Abstract
Spontaneous intracerebral hemorrhage (sICH) is associated with high mortality and disability, with early hematoma expansion (EHE) being a key factor in poor prognosis. The most effective strategies for predicting EHE and improving patient outcomes remain unclear. This study systematically reviewed clinical studies on EHE in sICH from the PubMed database (2013–2023) to identify key predictors for a prediction model. Key factors included: Hematoma hypodensity, Island sign, Glasgow Coma Scale, Hematoma in ventricular, and Taking anticoagulant(H-I-G-H-T). The HIGHT model was developed based on these predictors and validated using receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis. A dynamic nomogram (DN) was created for clinical use. Results: The model was tested in a retrospective cohort of 532 patients, yielding a good fit (AUC = 0.706, P < 0.0001). In a prospective cohort of 83 patients, the AUC was 0.642 (P = 0.048), with sensitivity of 93.75% and specificity of 32.84%.Conclusion: The HIGHT prediction model demonstrated It has shown a certain extent predictive effectiveness in both retrospective and prospective evaluations. And the online DN is now available for clinical use.
Similar content being viewed by others
Introduction
Spontaneous intracerebral hemorrhage (sICH) is a severe subtype of stroke, associated with high early mortality and long-term disability. Despite representing only 10–20% of all strokes, sICH results in a disproportionate share of stroke-related deaths, with one-month mortality rates approaching 50% and poor functional outcomes common among survivors1. Among the factors contributing to this poor prognosis, early hematoma expansion (EHE) is considered one of the most important and modifiable predictors of neurological deterioration and death2. EHE occurs in approximately one-third of patients within the first 24 h, and it can exacerbate damage by inducing necrosis, causing permanent changes in nerve cells, and rapidly deteriorating nervous system function through the activation of M1-type microglia. This makes EHE a major contributor to the high rates of mortality and disability associated with sICH3,4. So making early identification and intervention a critical window for improving outcomes5. Consequently, substantial efforts have been devoted to developing clinical and imaging-based tools to predict hematoma expansion and stratify patients for timely treatment.
Nevertheless, existing predictive strategies face several limitations. Many rely on computed tomography angiography (CTA) markers such as the “spot sign”, which, although validated, require intravenous contrast and advanced imaging infrastructure not available in all centers2. In response, non-contrast computed tomography (NCCT) markers—including blend sign, island sign, hypodensity, and BAT score—have emerged as alternative predictors of EHE2, with comparable accuracy to CTA-based methods. However, many of these models have been developed using small, single-center cohorts, lack external validation, and often fail to integrate clinical factors or emerging computational techniques. Moreover, few studies have systematically addressed the combined predictive value of radiological and clinical features in a comprehensive model.
To address these gaps, we present a predictive model for EHE that integrates both imaging and clinical parameters. Compared to previous approaches, our study introduces several improvements: (1) exclusive reliance on NCCT imaging enhances generalizability in low-resource settings; (2) incorporation of routinely collected clinical variables increases practical applicability; and (3) application of modern statistical frameworks improves model robustness. This integrative approach may offer a more accessible and accurate tool for early identification of high-risk patients, facilitating prompt interventions to improve clinical outcomes.
Methods
Study population
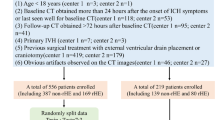
This research is a combined retrospective and prospective clinical study conducted at a single site. Between January 1, 2013 and December 31, 2022, 755 patients with sICH were retrospectively identified from the Neurosurgery Department of the Second Affiliated Hospital of Fujian Medical University. After applying specific criteria, a total of 532 cases were included in the final retrospective analysis. The inclusion criteria were as follows: (1) patients aged ≥ 18 years; (2) hematoma volume > 1 mL; and (3) hospitalization for more than 48 h. The exclusion criteria included: (1) time from onset to first CT ≥ 24 h; (2) interval between two CT scans ≥ 24 h; (3) patients who underwent surgery before the CT review; (4) hemorrhage due to other causes, such as brain tumors, cerebral arteriovenous malformations, aneurysms, moyamoya disease; (5) concurrent severe cardiac, pulmonary, hepatic, or renal failure; (6) acute or chronic kidney disease; and (7) the presence of malignant tumors or liver cirrhosis.
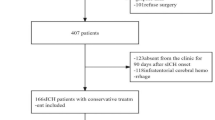
Additionally, 447 patients with sICH were prospectively enrolled at our facility between January 1, 2023, and August 31, 2023, based on the following inclusion criteria: (1) increase in hematoma size by > 6 mL or > 33% on follow-up CT; (2) hematoma volume > 1 mL; (3) diagnosis of sICH confirmed by head CT; (4) hospitalization for > 48 h; and (5) patients aged ≥ 18 years. The exclusion criteria were similar to those used in the retrospective analysis, with the addition of exclusion for surgery performed at non-hospital facilities. Ultimately, 83 cases were included in the prospective analysis. For patients who met surgical criteria and experienced preoperative hemorrhage expansion, surgery could be performed within 72 h after expansion of the hematoma.(Fig. 1). Informed consent was waived by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University for the retrospective study[(2023) The Second Affiliated Hospital of Fujian Medical University Ethical Review (685)], in accordance with the Declaration of Helsinki, revised in 2008, as Chinese laws and regulations do not require it for retrospective analyses. And all 447 patients in the prospective study signed informed consent, either personally or through their families. All that was approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University [(2023) The Second Affiliated Hospital of Fujian Medical University Ethical Review (685)], in accordance with the Declaration of Helsinki, revised in 2008.
Data collection
Clinical baseline data and demographic characteristics were collected for patients with sICH, including age, sex, admission blood pressure, history of hypertension, diabetes mellitus, anticoagulant use, smoking history, time from disease onset to first CT examination, GCS, imaging data from the first and second CT scans, and laboratory tests performed on peripheral venous blood drawn within an hour of hospitalization by the neurosurgery department. Sedation was also documented not to be administered after hemorrhage.
Definition of EHE
EHE was defined as an increase in hematoma of > 6 mL or > 33% from the previous evaluation on CT within 24 h, based on prior literature6,7,8,9,10.
Statistical analysis
Normally distributed continuous variables were expressed as mean ± standard deviation (M ± SD), while non-normally distributed variables were presented as quartiles. Categorical variables were reported as percentages (%). For between-group comparisons, Student’s t-test or analysis of variance was used for normally distributed variables, while the Mann−Whitney U test and Kruskal−Wallis test were applied for non-normally distributed variables. The chi-square test was used for categorical variables and Pearson’s correlation analysis was performed for correlation assessments.
In the final model, the receiver operating characteristic (ROC) curve and the area under the curve (AUC) were used to evaluate the sensitivity and specificity of the model in predicting rebleeding. The calibration curve and Hosmer−Lemeshow goodness of fit (HLGOF) test were applied to assess the model’s goodness of fit, and decision curve analysis (DCA) was used to evaluate its clinical utility. Statistical analyses were performed with the R language version 4.3.1 and Graphs were produced with GraphPad Prism version 8.0 and MedCalc version 20.0.4. The P-value was set at < 0.05.
Results
Clinical and demographic characteristics of participants
Following a retrospective analysis of patient data, 755 patients with sICH were initially identified, of whom 532 met the inclusion criteria. The mean age was 59.51 ± 10.73 years, with 36.7% of the patients being female. Among these, 57 patients (10.71%) experienced EHE (Table 1).
For prospective analysis, baseline characteristics, including general data, laboratory tests, and imaging data, were collected from 447 patients with sICH. Based on inclusion and exclusion criteria, a total of 83 patients were ultimately enrolled, with a mean age of 60.04 ± 13.50 years; 42.2% were female, and 16 patients (19.28%) developed EHE (Table 2).
The construction of the HIGHT model
A total of 162 clinical studies on the prediction of EHE in patients with sICH were identified from the PubMed database between January 1, 2013 and June 5, 2023. Of these, 41 articles were ultimately included in the systematic evaluation. The risk factors with fixed effects, low heterogeneity, and strong correlation to predict EHE were selected for further analysis. These risk variables were used to develop the HIGHT prediction model for EHE.
Construction of the HIGHT score
The reference value Wij for each group of risk variables was determined by research by grouping them based on their clinical significance or use. Typically, the reference value Wij is selected from the middle of the group. Continuous variable GCS score: light (13–15) Wij: 13 + 15 / 2 = 14, medium (9−12) Wij: 9 + 12 / 2 = 10.5, and heavy (3−8) Wij: 3 + 8 / 2 = 5.5; dichotomous variables whose Wij are all dominated by negative results take the value of 0. The setting of the reference value WiREF for risk factors was chosen according to clinical habit: “GCS of 13–15, no taking anticoagulants, no island sign, no hematoma in ventricle, no hypodensity in hematoma” was chosen as the reference value (GCS: WiREF = 14; island sign: WiREF = 0; hematoma in the ventricle: WiREF = 0; and hypodensity in the hematoma: WiREF = 0). The OR values for each risk factor in Table 3 were transformed into β values (β = log (OR)). The distance D-value between the grouping of each risk factor and the base risk reference value was calculated: D = (Wij-WiREF)* β. The HIGHT model had only the GCS score as a continuous variable, and the constant B corresponding to 1 point in the scoring tool was set to be dominated by the GCS score, with each decrease of -1 point counted as 1 point, B = -1*(-0.06) = 0.06. The final score for each variable is Point = D/B, and finally the calculated values are rounded to the nearest integer11.(Table 4).
Acquisition of imaging data
The “island sign” is defined as three or more small, round, or oval hematomas distributed around the main hematoma that are not connected to it, and four or more small, lobulated, or bubble-shaped hematomas distributed around the main hematoma that may be connected to it12. “Hypodensity in hematoma” is defined as a hypodense area of any shape, size, and density that is encapsulated within the hemorrhagic region and does not require density measurement13. “Hematoma in the ventricle” refers to a hematoma that extends into the ventricular system following a cerebral hemorrhage. CT examinations for all patients were performed using a Philips ICT 128-row, 256-slice Brilliance CT scanner in our imaging department, with a slice thickness of 0.5 mm, a window width of 160 mm, a Dose Right Index of 37, kV = 120, mAs = 337, and a scan duration of 6.6 s. Two radiologists, blinded to clinical information, independently assessed the location of the hematoma, island sign, hypodense areas within the hematoma, black hole sign, vortex sign, hematoma extension into the ventricles, and other non-enhanced imaging signs. Inter-rater reliability was evaluated using the Cohen’s Kappa test, yielding an κ-value of 0.680, indicating good agreement.
A retrospective study validates the HIGHT prediction model
Through a retrospective analysis of 532 patients with sICH, a nomogram for the HIGHT prediction model was established (Fig. 2). The diagram shows that “hypodensity in the hematoma” had the greatest weight in the model, as indicated by the longest line, while “hematoma in the ventricle” had the least weight. The probability of a patient experiencing EHE can be assessed by scoring the clinical data using the markers in the nomogram, with the total score corresponding to the risk probability of EHE occurrence14. The ROC curve (Fig. 3) was used to validate the accuracy of the HIGHT prediction model: AUC = 0.706 (95% CI: 0.665–0.744), P < 0.001, Z = 5.764, HLGOF test X² = 6.361, P = 0.607. The optimal cut-off value was 0.092, with a Youden index of 0.302, sensitivity of 64.91%, specificity of 65.26%, and overall good accuracy. All test indices were excellent. The calibration curve further validated the predictive capability of the HIGHT model, with a C-index of 0.706 and an R² of 0.108, indicating strong predictive ability in the retrospective analysis (Fig. 4)15. Additionally, the DCA curve was used to validate the clinical applicability of the model (Fig. 5). The threshold range was approximately 0.05 to 0.55, and the optimal cut-off value obtained from the ROC analysis (0.092) fell within this range, confirming the good clinical utility of the model16.
HIGHT model nomogram The hematoma that breaks into the ventricle has the smallest straight line, and the longest straight line occupied by a hypodense shadow within it shows that it is the most heavily weighted in the model. Using a column-line diagram and the values of clinical data for each patient, the probability of EHE in that patient was determined. The overall score that resulted was related to the linear predictive value and the likelihood that the hazard would materialize.
HIGHT model calibration curve. The data from the study’s patients is represented by the dashed line in the curve, and the ideal prediction curve—that is, the patients who were really expected to develop EHE—is shown by the gray line. The higher the accuracy of the forecast, the closer the dashed and gray lines are to one another.
HIGHT model DCA curve. The red curve represents the outcome predicted by the patient’s actual data, with patients above the gray and black lines having a benefit and the threshold ranging from approximately 0-0.55, with approximately 55% of the patients having a benefit. The diagonal gray line indicates that all patients from the retrospective analysis of the data were predicted to have EHE, the horizontal black line indicates that all patients were predicted to have non-EHE, the vertical coordinate indicates the net benefit, and the horizontal coordinate indicates the degree of risk.
Prospective study validates HIGHT score
In a prospective cohort, 447 patients with spontaneous intracerebral hemorrhage (ICH) were initially screened, and 83 met the inclusion criteria for final analysis. Among these, early hematoma expansion (EHE) occurred in 16 patients (19.28%). The previously developed HIGHT score was applied to this validation cohort. The receiver operating characteristic (ROC) analysis yielded an area under the curve (AUC) of 0.642 (95% CI: 0.529–0.744; Z = 1.980; P = 0.048), indicating modest discriminative ability. The optimal cut-off value determined by the Youden index was > 7, corresponding to a sensitivity of 93.75% and a specificity of 32.84% (Fig. 6, A).
Prospective analysis HIGHT score ROC. (A) Receiver operating characteristic (ROC) curve analysis of the HIGHT score yielded an AUC of 0.642 (95% CI: 0.529–0.744; P = 0.048), with a Youden index of 0.266. The optimal cut-off value was > 7, corresponding to a sensitivity of 93.75% and a specificity of 32.84%. (B) Calibration curve showing the agreement between predicted probabilities and observed outcomes. The bias-corrected curve (red) closely approximates the ideal diagonal line, suggesting good model calibration. The model was validated using 300 bootstrap resamples, with a mean absolute error of 0.031. (C) Decision curve analysis (DCA) demonstrating the net clinical benefit of using the HIGHT score across different threshold probabilities. The HIGHT model (blue line) showed superior net benefit compared to “treat-all” and “treat-none” strategies, particularly in the lower risk threshold range.
Of the 83 patients, 15 scored above the threshold (> 7), capturing 93.75% (15/16) of those who experienced EHE, suggesting strong sensitivity despite limited specificity. The calibration curve (Fig. 6, B) demonstrated good agreement between predicted and observed probabilities, with minimal mean absolute error across 300 bootstrap repetitions. Decision curve analysis (DCA) (Fig. 6, C) showed a net clinical benefit of the HIGHT model across a wide range of threshold probabilities, particularly when compared with “treat-all” and “treat-none” strategies.
Establishment of an online dynamic nomogram and method of clinical application
The fitted HIGHT predictive model is produced as an online dynamic nomogram. Clinicians simply enter the URL (https://wjy-article.shinyapps.io/dynnomapp/) on their mobile devices to enable the rapid application of the HIGHT predictive model for patient assessment.
Discussion
Fitting predictive models using data from a single site is a common approach in many clinical investigations on the prediction of EHE17,18,19,20,21. Despite this limitation, our model showed high goodness of fit, clinical usability, and predictive performance between different study centers. However, the validation using data exclusively from a single research center remains questionable. By reviewing 10 years of clinical research in this area from the PubMed database, we identified and summarized all relevant risk factors to predict EHE. The HIGHT prediction model was then developed by systematically analyzing these data using Review Manager 5.4 software, focusing on fixed effects to identify risk factors with strong correlations and low variability. The model showed considerable flexibility. The clinical applicability, goodness of fit, and predictive performance of the model were confirmed through retrospective analysis using data from our institution. Additionally, more data were collected for prospective validation, which showed exceptional predictive sensitivity, reinforcing the superiority of the model.
Previous research has developed the model and highlighted its advantages; however, it has not shown ease of use in clinical settings, resulting in limited clinical generalizability and dissemination. Although several studies have created scoring systems, such as the HEMRICH, BAT, and HEP scores, they have not provided an easy-to-use platform for physicians22,23. Considerations include the number of variables required, the challenges associated with acquiring these variables and the complexity of evaluating them. To address this gap, a web page was developed to establish an online prediction tool that allows clinicians to assess the probability of EHE in patients with sICH accurately and promptly. This tool is designed to facilitate early intervention by physicians, thus improving survival rates and quality of life for patients with sICH. In this study, the HIGHT prediction model was applied to construct the foundation of the model using a DN.
In the current retrospective analysis to validate the HIGHT prediction model, the ROC test indicated a prediction accuracy of 70.6%, with good sensitivity (64.91%) and specificity (65.2%). Several previous clinical studies demonstrated good specificity but were inadequate in sensitivity24. The findings of this study support the generalizability of the model and its enhanced balance between sensitivity and specificity, derived from a rigorous review of the literature. The HIGHT prediction model was further developed into the HIGHT scoring scale, which was prospectively collected from our center and validated with an accuracy of 64.2%, a sensitivity of 93.75%, and a specificity of 32.84%. This process was carried out to further confirm the reliability of the model and highlight the high predictive efficiency of the HIGHT model.
The HIGHT prediction model includes factors such as hypodensity in the hematoma, the island sign, GCS scores, hematoma in the ventricle, and taking anticoagulants. These factors can be easily assessed when a patient is admitted to the hospital. The GCS is one of the most widely used clinical scoring systems in neurosurgery, assessing limb movement, verbal response, and eye-opening reactions, with scores ranging from 3 to 15. This scale quickly evaluates the severity of the condition, the patient’s level of consciousness, and guides specific treatments25. The mechanism by which the GCS predicts EHE remains under investigation. As a widely used clinical metric, it has been incorporated into numerous studies as a crucial predictor of EHE in patients with sICH26,27,28. A declining GCS score may indicate an expanding hematoma and worsening consciousness, and it is also a more precise indicator of the risk factors for profound hypotension. Lower GCS scores suggest a poorer state of consciousness and more severe disease, predicting larger intracranial hematomas and more extensive neurological damage27,29. Advances in cardiac and cerebrovascular interventional surgeries have led to an increased number of patients who require long-term oral anticoagulants. Post-intervention, anticoagulants reduce blood viscosity and prevent infarction symptoms caused by interventional materials. However, they also pose a risk of bleeding complications, with an increasing number of sICH cases attributed to anticoagulant use reported in recent years30,31. Moreover, recent studies have highlight the association between taking anticoagulants and hematoma growth. The risk of EHE associated with anticoagulant use has been documented in various studies. Anticoagulants reduce the production of coagulation factors and inhibit platelet aggregation, which is essential to prevent myocardial infarction. However, this impaired coagulation capacity can lead to larger hematomas following vascular ruptures and hemorrhage32. Enhanced inhibition of anticoagulants during the initial stages of bleeding has been suggested to reduce the likelihood of EHE in patients with ICH33.
With the ongoing advances in imaging technology, many clinical studies have used imaging signs as predictors of EHE in patients with sICH. Demchuk et al.34 identified the “spot sign” on CT angiography (CTA) as an independent risk factor for EHE, a finding that has gained wide acceptance among researchers. However, the urgency and severity of sICH, coupled with the limited availability of CTA in emergency settings at many institutions, pose challenges to its broad implementation. In recent years, imaging signs from non-enhanced CT scans have increasingly been the focus of research. In this study, the “island sign,” “hypodensity in the hematoma,” and “hematoma in the ventricle” were selected for inclusion in the HIGHT prediction model. Qi et al.12 described the “island sign” as one or more hemorrhagic dots around the main hematoma, indicating ruptures in small peripheral blood vessels leading to active bleeding and possible fusion and enlargement of the hematoma over time. Boulouis et al.34 highlighted “hypodensity in the hematoma” as reflecting various stages of bleeding. This imaging characteristic suggests that the hematoma is fragile and susceptible to further rupture and growth. The development of the hematoma continues until it stabilizes, counteracted by the mechanical resistance of the surrounding brain structures. A common physiological response to cerebral hemorrhage is hypoperfusion around the hematoma, which reduces tissue integrity and mechanical resistance to the expansion of the hematoma35.
Limitations
Although the systematic evaluation of the literature to date has supported the generalizability of the HIGHT prediction model, several limitations remain. First, the literature search was restricted to the PubMed database and covered the period from January 1, 2013 to June 5, 2023. This approach resulted in an incomplete search due to the limited time frame. Future research should include additional databases such as Web of Science, EMbase, Scopus, and the Cochrane Library to conduct more comprehensive systematic reviews. The search time frame should begin with the first published literature on the prediction of EHE in patients with sICH. Second, the model validation relied solely on single-center data, despite the implementation of retrospective and prospective analyses. This limitation introduced an unavoidable selection bias. Moreover, despite the strong predictive efficacy in the overall validation results, not all variables in the univariate analyses (Tables 1 and 2) were statistically significant, reducing statistical power and suggesting that the model might not be the best fit for the data from this center. The inclusion and exclusion criteria allowed two CT examinations to be performed in 24 h. However, patients who experienced hematoma expansion beyond this time frame were excluded, further limiting the data. Third, in this study, an increase in hematoma size within 24 h was defined as an increase of > 6 mL or > 33% from the previous CT examination. Although this definition is widely accepted among experts, there is considerable debate about the criteria and time frames that define EHE, with numerous studies using various markers. Fourth, the HIGHT prediction model constructed by systematic evaluation lacked key laboratory indicators, such as serum calcium36, blood glucose37 and INR38, which many researchers have identified as risk factors to predict EHE. This omission represents a significant weakness of the model. Finally, this study overlooked the elaboration of the relationship between the HIGHT model and the prognosis of patients. And the prognosis of EHE in sICH patients included in this study. Future studies aimed at predicting EHE in patients with ICH should expand their database searches, extend the time window for literature reviews, and perform stratified analyses by subgroups to improve the credibility of the model. Validation should include multicenter data to overcome geographic limitations and incorporate diverse racial groups to maximize generalizability of the model.
Conclusion
After a comprehensive review of the literature, the HIGHT prediction model was developed. It has shown a certain extent predictive effectiveness in both retrospective and prospective evaluations. Using web-based DN accessible via mobile devices connected to the Internet, physicians can quickly estimate the likelihood of EHE in patients with sICH. Early therapeutic interventions facilitated by this model have the potential to improve the quality of life and survival rates of patients.
Data availability
Our data and materials are derived from the Second Affiliated Hospital of Fujian Medical University. The data are authentic and reliable, if you need the original files you can email the corresponding author for them.
References
Magid-Bernstein, J. et al. Cerebral hemorrhage: pathophysiology, treatment, and future directions. Circ. Res. 130, 1204–1229 (2022).
Yogendrakumar, V. et al. Independent validation of the hematoma expansion prediction score: a non-contrast score equivalent in accuracy to the spot sign. Neurocrit Care. 31, 1–8 (2019).
Zhang, F. et al. Value of image biomarkers based on dual-energy computed tomography angiography material separation technique in predicting early hematoma expansion in spontaneous intracerebral hemorrhage. World Neurosurg. 183, e502–e511 (2024).
Chu, H., Huang, C., Tang, Y., Dong, Q. & Guo, Q. The stress hyperglycemia ratio predicts early hematoma expansion and poor outcomes in patients with spontaneous intracerebral hemorrhage. Ther. Adv. Neurol. Disord. 15, 17562864211070681 (2022).
Bakar, B. et al. In spontaneous intracerebral hematoma patients, prediction of the hematoma expansion risk and mortality risk using radiological and clinical markers and a newly developed scale. Neurol. Res. 43, 482–495 (2021).
Brouwers, H. B. & Greenberg, S. M. Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc. Dis. (basel Switz). 35, 195–201 (2013).
Demchuk, A. M. et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 11, 307–314 (2012).
Broderick, J. P., Brott, T. G., Duldner, J. E., Tomsick, T. & Huster, G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 24, 987–993 (1993).
Becker, K. J. et al. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke 30, 2025–2032 (1999).
Morotti, A. et al. Intracerebral haemorrhage expansion: definitions, predictors, and prevention. Lancet Neurol. 22, 159–171 (2023).
D’Agostino, R. B. et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation 117, 743–753 (2008).
Li, Q. et al. Island sign: an imaging predictor for early hematoma expansion and poor outcome in patients with intracerebral hemorrhage. Stroke 48, 3019–3025 (2017).
Morotti, A. et al. Standards for detecting, interpreting, and reporting Noncontrast computed tomographic markers of intracerebral hemorrhage expansion. Ann. Neurol. 86, 480–492 (2019).
Myers, J., Do, D., Herbert, W., Ribisl, P. & Froelicher, V. F. A nomogram to predict exercise capacity from a specific activity questionnaire and clinical data. Am. J. Cardiol. 73, 591–596 (1994).
Karnes, H. T., Shiu, G. & Shah, V. P. Validation of bioanalytical methods. Pharm. Res. 8, 421–426 (1991).
Van Calster, B. et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur. Urol. 74, 796–804 (2018).
Jia, Y. et al. Direct bilirubin: a predictor of hematoma expansion after intracerebral hemorrhage. Am. J. Emerg. Med. 71, 150–156 (2023).
Morotti, A. et al. Using Noncontrast computed tomography to improve prediction of intracerebral hemorrhage expansion. Stroke 54, 567–574 (2023).
Rodriguez-Luna, D. et al. Derivation and validation of three intracerebral hemorrhage expansion scores using different CT modalities. Eur. Radio. 33, 6045–6053 (2023).
Song, L. et al. Predicting hemorrhage progression in deep intracerebral hemorrhage: a multicenter retrospective cohort study. World Neurosurg. 170, e387–e401 (2023).
Yan, Y. et al. Clinical characteristics of spontaneous intracranial basal ganglia hemorrhage and risk factors for hematoma expansion in the plateaus of China. Front. Neurol. 14, 1183125 (2023).
Yao, X., Xu, Y., Siwila-Sackman, E., Wu, B. & Selim, M. The HEP score: a nomogram-derived hematoma expansion prediction scale. Neurocrit Care. 23, 179–187 (2015).
Sakuta, K. et al. The NAG scale: noble predictive scale for hematoma expansion in intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. : Off J. Natl. Stroke Assoc. 27, 3675 (2018).
Li, Z. et al. Hematoma expansion in intracerebral hemorrhage: an update on prediction and treatment. Front. Neurol. 11, 702 (2020).
Teasdale, G. et al. The Glasgow coma scale at 40 years: standing the test of time. Lancet Neurol. 13, 844–854 (2014).
Quintas-Neves, M. et al. Noncontrast computed tomography markers of outcome in intracerebral hemorrhage patients. Neurol. Res. 41, 1083–1089 (2019).
Zhang, X. et al. A predictive nomogram for intracerebral hematoma expansion based on non-contrast computed tomography and clinical features. Neuroradiology 64, 1547–1556 (2022).
Li, H., Xie, Y., Liu, H. & Wang, X. Non-contrast CT-based radiomics score for predicting hematoma enlargement in spontaneous intracerebral hemorrhage. Clin. Neuroradiol. 32, 517–528 (2022).
Huynh, T. J. et al. Validation of the 9-point and 24-point hematoma expansion prediction scores and derivation of the PREDICT a/b scores. Stroke 46, 3105–3110 (2015).
Juvela, S. & Kase, C. S. Advances in intracerebral hemorrhage management. Stroke 37, 301–304 (2006).
Tsivgoulis, G. et al. Direct oral anticoagulant- vs vitamin K antagonist-related nontraumatic intracerebral hemorrhage. Neurology 89, 1142–1151 (2017).
Boulouis, G. et al. Association of key magnetic resonance imaging markers of cerebral small vessel disease with hematoma volume and expansion in patients with Lobar and deep intracerebral hemorrhage. JAMA Neurol. 73, 1440–1447 (2016).
Small, C., Attridge, R. L., Franco-Martinez, C., Donnelly, J. & Barthol, C. Prothrombin complex concentrate use in intracranial hemorrhage patients with cirrhosis not on prior anticoagulation. J. Intensive Care Med. 37, 633–640 (2022).
Boulouis, G. et al. Association between hypodensities detected by computed tomography and hematoma expansion in patients with intracerebral hemorrhage. JAMA Neurol. 73, 961–968 (2016).
Fisher, C. M. Pathological observations in hypertensive cerebral hemorrhage. J. Neuropathol. Exp. Neurol. 30, 536–550 (1971).
Morotti, A. et al. Association between serum calcium level and extent of bleeding in patients with intracerebral hemorrhage. JAMA Neurol. 73, 1285–1290 (2016).
Yang, M., Du, C., Zhang, Q., Ma, Q. & Li, R. Nomogram model for predicting hematoma expansion in spontaneous intracerebral hemorrhage: multicenter retrospective study. World Neurosurg. 137, e470–e478 (2020).
Huang, Y., Zhang, Q. & Yang, M. A reliable grading system for prediction of hematoma expansion in intracerebral hemorrhage in the basal ganglia. Biosci. Trends. 12, 193–200 (2018).
Acknowledgements
We would like to express our gratitude to the doctors in the case room and the Department of Imaging Medicine of the Second Affiliated Hospital of Fujian Medical University for their selfless help. We would like to thank Editage(www.editage.cn) for English language editing.
Funding
Project of Science and Technology Program of Quanzhou City, Fujian Province, China (CQZ2023N001S0077).
Author information
Authors and Affiliations
Contributions
Jiayin Wang and Shaojie Li performed the experiments and collected the primary data. Baofang Wu, Liangqin Luo, and Hongzhi Gao wrote the main manuscript text and prepared the figures and tables. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Li, S., Wu, B. et al. Development and validation of the HIGHT prediction model for early hematoma expansion in spontaneous intracerebral hemorrhage. Sci Rep 15, 35827 (2025). https://doi.org/10.1038/s41598-025-19885-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19885-9