & Serum Vitamin, E. Levels of adults with nonalcoholic fatty liver disease: an inverse relationship with All-Cause mortality in Non-Diabetic but not in Pre-Diabetic or diabetic subjects. J. Clin. Med. 8 https://doi.org/10.3390/jcm8071057 (2019).
Li, J. et al. Visceral adiposity index is associated with arterial stiffness in hypertensive adults with normal-weight: the China H-type hypertension registry study. Nutr. Metabolism. 18 https://doi.org/10.1186/s12986-021-00617-5 (2021).
Guo, Z. et al. Free thyroxine, brain frailty and clock drawing test performance in patients with acute minor stroke or transient ischaemic attack. Clin. Endocrinol. 96, 175–183. https://doi.org/10.1111/cen.14564 (2022).
Feng, X. et al. Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics 9, 1923–1951, (2019). https://doi.org/10.7150/thno.30787
Sakakibara, B. M., Obembe, A. O. & Eng, J. J. The prevalence of cardiometabolic Multimorbidity and its association with physical activity, diet, and stress in canada: evidence from a population-based cross-sectional study. BMC Public. Health. 19, 1361. https://doi.org/10.1186/s12889-019-7682-4 (2019).
Jani, B. D. et al. Relationship between multimorbidity, demographic factors and mortality: findings from the UK biobank cohort. BMC Med. 17 https://doi.org/10.1186/s12916-019-1305-x (2019).
He, L. et al. Correlation of cardiometabolic index and sarcopenia with cardiometabolic Multimorbidity in middle-aged and older adult: a prospective study. Front. Endocrinol. 15, 1387374. https://doi.org/10.3389/fendo.2024.1387374 (2024).
Dong, T. et al. Association of adiposity indicators with cardiometabolic Multimorbidity risk in hypertensive patients: a large cross-sectional study. Front. Endocrinol. 15, 1302296. https://doi.org/10.3389/fendo.2024.1302296 (2024).
He, J. et al. The TG/HDL-C Ratio Might Be a Surrogate for Insulin Resistance in Chinese Nonobese Women. Int. J. Endocrinol. 105168. https://doi.org/10.1155/2014/105168 (2014).
Wang, J. et al. Comparison of several blood lipid-related indexes in the screening of non-alcoholic fatty liver disease in women: a cross-sectional study in the Pearl river delta region of Southern China. BMC Gastroenterol. 21, 482. https://doi.org/10.1186/s12876-021-02072-1 (2021).
Wang, H., Chen, Y., Guo, X., Chang, Y. & Sun, Y. Usefulness of cardiometabolic index for the Estimation of ischemic stroke risk among general population in rural China. Postgrad. Med. 129, 834–841. https://doi.org/10.1080/00325481.2017.1375714 (2017).
Wang, H. et al. Validity of cardiometabolic index, lipid accumulation product, and body adiposity index in predicting the risk of hypertension in Chinese population. Postgrad. Med. 130, 325–333. https://doi.org/10.1080/00325481.2018.1444901 (2018).
Pasquali, R. & Oriolo, C. Obesity and androgens in women. Front. Horm. Res. 53, 120–134. https://doi.org/10.1159/000494908 (2019).
Meredith-Jones, K. et al. Age- and sex-specific visceral fat reference cutoffs and their association with cardio-metabolic risk. Int. J. Obes. 45, 808–817. https://doi.org/10.1038/s41366-021-00743-3 (2021).
Park, J. H., Koo, B. K., Kim, W. & Kim, W. H. Histological severity of nonalcoholic fatty liver disease is associated with 10-year risk for atherosclerotic cardiovascular disease. Hep. Intl. 15, 1148–1159. https://doi.org/10.1007/s12072-021-10209-3 (2021).
Toh, J. Z. K. et al. A Meta-Analysis on the global prevalence, risk factors and screening of coronary heart disease in nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatology: Official Clin. Pract. J. Am. Gastroenterological Association. 20, 2462–2473e2410. https://doi.org/10.1016/j.cgh.2021.09.021 (2022).
Zhou, X. D. et al. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc. Diabetol. 21, 270. https://doi.org/10.1186/s12933-022-01697-0 (2022).
DeFilippis, A. P. et al. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic study of atherosclerosis. Atherosclerosis 227, 429–436. https://doi.org/10.1016/j.atherosclerosis.2013.01.022 (2013).
Borén, J. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European atherosclerosis society consensus panel. Eur. Heart J. 41, 2313–2330. https://doi.org/10.1093/eurheartj/ehz962 (2020).
Zou, Y. et al. Association of remnant cholesterol with nonalcoholic fatty liver disease: a general population-based study. Lipids Health Dis. 20 https://doi.org/10.1186/s12944-021-01573-y (2021).
Fukuda, Y. et al. Triglycerides to high-density lipoprotein cholesterol ratio is an independent predictor of incident fatty liver; a population-based cohort study. Liver International: Official J. Int. Association Study Liver. 36, 713–720. https://doi.org/10.1111/liv.12977 (2016).
Fan, N. et al. Triglycerides to high-density lipoprotein cholesterol ratio as a surrogate for nonalcoholic fatty liver disease: a cross-sectional study. Lipids Health Dis. 18 https://doi.org/10.1186/s12944-019-0986-7 (2019).
Chen, Z., Yu, Y., Cai, J. & Li, H. Emerging molecular targets for treatment of nonalcoholic fatty liver disease. Trends Endocrinol. Metab. 30, 903–914. https://doi.org/10.1016/j.tem.2019.08.006 (2019).
Hodson, L. & Gunn, P. J. The regulation of hepatic fatty acid synthesis and partitioning: the effect of nutritional state. Nat. Rev. Endocrinol. 15, 689–700. https://doi.org/10.1038/s41574-019-0256-9 (2019).
Nassir, F. NAFLD: mechanisms, treatments, and biomarkers. Biomolecules 12 https://doi.org/10.3390/biom12060824 (2022).
Avramoglu, R. K., Basciano, H. & Adeli, K. Lipid and lipoprotein dysregulation in insulin resistant States. Clin. Chim. Acta. 368, 1–19. https://doi.org/10.1016/j.cca.2005.12.026 (2006).
Gordts, P. L. et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J. Clin. Investig. 126, 2855–2866. https://doi.org/10.1172/jci86610 (2016).
Tilg, H. & Hotamisligil, G. S. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology 131, 934–945. https://doi.org/10.1053/j.gastro.2006.05.054 (2006).
Ridker, P. M. et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131. https://doi.org/10.1056/NEJMoa1707914 (2017).
Targher, G., Tilg, H. & Byrne, C. D. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 6, 578–588. https://doi.org/10.1016/s2468-1253(21)00020-0 (2021).
Acknowledgements
The data from NHANES collection were sponsored by the CDC.
Funding
Zhejiang Provincial Traditional Chinese Medicine Science and Technology Project (2024ZL181).
Author information
Authors and Affiliations
Contributions
JYY: Writing – original draft, Conceptualization, Methodology, Investigation, Formal analysis. YLX: Writing – review & editing, Supervision. YJG: Writing – review & editing, Supervision. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The studies involving humans were approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Consent for publication
All the authors listed have approved the manuscript that is enclosed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ye, J., Xie, Y. & Gong, Y. Association between cardiometabolic index and cardiometabolic multimorbidity in non-alcoholic fatty liver disease patients: evidence from a cross-sectional study. Sci Rep 15, 33622 (2025). https://doi.org/10.1038/s41598-025-19177-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19177-2