Abstract
This study addresses the limited noninvasive tools for Head and Neck Squamous Cell Carcinoma (HNSCC) progression-free survival (PFS) prediction by identifying Computed Tomography (CT)-based biomarkers for predicting prognosis. A retrospective analysis was conducted on data from 203 HNSCC patients. An ensemble feature selection involving correlation analysis, univariate survival analysis, best-subset selection, and the LASSO-Cox algorithm was used to select functional features, which were then used to build final Cox Proportional Hazards models (CPH). Our CPH achieved a 0.69 concordance index in an external indepedent cohort of 77 patients. The model identified five CT-based radiomics features, Gradient ngtdm Contrast, Logσ=33D-FirstorderRootMeanSquared, Logσ=0.13D-glszm SmallAreaLowGrayLevelEmphasis, Exponential-gldm LargeDependenceHighGrayLevelEmphasis, and Gradient ngtdm Strength as survival biomarkers (p-value < 0.05). These findings contribute to our knowledge of how radiomics can be used to predict the outcome so that treatment plans can be tailored for people with HNSCC to improve their prognosis.
Similar content being viewed by others
Introduction
As of 2022, head and neck cancer (HNC) has surpassed liver cancer to become the sixth most common cancer, with a mortality rate of 4.9%, exceeding that of prostate cancer at 4.1%1. Head and Neck Squamous Cell Carcinoma (HNSCC), which arises from the epithelial layers of the oral cavity, pharynx, and larynx, accounts for 90% of HNC cases2. Alarmingly, there has been a recent surge in the incidence of HNSCC, particularly among younger individuals3, showing an annual increase of 30%2,4. According to 2022 global statistics5, cancers of the oral cavity represent 389,485 new cases, comprising 2% of all cancer incidences, with a mortality rate of 1.9%. Laryngeal cancer reports 188,960 new cases, making up 0.9% of all cancer incidences and a mortality rate of 1.1%. Pharyngeal cancers contribute 313,008 new cases, translating to 1.5% of all cancer incidences, with a mortality rate of 1.7%. These increases are largely attributed to risk factors such as smoking, alcohol, and areca nut consumption—particularly affecting the oral cavity and larynx—as well as the escalating incidence of human papillomavirus (HPV) infections impacting the oropharynx1,3. Despite advancements in surgical techniques and adjuvant therapies, the 5-year overall survival rate remains around 50%6,7. Integrating prognostic and predictive biomarkers into clinical care could help surmount challenges associated with targeted therapies, leading to extended patient survival4.
Tobacco smoking and alcohol consumption significantly increase the risk of HNSCC, especially when combined, affecting the larynx most8. The other HNSCC sites, such as the oral cavity and oropharynx, are also affected by exposure to those carcinogens and their combination9. Human Papillomaviruses 16 and 18 (HPV16 and HPV18) account for around 85% of HPV-associated HNSCC. HPV is the key risk factor for oropharyngeal cancer, with the effect amplified by smoking10. Histological biopsies are widely utilized for diagnosing and assessing the prognosis of HNSCC11. Additionally, diagnostic and prognostic biomarkers for HNSCC have been investigated in biological fluids such as saliva, blood, serum, and plasma. Various studies12,13,14 have confirmed the altered expression of specific microRNAs (miRNAs) in HNSCC, including miRNA-18412, miR-3115, and miR-27b16, which are linked to cancerous transformation and the advancement of the disease. Moreover, patients with HNSCC have shown higher levels in biomarkers like CA12517,18, tissue polypeptide antigen, and Cyfra21−1 compared to those in control groups. Preclinical and clinical molecular biomarkers, including p1619, EGFR20, TP5321, and Cyclin D122, have also demonstrated their ability to distinguish between patients and control groups and show disparities in survival based on cutoff values.
Various imaging modalities, including Computed Tomography (CT), Positron Emission Tomography (PET), and Magnetic Resonance Imaging (MRI), have been explored for their potential as biomarkers by extracting quantitative imaging features known as radiomics features. Unlike biomolecule-based assays, imaging techniques offer a noninvasive approach. They utilize readily available imaging data from routine diagnostic scans without additional costs. Additionally, 3D imaging provides unique intratumor heterogeneity information about the tumor and its parenchyma23,24. These high throughput radiomics features can be utilized to develop predictive models for survival and treatment failure. The rationale behind this approach is that imaging captures crucial information about tumor phenotype and microenvironment. For instance, the American College of Radiology has established a standardized Neck Imaging Reporting and Data System (NI-RADS) to monitor posttreatment progress. Studies have demonstrated a strong correlation between the NI-RADS category and treatment failure in patients with HNSCC. Imaging factors have proven to be reliable in providing accurate prognostic information for posttreatment recurrence screening. In our preliminary study, two potential radiomic biomarkers for overall survival were identified25,26. However, there remains a gap in identifying noninvasive factors for progression-free survival after treatment in patients with HNSCC. Our study differs from previous research by focusing on the susceptibility or risk associated with the biomarker, specifically the increased likelihood of progression within 2 years post-treatment. Consequently, our emphasis lies on achieving high specificity and sensitivity to minimize the occurrence of false positives and false negatives.
Progression-free survival (PFS) encompasses the duration from the start of treatment to either the progression or death for any reason, with patients lost during follow-up treated as censored observations, contributing less weight to the survival analysis. PFS stands as a critical surrogate endpoint in oncology clinical trials due to its capacity to provide early indications of treatment efficacy. This measure is particularly valuable for its ability to suggest therapeutic benefits without the long follow-up required for observing changes in overall survival (OS), thereby accelerating the decision-making process in drug development. PFS is also pertinent when subsequent treatments may confound survival outcomes, as it more directly reflects the benefits of the treatment being tested. By potentially correlating with symptom improvement, PFS offers insights into a therapy’s impact on disease progression and patient quality of life, a central goal of cancer treatment. Its use necessitates careful validation to confirm its predictive power for OS, thereby embodying the balance between timely patient-centric research and the need for methodological precision.
A single academic health network serving a diverse population has a varied cohort of HNSCC cases. This study aims to identify imaging biomarkers associated with PFS in these patients. We conducted a retrospective study using high-dimensional radiomics data and clinical follow-up information, with PFS as the primary endpoint. To achieve this, we utilized the Cox Proportional Hazard (CPH) model. Due to the high dimensionality of the imaging data, feature selection methods and the Model Selection strategy were employed to avoid overfitting and select a concise set of candidate factors. Additionally, we examined variations in PFS outcomes by stratifying individuals based on different levels of identified radiomics biomarkers. Finally, the results of the CPH model were summarized in a nomogram to aid in treatment decision-making. The generalizability of the bimarker was evaluated through external independent patient cohorts.
Methods
Endpoints of interest and study cohorts
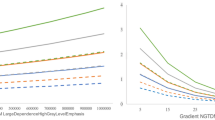
This retrospective cohort study examines a group of HNSCC patients who took contrast-enhanced CT scans before treatment between 2003 and 2022. The training data consisted of 203 patients from the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center treated during 2003–2022, and the testing data consisted of 77 patients from MD Anderson Cancer Center treated during 2003–2013. We collected six clinical attributes in the training set, including race, gender, tobacco use, alcohol consumption, stage, and treatment modality, summarized in Table 1. Patients were categorized into four stages (I, II, III, and IV) based on the pathological assays of tumor specimens. Smoking and alcohol status were self-reported. Seven treatment modalities, including chemoradiotherapy (CRT), chemotherapy (CT), radiotherapy (RT), surgery (Sx), radiotherapy plus surgery (RT + Sx), CRT + Sx, and CT + Sx, were administered to patients as their initial treatment. The primary endpoint in this study was Progression-free survival (PFS), which, according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.127, is defined as the time from initiation of treatment until the first progression of the disease or death from any cause. A total of 34 patients died within 1 year after treatment, and 53 patients died within 2 years after treatment. The average survival time among all 203 patients was 40 months (ranging from 1 to 154 months). Among the patients who died, the average survival time was 19 months (ranging from 2 to 154 months), whereas among patients alive at the last follow-up, the average survival time was 58 months (ranging from 1 to 137 months). Kaplan-Meier (KM) curve plots for progressive free survival (PFS) at 12 months, 24 months, 48 months, and 60 months are presented in each row in Fig. 1. The first column of the plots is stratified by race, smoker or non-smoker status, drinking or non-drinking status, and the stage of the disease in the last column. The log-rank test shows significant results for race and drinking status at PFS12, PFS24, PFS48, and PFS60. However, smoking status is not significant across all four PFS periods. The stage of the disease is consistently significant across all four PFS intervals. In summary, African Americans exhibit worse PFS after two years of treatment compared to European Americans. Drinkers show poorer long-term PFS and advanced stages are associated with worse progression-free survival.
A total of 2840 consecutive patients with head and neck squamous cell carcinoma (HNSCC) treated with curative-intent radiotherapy (RT) at MD Anderson Cancer Center from 2003 to 201328 were initially screened. Of these, patients who had undergone whole-body CT scans before RT were included in the study (n = 215). Clinical data were extracted from the MD Anderson Cancer Center’s custom electronic medical record system. The clinical factors available for analysis include gender, smoking status, and AJCC stage29. The symbol “/” in Table 1 signifies the absence of information. Due to the extensive time required for data reconciliation, 77 of these patients were used for external validation.
Overall workflow
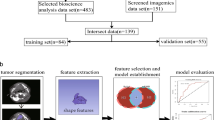
The workflow outlining our approach is illustrated in Fig. 2. In this workflow, the neoplasm volume serves as the region of interest (ROI) from which all radiomics features are computed. The contouring of the ROI was performed manually by experienced Radiation Oncologists, who were not directly involved in the study, using the Varian Medical System Eclipse software environment. These features underwent a selection process to minimize redundancy and were combined with clinical data. A Cox Proportional Hazard model, optimized via the best subset approach and fivefold cross-validation, was then applied to independent public data30. The model’s predictive performance was evaluated using the concordance Index (CI). All statistical analyses were performed using R programming language, with a significance level (alpha) set at 0.05 for all tests. All procedures included in this application have been approved previously by the Institutional Review Boards (IRB) of the University of Maryland School of Medicine.
Feature extraction
In this study, feature extraction was performed in each set of images using the Python library PyRadiomics31. Features described by the Imaging Biomarker Standardization Initiative (IBSI)32 were extracted in six families: shape, first-order, gray level co-occurrence matrix (GLCM), gray level run length matrix (GLRLM)33, grey level size zone matrix (GLSZM)34,35, gray level dependence matrix (GLDM)36 and neighborhood grey tone difference matrix (NGTDM)37. Additionally, features are calculated from wavelet, Laplacian of Gaussian (Log) with sigma values of 0.1, 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0, square, square root, logarithm, exponential, and gradient-filtered images as well as original, making the total number of radiomic features 1,925.
It is important to note that previous research has demonstrated that radiomic features are sensitive to voxel size38. Therefore, maintaining consistent voxel sizes across patients is essential for obtaining accurate and reliable radiomics feature calculations. In this study, CT resolution sizes varied from 0.3 × 0.3 × 0.5 mm to 1.3 × 1.3 × 5 mm. To address this variability, all CT images were resampled to a resolution of 1 × 1 × 1 mm3 using the basis spline algorithm (Bspline) to interpolate the HU values in the resampled voxels. Correspondingly, the nearest neighbor algorithm was employed to resample the masks to the same resolution. Subsequent to this, gray level normalization, a critical step for enhancing the comparability and robustness of radiomics features across different settings and patient variations, was applied. Previous studies have shown that gray level normalization reduces variance and enhances the robustness of radiomic features, particularly with respect to varying discretization levels38. Normalization was achieved by scaling the HU values to a uniform range across patients, specifically by subtracting the mean and dividing the voxel values by the standard deviation. Typically, this process results in a region of interest (ROI) with intensities approximately in the range of [-3, 3] after excluding outliers beyond three standard deviations. These values were then further scaled using a normalized scale parameter, such as a value of 100, resulting in an approximate range of [-100, 100]. Finally, the intensities within the ROI were discretized using a unified bin-width of 5, starting from the minimum normalized HU value of 0. In this study, a bin width of 5 was chosen to ensure an adequate number of bins (between 1 and 400), thereby capturing more granular textural information39. This discretization step assigns a new value to each voxel using the formula \(floor\left(\frac{origin\:intesity}{5}\right)+1.\) This discretization approach offers the advantage of noise suppression and improved robustness of radiomic features.
Feature selection
Pearson correlation analysis
In this study, we utilized an ensemble feature selection method to refine the input dataset for Cox proportional hazard modeling, addressing challenges associated with high-dimensional data. One big hurdle in high-dimensional data is multicollinearity, which occurs when there is a high correlation between two or more measurements in the data. Our previous study shows examples of multicollinearity among features25. There is now a substantial body of research on mitigating multicollinearity, such as Principal Component Analysis (PCA), Sparse PCA40, and Kernel PCA (KPCA)41,42. To mitigate multicollinearity, we first employed Pearson’s correlation coefficient to detect linear dependencies among radiomics features. The Pearson correlation ranges from − 1 to 1, with a value of 0 indicating no linear correlation. In the medical field, a Pearson’s score of 0.7 suggests a moderate agreement between two features based on previous studies43,44. Features with Pearson’s score above 0.7 in absolute value (highly correlated) were removed, resulting in 114 features for subsequent analysis. Figure 3 uses a color scheme where white represents no correlation, blue represents a perfect negative correlation, and red represents a perfect positive correlation. The left diagonal map illustrates the correlation coefficients prior to the feature selection process, revealing the initial relationships between features. The right diagonal map presents the correlation coefficients after highly correlated features have been removed, demonstrating the outcome of the feature selection process. The left diagonal map initially revealed numerous red and blue shades, indicating strong positive and negative correlations, respectively, among the data. By comparison, the right diagonal map is lighter, indicating these highly correlated features were subsequently removed from the data. These correlation measures provide insights into the relationships among radiomics features and aid in addressing multicollinearity to ensure more robust and accurate modeling in our study.
Correlation coefficient heatmaps: The diagonal heatmap illustrates the pairwise correlations among radiomics features before pruning. The color scale represents the strength of the correlation, with blue indicating negative correlation, red indicating positive correlation, and white representing no correlation. Panels A and B depict the feature pairs before and after pruning, respectively.
Feature filtering
The univariate Cox score is the most straightforward method for identifying features associated with variability in survival time in time-to-event data analysis. The screening procedure consists of two steps: fitting 114 univariate Cox proportional hazards models for all radiomics features and using either the Wald or score test statistic to assess the strength of association between each feature and the outcome. For a \(k\)-th radiomic feature of a patient, we have a hazard ratio
\({h}_{k}\) is the hazard function for \(k\)-th feature and \({\bar{h}}_{k}\) is the baseline hazard function. The radiomic feature filtering is based on the null hypothesis test \({\beta}_{k}=0\) (score or Wald test) for all 114 radiomic features, respectively. This technique selects features with P-values below a certain threshold long established in microarray data analysis45,46. We chose a threshold that maximizes predictive accuracy using cross-validated log-likelihood (CVL)47. Figure 4 shows the CVL curve for the data; it indicates that the Wald and score tests show maximum CVL (red triangle) at optimal P-values of 0.004 and 0.01, respectively. The Wald test selected 17 radiomic features with a P-value < 0.004, resulting in a CVL of -629 and a false discovery rate (FDR) of 0.027, meaning there are 17 × 0.027 = 0.459 falsely selected features. Similarly, the score test picked 24 radiomic features with a P-value < 0.01, resulting in a CVL of -642 and a false discovery rate (FDR) of 0.0475, with 24 × 0.0475 = 1.14 falsely selected features. In summary, the Wald test has better CVL and FDR values than the score test. RCVL1 and RCVL2, referred to as “re-substitution CVL”, establish an upper bound for the variation in the CVL value caused by random cross-validation. If the CVL value is below both RCVL1 and RCVL2, the CVL variation is considered to be within control. In our results, we determine that both CVLs are within control since they are less than both RCVL1 and RCVL2, respectively. It is worth noting that the maximum CVL of the Score test is close to the RCVL2.
Cox proportional hazards modelling
After feature selection, a multivariate Cox proportional hazards model was used to model patient prognosis. The model estimates the coefficients in the linear equation:
where \(h\left(t|{X}_{i}\right)\) is the hazard function for patient\(i\), assuming a constant hazard ratio over time. The coefficients \(\beta\) were determined by maximizing the partial likelihood function:
with \(E\) representing the indices of deceased or progressed patients and \({\bar{E}}_{r}\) the indices of surviving and non-progressed patients at the time \({t}_{r}\). A 10-fold cross-validation was then performed to assess model generalizability. The dataset was divided into 10 folds, ensuring that each fold maintains the same distribution of race and status as the overall dataset. The training model \({\widehat{\beta}}_{train}\) predicted biomarkers for the testing set, and the concordance index was averaged across all folds. We validated the model using cross-institution external data. The Log-rank test is used to assess the significance of the Cox model. The concordance index was primarily used to evaluate prognostic accuracy, and the univariate Wald score was employed to gauge the significance of individual features. All analyses were conducted using R version 4.3.0. The alpha level for significance was set at a two-sided P-value of 0.05.
Results
Feature selection
Based on the aforementioned ensemble feature selection methods, we identified a subset of 17 radiomic features for further analysis. Table 2 presents a detailed list of these 17 features grouped by their respective families.
To identify the most fitting subset of the 17 selected radiomic features, we performed a Best Subset selection on the training set using Cox regression analysis, with the concordance index (C-index) serving as the evaluation metric. Table 3 presents the summary of model selection for the Cox proportional hazards model of degrees of freedom ranging from 1 to 17. Each row represents the optimal model with the highest CI at that degree of freedom (d.f.), detailing the features selected, log-rank test statistic, score test statistic, training CI (concordance index), and independent CI. For example, the one-degree-of-freedom model reaches the highest training CI of 0.657 at Gradient-NGTDM-Busyness and a independent CI of 0.636 among all one-degree-of-freedom models, while the model with all 17 features has a training CI of 0.712 and a independent CI of 0.697. The highest training CI of 0.715 is observed in models with 5 degrees of freedom, with a corresponding independent CI of 0.69. We opted for model 5 due to its best performance. To fit a CPH model, it is critical to choose an appropriate degree of freedom that balances the complexity and accuracy of the model. According to48, a useful heuristic is to limit the number of predictors used in the fitting, which should be at most 10% of the events in the training sample. In our study, we observed 55 out of 77 events in MD Anderson data. Therefore, our target degree of freedom in the final model should be at most 5. This result also aligns with the regularized Cox regression (Lasso-Cox)49,50 10-fold cross-validation optimal model selection result, depicted in Fig. 5.
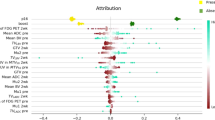
The optimal model, identified by the minimum lambda (the left vertical line) by Lasso-Cox, corresponds to 5 degrees of freedom in terms of partial likelihood (deviance) and concordance index (CI). The selected model in Table 4 revealed several notable associations. The features Logσ=0.1 3D glszm SmallAreaLowGrayLevelEmphasis and Exponential gldm LargeDependenceHighGrayLevelEmphasis have hazard ratios of 1.3 and 1.2, respectively. There is a 30% increase in the expected hazard relative to a one-unit increase in Logσ=0.1 3D glszm SmallAreaLowGrayLevelEmphasis, holding others constant. The expected hazard is 1.2 times higher in patients with one unit higher in Exponential gldm LargeDependenceHighGrayLevelEmphasis. Two other features, Gradient ngtdm contrast, and Logσ=3 3D Firstorder RootMeanSquared, have hazard ratios of 0.48 and 0.73, meaning a 52% and 27% drop in hazard for every one unit increase in them, respectively. Overall, the model’s performance was robust, achieving a concordance index of 0.72 (se = 0.028). In comparison, the model incorporating stage and smoking status, as shown in Supplementary Table ST1, achieved a slightly higher concordance index of 0.74 (se = 0.027), demonstrating good predictive discrimination. The likelihood ratio test yielded a chi-square value of 48.6 on 5 degrees of freedom (p = 3e-09). In contrast, the Wald test produced a chi-square value of 35.5 (p = 1e-06), and the score test resulted in a chi-square value of 37.1 (p = 6e-07), all indicating the model’s overall significance.
When testing the trained model on the external MD Anderson cancer dataset, the concordance index was 0.68, compared to 0.71 for the mixed model shown in Supplmentary Table ST2. Notably, one feature, Gradient Ngtdm Strength, remained significant with a hazard ratio of 0.54 and a significant likelihood ratio test, as presented in Table 5. This indicates the good generalization of this 5-degree freedom model.
Radiomics-based nomogram
Based on our model, we developed a nomogram in Fig. 6 that visually represents the CPH model presented in Table 4. This nomogram allows the estimation of progression survival for HNSCC patients after treatment. To use the nomogram, one simply needs to input the values of four variables and mark them on their respective axes. Connecting these marked values with vertical lines to the top scale (points scale) determines the points for each variable. Adding these points together and marking them on the total points axis provides the total points. By connecting the position of total points with the corresponding survival probability, one can estimate the overall outcomes based on the Linear Predictor.
The purpose of the nomogram in this study is to provide a practical and user-friendly tool for estimating overall survival in HNSCC patients after treatment. By integrating multiple prognostic factors into a graphical representation, the nomogram allows healthcare professionals to easily assess individual patient outcomes and make informed decisions regarding treatment strategies. The benefit of using a nomogram lies in its ability to incorporate complex statistical models into a visually intuitive format, enabling personalized risk prediction. It offers improved prognostic accuracy, individualized treatment planning, and enhanced communication between healthcare providers and patients. The nomogram serves as a valuable addition to clinical practice by facilitating shared decision-making and promoting precision medicine approaches in the management of HNSCC.
Discussion
This study aimed to assess the prognostic value of radiomics features in patients with HNSCC. Our results indicate that analyzing radiomics features from pre-treatment CT scans can offer valuable insights into the factors affecting progression-free survival and can serve as important prognostic biomarkers for this patient cohort.
Notable findings from our study include significant hazard ratios for radiomics features in relation to progression-free survival. The concordance index (CI) maintained a stable and high average value of 0.7, demonstrating good predictive accuracy of the prognostic model. These results underscore the potential of radiomics as a noninvasive and quantitative tool for treatment prognosis. To ensure accurate feature calculation, we standardized the voxel spacing across patients by resampling CT images and applied gray-level normalization to enhance feature comparability and robustness across different settings. These steps are essential for conducting reliable and reproducible radiomics analysis.
The Cox proportional hazards modeling revealed several significant radiomics features associated with survival in HNSCC patients. The continuous variables, Logσ=0.1 3D glszm SmallAreaLowGrayLevelEmphasis, Exponential gldm LargeDependenceHighGrayLevelEmphasis, Gradient ngtdm contrast and Logσ=3 3D Firstorder RootMeanSquared showed varying levels of significance in the model, indicating their potential as prognostic biomarkers. The performance of our Cox model was assessed using log-likelihood ratio and score tests. These results suggest that our Cox model provides robust predictive performance for survival in HNSCC patients.
Despite highlighting the potential of radiomics in HNSCC prognostication, it is important to acknowledge the study’s limitations. A limitation of this study is the difference in clinical distributions between the training and independent set, such as the absence of AJCC stage IV patients in the independent cohort. While this highlights the biomarkers’ potential generalizability across heterogeneous populations, it limits direct comparability of the results. The relatively small sample size might affect the generalization of the validated radiomics features in our model. To support a low-biased and variance survival model with five biomarkers, it is recommended to have at least 50 events in the training set, necessitating a sample size containing 84 events if 60% is allocated for the training set. This criterion limits the degrees of freedom our model can reach, potentially impacting the prognostic ability of the radiomics features. Furthermore, the current analysis focused on extracting radiomics features from a single imaging modality, namely CT. Future research should explore radiomics features from multiple modalities, such as CT and MRI, to potentially enhance prediction accuracy. Additionally, the significant association between certain radiomics features and progression-free survival suggests that imaging features might reflect some underlying molecular characteristics of the tumors. Last but not least, smoking status is not significant across all four PFS periods. We hypothesize that the smoking status captures only a history of smoking, without accouting for cessation during treatment. Since smoking cessation is believed to improve treatment outcomes51,52, this may diminish the observed prognostic impact of baseline smoking status.
Future studies should aim to integrate genetic data, such as TP53 mutations and P16 overexpression, with radiomics data to provide a noninvasive, multi-modal approach to predicting outcomes in HNSCC.
Conclusion
In summary, our study demonstrated the potential of radiomics as a valuable tool for predicting treatment response in HNSCC patients. Incorporating radiomics analysis into clinical practice could improve decision-making and patient stratification, thereby reducing both over-treatment and under-treatment to enhance outcomes. The findings from this study pave the way for future investigations through larger clinical trials to further evaluate the clinical efficacy of radiomics biomarkers for progression-free survival prediction in HNSCC patients.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to institutional policy but are available from the corresponding author upon reasonable request.
Change history
21 February 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-90567-2
References
Barsouk, A., Aluru, J. S., Rawla, P., Saginala, K. & Barsouk, A. Epidemiology, risk factors, and prevention of head and neck squamous cell carcinoma. Med. Sci. 11, 42 (2023).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 71, 209–249 (2021).
Gormley, M., Creaney, G., Schache, A., Ingarfield, K. & Conway, D. I. reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. Br. Dent. J. 233, 780–786 (2022).
Johnson, D. E. et al. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers. 6, 92 (2020).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. (2024).
Licitra, L., Locati, L. & Bossi, P. Head and neck cancer. Ann. Oncol. 15, iv267–iv273 (2004).
Duprez, F. et al. Distant metastases in head and neck cancer. Head Neck. 39, 1733–1743 (2017).
Hashibe, M. et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidemiol. Biomark. Prev. 18, 541–550 (2009).
Gormley, M. et al. A multivariable mendelian randomization analysis investigating smoking and alcohol consumption in oral and oropharyngeal cancer. Nat. Commun. 11, 6071 (2020).
Tumban, E. A current update on human papillomavirus-associated head and neck cancers. Viruses 11, 922 (2019).
Fuller, C. et al. Adjunctive diagnostic techniques for oral lesions of unknown malignant potential: Systematic review with meta-analysis. Head Neck 37, 755–762 (2015).
Menini, M. et al. Salivary micro-RNA and oral squamous cell carcinoma: A systematic review. J. Pers. Med. 11, 101 (2021).
Al Rawi, N. et al. The role of differentially expressed salivary microRNA in oral squamous cell carcinoma. A systematic review. Arch. Oral Biol. 125, 105108 (2021).
D’Souza, W. & Kumar, A. microRNAs in oral cancer: Moving from bench to bed as next generation medicine. Oral Oncol. 111, 104916 (2020).
Liu, C. J., Lin, S. C., Yang, C. C., Cheng, H. W. & Chang, K. W. Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck. 34, 219–224 (2012).
Momen-Heravi, F., Trachtenberg, A., Kuo, W. & Cheng, Y. Genomewide study of salivary microRNAs for detection of oral cancer. J. Dent. Res. 93, 86S–93S (2014).
Nagler, R., Bahar, G., Shpitzer, T. & Feinmesser, R. Concomitant analysis of salivary tumor markers—A new diagnostic tool for oral cancer. Clin. Cancer Res. 12, 3979–3984 (2006).
Balan, J. J., Rao, R. S., Premalatha, B. & Patil, S. Analysis of tumor marker CA 125 in saliva of normal and oral squamous cell carcinoma patients: A comparative study. J. Contemp. Dent. Pract. 13, 671–5 (2012).
Gillison, M. et al. Analysis of the effect of p16 and tobacco pack-years (py) on overall (OS) and progression-free survival (PFS) for patients with oropharynx cancer (OPC) in radiation therapy oncology group (RTOG) protocol 9003. J. Clin. Oncol. 28, 5510–5510 (2010).
Grandis, J. R. & Tweardy, D. J. TGF-α and EGFR in head and neck cancer. J. Cell. Biochem. 53, 188–191 (1993).
Poeta, M. L. et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 357, 2552–2561 (2007).
Michalides, R. et al. Overexpression of cyclin D1 correlates with recurrence in a group of forty-seven operable squamous cell carcinomas of the head and neck. Cancer Res. 55, 975–978 (1995).
Fan, M. et al. Radiomic analysis of imaging heterogeneity in tumours and the surrounding parenchyma based on unsupervised decomposition of DCE-MRI for predicting molecular subtypes of breast cancer. Eur. Radiol. 29, 4456–4467 (2019).
Aerts, H. J. et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 5, 4006 (2014).
Ling, X. et al. Identification of CT-based non-invasive radiomic biomarkers for overall survival prediction in oral cavity squamous cell carcinoma. Sci. Rep. 13, 21774 (2023).
Ren, L. et al. Radiomic biomarkers of Locoregional recurrence: Prognostic insights from oral cavity squamous cell carcinoma preoperative CT scans (2024).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Grossberg, A. J. et al. Imaging and clinical data archive for head and neck squamous cell carcinoma patients treated with radiotherapy. Sci. Data 5, 1–10 (2018).
Amin, M. B. et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more personalized approach to cancer staging. Cancer J. Clin. 67, 93–99 (2017).
Grossberg, A. et al. Data from head and neck cancer CT atlas. Cancer Imaging Arch. 10, K9 (2017).
van Griethuysen, J. J. M. et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 77, E104–E107 (2017).
Zwanenburg, A. et al. The image biomarker standardization initiative: Standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295, 328–338 (2020).
Galloway, M. M. Texture analysis using gray level run lengths. Comput. Graphics Image Process. 4, 172–179 (1975).
Thibault, G., Fertil, B. & Navarro, C. Texture indexes and gray level size zone matrix: Application to cell nuclei classification in proceedings of the pattern recognition and information processing 2009. In International Conference on Pattern Recognition and Information Processing (PRIP’09) 140–145.
Thibault, G., Angulo, J. & Meyer, F. Advanced statistical matrices for texture characterization: Application to cell classification. IEEE Trans. Biomed. Eng. 61, 630–637 (2013).
Sun, C. & Wee, W. G. Neighboring gray level dependence matrix for texture classification. Comput. Vis. Graphics Image Process. 23, 341–352 (1983).
Amadasun, M. & King, R. Textural features corresponding to textural properties. IEEE Trans. Syst. man. Cybern. 19, 1264–1274 (1989).
Shafiq-ul-Hassan, M. et al. Intrinsic dependencies of CT radiomic features on voxel size and number of gray levels. Med. Phys. 44, 1050–1062 (2017).
Larue, R. T. et al. Influence of gray level discretization on radiomic feature stability for different CT scanners, tube currents and slice thicknesses: A comprehensive phantom study. Acta Oncol. 56, 1544–1553 (2017).
Ling, X. & Brooks, J. P. L1-norm regularized L1-norm best-fit line problem. arXiv:2010.04684 (2020).
Bui, A. T., Im, J. K., Apley, D. W. & Runger, G. C. Projection-free kernel principal component analysis for denoising. Neurocomputing 357, 163–176 (2019).
Ling, X., Bui, A. & Brooks, P. Kernel l1-norm principal component analysis for denoising. Optim. Lett. https://doi.org/10.1007/s11590-023-02051-3 (2023).
Akoglu, H. User’s guide to correlation coefficients. Turkish J. Emerg. Med. 18, 91–93 (2018).
Chan, Y. Biostatistics 104: Correlational analysis. Singap. Med. J. 44, 614–619 (2003).
Lazar, C. et al. A survey on filter techniques for feature selection in gene expression microarray analysis. IEEE/ACM Trans. Comput. Biol. Bioinf. 9, 1106–1119 (2012).
Tusher, V. G., Tibshirani, R. & Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. 98, 5116–5121 (2001).
Matsui, S. Predicting survival outcomes using subsets of significant genes in prognostic marker studies with microarrays. BMC Bioinform. 7, 1–9 (2006).
Harrell, F. E. Jr, Lee, K. L. & Mark, D. B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 15, 361–387 (1996).
Simon, N., Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for Cox’s proportional hazards model via coordinate descent. J. Stat. Softw. 39, 1 (2011).
Hastie, T., Qian, J. & Tay, K. An introduction to glmnet. CRAN R Repos.. 5, 1–35 (2021).
Smaily, H. et al. Smoking cessation intervention for patients with head and neck cancer: A prospective randomized controlled trial. Am. J. Otolaryngol. 42, 102832 (2021).
McCarter, K. et al. Smoking cessation care among patients with head and neck cancer: A systematic review. BMJ Open. 6, e012296 (2016).
Acknowledgements
Xiao Ling and Lei Ren were supported by the National Institutes of Health (NIH) grants R01DE033426, R01EB032680 and U54CA273956. Daria A. Gaykalova was supported by a Research Scholarship Grant, RSG-21-020-01-MPC from the American Cancer Society, and R01DE027809 from the National Institute of Health.
Author information
Authors and Affiliations
Contributions
X.L: Methodology, Software, Formal analysis, Writing- Original draft S.B. , M.F., J.M., E.A., H.T., D.A.: Resources, Writing- Reviewing and Editing L.S., R.k., W.S.M.: Resources, Data curation, Writing- Reviewing and Editing R.M.: Investigation D.A.G.: Investigation, Validation, Conceptualization, Writing- Reviewing and Editing P.T.H, A.S., L.R.: Resources, Conceptualization, Supervision, Project administration, Writing- Reviewing and Editing, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study protocol complied with the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Maryland School of Medicine (approval no. 00007145). All patients provided written informed consent prior to enrollment.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The Acknowledgments section in the original version of this Article was incomplete. It now reads: “Xiao Ling and Lei Ren were supported by the National Institutes of Health (NIH) grants R01DE033426, R01EB032680 and U54CA273956. Daria A. Gaykalova was supported by a Research Scholarship Grant, RSG-21-020-01-MPC from the American Cancer Society, and R01DE027809 from the National Institute of Health.”
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ling, X., Bazyar, S., Ferris, M. et al. Identification of CT based radiomic biomarkers for progression free survival in head and neck squamous cell carcinoma. Sci Rep 15, 1279 (2025). https://doi.org/10.1038/s41598-025-85498-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85498-x