Abstract
Atrial fibrillation (AF), the most prevalent critical care arrhythmia, demonstrates substantial mortality associations where renal dysfunction management plays a pivotal therapeutic role. We examined the prognostic capacity of admission blood urea nitrogen-to-creatinine ratio (BUN/Cr) - a low-cost renal biomarker - for 28-/365-day mortality prediction in AF through multidimensional survival analyses leveraging the MIMIC-IV 3.1 database. Data relevant to AF patients were extracted from the publicly available MIMIC-IV 3.1 database based on predefined inclusion and exclusion criteria. Cox proportional hazards regression, Kaplan-Meier survival analysis, and Restricted Cubic Spline (RCS) models were used to assess the association between the BUN/Cr and the risk of 28-day and 365-day mortality. Subsequently, a short-term and long-term mortality risk prediction model for AF patients was developed using interpretable machine learning algorithms, incorporating the BUN/Cr and other clinical features. The MIMIC-IV analysis included 14,725 AF patients (72.9 ± 11.7 years, 60.3% male). Cox regression identified BUN/Cr as an independent predictor of 28-day and 365-day mortality, with risk quintiles showing a non-linear pattern: Q5 (> 27.8), Q4 (22.0–27.8), Q1 (≤ 15.0), Q3 (18.5–22.0), and Q2 (15.0–18.5). Kaplan-Meier curves confirmed decreasing survival with elevated BUN/Cr. Restricted cubic splines revealed U-shaped mortality relationships (P < 0.001), with inflection points at BUN/Cr = 16.49 (28-day) and 16.67 (365-day). Among machine learning models, XGBoost outperformed others in predicting mortality (28-day: AUC = 0.793 [0.776–0.810], Accuracy = 73.1%; 365-day: AUC = 0.778 [0.764–0.793], Accuracy = 69.8%). SHAP analysis ranked BUN/Cr fourth among predictors for both endpoints. The BUN/Cr emerged as a robust independent predictor of short- and long-term mortality in AF. The interpretable XGBoost model, integrating BUN/Cr with clinical variables, achieved superior predictive accuracy for 28-/365-day outcomes while maintaining generalizability. BUN/Cr constituted a fourth-ranked feature across mortality timelines. These findings underscore its clinical utility for AF risk stratification and treatment optimization, supporting biomarker-guided therapeutic interventions.
Similar content being viewed by others
Introduction
The occurrence and frequency of atrial fibrillation (AF), a widespread arrhythmia in clinical practice, have been on the rise globally, posing a substantial public health concern1,2. Projections indicate that by 2050, the number of AF patients in the United States could reach between 6 and 12 million, while in Europe, it may climb to approximately 17.9 million1,3. In China, epidemiological studies have also shown a comparable trend, with the prevalence of AF steadily increasing and the patient population approaching 20 million, which is likely attributable to the accelerated aging of the population in recent years4,5. AF diminishes the quality of life for patients and substantially raises the risk of stroke, serving as a primary cause of ischemic stroke6,7. Additionally, AF is linked to high rates of heart failure (HF) incidence and mortality, significantly impacting patient prognosis8. Blood urea nitrogen (BUN) and creatinine (Cr) are commonly used indicators for evaluating renal function, with their ratio reflecting kidney filtration and excretion efficiency. Research has demonstrated that the BUN/Cr is closely associated with adverse outcomes in heart failure patients. Both acute heart failure (AHF) and chronic heart failure (CHF) patients with a higher BUN/Cr face an increased risk of all-cause mortality and readmission9,10. For instance, the Matsue study found that in AHF patients, a higher BUN/Cr ratio was significantly linked to increased mortality and readmission rates during hospitalization11. Similar findings suggest that in CHF patients, an elevated BUN/Cr serves as a crucial biomarker for predicting long-term prognosis12. However, the role of BUN/Cr in predicting the prognosis of AF patients is largely unexplored. Considering the significant link between AF and heart failure, along with the impact of the BUN/Cr ratio on heart failure outcomes, examining its effect on AF patients is crucial. Studies conducted recently have pointed out that compromised renal function might significantly contribute to the emergence and progression of atrial fibrillation13,14,15. Thus, the BUN/Cr ratio serves as an affordable and straightforward biomarker, offering fresh perspectives on risk evaluation and prognosis forecasting for AF patients in clinical environments. Examining Studying how the BUN/Cr affects AF prognosis can give important guidance for treating and managing patients with AF. The main goal of this study is to evaluate the link between the blood urea nitrogen to serum creatinine ratio in AF patients and the mortality rate of ICU inpatients. Simultaneously, a machine learning model was created to predict mortality risk in atrial fibrillation patients, utilizing BUN/Cr along with additional clinical indicators.
Materials and methods
Data source
This retrospective cohort study utilized version 3.1 of the MIMIC-IV database. Dr. Yang Shu (Credential ID:62274870) curated AF patient data through privileged access. The investigation strictly complied with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines, maintaining methodological rigor and transparency throughout all analytical processes.
Participants
AF patients were identified using ICD-9 (42731) and ICD-10 (I48) codes. Inclusion criteria included: (i) initial ICU admission, (ii) age ≥ 18 years, and (iii) ICU stay ≥ 24 h. Patients with missing BUN/Cr data were excluded. From 14,725 eligible AF cases meeting these criteria, all clinical parameters were systematically extracted for analysis.
Data extraction
Clinical data extraction employed Navicat Premium with SQL-structured querying, systematically retrieving demographic profiles, vital parameters, laboratory markers, comorbidity burdens, and therapeutic regimens. Primary outcome measures focused on 28-day/365-day mortality post-ICU admission, capturing post-admission survivorship trajectories.
Statistical analysis
Patient groups: AF patients were stratified by BUN/Cr quintiles: Q1 (≤ 15.0), Q2 (15.1–18.5), Q3 (18.6–22.0), Q4 (22.1–27.8), and Q5 (> 27.8). This quintile-based categorization facilitated granular renal functional analysis: Q1 (very low), Q2 (low), Q3 (moderate), Q4 (high), Q5 (very high).
Baseline data analysis: Baseline characteristics of AF patients across BUN/Cr quintiles were analyzed. Demographic data, vital signs, laboratory parameters, comorbidities, severity scores and treatment modalities were stratified by group. Categorical variables were expressed as frequencies (percentages) with Chi-square/Fisher’s exact tests for intergroup comparisons. Continuous variables followed normal distributions (mean ± SD, independent t-test) or non-normal distributions [median (IQR), Mann-Whitney U test]. All statistical assessments maintained a two-tailed α = 0.05 significance threshold.
Association analysis between BUN/Cr and prognosis: (i) Cox multivariate regression analysis was conducted to explore the relationship between different BUN/Cr levels (Q1 ~ Q5) and mortality risks. (ii) Kaplan-Meier survival curves were used to visualize the survival outcomes of patients with different BUN/Cr levels during the 28-day and 365-day periods after ICU admission. (iii) RCS analysis were employed to visualize potential nonlinear relationships between BUN/Cr and 28-day and 365-day mortality rates. Following the identification of a nonlinear relationship, two-segment Cox regression models were used to assess the threshold effects of BUN/Cr on short-term and long-term patient prognosis.
Interpretability of clinical prediction model analysis: Clinical prediction models are employed to predict both short-term and long-term mortality risks in AF patients. (i) Feature Selection: Initially, the LASSO regression algorithm is utilized to select key predictive features. Redundant features are subsequently removed through multicollinearity analysis. (ii) Model Construction and Evaluation: The models are built using five algorithms (XGBoost, Logistic Regression, LightGBM, Random Forest, and AdaBoost). The models are evaluated via a five-fold, five-times cross-validation approach, with performance assessed using metrics such as ROCAUC, Accuracy, and F1 score to select the optimal algorithm. The dataset is split into a 7:3 ratio for the training and testing sets. Within the training set, model performance is optimized via “five-fold cross-validation combined with hyperparameter grid search.” Finally, the best-performing model is evaluated on the test set to assess its predictive performance and generalizability in predicting mortality risks. (iii) SHAP Interpretability Analysis: Feature importance and summary plots are generated to illustrate the impact of key features on adverse outcomes. Scatter plots are utilized to explore and confirm the non-linear relationship between the key feature BUN/Cr and the outcome variable. Additionally, SHAP force plots are used to visually demonstrate the contribution of features in predicting mortality risks, offering an intuitive understanding of individualized predictions.
This study used a two-tailed test, with P < 0.05 considered statistically significant. All analyses were conducted using R 4.1.2 and Free Statistics software version 1.5.
Results
Population
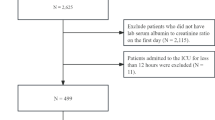
The MIMIC-IV analysis initially identified 83,468 AF patients. After excluding 65,809 non-ICU admissions, 17,659 first-time ICU admissions were retained. Subsequent exclusions applied: age < 18 (n = 0), ICU stays < 24 h (n = 2528) and those whose time of death was recorded before the time of admission (the survival time was negative, n = 1). Missing variables were addressed via median imputation. These criteria yielded a final cohort of 14,725 AF patients (Fig. 1).
Baseline characteristics
The study cohort included 14,725 atrial fibrillation (AF) patients with a median age of 74.0 years (IQR: 65.0–82.0; 60.3% male). A dose-response relationship was observed across increasing BUN/Cr quintiles, with progressively higher heart rates (P < 0.001), elevated blood glucose levels (P < 0.001), and increased severity scores (APS III, SAPS II, OASIS; all P < 0.001). Mortality analysis revealed a significant U-shaped association: the Q2 group demonstrated the most favorable outcomes, with the lowest 28-day mortality (11.4% vs. Q1:15.0%, Q3:14.5%, Q4:18.3%, Q5:27.3%; P < 0.001) and 365-day mortality rates (23.4% vs. Q1:28.8%, Q3:27.1%, Q4:34.9%, Q5:47.9%; P < 0.001), highlighting the non-linear mortality risk associated with BUN/Cr ratios (Table 1).
Cox multivariate regression analysis
For 28-day mortality, unadjusted analysis (Model 1) revealed elevated risks in Q1 (≤ 15.0, P < 0.001), Q3 (HR = 1.28, P = 0.001), Q4 (HR = 1.66, P < 0.001), and Q5 (HR = 2.60, P < 0.001) versus Q2. Similarly, 365-day mortality analyses showed increased risks for Q1 (P < 0.001), Q3 (HR = 1.19, P = 0.001), Q4 (HR = 1.61, P < 0.001), and Q5 (HR = 2.46, P < 0.001). Consistent trends persisted in adjusted models: Model 2 (sex, SpO2, WBC, glucose, potassium) and Model 3 (Model 2 + statins + hyperlipidemia) yielded concordant results (Table 2.).
BUN/Cr ratios demonstrated graded associations with mortality, showing: Short-term risk hierarchy: Q5 > Q4 > Q1 > Q3 > Q2; (ii) Long-term pattern: Identical to short-term stratification.
K-M survival curve analysis
Kaplan-Meier curves assessed associations between BUN/Cr quintiles and 28-day/365-day mortality in AF patients, using ICU survival duration as the timeline (Fig. 2). The results indicated that, on day 28 after ICU admission, the mortality risk for AF patients ranged from highest to lowest as follows: high BUN/Cr levels (Q5, BUN/Cr > 27.8), relatively high BUN/Cr levels (Q4, 22.0 < BUN/Cr ≤ 27.8), low BUN/Cr levels (Q1, BUN/Cr ≤ 15.0), moderate BUN/Cr levels (Q3, 18.5 < BUN/Cr ≤ 22.0), and relatively low BUN/Cr levels (Q2, 15.0 < BUN/Cr ≤ 18.5).
Similarly, on day 365 after ICU admission, the mortality risk for AF patients was ranked from highest to lowest as: high BUN/Cr levels (Q5, BUN/Cr > 27.8), relatively high BUN/Cr levels (Q4, 22.0 < BUN/Cr ≤ 27.8), low BUN/Cr levels (Q1, BUN/Cr ≤ 15.0), moderate BUN/Cr levels (Q3, 18.5 < BUN/Cr ≤ 22.0), and relatively low BUN/Cr levels (Q2, 15.0 < BUN/Cr ≤ 18.5).
The results indicated that both high and low levels of BUN/Cr were associated with increased short- and long-term mortality risks in atrial fibrillation patients. The mortality risk was ranked from highest to lowest as follows: Q5, Q4, Q1, Q3, and Q2.
Restricted cubic spline analysis
RCS analysis demonstrated a U-shaped association between BUN/Cr and 28-/365-day all-cause mortality in AF patients (P < 0.01). Mortality risks initially decreased then increased with rising BUN/Cr levels (Fig. 3).
Segmented Cox regression analysis identified threshold BUN/Cr values at 16.49 (28-day) and 16.67 (365-day) in AF patients (Table 3). Below these thresholds, each 1 unit BUN/Cr increase corresponded to reduced mortality risks (28-day: HR = 0.938; 365-day: HR = 0.937). Conversely, above thresholds, equivalent BUN/Cr elevation predicted increased mortality (28-day&365-day: HR = 1.031). This bidirectional pattern demonstrated a over 3% mortality risk transition (P < 0.001 for slope comparisons).
Subgroup analysis
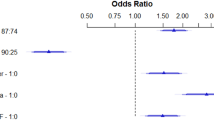
Cox proportional hazards models revealed significant interactions between elevated BUN/Cr and two factors: pre-existing renal disease (28-day: P = 0.001; 365-day: P < 0.001) and early aspirin therapy (28&365-day: P < 0.001) regarding mortality risks in AF patients (Fig. 4).
Renal dysfunction substantially modified BUN/Cr prognostic implications. Each 1-unit BUN/Cr increase conferred differential mortality increments: 2.0% in renal patients versus 3.0% in non-renal counterparts. Despite lower HR escalations, absolute mortality was higher in renal patients (28-day: 21.8% vs. 15.6%; 365-day: 43.2% vs. 28.5%). This paradox reflects divergent pathophysiology: impaired kidneys exhibit reduced capacity to compensate for uremic toxin accumulation, whereas BUN/Cr fluctuations in preserved renal function may represent transient stressors mitigated by intact nephron adaptability.
Aspirin administration within 24 h of ICU admission demonstrated therapeutic modulation. While BUN/Cr-associated mortality increments remained identical (4.0%/unit) between aspirin-treated and untreated groups, absolute mortality diverged markedly (28-day: 11.2% vs. 20.8%; 365-day: 22.4% vs. 38.2%, P < 0.001). Mechanistically, aspirin’s dual actions are plausible: (i) platelet inhibition improves renal microcirculation by preventing platelet-fibrin thrombosis in peritubular capillaries, attenuating ischemic tubular injury; (ii) cyclooxygenase-2 suppression mitigates inflammatory cytokine-mediated glomerular hypofiltration, disrupting the BUN/Cr-inflammation multi-organ failure cycle. This protective synergy proves critical in acute critical illness where hemodynamic instability amplifies uremic toxicity.
Notably, BUN/Cr interactions with age, chronic pulmonary disease, diabetes, clopidogrel, statins or mechanical ventilation showed no statistical significance (P > 0.05). These findings emphasize the specificity of renal function and aspirin therapy as key modifiers of BUN/Cr prognostic value.
Feature selection
Feature selection using LASSO regression
Using the LASSO regression algorithm, we selected 16 key features for the 28-day mortality risk prediction model, which include: Age, Gender, Heart rate, SpO2, WBC, Glucose, TC, TG, Hypertension, Hyperlipidemia, Renal disease, Severe liver disease, APS III, Aspirin, Statins, and BUN/Cr, as shown in Fig. 5A. Similarly, using the LASSO regression algorithm, we selected 17 key features for the 365-day mortality risk prediction model, which include: Age, Gender, Heart rate, SpO2, WBC, Glucose, TC, TG, Potassium, Hypertension, Hyperlipidemia, Renal disease, Severe liver disease, APS III, Aspirin, Statins, and BUN/Cr, as shown in Fig. 5B.
Diagnosis of collinearity
For the 28-day mortality risk prediction model, the variance inflation factor (VIF) was used to diagnose multicollinearity and assess the independence among the 16 factors. The results showed that the maximum VIF value was 1.4, which is less than 5 (specific results are shown in Table 4). This indicated that the selected key features had high independence, with no significant multicollinearity issues, allowing the analysis results to be accurately reflected. For the 365-day mortality risk prediction model, the variance inflation factor was also used to assess the independence of the 17 factors. The results showed that the maximum VIF value was 1.402, which is less than 5 (specific results are shown in Table 5). This suggested that the selected key features had high independence and no significant multicollinearity issues. For the specific input list of the binary classification model, please refer to Supplementary TableS1.
Machine learning construction and evaluation
Prediction model for 28-day mortality risk
Selection of optimal algorithm
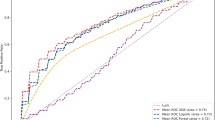
In this study, we used the 28-day mortality rate as the model output, employing key predictive features selected through feature screening as inputs. Five machine learning algorithms–XGBoost, Logistic Regression, LightGBM, Random Forest, and AdaBoost–were utilized to construct the models. To compare and optimize the algorithms, we employed a five-fold, five-time cross-validation method and evaluated model performance using metrics such as ROCAUC, Accuracy, and F1 score. According to the experimental results (see Fig. 6; Table 6), the XGBoost algorithm demonstrated the best short-term mortality risk prediction performance on the validation set, with an AUC of 0.810, Accuracy of 74.0% (95% CI: 72.2 ~ 75.7%), and an F1 score of 0.490.
Evaluation of the optimal algorithm
The dataset was partitioned into training (70%) and independent test (30%) sets. Through 5-fold cross-validation with grid search optimization (learning_rate = 0.3, max_depth = 4, min_child_weight = 6, reg_lambda = 1), XGBoost demonstrated superior predictive performance, achieving an AUC of 0.793 (95% CI: 0.75–0.83), accuracy of 73.1%, and F1-score of 0.479 on the held-out test set. These results indicate robust discriminative ability for short-term mortality risk prediction while maintaining clinically relevant performance metrics.
Prediction model for 365-day mortality risk
Selection of the optimal algorithm
In this study, key predictive features, selected through feature screening, were used as inputs for model construction based on five machine learning algorithms: XGBoost, Logistic Regression, LightGBM, Random Forest, and AdaBoost. To compare and optimize the algorithms, we employed a five-fold, five-time cross-validation method and evaluated model performance using metrics such as ROCAUC, Accuracy, and F1 score. According to the experimental results (see Fig. 7; Table 7), the XGBoost algorithm demonstrated the best performance for long-term mortality risk prediction on the validation set, with an AUC of 0.786, Accuracy of 70.8% (95% CI: 72.2 ~ 75.7%), and an F1 score of 0.614.
Evaluation of the optimal algorithm: The dataset was partitioned into training and testing sets (7:3 ratio), with model development conducted using five-fold cross-validation on the training set. Through grid search optimization, we identified optimal hyperparameters (learning_rate = 0.3, max_depth = 4, min_child_weight = 6, reg_lambda = 0.5) for the XGBoost algorithm. When evaluated on the independent test set for 365-day mortality prediction, the optimized XGBoost model demonstrated robust performance, achieving an AUC of 0.778 (95% CI), with corresponding accuracy of 69.8% and F1-score of 0.613. These results indicate that our carefully tuned XGBoost algorithm provides clinically meaningful predictive capability for long-term mortality risk assessment.
Interpretable analysis based on the SHAP method
Prediction model for 28-day mortality risk
This study utilized the SHAP method to conduct a detailed interpretative analysis of the XGBoost-based short-term mortality risk prediction model for AF patients. We sequentially generated the importance ranking plot, summary plot, scatter plot, individual force plots of the survivor and non-survivor (as shown in Fig. 8). Compared to other key predictive features, BUN/Cr made a considerable contribution to mortality risk prediction, ranking fourth in importance (as shown in Fig. 8(A ~ B)). For AF patients in the test set, a non-linear relationship between BUN/Cr and mortality risk was observed, with both lower and higher levels of BUN/Cr potentially increasing the short-term mortality risk. This finding was consistent with the results from the RCS analysis in Sect. 3.5, which examined the association between BUN/Cr and short-term prognosis in all patients, and revealed a potential “U”-shaped curve relationship (as shown in Fig. 8 C).
According to the force plot for a surviving AF patient (as shown in Fig. 8D), the XGBoost-based 28-day mortality risk prediction model for AF patients demonstrated good accuracy, with a predicted mortality probability of 13%, consistent with the patient’s survival outcome after ICU admission. Heart rate within the normal range and a lower APS III score contributed to a reduced short-term mortality risk for AF patients, while higher age was associated with an increased mortality risk. Similarly, based on the force plot for a deceased AF patient (as shown in Fig. 8E), the XGBoost-based 28-day mortality risk prediction model for AF patients was also accurate, with a predicted mortality probability of 58.0%, aligning with the patient’s survival outcome within 28 days of ICU admission. Higher disease severity scores (APS III), elevated heart rate, higher blood glucose levels, and increased BUN/Cr all contributed to an increased short-term mortality risk for AF patients.
Prediction model for 365-day mortality risk
This study utilized the SHAP method to conduct a detailed interpretative analysis of the XGBoost-based long-term mortality risk prediction model for AF patients. We sequentially generated the importance ranking plot, summary plot, scatter plot, force plot of the survivor and non-survivor (as shown in Fig. 9). Compared to other key predictive features, BUN/Cr made a significant contribution to the 365-day mortality risk prediction, ranking fourth in importance (as shown in Fig. 9(A ~ B)). For AF patients in the test set, a non-linear relationship between BUN/Cr and mortality risk was observed, with both lower and higher levels of BUN/Cr potentially increasing the long-term mortality risk. This result was consistent with the findings from the restricted cubic spline analysis in Sect. 3.5, which examined the relationship between BUN/Cr and long-term prognosis in all patients, showing similar trends (as shown in Fig. 9C).
According to the force plot for a surviving AF patient (as shown in Fig. 9D), the XGBoost-based 365-day mortality risk prediction model for AF patients demonstrated good accuracy, with a predicted mortality probability of 20.0%, which was consistent with the patient’s survival outcome after ICU admission. Heart rate within the normal range and a lower APS III score contributed to reducing the long-term mortality risk for AF patients, while higher age increased the patient’s mortality risk. Similarly, based on the force plot for a deceased AF patient (as shown in Fig. 9E), the XGBoost-based 365-day mortality risk prediction model for AF patients was also accurate, with a predicted mortality probability of 62.0%, aligning with the patient’s survival outcome within 365 days of ICU admission. Higher age, higher disease severity scores (APS III), and elevated BUN/Cr all contributed to an increased long-term mortality risk for AF patients.
Discussion
In this large-scale retrospective cohort study based on the MIMIC-IV database, we investigated the impact of the BUN/Cr on the 28-day and 365-day all-cause mortality of critically ill patients with atrial fibrillation and established a predictive model to assess the mortality risk of these patients. The main findings are as follows: (i) A non-linear, U-shaped relationship exists between BUN/Cr and the mortality risk at 28 and 365 days for atrial fibrillation patients, with both low and high BUN/Cr levels increasing the risk; (ii) In our analysis, BUN/Cr levels of 16.49 and 16.67 mg/dl, respectively, serve as threshold turning points for short-term and long-term mortality risk in atrial fibrillation patients, which can assist clinicians in better monitoring patients; (iii) BUN/Cr significantly influences the XGBoost model, indicating its potential for machine learning applications in predicting all-cause mortality among ICU patients with atrial fibrillation. To sum up, BUN/Cr is crucial for assessing risk in critically ill patients with atrial fibrillation, and healthcare providers should monitor changes in BUN/Cr levels in these patients.
The occurrence and progression of AF were closely associated with adverse prognosis, particularly increased mortality. AF increased the likelihood of cardiovascular incidents and is linked to higher death rates. It often coexisted with heart failure, with both conditions affecting each other. In individuals with HF, the occurrence of AF was associated with increased mortality and poorer clinical outcomes16. This connection was especially evident in patients with heart failure with preserved ejection fraction (HFpEF), where atrial fibrillation was linked to worse clinical outcomes. Findings from sub-Saharan Africa revealed that AF was connected to an increase in mortality among HF patients in this region17. Research showed that the ten-year mortality rate for patients with AF was 46.8%, while it was 36.8% for those with a normal sinus rhythm18. A different study revealed that AF considerably elevated the risk of death in patients hospitalized with acute decompensated heart failure (ADHF)19. The impact of new-onset AF versus existing AF on in-hospital and 90-day mortality remained controversial and required further investigation20. Importantly, the prognostic implications of AF extended beyond HF patients. ICD therapy is also linked to the prognosis of AF, especially in those with heart failure and decreased ejection fraction21. Additionally, AF often coexisted with myocardial infarction (MI), leading to higher morbidity and necessitating comprehensive risk management strategies22.
The BUN/Cr ratio was a common clinical indicator used to evaluate kidney function, and its link to overall mortality has been thoroughly studied. Analysis using a multivariate Cox proportional hazards model showed that a higher BUN/Cr ratio was significantly linked to a greater risk of death from any cause (HR = 1.52, 95% CI: 1.21–1.91; P-Value < 0.001)23. In a similar study, a higher BUN/Cr ratio was linked to worse outcomes in AHF patients, as shown by higher mortality rates10. In various disease populations, the BUN/Cr has shown prognostic significance. For instance, in patients with acute myocardial infarction (AMI), a higher BUN/Cr was linked to greater in-hospital mortality24. In those with acute decompensated heart failure (ADHF), an elevated BUN/Cr was predictive of long-term mortality25. Additionally, in patients with acute ischemic stroke (AIS), a higher BUN/Cr was associated with increased in-hospital mortality26. The prognostic value of the BUN/Cr might vary across different patient populations.An elevated BUN/Cr ratio in individuals suffering from chronic heart failure and renal dysfunction was linked to a higher chance of in-hospital death9. Additionally, for patients experiencing acute coronary syndrome (ACS), the BUN/Cr ratio could give significant insights into potential complications and mortality risks27.
As a clinical indicator for evaluating renal hemodynamics and metabolic balance, the BUN/Cr might influence the pathological progression of atrial fibrillation through multiple mechanisms, thereby affecting prognosis. A higher BUN/Cr ratio is closely associated with a reduced glomerular filtration rate (GFR) and abnormal intrarenal blood flow distribution. These pathophysiological alterations may indirectly contribute to the development of atrial electrical and structural remodeling through mechanisms such as fluid retention, electrolyte disturbances (e.g., hypokalemia), and the accumulation of uremic toxins (e.g., indoxyl sulfate)28,29. Importantly, renal dysfunction has been shown to exacerbate atrial endothelial cell injury and interstitial fibrosis by activating the MAPK/NF-κB inflammatory pathway and mitochondrial oxidative stress response, processes that were particularly pronounced in AF patients29,30. Furthermore, an elevated BUN/Cr might indicate subclinical dehydration or a hypercatabolic state, which could increase the risk of a prothrombotic state through sympathetic nervous system activation and blood concentration effects31,32. Our findings align with these mechanisms, showing that higher BUN/Cr quintiles are associated with greater disease severity scores and comorbidities, including heart failure and kidney disease. The interaction among multiple organs may synergistically amplify the risk of mortality.
Serum creatinine and estimated glomerular filtration rate (eGFR) were traditional biomarkers that have been commonly used to assess kidney function. However, the BUN/Cr offered distinct advantages. Compared with creatinine alone, BUN/Cr could simultaneously reflect both glomerular and tubular injury as well as metabolic stress, which were particularly prevalent in critically ill patients33. Recent cohort studies have demonstrated that BUN/Cr has superior predictive ability for mortality in heart failure patients compared to eGFR34. Our study further shows that BUN/Cr is superior in predicting mortality in atrial fibrillation patients, likely due to its greater sensitivity in capturing early hemodynamic changes and metabolic disturbances. Additionally, a retrospective analysis highlighted the prognostic value of BUN/Cr in cardiogenic shock patients, further validating its potential for broad application in cardiovascular intensive care35.
The prognostic significance of the BUN/Cr ratio in patients with AF is underpinned by a distinct pathophysiological rationale, which is predominantly manifested in the AF - specific mechanisms of cardio - renal interaction. Firstly, the loss of effective atrial contraction and the subsequent reduction in cardiac output resulting from AF lead to a notable decline in renal blood flow36. Specifically, atrial dysfunction, as exemplified by a decreased left atrial ejection fraction, is independently associated with an elevated BUN/Cr ratio12. Moreover, Mendelian randomization has established a causal link between urea nitrogen levels and AF ([OR] = 1.505)37indicating that both conditions share the pathological processes of hemodynamic deterioration and prerenal azotemia. Secondly, AF triggers the release of arginine vasopressin (AVP) through the activation of the sympathetic nervous system and the renin - angiotensin - aldosterone system (RAAS). This results in the BUN/Cr ratio reflecting an increase in AVP - mediated tubular urea reabsorption rather than being solely indicative of renal injury. This mechanism is particularly prominent in the AF patient population because atrial stretch receptors can further stimulate AVP release12,38. Thirdly, AF - mediated left atrial remodeling, encompassing fibrosis and dilation, not only directly impairs atrial mechanical function but also, in conjunction with the BUN/Cr ratio, synergistically elevates the risk of mortality36,38. Shared pathological mechanisms such as inflammation and oxidative stress, as evidenced by the synergistic effect between the red blood cell distribution width - to - albumin ratio and BUN/Cr, exacerbate the vicious cycle of cardio - renal interactive injury39,40. In addition, patients with AF require long-term anticoagulation therapy, and renal insufficiency represents a key risk factor for both bleeding and thrombotic events. Accumulating evidence indicates that a reduction in estimated glomerular filtration rate (eGFR) is significantly correlated with the presence of fine fibrillation waves in AF, which serve as markers of atrial fibrosis14,41. This association suggests a pathophysiological interaction between the heart and kidneys that contributes to disease progression. In this context, the BUN/Cr ratio demonstrates enhanced sensitivity in detecting subclinical renal hypoperfusion among AF patients, thereby offering a valuable tool for early warning of anticoagulation-related complications42. In conclusion, the superiority of the BUN/Cr ratio in AF patients lies in its ability to comprehensively reflect AF - specific pathologies, thereby establishing it as a crucial biomarker for multi - organ damage in clinical settings.
This study achieved an innovative transformation in the risk assessment of atrial fibrillation patients by incorporating the serum BUN/Cr into a machine learning model. The experimental results demonstrated that the XGBoost-based prediction model exhibited superior performance, with an area under the curve (AUC) of 0.79, significantly outperforming traditional univariate analysis methods and existing scoring systems. This finding aligns with the current trend of applying machine learning techniques in AF research, particularly in integrating biomarkers related to multi-organ dysfunction, which enhances predictive accuracy43. SHAP value analysis revealed that the serum BUN/Cr was one of the most influential predictors in the model, further confirming its critical role in mortality risk assessment. This model not only enables real-time monitoring but also provides a reference for individualized treatment strategies, such as diuretic dose adjustment or renal replacement therapy, thereby advancing clinical translational research.
Limitations and future prospects
The MIMIC-IV database was the source of data for this study. While these databases provide extensive clinical information, they may contain data gaps or incomplete records, restricting the generalization of our results to settings outside the ICU and geographically diverse populations. This limitation may affect the comprehensive evaluation of the correlation between BUN/Cr and mortality. As an inherent characteristic of observational studies, this design is susceptible to potential biases, including inadequate control for confounding factors like inflammatory markers (for instance, C-reactive protein) and nutritional status. Additionally, the heterogeneity in laboratory test data collection can impact the interpretability of the results. BUN/Cr levels are influenced by multiple factors, including renal function, liver function, and protein intake, which may not have been measured under standardized conditions. Although the machine learning models demonstrated strong performance in predicting mortality, their generalization ability may be constrained by the quality and quantity of the training data.
Among the clinical indicators for evaluating renal function impairment, cystatin C has garnered significant attention due to its unique advantages. As an endogenous metabolite, cystatin C can sensitively reflect early changes in glomerular filtration rate (GFR) and offers notable specificity advantages over traditional markers. Unlike conventional biomarkers, cystatin C levels are not influenced by inflammatory responses or infectious diseases, thereby demonstrating superior diagnostic performance44. In monitoring acute kidney injury (AKI), neutrophil gelatinase-associated lipocalin (NGAL) has been clinically validated as a new biomarker. Research data indicate that NGAL achieves 81.5% specificity and 38.7% sensitivity in predicting postoperative AKI45. Notably, the pathological mechanisms underlying atrial fibrillation are closely intertwined with heart-kidney interactions. The vicious cycle formed by heart failure (HF) and chronic kidney disease (CKD) exacerbates patient conditions, leading to significantly increased mortality. These comorbidities not only elevate the risk of AF but also intensify clinical symptoms and adversely affect prognosis. Based on these insights, future research should focus on elucidating the collaborative diagnostic potential of traditional renal function indicators (like the BUN/Cr ratio) and novel biomarkers (such as cystatin C and NGAL). Additionally, efforts should be directed toward developing a multi-dimensional heart-kidney interaction biomarker assessment system to enhance the accuracy of early diagnosis and prognosis prediction for AF patients.
Conclusion
BUN/Cr is an important predictor of short-term and long-term mortality risk in AF patients. The XGBoost model combined with BUN/Cr showed high predictive performance and good generalization ability in predicting short-term mortality risk, and BUN/Cr ranked higher in the model. These results underscored the usefulness of BUN/Cr as an important biomarker for guiding clinical decisions and enhancing outcomes in patients with AF.
Data availability
The data were obtained from the MIMIC-IV database. Access requests can be made through the PhysioNet website: https://physionet.org/content/mimiciv/3.1/.
References
Lippi, G., Sanchis-Gomar, F. & Cervellin, G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int. J. Stroke. 16, 217–221 (2021).
Heeringa, J. et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur. Heart J. 27, 949–953 (2006).
Xiao, X. et al. Prevalence of atrial fibrillation in hospital encounters with End-Stage COPD on home oxygen: National trends in the united States. Chest 155, 918–927 (2019).
Shi, S. et al. Prevalence and risk of atrial fibrillation in china: A National cross-sectional epidemiological study. Lancet Reg. Health - West. Pac. 23, 100439 (2022).
Wong, C. X. et al. The burden of atrial fibrillation in the Asia-Pacific region. Nat. Rev. Cardiol. 21, 841–843 (2024).
Melidoro, P. et al. Enhancing stroke risk stratification in atrial fibrillation through non-Newtonian blood modelling and Gaussian process emulation. J. Physiol. https://doi.org/10.1113/JP287283 (2024).
Sposato, L. A. & Seiffge, D. J. Atrial fibrillation detected after stroke and increased risk of death. Neurology 96, 557–559 (2021).
Bonini, N. et al. Optimal medical therapy for heart failure and integrated care in patients with atrial fibrillation: A report from the ESC-EHRA EORP atrial fibrillation Long-Term general registry. J. Am. Heart Assoc. 14, e030499 (2025).
Wang, Y. et al. Blood Urea nitrogen to creatinine ratio and long-term survival in patients with chronic heart failure. Eur. J. Med. Res. 28, 343 (2023).
Zhu, X. et al. Blood Urea nitrogen to creatinine ratio and Long-Term mortality in patients with acute heart failure: A prospective cohort study and Meta-Analysis. Cardiorenal Med. 10, 415–428 (2020).
Matsue, Y. et al. Blood Urea nitrogen-to-creatinine ratio in the general population and in patients with acute heart failure. Heart 103, 407–413 (2017).
Tolomeo, P. et al. Independent prognostic importance of blood Urea nitrogen to creatinine ratio in heart failure. Eur. J. Heart Fail. 26, 245–256 (2024).
Ding, W. Y., Gupta, D., Wong, C. F. & Lip, G. Y. H. Pathophysiology of atrial fibrillation and chronic kidney disease. Cardiovasc. Res. 117, 1046–1059 (2021).
Lan, Z. et al. Predictive value of fine fibrillatory wave for declining eGFR in patients with persistent atrial fibrillation: Long-term follow-up study. Int. J. Cardiol. 417, 132521 (2024).
Shinohara, K. Renal denervation for hypertensive heart disease and atrial fibrillation. Hypertens. Res. 47, 2665–2670 (2024).
Liu, G., Long, M., Hu, X., Hu, C. H. & Du, Z. M. Meta-Analysis of atrial fibrillation and outcomes in patients with heart failure and preserved ejection fraction. Heart Lung Circ. 30, 698–706 (2021).
Chen, Y. et al. Atrial fibrillation and mortality in outpatients with heart failure in tanzania: a prospective cohort study. BMJ Open. 12, e058200 (2022).
Laenens, D. et al. The impact of atrial fibrillation on prognosis in aortic stenosis. Eur. Heart J. - Qual. Care Clin. Outcomes. 9, 778–784 (2023).
Clavel-Ruipérez, F. G. et al. Mortality and atrial fibrillation in the FIACA study: evidence of a differential effect according to admission diagnosis. Rev. Esp. Cardiol. (Engl Ed). 71, 155–161 (2018).
Qian, J., Kuang, L., Chen, F., Liu, X. & Che, L. Prognosis and management of new-onset atrial fibrillation in critically ill patients. BMC Cardiovasc. Disord. 21, 231 (2021).
Naka, K. K. et al. Association between atrial fibrillation and patient-important outcomes in heart failure patients with implantable cardioverter-defibrillators: a systematic review and meta-analysis. Eur. Heart J. - Qual. Care Clin. Outcomes. 5, 96–104 (2019).
Sadat, B., Taii, A., Sabayon, H., Narayanan, C. A. & M. & Atrial fibrillation complicating acute myocardial infarction: prevalence, impact, and management considerations. Curr. Cardiol. Rep. 26, 313–323 (2024).
Zhen, Z. et al. Prognostic significance of blood Urea nitrogen/creatinine ratio in chronic HFpEF. Eur. J. Clin. Invest. 52, e13761 (2022).
Huang, S. et al. Association between the blood Urea nitrogen to creatinine ratio and in–hospital mortality among patients with acute myocardial infarction: A retrospective cohort study. Exp. Ther. Med. 25, 36 (2022).
Murata, A. et al. Relationship between blood Urea nitrogen-to-creatinine ratio at hospital admission and long-term mortality in patients with acute decompensated heart failure. Heart Vessels. 33, 877–885 (2018).
Li, B. et al. The association of blood Urea nitrogen-to-creatinine ratio and in-hospital mortality in acute ischemic stroke patients with atrial fibrillation: data from the MIMIC-IV database. Front. Neurol. 15, 1331626 (2024).
Adam, A. M. et al. Efficacy of serum blood Urea nitrogen, creatinine and electrolytes in the diagnosis and mortality risk assessment of patients with acute coronary syndrome. Indian Heart J. 70, 353–359 (2018).
Chen, T. K., Knicely, D. H. & Grams, M. E. Chronic kidney disease diagnosis and management: A review. JAMA 322, 1294 (2019).
Yamagami, F., Tajiri, K., Yumino, D. & Ieda, M. Uremic toxins and atrial fibrillation: mechanisms and therapeutic implications. Toxins 11, 597 (2019).
Wallentin, L. et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Circulation 130, 1847–1858 (2014).
Bae, S. J., Lee, S. H., Yun, S. J. & Kim, K. Comparison of IVC diameter ratio, bun/creatinine ratio and bun/albumin ratio for risk prediction in emergency department patients. Am. J. Emerg. Med. 47, 198–204 (2021).
Thongprayoon, C., Cheungpasitporn, W. & Kashani, K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J. Thorac. Dis. 8, E305–E311 (2016).
Li, H. et al. Association of bun/cr ratio-based dehydration status with infarct volumes and stroke severity in acute ischemic stroke. Clin. Neurol. Neurosurg. 229, 107741 (2023).
Sakr, A. R. M., Gomaa, G. F. E., Wasif, S. M. E. & Eladawy, A. H. H. The prognostic role of urea-to-creatinine ratio in patients with acute heart failure syndrome: a case-control study. Egypt. Heart J. 75, 78 (2023).
Sun, D., Wei, C. & Li, Z. Blood Urea nitrogen to creatinine ratio is associated with in-hospital mortality among critically ill patients with cardiogenic shock. BMC Cardiovasc. Disord. 22, 258 (2022).
Yano, M. et al. Clinical impact of blood Urea nitrogen, regardless of renal function, in heart failure with preserved ejection fraction. Int. J. Cardiol. 363, 94–101 (2022).
Zhou, X. et al. Causal links between renal function and cardiac structure, function, and disease risk. Glob Heart. 19, 83 (2024).
Nakano, Y. et al. Predictors of hypotension after angiotensin Receptor-Neprilysin inhibitor administration in patients with heart failure. Int. Heart J. 65, 658–666 (2024).
Pan, L. Y. & Song, J. Association of red cell distribution width/albumin ratio and in hospital mortality in patients with atrial fibrillation base on medical information Mart for intensive care IV database. BMC Cardiovasc. Disord. 24, 174 (2024).
Fu, Y., Wei, X., Xu, C. & Guifu Wu. Independent effects of the glucose-to-glycated hemoglobin ratio on mortality in critically ill patients with atrial fibrillation. Diabetol. Metab. Syndr. 16, 171 (2024).
Shantsila, A., Lip, G. Y. H. & Lane, D. A. Relationship between systolic blood pressure and renal function on clinical outcomes in patients with atrial fibrillation: a report from the prospective AF-GEN-UK registry. J. Hypertens. 42, 2148–2154 (2024).
Malagutte, K. N. D. S. et al. Quality of oral anticoagulation in atrial fibrillation patients at a tertiary hospital in Brazil. Arq. Bras. Cardiol. 119, 363–369 (2022).
Wu, J., Lu, A. D., Zhang, L. P., Zuo, Y. X. & Jia, Y. P. [Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia]. Zhonghua Xue Ye Xue Za Zhi. 40, 52–57 (2019).
Sheikh, M. S. & Kashani, K. B. Beyond creatinine: New methods to measure renal function?. Eur. J. Intern. Med. https://doi.org/10.1016/j.ejim.2025.01.015 (2025).
Perry, T. E. et al. Plasma neutrophil gelatinase-associated Lipocalin and acute postoperative kidney injury in adult cardiac surgical patients. Anesth. Analg. 110, 1541–1547 (2010).
Funding
This research was funded by the Jiangsu Hospital Association’s Hospital Management Innovation Research Program (JSYGY-3-2024-123), the Wuxi Association for Science and Technology’s Key Soft Science Project (KX-24-C039), and the Wuxi Municipal Health Commission’s Science and Technology Achievements and Appropriate Technology Promotion Project (T202325).
Author information
Authors and Affiliations
Contributions
SY: Conceptualization, Writing-original draft. SZ: Conceptualization, Writing-review & editing. GZhu: Writing-review & editing. JLiu: Data curation. SWu: Formal analysis. HFu: Supervision, Methodology. MZhu: Supervision, Project administration, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Ethics approval and consent to participate
This study was carried out in full compliance with the principles of the Helsinki Declaration. The creation of the database was approved by the Massachusetts Institute of Technology, and informed consent was obtained for the original data collection. As a result, ethical approval and informed consent were not necessary for this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, S., Zhang, S., Zhu, G. et al. Development and validation of a machine learning model integrating BUN/Cr ratio for mortality prediction in critically ill atrial fibrillation patients. Sci Rep 15, 35157 (2025). https://doi.org/10.1038/s41598-025-19207-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19207-z