Abstract
The present study used an in-vivo inflammation-induced colorectal cancer (CRC) model to evaluate the additive effect of thapsigargin (TG) with the standard chemotherapy drug irinotecan (IRN). CRC was induced by AOM/DSS, and after 10th weeks, animals were treated with five weekly cycles of either IRN or TG or a combination of both drugs. All the animals were sacrificed after the 16th week, and the data were analysed. Coadministration of both IRN and TG substantially reduced both tumor numbers and occurrence of aberrant crypt foci (ACF) in the colon tissues as compared to only IRN/TG-treated animal groups. Further analyses revealed that IRN and TG together alleviated ultrastructural abnormalities of the colon with a recovered overall histoarchitecture, enhanced ER stress and mitochondrial dysfunction, reduction of PCNA positive cells indicating low rate of cellular proliferation, increased DNA fragmentation and apoptosis supported by higher intensity of γH2AX and cleaved caspase-3 immunohistochemistry. Gene expression analyses of key oncogenic biomarkers also suggest that the addition of TG with IRN is more effective in inhibiting carcinogenic transformation in the colon of AOM/DSS-treated mice. This study provides direct evidence of the superior therapeutic potential of a combination of both drugs compared to conventional monotherapy in the management of CRC.
Similar content being viewed by others
Introduction
Cancer is a group of diseases manifested due to the dysregulated proliferation of transformed cells supported by natural selection1. According to the World Health Organization (WHO, 2020), there was an emergence of ~ 20 million new cancer cases and 10 million cancer deaths across the world, of which colorectal cancer (CRC) ranked the 3rd leading cause of cancer deaths2. Despite having various treatment options, the outcome of individuals with advanced grade CRC is not satisfactory due to different stages of treatment failures3. To resolve such therapeutic inefficiencies, refined modern therapies are being developed constantly to formulate novel molecules and to enhance the efficacy of already existing drugs for improvement of the overall quality of life of the patients4,5. For example, a combination of a standard chemotherapeutic drug with another cytotoxic agent provided a strong rationale to achieve higher efficiency in the management of various malignancies6.
Numerous studies reported the role of endoplasmic reticulum (ER) stress in the management of different malignancies by triggering apoptosis via sustained elevation of intracellular calcium and thereby activating the unfolded protein response (UPR) pathways7. Thapsigargin (TG), an irreversible, non-competitive inhibitor of sarco/endoplasmic reticulum calcium ATPase (SERCA), has emerged as a potential therapeutic agent showing successful results in improving treatment outcomes in different cancer models8. Mispagargin (G202), a derivative prodrug of TG, has been demonstrated to have on-target anti-tumorigenic activity against prostate cancer9. TG has been reported to reduce proliferation, migration and invasion in adrenocortical carcinoma cells by increasing apoptosis and ER-stress10. TG, in combination with certain chemotherapeutic agents, has been shown to enhance the efficacy of the overall treatment. For instance, a Combination of SERCA inhibitors, including TG, with rapamycin was found to trigger rapid tumour regression in RAS-driven tumours and prevent their reappearance, suggesting ER-stress as a prominent driver of this event11. Hence, it is hypothesized that the mechanistic complementarity of IRN with TG would be a potential therapy to manage CRC. The deliberate amplification of prolonged ER stress can potentially enhance the cytotoxicity of IRN by lowering the apoptotic threshold. The TG-mediated dysregulation of calcium elevates the apoptotic response, which has already been triggered by IRN-induced DNA damage12,13. The present study, therefore, evaluates the anti-tumour efficacy of combined therapy of IRN with SERCA inhibitor, TG, in an AOM/DSS-induced inflammation-associated colon cancer model in mice.
Results
TG combined with IRN improved weight gain, DAI score, and reduced both tumour numbers and formation of ACF in the colon
The body weights were found to have declined considerably in the PC group of mice with inflammation-associated colorectal cancer, which were reversed back to the range of normal control after combined treatment with IRN and TG (Fig. 1b). The DAI scores were also found to be consistently high in both the PC as well as IRN groups of animals throughout the experiment. However, these scores were improved substantially on administration of TG alone and in combination with IRN (Fig. 1c). The weight: length ratio of the colon showed a prominent increase only in the PC group; however, combined treatment of IRN and TG was found to decrease the same significantly (**p < 0.01, Fig. 1d and e). The numbers of tumours were found to be significantly higher in the PC group, which was partially reduced in both the IRN and TG-treated groups of animals. However, the most significant decrease (***p < 0.001) in the tumour burden was noticed in the IRN + TG group (Fig. 1f). On evaluating the colon tissues for the formation of ACF, a significantly higher ACF count was observed in the PC group of animals compared to NC (***p < 0.001, Fig. 1g,h). Though different treatments significantly reduced the formation of ACF in colon tissues, the addition of TG with IRN resulted in a more effective reduction of the formation of ACF (Fig. 1g,h).
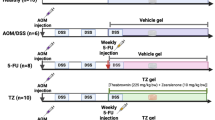
(a) Experimental design, (b) Changes in the weight gain (***p < 0.001, **p < 0.01, *p < 0.05 when compared to NC, ap<0.01 when compared to PC, bp< 0.05 for PC V/S IRN), (c) DAI scores, (d) Lengths of colon (arrows indicate tumor and inflammation), (e) Weight per unit length ratio of colon (**p < 0.01 when compared NC v/s PC; *p < 0.05, when compared PC v/s IRN and PC V/S TG; and **p < 0.01, when compared PC v/s IRN + TG), (f) Changes in the number of tumors (***p < 0.001, when NC v/s PC; **p < 0.01, when PC v/s IRN and PC v/s TG; and ***p < 0.001, when PC v/s IRN + TG), (g and h). Formation and quantification of ACF in 5 different groups of animals (***p < 0.001, when NC v/s PC; **p < 0.01, when PC v/s IRN and PC v/s TG; and ***p < 0.001, when PC v/s IRN + TG). One way ANOVA followed by post hoc Tukey test was performed for comparison among the groups.
TG combined with IRN improved colon ultrastructure in AOM/DSS-induced CRC
SEM micrographs illustrated ACF-like remodeling in the colon with enlarged, irregular pits, thick pericryptal rims, ulcer and erosions, etc. in the PC group when compared to NC group (Fig. 2b). The colons from the IRN treated group showed thin and patchy film with exposed corrugations, supportive of irinotecan-induced mucositis (Fig. 2c). The TG group of animals demonstrated a continuous surface overlain by condensed adherent mucus (Fig. 2d). The SEM images of IRN + TG group showed improved architecture with uniform crypt orifices, thin rims and intact intercrypt reliefs like NC group (Fig. 2e).
Representative SEM micrographs of (a) Normal colon showing intact mucosal corrugation with adherent mucus, no denudation, (b) AOM/DSS-induced tumour colon showing ACF with irregular, enlarged pits (white arrow), thickened pericryptal rims (yellow arrow), (c) Colon with IRN monotherapy showing patchy film, (d) Colon with TG alone, demonstrating a continuous surface overlain by adherent mucus, (e) Colon with administration of both IRN and TG, illustrating a recovered architecture with uniform crypt orifices (yellow arrow), thin rim (white arrow), and intact intercrypt relief. Representative TEM micrographs of Normal colon with classic double-membrane structure (N: Nucleus, ER: endoplasmic reticulum, M: Mitochondria), (g) AOM/DSS-treated colon showing irregular disrupted nucleus (DN), chromatin marginalisation (CM), junctional failure (JF), and inflamed and discontinuous basement membrane (BM) during CRC, (h) Colon from the IRN group showing drug-mediated toxicity and stromal remodeling with slightly swollen mitochondrion (sM) and autophagic vesicles (A), (i) Colon from the TG-only group depicting swollen mitochondrion (sM), dilated and vesiculated ER (Dil./Ves. ER), electron-dense lysosomes and areas with cytoplasmic clearing and vacuolization (V), (j) The colon from the IRN + TG group illustrates swollen, fragmented, or disrupted mitochondria (DM), autophagic vesicles (A), severe ER stress with dilated and vesiculated ER (dil ER).
TEM analysis of colon tissues from PC groups revealed cellular disintegration with abnormal nuclear morphology, marginalized chromatin distribution leading to karyorrhectic fragmentation, widened intercellular area with loss of junction integrity when compared to NC (Fig. 2g). The colon from IRN treated group showed drug-associated heterogenous mitochondrial status indicating cell stress and stromal remodeling (Fig. 2h). However, the colon from TG group demonstrated ER stress with dilated RER cisternae, swollen mitochondria, vacuolization and cytoplasmic clearing (Fig. 2i). The most prominent cellular stress was observed in the IRN + TG treated group evidenced by presence of swollen, vacuolated, or fragmented mitochondria, severe ER stress with dilated and vesiculated ER, corresponding to the additive effects of both the drugs (Fig. 2j).
TG combined with IRN improved colon histoarchitecture and restoration of goblet cells in the colon
Histopathological examination of the colon tissues of PC group of animals clearly indicates CRC progression including distorted crypts, pseudostratified and hyperchromatic nuclei, ulcerations accompanied by infiltration of neutrophils into the lamina propria and sub-mucosa, presence of adenoma and several adenocarcinoma lesions which were absent in NC group of animals (Fig. 3b). These structural abnormalities were partially reversed in both IRN-alone and TG-alone groups (Fig. 3c and d), however, the most remarkable improvement in colon histoarchitecture was noticed in IRN + TG treated group (Fig. 3e). Histopathological scoring also indicated significantly better treatment outcomes in IRN + TG treated group compared to only IRN and TG treated group of animals (***p < 0.001, Fig. 3f). The quantification of adenocarcinoma indicated no adenocarcinoma incidence in the NC group, whereas all AOM/DSS-induced mice (6/6; 100%) developed adenocarcinoma lesions. In the groups receiving monotherapy with either IRN or TG, the incidence of adenocarcinoma was reduced to 50% (3/6) and 66.67% (4/6) respectively. However, combined treatment of IRN with TG reduced the incidence of adenocarcinoma to 16.7% (1/6) only. Statistical comparison using Fisher’s exact test confirmed a significant reduction in adenocarcinoma incidence in the IRN + TG group when compared with the AOM/DSS control (p < 0.01, supplementary table S4). These results quantitatively substantiated the histological observations and demonstrated that TG co-administration effectively suppressed the incidence of adenocarcinoma in AOM/DSS induced CRC model in mice. Further, Alcian blue/nuclear fast red staining of colon tissues from the PC group demonstrated severe depletion of goblet cells compared to the NC group (Fig. 3p,q and u). However, the goblet cells were found to be restored significantly both in TG (***p < 0.001, Fig. 3s and u) as well as in the IRN + TG-treated group of animals (**p < 0.01, Fig. 3t and u). In contrast to the TG group, the IRN-treated group demonstrated severe depletion of the goblet cells, as was observed in the PC group. (Fig. 3r and u).
Representative histological pictomicrographs of H&E staining in (a) Normal colon, (b) colon after AOM/DSS-induced CRC, (c) colon after IRN-monotherapy, (d) colon after TG-monotherapy, (e) colon after administration of both IRN and TG, and (f) histological scoring of colon per high power field (HPF) (**p < 0.01 in PC v/s IRN, and PC v/s TG; and ***p < 0.001, when compared to PC v/s IRN + TG). AB/NFR staining in (p) Normal colon, (q) colon after AOM/DSS-induced CRC, (r) colon after IRN-monotherapy, (s) colon after TG-monotherapy, (t) colon after co-administration of both IRN and TG, and (u) Quantification of goblet cells per crypts in 5 groups of animals (***p < 0.001, when PC compared to TG; **p < 0.01, when PC v/s IRN + TG, and IRN v/s IRN + TG; and *p < 0.05, when TG v/s IRN + TG). One way ANOVA followed by post hoc Tukey test was performed for comparison among the groups.
TG combined with IRN reduced proliferative index, increased DNA fragmentation and apoptosis in colon tumorigenesis
To analyze the effect of different treatment options on cellular proliferation and cell death in the AOM/DSS model of after different treatment options, colon tissues were subjected to immunohistochemistry (IHC) to quantify the number of PCNA, γH2AX, and cleaved caspase-3 positive cells. In case of PCNA, the highest level of expression was observed in the PC group (Fig. 4b), which was significantly reduced after IRN + TG treatment (***p < 0.001, Fig. 4e and p). Similarly, IHC of γH2AX indicated a significantly higher percentage of γH2AX positive cells in the IRN + TG group (***p < 0.001, Fig. 4j and q) and in the IRN-only group (**p < 0.01, Fig. 4h and q). Further, the expression of cleaved caspase-3 was reduced in both NC and PC groups but significantly elevated in all other treatment groups (**p < 0.01, ***p < 0.001, Fig. 4m,o and r).
Representative immunohistochemical images of PCNA, γH2AX and cleaved caspase-3, respectively, in the normal colon (a, f, k), PC colon after AOM/DSS induced CRC (b, g, l), colon after IRN-monotherapy (c, h, m), colon after TG-monotherapy (d, i, n), and colon after administration of both IRN + TG (e, j, o); IHC scoring of colon for (p) PCNA positive cells per HPF (***p < 0.001, when NC v/s PC; **p < 0.01, when PC v/s IRN and PC v/s TG; and ***p < 0.001, when PC v/s IRN + TG), (q) γH2AX positive cells per HPF (*p < 0.05, when NC v/s PC and PC v/s TG; **p < 0.01, when PC v/s IRN; and ***p < 0.001, when PC v/s IRN + TG), and (r) cleaved caspase-3 positive cells per HPF (*p < 0.05, when NC v/s PC; **p < 0.01, when PC v/s IRN; and ***p < 0.001, when PC v/s TG, and PC v/s IRN + TG). One way ANOVA followed by post hoc Tukey test was performed for comparison among the groups.
TG combined with IRN alters the relative gene expressions of cyclin D1, β-catenin and E-cadherin in AOM/DSS-induced CRC
Further, we analyzed the relative expression of key oncogenic biomarkers in the colon tissues of all experimental groups. The qRT-PCR analysis of cyclin D1 and β-catenin mRNA, the marker of oncogenic proliferation, indicated a significant overexpression in PC groups compared to the NC (***p < 0.001, Fig. 5a,b). The expression level of both mRNAs (cyclin D1 & β-catenin) was only partially suppressed in IRN-alone and TG-alone groups, while a significant suppression of expression was noticed in the combined IRN + TG group (***p < 0.001, Fig. 5a and b). In contrast to this, the expression level of EMT-marker gene E-cadherin mRNA was significantly downregulated in the PC group compared to the NC (***p < 0.001, Fig. 5c). This abnormal downregulation of E-cadherin was reversed in both IRN and TG treated groups, however the most significant reversal was found in the IRN + TG treated group of animals (***p < 0.001, Fig. 5c).
Relative mRNA expression of (a) Cyclin D1 (***p < 0.001, when NC v/s PC; **p < 0.01, when PC v/s IRN and PC v/s TG; and ***p < 0.001, when PC v/s IRN + TG), (b) β-catenin (***p < 0.001, when NC v/s PC; **p < 0.01, when PC v/s IRN; *p < 0.05 in PC v/s TG; and ***p < 0.001, when PC v/s IRN + TG) and (c) E-cadherin(***p < 0.001, when NC v/s PC; **p < 0.01, when PC v/s IRN; *p < 0.05 in PC v/s TG; and ***p < 0.001, when PC v/s IRN + TG) in terms of GAPDH in the colons of 5 different groups. One way ANOVA followed by post hoc Tukey test was performed for comparison among the groups.
Discussion
Numerous studies have suggested poor treatment outcomes in conventional chemotherapies for CRC4,5,6. Irinotecan, though widely used as a standard chemotherapy candidate, was found to be responsible for the increased incidence of grade≥3 adverse effects. When used as second-line monotherapy, it resulted in many unsatisfactory outcomes due to drug resistance attributed to overexpression of drug efflux transporter ABCG2, downregulated Top1 mRNA, a missense mutation of Top1, high resistance of cancer stem cells to IRN, etc14. For these reasons, this study aims to investigate the potential combination therapy of TG with IRN. The rationale behind combining IRN with TG was primarily due to their non-overlapping modes of action, where IRN generates DNA breaks and thereby activates apoptosis, while TG depletes ER-calcium levels, triggering the caspase-mediated apoptosis15,16. Furthermore, if the addition of TG enhances the therapeutic efficacy of IRN at lower concentrations, then each drug could be administered at reduced doses, minimizing its dose-limiting toxicities while overcoming chemoresistance. In this model of CRC, the addition of TG with IRN improved the overall treatment outcomes compared to single-agent conventional chemotherapy. AOM/DSS exposure caused the development of severe disease symptoms, including reduction in body weight, rise in the DAI scores, formation of numerous ACFs, and tumors, as reported previously17. These morphometric indicators of CRC were significantly reduced in the IRN + TG co-treatment group. The animals in the combination-therapy group maintained a stable range of body weight and colon parameters like those of the healthy controls, while the IRN monotherapy group showed a partial improvement only. SEM analysis data showed a significant reversal of colonic epithelial architecture back to normalcy in the IRN + TG group with uniform crypt orifices, compared to ACF formation with irregular crypts in PC. The pictographs of TEM revealed abnormal nucleus, irregular chromatin distribution and loss of junction integrity, etc. in the PC group, indicating oncogenic transition. The colon from IRN + TG animals depicted severe cell stress, leading to enhanced mitochondrial dysfunction and dilated, vesiculated ER. This kind of stress is primarily attributed to TG, enhanced by IRN-associated mitochondrial damage.
Consistent with earlier findings, mice with AOM/DSS have undergone marked neoplastic transformations18. These transformations were significantly reduced in the mice treated with a regimen of both TG and IRN. The mice receiving a combination of both IRN and TG showed significant restoration of colonic epithelial architecture with well-organised crypts and mild inflammatory cells, indicating overall reduction of tumour load. Both the monotherapy groups demonstrated lower-grade lesions and reduced carcinogenic changes compared to the PC. Similar results were also obtained previously when IRN was combined with melatonin and other herbal molecules, including wogonin or celastrol19. The number of goblet cells, which was highly reduced in both PC as well as IRN monotherapy mice, was found to be well-preserved in both TG-alone and combination group of animals, indicating improvement of mucosal health20. The treatment-associated diarrhoea and mucositis by IRN could be minimized by the addition of TG, as it preserved the mucus-secreting goblet cells in the colon after treatment.
The immunohistochemical analysis of the present study showed that both TG and IRN were involved in complementary anti-neoplastic pathways of apoptosis, DNA damage and proliferation inhibition to reduce the disease severity. The addition of TG with IRN demonstrated a dramatic increase in apoptosis with the most intense cleaved caspase 3 signal than either of the treatment monotherapies, suggesting severe cell death [28; 29]. This effect may occur due to robust amplification of the IRN-triggered apoptosis by TG. Similar to this, a combination of EGCG with NSAIDs was reported to inhibit tumorigenesis in rodents via apoptosis21. In the diseased animals, the cell proliferation marker PCNA showed marked elevation than any other group, indicating rampant proliferative activity during carcinogenesis, with suppression of cell death22. This was reversed partially in both IRN-only and TG-only groups, the IRN + TG group showing the highest anti-proliferative effect, corresponding to its lowest PCNA signal due to the additive effect of both TG and IRN. A similar effect was observed when quercetin was co-administered with docetaxel in prostate cancer cells23. However, the γ-H2AX signal in the TG co-treatment group did not exceed that of the IRN monotherapy group. IRN caused its chemotherapeutic effects primarily via DNA damage, which ultimately triggered cell death15. The highest signal intensity of γH2AX in the IRN + TG group was therefore attributed primarily to IRN.
The qRT-PCR studies of this experiment showed significant upregulation of cyclin D1 and β-catenin in CRC animals of the PC group, suggesting rigorous proliferation of cancer cells, consistent with the earlier findings24. Treatment with either IRN or TG alone reversed these effects. The most pronounced reversal was found in the IRN + TG group. IRN could arrest the cell cycle by initiating DNA damage, and TG could induce calcium homeostasis modulated cellular differentiation15,16. When both drugs were combined, it showed a notably huge additive effect, where both markers were expressed in a near-normal range. In contrast, the expression level of E-cadherin mRNA was markedly suppressed in the PC group, indicating rapid epithelial-mesenchymal transition during oncogenesis25. Near-normal range of expression of this gene was observed under co-administration of TG with IRN. Similar results were obtained using 17-DMCHAG, an Hsp90 inhibitor, that managed prostate cancer in vitro by upregulating the E-cadherin levels while inhibiting cellular proliferation and triggering apoptosis26. The restoration of goblet cells and near-normal expression of E-cadherin together in TG-treated animals suggested that the oncogenic epithelial conditions were reversed with the administration of TG. The novelty of this experiment lies in exploiting the complementary actions of both TG and IRN to enhance the efficacy of IRN monotherapy in CRC. These pre-clinical data showed that TG can substantially improve therapeutic outcomes when administered together with IRN. However, direct use of TG is restricted in previous literature due to its systemic toxicity and lack of selectiveness and therefore targeted prodrug development was encouraged for TG. In our model, the dose of TG (1 mg/kg) was well-tolerated, as evidenced by the gross morphometric parameters and no mortality was recorded in any group, including the TG groups. By optimising the dose, this study exploited the therapeutic benefit of TG without any proportional increase of its systemic side effects. The present study demonstrated that the addition of TG with IRN significantly magnified the chemotherapeutic effect by a marked increase in apoptosis and DNA fragmentation, and modulation of the proliferative index of the cells. By enhancing the overall anti-tumorigenic potential of the IRN, TG could provide a newer avenue to the translational combination therapy trials in CRC.
Limitations of the present study
While TG effectively enhanced IRN sensitivity, its non-selective SERCA inhibition may cause off-target effects. In the present study a very low dose (1 mg/kg, weekly) of TG was selected to ensure safety, as evidenced by the absence of mortality or severe observable adverse effects in the TG-only group. Future translation would benefit from tumour-selective TG formulations, such as tumor-selective or colon-targeted, microbiota-activated prodrugs, to achieve localized delivery with reduced systemic toxicity. This study only focused on molecular and histoarchitectural validation using qRT-PCR and immunohistochemistry, however, incorporation of western blot analysis of ER stress marker proteins and quantification of intracellular calcium level in future work will further substantiate the present findings. Moreover, in the present study only male mice were used to arrive at a potential conclusion about combination of TG with conventional IRN therapy, hence potential sex-specific effects remain to be explored.
Methods
Drugs and chemicals
Azoxymethane (AOM, CAS NO: 25843-45-2), Dextran Sulphate Sodium (Mol. Weight: 35–50 kDa, DSS, CAS NO: 9011-18-1) and Thapsigargin (TG, Cat No. T7459) were purchased from Sigma-Aldrich, USA, SRL, Mumbai, India and Invitrogen, USA, respectively. Irinotecan Hydrochloride injection IP (FOIRI-40) was purchased from Zydus Oncosciences, India. Kits and primary antibodies for the immunohistochemical detection were purchased from Proteintech, IL, USA, and were mentioned in Table 1. All the other essential chemicals were of analytical grade and bought by local distributors. The list of antibodies and kits for IHC is mentioned in Table 1.
Experimental animals and experimental design
Thirty male Swiss albino mice (20 ± 5 g), aged 10 weeks, were used in the present experiment and obtained from the animal house facility of the Department of Zoology, Gauhati University. The animals were housed under controlled laboratory conditions (T: 24 ± 2℃, Humidity: 56–65 mm of Hg) with free access to food and water ad libitum throughout the experimental period. All the experimental procedures were in accordance with the Institutional guidelines and approved by the Institutional Animal Ethical Committee (IAEC), Gauhati University (Ref: IAEC/Per/2024/PP-IAEC/2024-3/8/10). The experiment was conducted and reported, strictly adhering to ARRIVE guidelines for reporting experiments on animals. The animals were randomly assigned to five different groups (n = 6, each): (1) Negative Control (NC), (2) Positive control (PC), (3) Irinotecan (IRN), (4) Thapsigargin (TG) and (5) IRN + TG group (Fig. 1a). CRC was induced as described previously with little modification27. Briefly, the mice were treated with a single intraperitoneal injection of the azoxymethane (AOM, 10 mg/Kg) followed by three alternate weekly cycles of dextran sulphate sodium (DSS, 1.5% w/v) in drinking water. A group (IRN) of AOM/DSS-induced CRC mice were treated with standard chemotherapy of irinotecan (35 mg/Kg, i.p), and another group (TG) was treated with 1 mg/kg of thapsigargin alone18,28, both for 5 once-weekly cycles. The last group (IRN + TG) of AOM/DSS-induced CRC animals were treated with a combination of both IRN (35 mg/kg) and TG (1 mg/kg) for 5 once-weekly cycles from week 10–14. The experiment was continued for 16 weeks, including 7 weeks for the tumour induction and the remaining 9 weeks of the treatment period. Body weights and the disease activity index (DAI) were recorded every week till the termination of the experiment. All animals were euthanised after the 16th week by cervical dislocation under xylazine and ketamine hydrochloride anaesthesia (10 mg/kg & 90 mg/Kg, i.p). Colon from each animal was excised, and colon lengths and weights were recorded. The colonic tissues were processed for ultrastructural, histological preparation and for qRT-PCR analysis of gene expression.
Disease activity indexing (DAI)
The severity of the disease in five different groups of animals was compared using the DAI scores. Each parameter, including movement, diet, defecation rates, stool consistency, and rectal bleeding, was scored according to the standard diagnostic criteria as reported previously (Table 2)29.
Quantification of tumour number and ACF in the colon
Colon from each animal was taken longitudinally and cleared of any debris; tumours were identified and counted under the microscope18. The whole mount sections of the colon were fixed in 10% neutral buffered formalin (NBF) overnight at room temperature, followed by washing, dehydration and staining with 0.05% methylene blue. Stained sections were observed under a microscope for the counting of the number of ACF per section as reported previously30.
Ultrastructural analysis of colon tissue using SEM and TEM
Sample processing for SEM
Colon segments (~ 2 × 2 mm2) were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer saline (PBS) overnight at 4 °C. The tissues were then washed in PBS, post-fixed in 1% osmium tetroxide (1 h.), rinsed and sequentially dehydrated with grades of alcohol followed by critical point drying using CO2. Samples were mounted with carbon tape and imaged using the scanning electron microscope (FESEM, Carl Zeiss, system: Sigma 300 VP, resolution: 1.2 nm at 15 kV).
Sample processing for TEM
The colons were cut into a 1 × 1 mm2 dimension and immersed in 2.5% glutaraldehyde in 0.1 M PBS at 4 °C for 4 h. The sections were then washed, post-fixed in 1% osmium tetroxide, sequentially dehydrated with grades of alcohol, cleared in xylene, infiltrated with epoxy resin, embedded and polymerized at 50–60 °C. Ultra-thin sections were then imaged by transmission electron microscope (Talos HR-TEM, Cryo-TEM, 200 kV FEG).
Histopathological analysis of colon tissue
Colon tissues were fixed in 10% NBF for 24 h. at room temperature, dehydrated, cleared in xylene, and finally embedded in paraffin. The tissues were then sectioned at 4 μm and processed for H&E staining. Histological scores were calculated in a blinded manner by examining 5 slides per animal per group in high-power microscopic fields (HPF) based on the scoring criteria of 0 = no changes, 1 = nuclear hyperplasia, 2 = dysplasia, 3 = adenoma, 4 = adenocarcinoma. Additionally, the incidence of adenocarcinoma in colon tissues of each animal per group was quantified and data were presented. Tissue sections were further stained with AB/NFR for evaluation of goblet cell density per crypt as reported previously20. All the histopathological analyses, wherever required, were performed using the Leica Microsystems (Switzerland) Limited. Included Application Information: Leica Application Suite, Version 4.2.0. The objective lenses of the system are Leica N PLAN 10X (NA: 0.25 mm) and Leica N PLAN 40X (NA: 0.65 mm). Camera included in the microsystem: Leica DFC295-500424310, acquisition software: Leica Application Suite (LAS v4.x).
Quantification of PCNA, γH2AX & cleaved caspase 3 by IHC
Paraffinized sections from the colon of were subjected to immunohistochemical analysis to evaluate the intensity of cellular proliferation (PCNA), DNA fragmentation (γH2AX) and apoptosis (Cleaved caspase 3). Slides were deparaffinized, rehydrated, and then antigen retrieval was performed at 90 °C for 40 min. The sections were then blocked with 3% BSA for 30 min and incubated overnight with primary antibody at 4 °C. Afterwards, sections were washed to remove excess primary antibody, treated with HRP-conjugated secondary antibody for 30 min. The signal development was done with DAB and H2O2, followed by counterstaining with hematoxylin. Tissues were then dehydrated with graded alcohol, cleared in xylene, mounted and observed under a microscope. The immunohistochemical results for quantification of PCNA, γH2AX & Cleaved caspase-3 were expressed as the number of positive cells per crypt after analysing at least 10 high-power microscopic fields (HPF) per animal. In our study, we confirmed specificity controls through the following criteria: (i) No primary antibody control: Omission of the primary antibodies resulted in the total elimination of signal development in the colon tissues of the same region, confirming that the staining was not due to non-specific binding. (ii) Positive control tissue: Positive staining was already being shown by the manufacturers in tissues known to express the particular antigens (Table 1). (iii) The distribution pattern of the antigens was consistent with their reported subcellular localisations. Wherever applicable, published studies employing the mentioned antibodies are cited to support their specificities further31,32,33.
Cyclin D1, β-catenin and E-cadherin gene expression analysis by qRT-PCR
The gene expression analysis of key oncogenic biomarkers, including Cyclin D1, β-catenin and E-cadherin in the mouse colon was performed as described below. Relative quantification of gene expression by RT-qPCR was done using a Tata MD CHECK Express real-time PCR machine with the following program: (95 °C–30 s), 40 cycles (95 °C–5 s, 58 °C–30 s) followed by melting curve 60°-99 °C. Each reaction comprised 20 ng cDNA, 10 pm of forward and reverse primers, and 2X Fast SYBR Green PCR Master Mix (TB Green Premix Ex Taq II, #RR820A), and the total volume of the reaction was 10 µl. Each sample was tested in triplicate, and Cq values were averaged. Cq values were determined by the qPCR data for target genes. To confirm equal loading, the amplification of GAPDH was tested in parallel as a reference gene. MyGoPro PCR Software 3.6 was used for experimental setup and data analysis. The forward and reverse primer sequences of different genes of mouse cDNA were taken from previously published literature after being verified in the Primer-BLAST software of NCBI. The list of different primers used in this study is summarised below in Table 3.
Statistical analysis
All data were presented as mean ± SEM and statistical differences were analyzed using one-way ANOVA, followed by post hoc Tukey test to compare the differences of means among the groups. The incidence of adenocarcinoma among the groups was analyzed with Fisher’s exact test and data were presented. The level of difference was considered significant at p < 0.05. All statistical procedure was computed using GraphPad Prism (version 10.0).
Data availability
All the necessary data is provided within the manuscript.
Abbreviations
- ACF:
-
Aberrant crypt foci
- AOM:
-
Azoxymethane
- CRC:
-
Colorectal cancer
- DSS:
-
Dextran sulphate sodium
- IRN:
-
Irinotecan
- SERCA:
-
Sarco/endoplasmic reticulum calcium ATPase
- TG:
-
Thapsigargin
References
Brown, J. S. et al. Updating the definition of cancer. Mol. Cancer Res. 21 (11), 1142–1147. https://doi.org/10.1158/1541-7786.MCR-23-0411 (2023).
Hossain, M. S. et al. Colorectal cancer: A review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers 14 (7), 1732. https://doi.org/10.3390/cancers14071732 (2022).
Kumar, A. et al. Current and emerging therapeutic approaches for colorectal cancer: A comprehensive review. World J. Gastrointest. Surg. 15 (4), 495–519. https://doi.org/10.4240/wjgs.v15.i4.495 (2023).
de With, M. et al. Irinotecan-induced toxicity: A Pharmacogenetic study beyond UGT1A1. Clin. Pharmacokinet. 62 (11), 1589–1597. https://doi.org/10.1007/s40262-023-01279-7 (2023).
Puzzo, M. et al. Colorectal cancer: current and future therapeutic approaches and related technologies addressing multidrug strategies against multiple level resistance mechanisms. Int. J. Mol. Sci. 26 (3), 1313. https://doi.org/10.3390/ijms26031313 (2025).
Bayat Mokhtari, R. et al. Combination therapy in combating cancer. Oncotarget 8 (23), 38022–38043. https://doi.org/10.18632/oncotarget.16723 (2017).
Yadav, R. K., Chae, S. W., Kim, H. R. & Chae, H. J. Endoplasmic reticulum stress and cancer. J. Cancer Prev. 19 (2), 75–88. https://doi.org/10.15430/JCP.2014.19.2.75 (2014).
Jaskulska, A., Janecka, A. E. & Gach-Janczak, K. Thapsigargin—from traditional medicine to anticancer drug. Int. J. Mol. Sci. 22 (1), 4. https://doi.org/10.3390/ijms22010004 (2020).
Boinapally, S. et al. A prostate-specific membrane antigen (PSMA)-targeted prodrug with a favorable in vivo toxicity profile. Sci. Rep. 11 (1), 7114. https://doi.org/10.1038/s41598-021-86551-1 (2021).
Wu, L. et al. Thapsigargin induces apoptosis in adrenocortical carcinoma by activating Endoplasmic reticulum stress and the JNK signaling pathway: an in vitro and in vivo study. Drug. Des. Devel. Ther. 13, 2787–2798. https://doi.org/10.2147/DDDT.S209947 (2019).
De Raedt, T. et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell. 20 (3), 400–413. https://doi.org/10.1016/j.ccr.2011.08.014 (2011).
Lindner, P. et al. Cell death induced by the ER stressor Thapsigargin involves death receptor 5, a non-autophagic function of MAP1LC3B, and distinct contributions from unfolded protein response components. Cell. Communication Signal. 18, 12. https://doi.org/10.1186/s12964-019-0499-z (2020).
Rudolf, E., Kralova, V., Rudolf, K. & John, S. The role of p38 in irinotecan-induced DNA damage and apoptosis of colon cancer cells. Mutat. Research/Fundamental Mol. Mech. Mutagen. 741–742, 27–34. https://doi.org/10.1016/j.mrfmmm.2013.02.002 (2013).
Ozawa, S., Miura, T., Terashima, J. & Habano, W. Cellular Irinotecan resistance in colorectal cancer and overcoming Irinotecan refractoriness through various combination trials including DNA methyltransferase inhibitors: a review. Cancer Drug Resist. (Alhambra Calif). 4 (4), 946–964. https://doi.org/10.20517/cdr.2021.82 (2021).
Saurav, S. et al. Overcoming Irinotecan resistance by targeting its downstream signaling pathways in colon cancer. Cancers 16 (20), 3491. https://doi.org/10.3390/cancers16203491 (2024).
Sehgal, P. et al. Inhibition of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by Thapsigargin analogs induces cell death via ER Ca2 + depletion and the unfolded protein response. J. Biol. Chem. 292 (48), 19656–19673. https://doi.org/10.1074/jbc.M117.796920 (2017).
Garg, A. et al. SelSA-1, a novel HDAC inhibitor demonstrates enhanced chemotherapeutic potential by redox modulation. Sci. Rep. 13 (1), 9301. https://doi.org/10.1038/s41598-023-36555-w (2023).
Borah, G. & Bharali, M. K. Green tea catechins in combination with Irinotecan attenuates tumorigenesis and treatment-associated toxicity in an inflammation-associated colon cancer mice model. J. Egypt. Natl Cancer Inst. 33, 17. https://doi.org/10.1186/s43046-021-00074-4 (2021).
Radajewska, A. et al. Combination of Irinotecan and melatonin with the natural compounds Wogonin and Celastrol for colon cancer treatment. Int. J. Mol. Sci. 24 (11), 9544. https://doi.org/10.3390/ijms24119544 (2023).
Wang, M. et al. Amelioration of AOM/DSS-induced murine colitis-associated cancer by Evodiamine intervention is primarily associated with gut microbiota-metabolism-inflammatory signaling axis. Front. Pharmacol. 12, 797605. https://doi.org/10.3389/fphar.2021.797605 (2021).
Fujiki, H., Sueoka, E., Watanabe, T. & Suganuma, M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J. Cancer Res. Clin. Oncol. 141 (9), 1511–1522. https://doi.org/10.1007/s00432-014-1899-5 (2015).
Zhou, H. et al. The prognostic value of proliferating cell nuclear antigen expression in colorectal cancer: A meta-analysis. Medicine 97 (50), e13752. https://doi.org/10.1097/MD.0000000000013752 (2018).
Sharma, S., Cwiklinski, K., Mahajan, S. D., Schwartz, S. A. & Aalinkeel, R. Combination modality using Quercetin to enhance the efficacy of docetaxel in prostate cancer cells. Cancers 15 (3), 902. https://doi.org/10.3390/cancers15030902 (2023).
Kim, Y. et al. Overexpression of β-Catenin and Cyclin D1 is associated with poor overall survival in patients with stage IAâ€IIA squamous cell lung cancer irrespective of adjuvant chemotherapy. J. Thorac. Oncol. 11 (Issue 12), 2193–2201. https://doi.org/10.1016/j.jtho.2016.07.021 (2016). ISSN 1556 – 0864.
Wendt, M. K., Taylor, M. A., Schiemann, B. J. & Schiemann, W. P. Down-regulation of epithelial Cadherin is required to initiate metastatic outgrowth of breast cancer. Mol. Biol. Cell. 22 (14), 2423–2435. https://doi.org/10.1091/mbc.E11-04-0306 (2011).
Wang, J. et al. 17-DMCHAG, a new geldanamycin derivative, inhibits prostate cancer cells through Hsp90 Inhibition and surviving downregulation. Cancer Lett. 362 (1), 83–96. https://doi.org/10.1016/j.canlet.2015.03.025 (2015).
Tanaka, T. et al. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 94 (11), 965–973. https://doi.org/10.1111/j.1349-7006.2003.tb01386.x (2003).
Ma, Z. et al. Thapsigargin sensitizes human esophageal cancer to TRAIL-induced apoptosis via AMPK activation. Sci. Rep. 6, 35196. https://doi.org/10.1038/srep35196 (2016).
Deng, J. et al. Pre-administration of Berberine exerts chemopreventive effects in AOM/DSS-induced colitis-associated carcinogenesis mice via modulating inflammation and intestinal microbiota. Nutrients 14 (4), 726. https://doi.org/10.3390/nu14040726 (2022).
McGinley, J. N., Thompson, M. D. & Thompson, H. J. A method for serial tissue processing and parallel analysis of aberrant crypt morphology, mucin depletion, and beta-catenin staining in an experimental model of colon carcinogenesis. Biol. Procedures Online. 12, 118. https://doi.org/10.1007/s12575-010-9032-x (2010).
Fang, Y. et al. CD36 inhibits β-catenin/c-myc-mediated Glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat. Commun. 10 (1), 3981. https://doi.org/10.1038/s41467-019-11662-3 (2019).
Shen, M. et al. DNAJC12 causes breast cancer chemotherapy resistance by repressing doxorubicin-induced ferroptosis and apoptosis via activation of AKT. Redox Biol. 70, 103035. https://doi.org/10.1016/j.redox.2024.103035 (2024).
Wu, C. et al. Trypsin-instructed bioactive peptide nanodrugs with cascading transformations to improve chemotherapy against colon cancer. J. Nanobiotechnol. 23 (1), 66. https://doi.org/10.1186/s12951-025-03143-1 (2025).
Larissa Lipskaia, Z. et al. Expression of sarco (endo) plasmic reticulum calcium ATPase (SERCA) system in normal mouse cardiovascular tissues, heart failure and atherosclerosis. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1843 (11), 2705–2718. https://doi.org/10.1016/j.bbamcr.2014.08.002 (2014).
Alotaibi, B. S., Selim, A. T., Makhlof, H. M. M. M. M. & M. E., & F., M. M New insights into the anticancer effects of polycladia crinita aqueous extract and its selenium nanoformulation against the solid Ehrlich carcinoma model in mice via VEGF, Notch 1, NF-кB, Cyclin D1, and caspase 3 signaling pathway. Front. Pharmacol. 15, 1345516. https://doi.org/10.3389/fphar.2024.1345516 (2024).
Wang, K. et al. Comparison of gene expression of the oncogenic Wnt/β-catenin signaling pathway components in the mouse and human epididymis. Asian J. Androl. 17 (6), 1006–1011. https://doi.org/10.4103/1008-682X.157396 (2015).
Song, W. et al. E-cadherin maintains the undifferentiated state of mouse spermatogonial progenitor cells via β-catenin. Cell. Biosci. 12, 141. https://doi.org/10.1186/s13578-022-00880-w (2022).
Acknowledgements
The authors acknowledge University Grants Commission (UGC) for providing SB with the Junior Research Fellowship (UGC-JRF), Ref No.- 221610241975. The authors are also grateful to CIF, Department of Chemistry, Gauhati University for SEM facility, and to SAIF, AIIMS, New Delhi for generation of the TEM data.
Author information
Authors and Affiliations
Contributions
Conceptualization- MKB; Methodology- MKB and SB; Investigation – SB; Data acquisition, interpretation and analysis- MKB, SB, NA, JD, SSR, SR, MB, RM, and SK; Writing – original draft – SB; Review– MKB, SB, NA, JD, SSR, SR, MB, RM, SK; Final proof-reading- MKB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The experiment was conducted and reported, strictly adhering to ARRIVE guidelines for reporting experiments on animals. All the procedures of experimentation involving animal study were approved by the Institutional Animal Ethical Committee (IAEC), Department of Zoology, Gauhati University, Assam, 781014, India (Ref: IAEC/Per/2024/PP-IAEC/2024-3/8/10).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Baruah, S., Bharali, M.K., Akhtara, N. et al. Thapsigargin enhanced chemotherapeutic sensitivity of irinotecan in the inflammation-induced colorectal cancer model in mice. Sci Rep 16, 4551 (2026). https://doi.org/10.1038/s41598-025-34567-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-34567-2