Abstract
Cirrhosis is a healing response to persistent liver injury, and the widely used clinical indicators of cirrhosis severity are limited. The lactate/albumin ratio (LAR) can be used as a marker of early prognosis in critically ill patients. The aim of this study was to explore the clinical significance of LAR for predicting the prognosis of patients with cirrhosis and to provide new insights. Patients diagnosed with liver cirrhosis were extracted from the MIMIC- IV database. The cox regression models were used to analyze the association between the LAR and all-cause mortality in cirrhosis patients. We analyzed potential nonlinear relationships between LAR and outcome indicators using restricted cubic spline cures (RCS). The predictive power was investigated using receiver operating characteristic(ROC) analysis. Additionally, we calculated the incremental effect of the LAR index. The study included a total of 2402 patients with cirrhosis. According to the Kaplan-Meier curves and cox regression analysis indicated that the risk of 28-day, 90-day, 180-day, 1-year all-cause mortality was significantly higher in the highest quartile of LAR. The result of the multivariable RCS model showed the LAR index have a linear relationship (p for nonlinear = 0.115) with 28d all-cause mortality. The area under the ROC curve (AUC) of LAR (66.44% [95% CI 0.64–68.88%]) was superior to lactate (63.51% [95% CI 61.01–66.01%]) and albumin (64.46% [95% CI 62.01–66.92%]). The predictive ability of scoring tools for 28d mortality was significantly improved after considering either the LAR index using integrated discrimination improvement index (IDI). The LAR index can serve as a significant predictor for all-cause mortality in critically ill patients with liver cirrhosis.
Similar content being viewed by others
Introduction
Cirrhosis is the 11th most common cause of death in the world, with an annual death toll of 1 million1. Cirrhosis is a healing response to persistent hepatic injury and chronic progressive disease caused by multiple etiological factors, which is histologically characterized by diffuse hepatocellular degeneration and necrosis, abnormal regeneration of hepatocytes, intrahepatic neovascularization, proliferation of hepatic fibrotic tissues, and pseudolobule formation2. Necrotic hepatocytes release intracellular substances such as nucleic acids, intracellular proteins, adenosine triphosphate, or nucleic acid compounds, which can stimulate nonparenchymal cells by damage-associated molecular pattern (DAMP) to release pro-fibrotic factors and promote the development of liver fibrosis3,4,5. Patients with cirrhosis are at increased risk of significant morbidity and mortality, which may arise from complications of portal hypertension or hepatic decompensation6.
In recent years, new predictors of prognosis in patients with cirrhosis have emerged. The gold standard for the diagnosis is liver biopsy, but it has certain limitations, such as invasive operation, high cost, and low acceptance by patients7. At present, the widely used clinical indicators for assessing the severity of cirrhosis are Child-Pugh classification and MELD score. The former is used to grade the severity of liver disease; the latter was initially used to priorities the waiting list for liver transplantation, and has subsequently been used to assess the prognosis of end-stage liver disease. Several studies have attempted to compare the utility of these two scores as prognostic markers in patients with cirrhosis, but all have been limited8,9. Therefore, there is an urgent need to propose new indicators to assess the prognosis of patients with cirrhosis.
Albumin is synthesized by the liver and is closely related to liver function. Cirrhosis is a consequence of long-term chronic damage to the liver, which reduces the rate and amount of albumin synthesis. Albumin administration has been shown to be associated with improved prognosis and complications in patients with cirrhosis10. Lactate is a product of anaerobic metabolism and is metabolized predominantly in the liver, and is an indicator of the severity of organ hypoperfusion and tissue hypoxia. Systemic inflammation and oxidative stress in cirrhotic patients may lead to mitochondrial dysfunction and reduced ATP synthesis, ultimately leading to the accumulation of lactate as an end product of anaerobic glycolysis11,12. Therefore, it seems safe to speculate that patients with cirrhosis will experience a rise in lactate and a fall in albumin due to the abnormal liver function, which could lead to an increased lactate to albumin ratio. The aim of this study was to explore the clinical significance of this simple, objective indicator for predicting the prognosis of patients with cirrhosis and to provide new insights.
Materials and methods
Database introduction
All data in this study are from The Medical Information Mart for Intensive Care IV (MIMIC-IV), a large and freely accessible database, which contains various information of ICU patients at Beth Israel Deaconess Medical Center (Boston, Massachusetts, USA) from 2008 to 2019, including baseline characteristics, vital signs, laboratory tests, medication treatment, surgical operations and follow-up survival status. The medical information is privatized and stored in MIMIC-IV, therefore we are not requested consent and ethical approval of patient. The first author (Ruoxi Zhang) completed the Collaborative Institutional Training Initative (CITI) and was qualified to use the database (ID: 13230609).
Study population
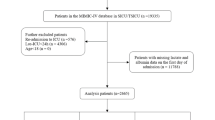
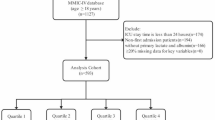
Patients with liver cirrhosis admitted to the ICU for their first hospitalization were included in the study. A total of 5929 patients diagnosed with liver cirrhosis were extracted, according to International Classification of Disease, 9th Revision and 10th Revision. The ICD 9 and 10 code included 5712, 5715, 5716, K703, K7030, K7031, K717, K74, K743, K744, K745, K746, K7460, K7469, P7881. The exclusion criteria were as follows: (1) patients stayed in ICU less than 24 h; (2) patients admitted multiple times for liver cirrhosis, for whom only the first admission data were extracted; (3) patients missing lactate and albumin data; (4) patients with malignant cancer, metastatic solid tumor.
Data extraction
The tool of data extraction was PostgreSQL software (v13.7.1) through running Structured Query Language (SQL). LAR was calculated by the formula: lactate(mmol/L)/albumin(g/L), which was identified as the primary variable. Blood lactate and serum albumin concentration were recorded after first admission in order to avoid the effect of subsequent treatment. A wealth of potential confounders about each patients at admission were extracted, covering demographic information (age, gender, race), vital sign (systolic blood pressure, diastolic blood pressure, BMI, heart rate, respiratory rate), medication and treatment (fibrates, statins, vasopressin, continuous renal replacement therapy, mechanical ventilation), comorbidities (acute kidney injury, atrial fibrillation, diabetes, heart failure, hypertension, obesity, respiratory failure, sepsis, viral hepatitis, nonalcoholic_steatohepatitis, alcoholic cirrhosis, autoimmune liver disease, acute and subacute hepatic failure, chronic hepatic failure, esophageal varices, hepatic encephalopathy), laboratory data (albumin, anion gap, alanine aminotransferase, aspartate aminotransferase, total bilirubin, c-reactive protein, lactate, glucose, serum urea [BUN], creatinine, calcium, sodium, potassium, international normalized ratio[INR], prothrombin time [PT], hematocrit, hemoglobin, platelets, white blood cell [WBC], lymphocyte count, red blood cell [RBC], red cell distribution width [RDW], triglyceride [TG]), sequential organ failure (SOFA), acute physiology score Ⅲ (APS Ⅲ), oxford acute severity of illness score (OASIS) and simplified acute physiology score (SAPS Ⅱ). And we calculated MELD, ARPI, Fib-4, ALBI based on the extracted data. When laboratory data were measured several times in 24 h of admission, the first result was recorded. To minimize reverse causation bias, data collected after the outcome event was considered invalid. Laboratory indications with missing data more than 20% were excluded and multiple interpolation was adopted to fill with missing data less than 20%.
Outcomes
The primary outcome was death from any cause within 28 days, 90 days, 180 days and 1 year of admission. The 28-day all-cause mortality rate was defined as the ratio of the total number of all-cause deaths during the 28-day hospitalization period to the average population of that population during the same period.
Statistical analysis
The Shapiro-Wilk test was used to assess whether the normal distribution is satisfied. Continuous variables were expressed as mean (standard deviation (SD)) or median (interquartile range (IQR)) and compared using Student’s t-test or nonparametric test as appropriate. Categorical variables were expressed in frequency and percentage (%) and tested the differences between groups using Pearson’s chi-square test or Fisher’s exact test. Univariate Cox analysis and multivariate Cox regression models were used to assess the relationship between LAR and 28 days, 90 days, 180 days, 1year mortality and estimated hazard ratios (HRs) with their 95% confidence intervals (95% CIs) and adjusted for several confounding variables respectively. The variables included in multivariate Cox regression models were clinically relevant and carefully chosen. Model 1 was unadjusted and variables including age, race and gender were adjusted in model 2. Model 3 was adjusted for Admission_age, Gender, Race, HR, RR, Alt, Anion_gap, Ast, Bun, Calcium, Creatinine, Plt, Glucose, Hemoglobin, INR, Potassium, RDW, Sodium, TB, WBC, Viral hepatitis, Acute and subacute hepatic failure, AKI, Alcoholic cirrhosis, Atrial fibrillation, Autoimmune liver disease, Chronic hepatic failure, CRRT, Diabetes, Esophageal varices blooding, Fibrates, Heart failure, Hepatic encephalopathy, Hypertension, nonalcoholic steatohepatitis, Obesity, Respiratory failure, Sepsis, Statins, Vasopressin, Mechanical ventilation. In order to check the incidence of outcome events according to different levels of LAR, we chose LAR segment as a categorical variable according to the quartile in the model (the lowest quartile of LAR values was used as the reference group). To prevent multicollinearity, variables with variance inflation factors greater than 5 were excluded from the model. The Kaplan–Meier method was used to tested the differences of survival data by the log-rank test. Restricted cubic splines (RCS) were adopted to reflect potential nonlinear correlations between LAR and outcomes. The predictive ability of LAR was evaluated through calculating the area under the ROC (AUC). Moreover, LAR cutoffs were established through optimal statistical threshold and Youden index was calculated by Sensitivity + Specificity-1. Integrated discrimination improvement(IDI) and net reclassification improvement(NRI) were calculated separately to evaluate the predictive ability and clinical value of the scoring tools added LAR index. Decision curve analysis (DCA) was performed assess the clinical utility improvement of LAR. Additionally, subgroup analysis was performed based on gender, race, AKI, heart failure, respiratory failure, sepsis, alcoholic cirrhosis, esophageal varices blooding, hepatic encephalopathy, acute and subacute hepatic failure. All statistical analysis were processed using the R language (R version 4.2.2) and P < 0.05 was considered statistically significant. All the original data have been uploaded as Supplementary files.
Result
Baseline demographic and clinical characteristics
The baseline characteristics of patients grouped by the quartiles of the LAR index were showed in Table 1. In the study, a total of 5929 patients with liver cirrhosis were enrolled. Detailed information about selection process is presented in Fig. 1. There were 883(36.8%) women and 1519(63.2%) men in the cohort, which the median age was 58.34 years. The study included multiple ethnicities, with white people accounting for the highest proportion, black people and Asians accounting for 8.1% and 1.2%, respectively. Based on the quartiles of the LAR index (Q1: 0.012–0.043, Q2: 0.043–0.064, Q3: 0.064–0.100, Q4: 0.100-1.838), patients were described in 4 groups (Table 1).
As the LAR index increased, patients were higher levels of HR, RR, AST, glucose, INR, lactate, PT, RDW, TB, WBC, lower level of albumin, calcium, Hematocrit, hemoglobin, PLT, RBC, higher prevalence of Atrial fibrillation, and higher usage rate of vasopressin, CRRT, mechanical ventilation. Meanwhile, we found that the mortality rate of liver cirrhosis patients in this study increased with longer follow-up time. 28d, 90d, 180d, 1year mortality were higher with the increasing LAR index. In other words, the mortality of 4 follow-up times was highest in Q4 group than Q1, Q2, Q3 group.
Relationship between LAR and 28 d, 90 d, 180 d, 1 year mortality
In order to access the effect of exposure variables on mortality, the Cox regression models were used and adjusted for several covariates. Model 1 was unadjusted. Model 2 was adjusted for age, race and gender. Model 3 was adjusted for admission_age, Gender, Race, HR, RR, Alt, Anion_gap, Ast, Bun, Calcium, Creatinine, Plt, Glucose, Hemoglobin, INR, Potassium, RDW, Sodium, TB, WBC, Viral hepatitis, Acute and subacute hepatic failure, AKI, Alcoholic cirrhosis, Atrial fibrillation, Autoimmune liver disease, Chronic hepatic failure, CRRT, Diabetes, Esophageal varices blooding, Fibrates, Heart failure, Hepatic encephalopathy, Hypertension, nonalcoholic steatohepatitis, Obesity, Respiratory failure, Sepsis, Statins, Vasopressin, Mechanical ventilation. The result of Cox regression model between LAR and 28d mortality showed patients with higher LAR (Q4: 0.1–1.838) have higher risk of mortality than those with low-value LAR (Q4: 0.012–0.043), in Model 1(HR [95% CI], 3.65 [2.869, 4.651]), Model 2(HR [95% CI], 3.693 [2.893, 4.741]) and Model 3(HR [95% CI], 2.078 [1.580, 2.733]). As the models were adjusted, the effect of LAR on 28d mortality gradually decreased. The same trend was seen in the analysis of 90 d, 180 d and 1 year mortality (Table 2).
Detection of nonlinear relationships
RCS curve analysis was preformed to access the potential nonlinear correlation between the LAR index and outcomes. According to the database result, there were 654 deaths out of 2402 patients (27.2%) at the 28d follow-up. The result of the multivariable RCS model showed the LAR index have a linear relationship (p for nonlinear = 0.115) with 28d all-cause mortality (Fig. 2). In addition, based on the quartiles of the LAR index, Kaplan–Meier survival curve showed a significant difference among various LAR index groups (Fig. 3).
The predictive ability and incremental effect of the LAR index
In evaluating the diagnostic performance of the LAR index, the maximum Youden index of 0.246 was identified at a cut-off value of 0.076, with a sensitivity of 57.34% and a specificity of 67.28%. It is important to note that this optimal cut-off value, derived from the Youden index in our cohort, is exploratory and specific to this dataset. External validation in independent, prospective populations is required to confirm its generalizability and clinical utility before it can be recommended for widespread clinical application. The ROC curves for the three indicators of LAR, lactate, albumin were plotted for predicting all-cause mortality within 28d of admission (Fig. 4). The area under the ROC curve (AUC) of LAR (66.44% [95% CI 0.64–68.88%]) was superior to lactate (63.51% [95% CI 61.01–66.01%]) and albumin (64.46% [95% CI 62.01–66.92%]). Moreover, we determined the optimal cut-off value for LAR to be 0.076, with sensitivity of 57.34% and specificity of 67.28% and Youden index of 0.246, indicating that LAR had a relatively good predictive ability for all-cause mortality in liver cirrhosis. Moreover, We observed that the predictive ability of LAR was superior to APRI, FIB-4, ALBI(AUC: 0.6644(0.64–0.6888) vs. 0.6126(0.5874–0.6379) vs. 0.6356(0.6108–0.6604) vs. 0.6438(0.6198–0.6678)). Altogether, the LAR index offers some value for predicting 28-day all-cause mortality in liver cirrhosis. In addition, we calculated the IDI and NRI of the scoring tools (APSIII, OASIS, SAPSII, MELD, SOFA) to analyze the impact of the LAR index on the predictive power of the scoring tools (Tables 3 and 4). The result demonstrated the predictive ability of the scoring tools (APSIII, OASIS, SAPSII, MELD) for 28 d mortality was significantly improved after considering either the LAR index according to the quartile classification (LAR[IQR]) or the numerical LAR index (LAR[numeric])(p < 0.05). Moreover, we drew clinical decision curves to assess the clinical utility improvement of LAR. The results showed the net clinical benefit of each scoring tool had an improvement after considering LAR (Fig. 5).
Subgroup analysis for LAR index on 28d all-cause mortality
Subgroup analysis were performed to explore the relationship between the 28d all-cause mortality and LAR in different conditions. Subgroup analysis were conducted for gender, race, AKI, heart failure, respiratory failure, sepsis, alcoholic cirrhosis, esophageal varices blooding, hepatic encephalopathy, acute and subacute hepatic failure. When stratified analysis were performed for AKI, respiratory failure, sepsis, alcoholic cirrhosis, hepatic encephalopathy, acute and subacute hepatic failure, the forest plots showed no significant interaction between LAR and most subgroups (p > 0.05). The results were showed in Fig. 6.
Discussion
The area under the ROC curve (AUC) for LAR (66.44%) was higher than that of lactate alone (63.51%) or albumin alone (64.46%), indicating its superior predictive performance for 28-day mortality. Furthermore, the incorporation of LAR into established scoring systems for liver cirrhosis and critically ill patients—such as APRI, FIB-4, ALBI, APSIII, OASIS, SAPSII, MELD, and SOFA—significantly enhanced their prognostic accuracy.
In recent years, it is clear that the pathophysiological background of liver cirrhosis is characterized by a systemic proinflammatory and pro-oxidant milieu13, which results in the development of multiorgan dysfunction by two paths. The first one is that triggers the release of pro-inflammatory mediators through the systemic spread of bacteria and/or bacterial products from the gut and danger-associated molecular patterns from the diseased liver through the activation of immune cells. The systemic proinflammatory is responsible for functional and structural changes in the albumin. The other one is mitochondrial oxidative phosphorylation dysfunction, which could lead to lactate metabolism disorder. Many researchers commit to develop indicators to predict the prognosis of patients with liver cirrhosis, including RDW14, CRP15,16, neutrophil to lymphocyte ratio(NLR)17,18, AST/PLT ratio(APRI)19, urokinase plasminogen activator receptor (uPAR)20, neutrophil gelatinase-associated lipocalin (NGAL)21, etc. The LAR has been employed as a novel predictor for critically ill patients22,23,24, with a notable capacity to predict mortality. Nevertheless, studies utilizing the LAR to forecast the prognosis of cirrhosis patients remain absent from the literature.
Lactate has been used to predict the prognosis of critically ill patients. The kinetics of lactate in patients with hepatic impairment diverge considerably from those observed in patients without hepatic impairment with regard to lactate metabolism25. It has been demonstrated that severe hepatic dysfunction has a significant detrimental impact on lactate clearance. Lactic acidosis in critically ill patients with cirrhosis is attributable to an increase in lactate production and a reduction in hepatic lactate disposal. This is due not only to tissue hypoxia, microcirculatory dysfunction, and increased glycolysis, but also to the underutilization of damaged mitochondrial oxidation26. While a healthy liver is capable of metabolizing approximately 70% of lactate, this capacity is impaired in chronic liver disease due to a reduction in functional hepatocyte mass27. Furthermore, a dysfunctional liver may even become a net lactate producer in critically ill patients.
Due to the persistent liver inflammatory state, serum albumin in cirrhosis patients undergoes structural and functional abnormalities that affect many of its properties, such as antioxidant, scavenging, immunomodulatory and endothelial protective functions. Consequently, the amount of circulating ‘effective’ albumin may be significantly reduced as a result of quantitative and qualitative changes13,28. Therefore, this explains the association between reduced albumin levels and poor prognosis. The systemic inflammatory response in patients with cirrhosis, including the production of inflammatory molecules, respiratory bursts, and processes such as cell migration and proliferation, requires large amounts of energy. In turn, states such as anorexia brought on by the inflammatory response reduce nutrient intake, which further promotes protein hydrolysis. In a metabolomics study of patients with liver failure, researchers found strong protein hydrolysis processes29.
In recent years, many researchers have reported the predictive value of LAR on different clinical settings. Cakir et al. found the LAR can be used to predict mortality in critically ill patients with sepsis30. Charipour et al. reported the LAR index is a prognostic marker in critically ill patients31. Our study showed that a high level of LAR index was strongly associated with 28-day all-cause mortality in critically ill patients with cirrhosis. This association held true even after potential confounders were considered. And all-cause mortality was linearly correlated with LAR index. A previous study on early prediction of mortality in patients with acute on chronic liver failure reported a significant correlation between LAR and in-hospital mortality (p < 0.001), which is similar to the present study24. This study also employed ROC curve analysis, which showed an area under the curve was 0.77. However, it should be noted that the study included patients with acute-on-chronic liver failure. In other words, the patients enrolled in this study exhibited a greater degree of illness severity than ours. Although LAR contributed to improved risk stratification and provided incremental prognostic value beyond conventional scoring systems, its AUC remained below 0.70, suggesting moderate discriminatory power. Thus, LAR may be best employed as a complementary, readily available biomarker rather than a stand-alone prognostic tool. In addition, LAR demonstrated a higher discriminative ability compared to other liver-specific indices including APRI, FIB-4, and ALBI, with AUC values of 0.6126(0.5874–0.6379) vs. 0.6356(0.6108–0.6604) vs. 0.6438(0.6198–0.6678), respectively. Moreover, the integration of LAR into established scoring systems (APSIII, OASIS, SAPSII, MELD, SOFA) significantly improved their predictive performance, as evidenced by IDI and NRI analyses (p < 0.05). Subgroup analyses revealed no significant interaction between LAR and 28-day all-cause mortality across most predefined subgroups. However, interactions were observed in subgroups defined by gender, race, heart failure, and esophageal variceal bleeding, though these may be influenced by limited sample sizes. Interestingly, among cirrhotic patients with sepsis, higher LAR quartiles (Q3 and Q4) were associated with significantly increased hazard ratios for mortality, whereas no such trend was observed in non-septic patients. This aligns with previous studies highlighting the strong predictive utility of LAR in septic critically ill patients30,32.
In summary, this study demonstrated the important role of LAR in predicting all-cause mortality of patients with liver cirrhosis. However, there still exists several limitations in our study. First of all, the results were lack of representation, because all of patients enrolled are from an American population. And it is necessitating large studied to improve statistical power in subgroup analysis. Secondly, our study reported a cutoff value (0.076) derived from the Youden index. However, it is important to note that this threshold was exploratory and cohort-specific. Therefore, external validation in independent populations is required prior to its implementation in clinical practice. Thirdly, as a study based on an electronic medical record database (MIMIC-Ⅳ database), it is inevitable that the database may not record some confounding factors. Therefore, the generalizability of the results requires further validation. Finally, since the study was single-center and retrospective, there is selection bias in the study design.
Conclusion
In conclusion, our study revealed that LAR index is a significant predictor of prognosis in critically ill patients with cirrhosis and has an approximately linear relationship with all-cause mortality. This will provide healthcare professionals with a simple, rapid and adjunction tool for early intervention for poor patient prognosis. LAR index still needs to be validated as a reliable biomarker for all-cause mortality in cirrhotic patients in large multicenter and prospective studies.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 396 (10258), 1204–1222. https://doi.org/10.1016/S0140-6736(20)30925-9 (2020).
Schuppan, D. & Afdhal, N. Liver cirrhosis. Lancet 371 (9615), 838–851. https://doi.org/10.1016/S0140-6736(08)60383-9 (2008).
Mueller, M. et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J. Hepatol. 62 (6), 1398–1404. https://doi.org/10.1016/j.jhep.2014.12.034 (2015).
Tsuchida, T. & Friedman, S. L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 14 (7), 397–411. https://doi.org/10.1038/nrgastro (2017).
Roehlen, N., Crouchet, E. & Baumert, T. F. Liver fibrosis: Mechanistic concepts and therapeutic perspectives. Cells 9 (4), 875. https://doi.org/10.3390/cells9040875 (2020).
D’Amico, G., Garcia-Tsao, G. & Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 44 (1), 217–231. https://doi.org/10.1016/j.jhep.2005.10.013 (2006).
European Association for the Study of the Liver. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis-2021 update. J. Hepatol. 75 (3), 659–689. https://doi.org/10.1016/j.jhep (2021).
Durand, F. & Valla, D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 28 (1), 110–122. https://doi.org/10.1055/s-2008-1040325 (2008).
European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 69 (2), 406–460. https://doi.org/10.1016/j.jhep.2018.03.024 (2018).
Wu, N. et al. Albumin, an interesting and functionally diverse protein, varies from native to effective. Mol. Med. Rep. 29 (2), 24. https://doi.org/10.3892/mmr.2023.13147 (2024).
Liang, J. et al. Liver metabolomics reveals potential mechanism of Jieduan-Niwan formula against acute-on-chronic liver failure(ACLF) by improving mitochondrial damage and TCA cycle. Chin. Med. 18 (1), 157. https://doi.org/10.1186/s13020-023-00858-x (2023).
Engelmann, C. et al. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J. Hepatol. 75 (Suppl 1), S49–S66. https://doi.org/10.1016/j.jhep.2021.01.002 (2021).
Bernardi, M. et al. Albumin in decompensated cirrhosis: New concepts and perspectives. Gut 69 (6), 1127–1138. https://doi.org/10.1136/gutjnl-2019-318843 (2020).
Turcato, G. et al. Red blood cell distribution width independently predicts 1-month mortality in acute decompensation of cirrhotic patients admitted to emergency department. Eur. J. Gastroenterol. Hepatol. 30 (1), 33–38. https://doi.org/10.1097/meg.0000000000000993 (2018).
Ho, K. M. & Lipman, J. An update on C-reactive protein for intensivists. Anaesth. Intensive Care. 37 (2), 234–241. https://doi.org/10.1177/0310057x0903700217 (2009).
Reinhart, K., Karzai, W. & Meisner, M. Procalcitonin as a marker of the systemic inflammatory response to infection. Intensive Care Med. 26 (9), 1193–1200. https://doi.org/10.1007/s001340000624 (2000).
Kalra, A. et al. Neutrophil-to-lymphocyte ratio correlates with proinflammatory neutrophils and predicts death in low model for end-stage liver disease patients with cirrhosis. Liver Transpl. 23 (2), 155–165. https://doi.org/10.1002/lt.24702 (2017).
Rice, J. et al. Neutrophil-to-lymphocyte ratio associates independently with mortality in hospitalized patients with cirrhosis. Clin. Gastroenterol. Hepatol. 16 (11), 1786–1791. https://doi.org/10.1016/j.cgh.2018.04.045 (2018).
Wai, C. et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38 (2), 518–526. https://doi.org/10.1053/jhep.2003.50346 (2003).
Zimmermann, H. W. et al. Circulating soluble urokinase plasminogen activator is elevated in patients with chronic liver disease, discriminates stage and aetiology of cirrhosis and predicts prognosis. Liver Int. 32 (3), 500–509. https://doi.org/10.1111/j.1478-3231.2011.02665.x (2012).
Gungor, G. et al. Neutrophil gelatinase-associated Lipocalin in prediction of mortality in patients with hepatorenal syndrome: A prospective observational study. Liver Int. 34 (1), 49–57. https://doi.org/10.1111/liv.12232 (2014).
Liu, Q. et al. Association between lactate-to-albumin ratio and 28-days all-cause mortality in patients with acute pancreatitis: A retrospective analysis of the MIMIC-Ⅳ database. Front. Immunol. 13, 1076121. https://doi.org/10.3389/fimmu.2022.1076121 (2022).
Wang, D. et al. Association between lactate/albumin ratio and all-cause mortality in critical patients with acute myocardial infarction. Sci. Rep. 13 (1), 15561. https://doi.org/10.1038/s41598-023-42330-8 (2023).
Krispin, I. et al. Elevated lactate/albumin ratio as a novel predictor of in-hospital mortality in hospitalized cirrhotics. Ann. Hepatol. 28 (3), 100897. https://doi.org/10.1016/j.aohep.2023.100897 (2023).
Gao, F. et al. Prognostic value of serum lactate kinetics in critically ill patients with cirrhosis and acute-on-chronic liver failure: A multicenter study. Aging (Albany NY). 11 (13), 4446–4462. https://doi.org/10.18632/aging.102062 (2019).
Scheiner, B. et al. Acid-base disorders in liver disease. J. Hepatol. 67 (5), 1062–1073. https://doi.org/10.1016/j.jhep.2017.06.023 (2017).
van Hall, G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. (Oxf). 199 (4), 499–508. https://doi.org/10.1111/j.1748-1716.2010.02122.x (2010).
Cremonese, C., Uschner, F. E. & Trebicka, J. News for usage of albumin in patients with liver disease. Dtsch. Med. Wochenschr. 145 (11), 722–726. https://doi.org/10.1055/a-1012-6991 (2020).
Moreau, R. et al. Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J. Hepatol. 72 (4), 688–701. https://doi.org/10.1016/j.jhep.2019.11.009 (2020).
Cakir, E. & Turan, I. O. Lactate/albumin ratio is more effective than lactate or albumin alone in predicting clinical outcomes in intensive care patients with sepsis. Scand. J. Clin. Lab. Invest. 81 (3), 225–229. https://doi.org/10.1080/00365513.2021.1901306 (2021).
Gharipour, A. et al. Lactate/albumin ratio: An early prognostic marker in critically ill patients. Am. J. Emerg. Med. 38 (10), 2088–2095. https://doi.org/10.1016/j.ajem.2020.06.067 (2020).
Shin, J. et al. Prognostic value of the lactate/albumin ratio for predicting 28-day mortality in critically ILL sepsis patients. Shock 50 (5), 545–550. https://doi.org/10.1097/SHK.0000000000001128 (2018).
Acknowledgements
We would like to express our sincere gratitude to Dr. Diangang Liu (Prof. Diangang Liu) for his valuable guidance and support during the preparation of this work.
Author information
Authors and Affiliations
Contributions
RXZ and DDL wrote the main manuscript text . HCL and JWYprepared Table 1. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, R., Yao, J., Li, H. et al. Lactate to albumin ratio as a novel predictor of short-term prognosis for liver cirrhosis in ICU. Sci Rep 15, 35754 (2025). https://doi.org/10.1038/s41598-025-19730-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19730-z
Keywords
This article is cited by
-
Coagulation risk in sepsis patients with elevated lactate-to-albumin ratio: a retrospective cohort study
BMC Infectious Diseases (2026)