Abstract
Mechanical stress is an important factor that induces intervertebral disc degeneration (IVDD). However, the established in vivo models are inadequate for simulating the natural progression of disc degradation induced by mechanical forces in daily life. This study aimed to explore an improved feeding approach to compensate for the inherent deficiencies of the traditional bipedal rat IVDD model. Bipedal rats in the experimental group (M group) were placed into cages specifically designed to induce them to walk upright following constantly sliding food to increase the intradiscal pressure on their lumbar spine. Traditional bipedal rats (T group) and intact homochromous rats (C group) were fed simultaneously. We then analyzed the outcome using general indicators (weight gain, ΔW), bipedal behavior (upright posture time) assessment, magnetic resonance imaging (MRI), and determination of disc histological grades from morphological observations and histological analyses (H&E and safranin O staining and immunohistochemical staining). Rats in the M group adapted to the upright posture more quickly and maintained their spines standing longer than those in the T group did (P < 0.05). The discs of rats in the M group exhibited a progressive decline in both the MRI index and signal intensity at 3 months, which was aggravated through 6 months (P < 0.05). Histological analyses with H&E and safranin O green staining of the discs of rats at 6 months revealed that IVDD in the M group was more severe than that in the C and T groups (P < 0.05). The upregulated expression of collagen I and typical inflammatory cytokines (COX-2, iNOS and TNF-α) was detected in the IVD tissues of the rats in the M group (P < 0.05). The present study provides a novel and appropriate approach for establishing a cumulative mechanical stress-triggered IVDD model.
Similar content being viewed by others
Introduction
Intervertebral disc degeneration (IVDD) is a major cause of chronic low back pain, and most epidemiological studies have suggested that excessive loading plays an important role in the progression of IVDD, which is more advanced in highly physically active populations1. As the precise pathogenesis of IVDD remains unknown, establishing animal models simulating this condition is essential for understanding disease progression and for the development of therapies aimed at early intervention. Previous studies have demonstrated that many experimental animals, including compression- or instability‑induced models, mimic mechanical stress applied to the spine2.
According to previous studies, great variations in intradiscal pressure (IDP)3, one of the determinants of axial spinal load, have been found in sheep during various daily activities. When the sheep stood up from a lying position, they detected 5- to sixfold greater IDP (approximately 3.73–4.78 MPa) than the value recorded at static standing (0.70–0.73 MPa)4. This finding indicated that IDP was largely affected by the position of the animal, which is considered an important element that induces degeneration of the lumbar intervertebral disc (IVD) to some extent.
Bipedal mouse and rat models were created through bilateral mid-humeral surgical and tail amputations by elevating the feeding trough and forcing them to adopt a bipedal standing posture to simulate lumbar IVDD caused by increased stress5. Nonetheless, subsequent studies revealed that bipedal rats failed to maintain a consistent upright posture for longer durations than did their quadrupedal peers, which challenged the effectiveness of this model6. Although the surgical approach for bipedal model construction was explored in 20067 and has been used to induce an upright posture in recent years8,9, it seems difficult for rodents to abandon the congenital habit of quadrupeds after amputation of forelimbs; instead, they usually support their weight above the waist with the remaining stump of the shoulder joints in both walking and resting stances (Fig. S1A).
In the present study, we modified a novel modeling approach by training forelimb-amputated rats to perform long-term bipedal walking with a special height-adjustable device that can keep water and food constantly moving. Thus, this model induced an overdosed spinal loading pattern that could vividly mimic the cumulative strain of occupational lumbar loading in daily life.
Methods
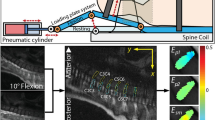
Forty-five 4-week-old Sprague‒Dawley (SD) rats purchased from the Experimental Animal Center of Qingdao University (Qingdao, China) were randomly divided into three groups: control (C group), traditional model (T group), and modified model (M group), with 15 rats in each group according to previous research8. Animals in the two model groups underwent amputation surgery, as previously described10, and were then transferred into two types of custom-made cages. Both were furnished with a food bucket and water bottle that could be freely height adjusted according to the length of the rats; however, the difference was that the device in the M group was connected to a stepping motor driver that allowed it to move laterally continuously at different speeds (Fig. 1), expecting the rats to walk with their lower limbs following the food. Thus, the rats were forced to maintain an upright position. The rats in the C group were housed in ordinary cages and received no treatment. All animals were housed in an environmentally controlled room with a 12‑h light cycle and a 20–26 °C temperature range. We ensured all the procedures adhered to the rules of 3R principle to treat animals kindly and reduce suffering and mortality rates. Animals occurring infection of incisional wound should be excluded during the experiment.
The schematic of this device is composed of a feeding bottom cage (thick black line), height adjustment box (thin red line), rods (blue cylinder), stepping motor (green circle), sliding rail (thin green line) and blocks (green rectangle), leading screw (thick green line), and trough (yellow line). Drawn by Meng Kong.
General indicators and bipedal behavior assessment
The time after surgery was used as the origin of the observation time for all rats, and the weight (W0) of the rats in each group at this time was recorded. To reduce unnecessary interference with rat habits caused by repeated operations, the body weights of the rats were monitored and recorded (Wx) once a month. The weight gain (ΔW) of the rats was used as the final monitoring index (Wx-W0).
An infrared camera monitoring system was used to determine whether the rats in the M group spent more time on their hindlimbs than did those in the other two groups in terms of diurnal activity. The percentages of time spent in the bipedal posture among rats in the three groups during the treatment sessions were then compared. The measurements were performed as follows: At selected time periods (baseline and 2 weeks and 1, 3, and 6 months thereafter), the video recording software was played back for the previous 24 h. One image of fixed time screenshots was obtained, and the upright posture of the rats was then determined (the angle between the long axis of the body and the ground ≥ 45°) every 4 h, and different scores were assigned according to the judgment criteria: if the rats were seen in an upright position, then this observation time accumulated one point; if they were in a recumbent position or failed to meet the upright standard, the score was recorded as 0 points. For example, one point means that this entire 4-h observation period was defined as an upright stance. The sum of the scores for all observation times was roughly taken as the measurement via tendency analysis, which assesses the daily upright time.
Magnetic resonance imaging (MRI)
Prior to 3 and 6 months after treatment, five rats from each group were randomly selected and examined with a same 3.0 Tesla (T) 3.0 T Varian MR scanner (MAGNETOM Skyra; Siemens AG) equipped with small animal-specific coils after intraperitoneal anesthesia with sodium pentobarbital (100 mg/kg). Representative T2-weighted compression lipid and midsagittal (referring to the spinous process as possible) plane images (Water: SAG IDEAL fat‑suppression sequence; repletion time, 3000 ms; echo time, 80 ms; field of view, 150 × 150 mm; slice thickness, 1.2 mm) of the lumbar spinal areas of the rats were obtained. MRI images of the lumbar 3/4, 4/5, and 5/6 segments were selected, and each disc was graded I‒V according to the Pfirrmann grading of spinal degeneration11. Furthermore, quantitative analysis of the images at the three previously selected levels was conducted using the polygon tool in ImageJ software (version 1.52, National Institutes of Health, USA), as shown in Fig. 2. The region of interest (ROI) around the nucleus pulposus (NP) of each disc was outlined on a screen with a graphic cursor, and then the area and average signal intensity (gray value) of this ROI were computed automatically. The MRI index, product of the NP area, and average signal intensity were calculated to serve as a complementary assessment method for IVDD changes. Finally, the lumbar 3/4, 4/5, and 5/6 segments of the rats were selected and read by two senior radiologists to minimize data errors induced by IVD heterogeneity. The mean values of the MRI indices were subsequently determined.
Sagittal T2 fat suppression weighted sequence (T2-STIR) of the median spine image demonstrating measurement of the NP area and signal intensity by creating an ROI with ImageJ software. The measurement parameters are shown at the upper right corner. The MRI index was defined as the product of the NP area and signal intensity. The mean MRI indices of L3/4, L4/5, and L5/6 were subsequently calculated, as shown at the lower right corner. Abbreviations: ROI, region of interest; NP, nucleus pulposus.
Histologic assessment
All the remaining mice in each group were sacrificed after MRI examination (6 months post-treatment), and the L4/5 IVD, along with the adjacent vertebrae dissected from five rats in each group, were harvested, fixed, decalcified, dehydrated, and paraffin embedded to produce coronal and cross-sectional continuous 4-mm sections. After deparaffinization and hydration, hematoxylin and eosin (H&E) and safranin O green staining were performed, and the sections were analyzed qualitatively to observe the extent of IVDD under a panoramic scanning microscope (magnification, × 20; Panoramic DESK, P‑MIDI, P250, P1000; 3DHISTECH, Ltd.) using CaseViewer software (version 2.3; 3DHISTECH, Ltd.). A modified grading system with rating scores ranging from 0 to 4, containing both the NP and annulus fibrosus (AF), was applied to assess the degree of IVDD according to H&E staining12. A modified Thompson grading scale for NP and AF was applied to assess the safranin O green staining results13. The scores for both AF and NP were summed for further analysis.
Immunohistochemical staining
Immunohistochemical staining was performed to examine the expression of cyclooxygenase 2 (COX-2) (1:200, Abcam, USA), inducible nitric oxide synthase (iNOS) (1:200, Abcam, USA), TNF-α (1:100) and collagen I (1:200, Abcam, USA) in the tissues. Following deparaffinization, each sample was incubated for 5 min at room temperature with 3% hydrogen peroxide to eliminate endogenous peroxidase activity. Subsequently, 20% goat serum (ZSGB-Bio, Beijing, China) was used to block nonspecific protein binding sites. This step was followed by incubation with the corresponding antibodies at 4˚C overnight. The following day, the tissue sections were incubated for 30 min with horseradish peroxidase‑conjugated goat anti‑rabbit IgG secondary antibodies (1:200, ZSGB-Bio, China) at 37 °C. The color was developed by incubation with the chromogen 3,3′-diaminobenzidine (DAB) tetrahydrochloride, followed by counterstaining with hematoxylin for 3 min at room temperature. An Olympus IX71 light microscope (Tokyo, Japan) was used to capture images of a total of five randomly selected regions in each immunohistochemical section, and the positive cells were counted with ImageJ software. NPCs in which the nucleus was stained and the cytoplasm exhibited brown staining were considered positive for the target proteins. All the histological analyses were conducted by two senior histopathologist to improve reliability of data.
Statistical analysis
All the experiments were performed in triplicate. The quantitative data are presented as the means ± standard errors. Statistical analysis was performed using GraphPad Prism 8.0.1 software (GraphPad Software, Inc.). Normality and homoscedasticity of data were all verified before analysing. Repeated-measures ANOVA was used to compare differences in standing time and body weight gain among different groups of rats and different time periods in the same group of rats. One-way ANOVA was used for comparisons of multiple sample means at each time point (including imaging and histologic scores), and two-by-two comparisons between multiple data points were performed using the LSD or Tamhane’s method (not chi-square) based on homogeneity of variance and Bonferroni correction was applied prevents inflated type I error rates. P < 0.05 was considered to indicate a statistically significant difference. Correction P value was 0.05/3.
Results
Upright posture time increased in rats in the M group
All SD rats that underwent forelimb disarticulation surgery did not experience surgical complications such as hemorrhage or accidental death from anesthesia overdose during the perioperative period and began to adapt to eating without forelimb holding of food within 3 days after surgery. Since the rats in the C group spent most of their time in the prone position and no practical standing posture was defined, we deleted these data. According to the results of repeated variance analysis by ANOVA (Fig. 3A), there was no statistically significant difference in upright posture time points between the two groups of bipedal rats at 2 weeks, and the rats in the M group were observed to stand upright at more time points than those in the T group at 1 month (1.73 ± 0.44 vs. 2.40 ± 0.61 points, P = 0.002); the differences were more visible at 3 months (1.71 ± 0.59 vs. 2.53 ± 0.50 points, P = 0.0006) and 6 months (1.86 ± 0.52 vs. 2.67 ± 0.60 points, P < 0.0001). The rats in the T group continued to walk forward in a prostrate state until the end of the observation period, whereas those in the M group were able to follow slow-moving food and continue to walk upright without prostrate reptiles (Video S1) and could maintain an upright posture for a long period even when they were not fed or watered (Fig. S1B, Video S2).
Changes in upright posture time and body weight variation among the three groups. (A) No statistically significant difference in upright posture time was found between the two groups of bipedal rats at 2 weeks, and the rats in the M group were observed to stand upright for a longer time than those in the T group at 1 month (P < 0.01); the differences were more apparent at 3 months (P < 0.001) and 6 months (P < 0.001). (B) All bipedal rats in both the M and T groups presented a lower ΔW than did those in the control group within 2 months, of which the M group presented a lower ΔW than did the T group. After 2 months, no statistically significant difference was identified among the three groups of rats in terms of ΔW. a, T versus M groups P > 0.05. ΔW, body weight gain since the initial observation. C, control; T, traditional; M, modified.
The weight gain (ΔW) of the rats in the three groups exhibited different trends (Fig. 3B). All bipedal rats in both the M and T groups presented a lower ΔW than did those in the control group within two months, whereas the ΔW of the M group was lower than that of the T group. Interestingly, after two months, no statistically significant difference was identified among the three groups of rats in terms of the ΔW from the original observation. One rat each in the traditional model and control groups died (due to incision infection and intestinal flatulence, respectively). These results suggest that the modified modeling method only affected the dietary habits of the rats in the early adaptation stage and that this effect disappeared after two months.
Modified modeling methods accelerate lumbar disc degeneration in rats
Manifestation on MRI
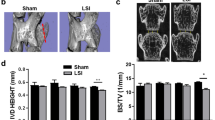
Typical images of the sagittal T2 fat-suppression weighted sequence (T2-STIR) of the median spine in the three groups of rats are shown in Fig. 4. The signal intensity of the NP in bipedal rats in the M group tended to diminish as early as 3 months and continued to decrease at 6 months (P = 0.0008), suggesting that IVDD was initiated earlier than 3 months and further aggravated within the 6-month experimental observation period. In rats in the T group, alterations in signal intensity were identified in the NP tissues from 6 months. The discs of rats in the M group exhibited a progressive decline in the MRI index (the product of the NP area and signal intensity from T2-STIR midsagittal plane images) initiated at 3 months and aggravated through 6 months, which was more acute than those of rats in the T group (initially at 6 months) and C group (no decline). A comparison of the Pfirrmann grades revealed that the rats in the T group presented only mild degeneration, whereas no significant signs of degeneration were detected in the C group at any of the observation points.
(A) Representative serial MR images of the lumbar spine in rats in the control, traditional and modified model groups, showing T2-STIR and midsagittal plane images obtained before and 3 and 6 months after the initiation of modeling. Note the progressive decrease in the NP area and signal intensity of the discs in the modified model group over the 6-month period. In contrast, MRI performance of the NP remained relatively intact in the discs of the control group over the same period, and decreased grayscale values were identified to a lesser extent in the traditional model group. (B) Trends in the Pfirrmann grade and MRI indices across the 3 groups (n ≥ 4). All the data are expressed as the means ± SDs. ***P < 0.001 a, c: Control vs. traditional groups P > 0.05; b, d: Control vs. traditional groups P < 0.05. MRI, magnetic resonance imaging; NP, nucleus pulposus.
Morphological changes in the intervertebral disc
Histological analysis with H&E and safranin O green staining of IVDs from the three groups of rats at 6 months revealed that IVDD in the M group was more severe than that in the C and T groups (Figs. 5 and 6). In the C group, the boundaries between the NP tissue and AF were clear. In the NP tissue, the content of matrix proteoglycans was high, and these proteoglycans were clustered into large vacuolated notochordal cells (Fig. 5, red arrowhead). The parallel-laminated AF also contained regular lamellar-arranged collagen fibers (Fig. 6, yellow arrow) mixed with small round chondrocyte-like cells (Fig. 6, black arrow). In the T group, the lamellar structure of the AF decreased, with some chondrocyte-like cells (Fig. 5, black arrow) appearing at the junction of the AF and NP, and the areas of NP tissue and proteoglycans in the extracellular matrix were correspondingly reduced. The number of notochordal cells in the NP is also reduced. The quantitative scoring results revealed that the T group had higher scores than the C group did (Fig. 5, P = 0.009). In the M group, AF lost its laminar structure, became infolded, and was structurally disordered. Collagen fibers invaded the NP tissue, and the boundaries became indistinct. In the NP area, the number of NP cells and amount of extracellular matrix decreased, and the apparent proliferation of fibroblasts in the matrix increased. A significantly higher histological score indicated more pronounced disc degeneration in the M group than in the T group (Fig. 5; P < 0.0001).
Representative histologic sections (H&E staining) of discs (L4/5) from rats in the C, T and M groups. The NP-AF border is approximately indicated by the solid oval green line. (A) A clear boundary (green solid line) between the NP and AF and a wider area of the NP were found in the C group. (B) In the T group, the area of the normal NP (green solid line) was reduced because of the expanded AF and structurally confused boundary. (C) In the M group, the normal NP structure was absent, and the boundary between the NP and the AF was indistinct. Locally enlarged images of selected regions of interest in A, B, and C are displayed in detail. (D) The NP in the C group presented a mix of large, vacuolated (notochordal) cells (red arrow). The junctional zone of NP-AF (E) and the original NP region (F) in the T and M groups presented a decreased number of notochordal cells and were increasingly occupied by proliferating fibroblasts (yellow arrow) and chondrocyte-like cells (black arrow). The degree of NP fibrosis was more severe in the M group. Abbreviations: C, control; T, traditional; M, modified; NP, nucleus pulposus; AF, annulus fibrosus. **P < 0.01, ***P < 0.001. Scale bars = 500 µm.
Representative images of safranin O green staining of discs (L4/5) in each group. (A) In the C group, a clear demarcation between the NP and AF (green solid line) was observed, and the content of matrix proteoglycans (reddish orange staining) was high. (D) Regularly lamellar arranged collagen fibers (pink staining, yellow arrow) were also found in the AF. (B) In the T group, pink-stained collagen fibers penetrated the NP area and were confounded by proteoglycans in the extracellular matrix, areas of NP tissue reduced (green solid line). (E) Lamellar-arranged collagen fibers still partly existed in the AF. (C) In the M group, indistinct boundaries were observed between the NP and AF, and the NP was infiltrated by disorganized collagen fibers, resulting in lower proteoglycan levels and fewer NP cells. (F) The disordered AF lost its fibrous lamellar structure, and a mass of chondrocyte-like cells (black arrow) proliferated. Normal, moderately degenerated and severely degenerated discs. In the discs of the modified group, the histologic grades were significantly greater than those of the internal control discs and the T group. AF, annulus fibrosus; NP, nucleus pulposus. C, control; T, traditional; M, modified. NP, nucleus pulposus; AF, annulus fibrosus. **P < 0.01. Scale bars = 500 µm.
The appearance of IVDD was also evaluated using a macroscopic axial view of freshly dissected IVD samples (Fig. 7). In the C group, transparent and jelly like fresh NP tissue was visible in the central area of the disc, and a clear boundary between the AF and NP was observed. In the T group, the outer AF was still arranged in layers, whereas the inner NP tissue exhibited a reduction in area but was still gelatinous, with a mildly blurred boundary between them. In the M group, the gelatinous NP tissue in the core area almost completely disappeared and was replaced by yellowish and tough fibrous connective tissue, the structure of which was also severely disordered, and the boundary between the NP and AF could not be clearly defined.
Typical axial views of the macroscopic appearance of the discs (L4/5) in each group. The black arrows point at the clear boundary between the normal NP and AF in the control group, whereas the red arrow indicates the mildly blurred boundary of the NP and AF in the traditional model group. The yellow arrow points to the fibrotic NP in the modified model group. AF, annulus fibrosus; NP, nucleus pulposus.
Degenerative and inflammatory biomarkers in the extracellular matrix of different groups
An imbalance in the synthesis and catabolism of the extracellular matrix is a major feature of IVDD.
Immunohistochemical staining (Fig. 8) revealed that the T group expressed more collagen I (P < 0.0001) than did the C group. Compared with those in the other two groups, the expression of collagen I (P = 0.0002) was greater in the IVD tissues of rats in the M group. In the pathogenesis of IVDD, inflammatory cytokines penetrated and caused destruction. The expression levels of typical inflammatory cytokines (COX-2, iNOS and TNF-α) were significantly increased in the M group. These findings suggest that the modified modeling method resulted in more dramatic extracellular matrix remodeling and secondary inflammatory responses in the intradiscal environment of the rats.
Expression levels of collagen I (Col I), COX-2, iNOS and TNF-α in the discs (L4/5) of each group. (A) Immunohistochemical staining analysis revealed that compared with the other two methods, the modified model method increased inflammatory cytokine expression and the Col I composition in the extracellular matrix in the nucleus pulposus of bipedal rats. (B–E) Quantitative analysis of the positive areas of Col I, COX-2, iNOS and TNF-α in different groups based on the results of immunohistochemical staining; the values are the means ± SDs of at least 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars = 100 µm. Abbreviations: C, control; T, traditional; M, modified.
Discussion
Mechanical stress is considered the main cause of IVDD14. Previous studies have suggested that mechanical stress could initiate and regulate IVDD occurrence and progression through many downstream biological signals, controlling IVD matrix metabolism, or affecting the biological behavior of IVD cells15. However, the regulatory mechanisms underlying the relationship between mechanical stress and IVDD remain unknown. The establishment of an effective mechanical animal model could be useful for investigating the mechanical and biological signal transduction mechanisms in discs and the pathogenesis of IVDD and for exploring effective treatment methods16.
As mentioned previously, researchers have established numerous modeling approaches, such as evoking intervertebral instability and puncturing the AF. However, these models have drawbacks in certain applications17. For example, the most commonly used stab wound-induced model involves injuring the disc and creating a wound that triggers IVDD by a surgical procedure18. However, the pathophysiology of this type of injury-induced model involves a frustrated healing response rather than the progression of natural disc degradation seen in human daily life19. To date, there is no widely accepted standardized method for establishing an ideal animal model.
Previous loading studies have attempted to apply long-term bending loads to the rat tail20 or add longitudinal load pressure by forcing on the rod that penetrates the spine21. Although these models do not damage the structure of the lumbar disc or maintain the original biochemical environment of the lumbar disc, from a bionic perspective, this approach does not seem to be successful. In most cases, the pressure that human IVDs withstand can be categorized into external and internal parts; the former is defined as the axial pressure exerted by the weight of the human body above the plane of the IVD segment, whereas the latter is the high load shear force, extrusion force, and tensile force generated by the lumbar paravertebral muscles to maintain balance when maintaining an upright stance or performing various daily activities, such as turning around, bending over, and heavy lifting22. Thus, the IVDD process is complex.
Studies on the differences in disc systems induced by quadrupedal and bipedal walking are important for understanding human IVDD. In the late 1950s, bilateral mid-humeral surgical amputations were used to create bipedal mouse or rat models, aiming to induce an altered vertical posture as a compensatory mechanism for the absence of forelegs and increasing the spinal undertaking load, which was observed to accelerate NP degeneration and frequent NP herniation5. However, subsequent experiments demonstrated that bipedal rats do not assume a more erect position than quadrupedal brethren6. In this study, rats in the C group showed scarcely any upright stances or change in dietary habit, thus 6 months were not sufficient to observe degeneration in their discs from the perspective of species development, and we also found that the rats in the T group did not develop more habitual assumptions of upright stances than those in the C group did. They failed to avoid their biogenetic behavioral habits easily when forward marching: most of them moved in a “prostrate” state with the front chest and shoulders as supporting points, then arched the spine, moved forward with both hind limbs, and finally spent time in an upright position only while eating and drinking. Unexpectedly, an increased incidence of scoliotic deformities was more often identified, limiting the utility of bipedal rats as a reliable IVDD model. Although previous studies have reported extended standing times in bipedal rats compared with their quadruped counterparts7, we can conclude that this method has at least one undesirable success ratio.
The principal purpose of this study was to demonstrate a modified feeding approach with constant sliding food, which seems to be a more effective procedure than the traditional method for inducing IVDD in bipedal rats. In this experiment, tendency analysis in which total time points were collected was applied to roughly compare the frequencies of individual rats in different groups in the upright position. We found that bipedal rats in the M group were induced to stand upright and continuously adjust their body posture to maintain their body balance while chasing food. To ensure that the rats were properly fed, the stepping motor allowed for an adjustment in fan speed (4 mm/s for every one-way distance in the early time) based on the familiarity of the rats with the continuously moving food. In the early stages, the amount of food intake (not statistically analyzed) in the M group was lower than that in the other two groups, which could be indirectly illustrated by the increase in body weight, indicating that the rats experienced minor adverse effects (e.g., eating deprivation due to the difficulty in obtaining access to food) but recovered in the short term. The height of the horizontal sliding feeding apparatus was gradually raised on top of the cage according to the increasing body length of the rats. Surprisingly, juveniles adapted to this mobile eating pattern within only two months. Interestingly, we found that as a species with strong novelty-seeking behavior23, traditional rearing cages usually limit this instinctive characteristic, but rats raised in special no-cover and hollowed-out fence-designed cages avoided this constraint. Even during feeding intermission, they spent less time moving in the “prostrate” state, exhibited increased locomotion and exploration of surrounding novel objects, and moved further, faster, and more continuously in the upright posture than did the rats in the T group. In this way, the lumbar paravertebral muscles could generate high load shear forces, extrusion forces and tensile forces from different planes to the disc, which are the internal parts of pressure mentioned above. From the perspective of etiology, the overdosed spinal loading pattern adopted in the current study vividly mimics the cumulative strain of occupational lumbar loading in daily human life, which has been demonstrated to have a positive dose–response association with IVDD.
In vivo, the balance and stabilization of the matrix, such as catabolism and anabolism of proteoglycans, the major components of extracellular matrix (ECM) macromolecules in natural NP tissues, significantly affect the characteristics of nucleus pulposus cells (NPCs)24. Notochordal cells, which are representative NPCs in native discs, play a vital role in maintaining disc integrity. In human IVD, it was previously demonstrated that the loss of notochordal cells and the proliferation of chondrocyte-like cells are highly consistent with disc degeneration25. In this study, we confirmed a more severe decline in the number of notochordal cells and the accompanying disorder in the ECM content in the M group. The release in inflammatory cytokines such as TNF-α, COX-2 and iNOS plays a critical role in the pathogenesis of IVDD26, and TNF-α directly induces iNOS and COX-2, which are considered markers of severe inflammation27. These molecular changes lead to increased catabolic enzymes activity and mediating oxidative stress and cellular apoptosis in NP cells, then resulting in the degradation of ECM. The corresponding findings were further demonstrated by immunohistochemical staining, which revealed that these inflammatory cytokines and Col I were expressed to different degrees in all groups of rats, indicating remarkable disc degeneration in the M group. Based on the sensitivity of T2-STIR in identifying the transformation of water, collagen II, and proteoglycan contents, MRI is an effective and objective method for IVDD examination. The quantitative MRI data of the spine in this study further support the evidence of degeneration in the lumbar discs of the M group, as the grayscale values of the NP areas were more marked than those in the C and T groups were. In clinical practice, discogenic low back pain was often encountered caused by IVDD, with changes in the disc signal while no height loss and no significant nerve compression identified. The findings of cellular molecular alteration in this study would contribute to investigate the mechanisms of human IVDD, and most importantly, facilitate to therapeutic strategies exploration by targeting these biomarkers, such as anti-inflammatory drugs or cytokine inhibitors.
All the aforementioned results convincingly indicate that a novel, more biomechanics-compliant modeling approach for bipedal rats was successfully constructed to simulate the pathogenesis of spinal degeneration induced by overloading mechanical stress, which appears to be effective and has several advantages over other models. First, this method led to the extension of the upright bipedal posture time and more multidimensional spinal motion in rats than did the traditional bipedal rat model. Second, this method simulated a more biologically rational and combined polytype loading that the disc withstood compared with the axial loading model through an external compression device, which was designed to exert static and dynamic compressive loading on the tail or lumbar IVDs28. Third, compared with the needle puncture injury model29, which involves an invasive procedure and cannot mimic excessive loading that the intervertebral disc undergoes during degeneration, the present model is noninvasive to the IVD and avoids the disturbance caused by acute structural trauma to the repair and degenerative process of the IVD, although additional trauma has occurred. In addition, it was as short as 3 months that light degeneration of the IVD could be identified, and the modeling cycle consumption was no more than 6 months, which appears to be more efficient than the classic older rat model. Moreover, because of their lower cost, ease of handling, and faster healing compared with large animal models, rodents are often more effective for biological research. The model used in this study involves an invasive procedure on animals, but from the point of view of animal welfare, no increased mortality or alimentary deficiency was identified, which is in accordance with modern society. Many previous molecular studies have investigated the role of several signal transduction pathways in the progression of mechanical stress-induced IVDD. However, relevant in vivo studies targeting intradiscal disruption and corresponding intervention measures are still underway.
Limitations
This study had several limitations. In addition, despite the sufficient exhibition of different tissue structures using a 3.0 Tesla (T) MRI scanner, H&E an S–O green staining, and immunoblotting analyses, more specialized testing techniques, e.g., electron microscopy and a 7.0 Tesla (T) MRI scanner, might be more sufficient to detect certain structural features and transformation of various cell clusters, such as notochordal, fibroblast-like cells, chondrocyte-like cells, and chondrocytes, in degenerative IVDs. Furthermore, the model animals used here were 1‑month-old rats, which were immature in the musculoskeletal system and might not be completely imitative to the mechanical loading of mature musculoskeletal animals or adults. However, a complicated and confusing topic in this field is that the initiation of IVDD may also occur before musculoskeletal maturity. Moreover, to make the conclusions more reliable, increased sample size and extended testing period might be preferable. The intervertebral joint system of rats does not have the characteristics of excessive coronal to sagittal articular processes like that of humans, nor does it have the physiological curvature of the human spine, so these inherent species distinctions make it difficult to clearly determine hybrid factors between the effects of gravity and changes in spinal muscle groups. Finally, since the upright position of the rats in this model is neither quantified nor uniform, there is a lack of precise control of the mechanical loading in this model. Although this model can demonstrate the relationship between upright posture and IVDD, there is also no direct biomechanical measurement of the spinal load or intradiscal load; without loading quantification, establishing a dose–response relationship between mechanical loading and IVDD is more meaningful.
Conclusion
In conclusion, the present study provides a novel and appropriate approach for establishing a cumulative mechanical stress-triggered IVDD model and is expected to become a prospective approach for further exploration of IVDD in humans.
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Hemanta, D., Jiang, X. X., Feng, Z. Z., Chen, Z. X. & Cao, Y. W. Etiology for degenerative disc disease. Chin. Med. Sci. J. Chung-kuo i hsueh k’o hsueh tsa chih. 31(3), 185–191 (2016).
Alini, M., Diwan, A. D., Erwin, W. M., Little, C. B. & Melrose, J. An update on animal models of intervertebral disc degeneration and low back pain: Exploring the potential of artificial intelligence to improve research analysis and development of prospective therapeutics. Spine 6(1), e1230 (2023).
Guehring, T. et al. Intradiscal pressure measurements in normal discs, compressed discs and compressed discs treated with axial posterior disc distraction: An experimental study on the rabbit lumbar spine model. Eur Spine J. Offic. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 15(5), 597–604 (2006).
Reitmaier, S. et al. Preliminary investigations on intradiscal pressures during daily activities: an in vivo study using the merino sheep. PLoS ONE 8(7), e69610 (2013).
Goff, C. W. & Landmesser, W. Bipedal rats and mice; laboratory animals for orthopaedic research. J Bone Joint Surg. Am. Vol. 39(3), 616–622 (1957).
Bailey, A. S., Adler, F., Min Lai, S. & Asher, M. A. A comparison between bipedal and quadrupedal rats: do bipedal rats actually assume an upright posture?. Spine 26(14), E308-313 (2001).
Xiao, J. et al. An improved operation approach for bipedal rat model construction. Zhonghua Yi Xue Za Zhi 86(39), 2781–2785 (2006).
Liang, Q. Q. et al. Prolonged upright posture induces degenerative changes in intervertebral discs of rat cervical spine. Spine 36(1), E14-19 (2011).
Liang, X. et al. Effect of axial vertical vibration on degeneration of lumbar intervertebral discs in modified bipedal rats: An in-vivo study. Asian Pac J Trop Med 10(7), 714–717 (2017).
Higuchi, M., Abe, K. & Kaneda, K. Changes in the nucleus pulposus of the intervertebral disc in bipedal mice. A light and electron microscopic study. Clin. Orthopaed. Relat. Res. 175, 251–257 (1983).
Pfirrmann, C. W., Metzdorf, A., Zanetti, M., Hodler, J. & Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26(17), 1873–1878 (2001).
Yang, F., Leung, V. Y., Luk, K. D., Chan, D. & Cheung, K. M. Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J. Pathol. 218(1), 113–121 (2009).
Han, B. et al. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine 33(18), 1925–1934 (2008).
Paul, C. P. et al. Dynamic and static overloading induce early degenerative processes in caprine lumbar intervertebral discs. PLoS ONE 8(4), e62411 (2013).
Navone, S. E. et al. Inflammatory mediators and signalling pathways controlling intervertebral disc degeneration. Histol. Histopathol. 32(6), 523–542 (2017).
Lotz, J. C. Animal models of intervertebral disc degeneration: Lessons learned. Spine 29(23), 2742–2750 (2004).
Bai, X. et al. Noninvasive cumulative axial load may induce intervertebral disc degeneration-A potential rabbit model. Exp. Ther. Med. 13(4), 1438–1446 (2017).
Elliott, D. M. et al. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine 33(6), 588–596 (2008).
Xi, Y. et al. Minimally invasive induction of an early lumbar disc degeneration model in rhesus monkeys. Spine 38(10), E579-586 (2013).
Lindblom, K. Intervertebral-disc degeneration considered as a pressure atrophy. J. Bone Joint Surg. Am. Vol. 39(4), 933–945 (1957).
Yao, T. et al. A new animal model of lumbar disc degeneration in rabbits. Spine J. Offic. J. North Am. Spine Soc. 24(8), 1519–1526 (2024).
Inoue, N. & Espinoza Orías, A. A. Biomechanics of intervertebral disk degeneration. Ortho. Clin. North Am. 42(4), 487–499 (2011).
Zhang, W. T. Z. L. & Li, X. W. Relationship between novelty seeking phenotype and impulsive behavior in rats. Chin. J. Behav. Med. Brain Sci. 11(4), 321–323 (2012).
Wu, B., Meng, C., Wang, H., Jia, C. & Zhao, Y. Changes of proteoglycan and collagen II of the adjacent intervertebral disc in the cervical instability models. Biomed. Pharmacother. Biomed. Pharmacother. 84, 754–758 (2016).
Aguiar, D. J., Johnson, S. L. & Oegema, T. R. Notochordal cells interact with nucleus pulposus cells: Regulation of proteoglycan synthesis. Exp. Cell Res. 246(1), 129–137 (1999).
Ding, H. et al. Progranulin derived engineered protein Atsttrin suppresses TNF-α-mediated inflammation in intervertebral disc degenerative disease. Oncotarget 8(65), 109692–109702 (2017).
Shukla, S. & Gupta, S. Suppression of constitutive and tumor necrosis factor alpha-induced nuclear factor (NF)-kappaB activation and induction of apoptosis by apigenin in human prostate carcinoma PC-3 cells: correlation with down-regulation of NF-kappaB-responsive genes. Clin. Cancer Res. Offic. J. Am. Assoc. Cancer Res. 10(9), 3169–3178 (2004).
MacLean, J. J. et al. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine 28(10), 973–981 (2003).
Rousseau, M. A. et al. Stab incision for inducing intervertebral disc degeneration in the rat. Spine 32(1), 17–24 (2007).
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Special Development Projects of Shandong Geriatric Medical Association (LKJGG2021W086), Qingdao Science and Technology Benefit the People Demonstration Project (23–2-8-smjk-7-nsh), Key Technology Research and Industrialization Demonstration Project of Qingdao (23–1-4-xxgg-11-nsh), and Medical Health Science and Technology Project of Shandong Province (202304070263).
Author information
Authors and Affiliations
Contributions
Kong M and Gao CT performed the experiments, analyzed data and drafted the manuscript. Han S performed some of the experiments. Jin CH and Hao M confirm the authenticity of all the raw data. Zhao JD, Luan J and Lin Y contributed to the data analysis and interpretation. Kong M, Li Q and Ma XX performed the in vivo experiments, data collection and analysis. Li Q and Ma XX designed the study, supervised data analysis and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflicts of interest related to this work.
Ethics approval and state of animal rights
All animal procedures were performed in accordance with the regulations approved by the Animal Experiment Ethics Committee of Qingdao Municipal Hospital (Approval on March 2nd, 2023. Nos. 182 and CT2023; Address: No. 5, Middle Donghai Road, Qing’dao) and complied with the relevant guidelines of ARRIVE standards.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary Material 2.
Supplementary Material 3.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kong, M., Gao, C., Han, S. et al. Establishing an intervertebral disc degeneration model in bipedal rats via a modified feeding approach. Sci Rep 15, 35319 (2025). https://doi.org/10.1038/s41598-025-19330-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19330-x