Abstract
Vibrio parahaemolyticus is pathogenic to both humans and marine animals. Antimicrobial-resistant (AMR) bacteria have been reported to cause mortalities in shrimp, with phage therapy presenting an alternative and eco-friendly biocontrol strategy for controlling bacterial diseases. Therefore, this study aimed to isolate and characterize phages for their applicability in lysing Vibrio parahaemolyticus. A novel phage vB_VpaS_BP15 (BP15) belonged to the subfamily Queuovirinae with an icosahedral head measuring 69.11 ± 5.38 nm in length and 65.40 ± 6.89 nm in width, and a non-contractile sheathed tail measuring 139.81 ± 14.79 nm. The one-step growth curve indicated a latent period of 30 min and a burst size of 120 PFUs per cell. Phage BP15 exhibited tolerance to a range of temperatures and pH values. Infection dynamic curves demonstrated that BP15 was highly effective against BCRC12959 at MOIs ranging from 0.01 to 10; even at a low multiplicity of infection (MOI) of 0.001, BP15 still caused growth retention. Phage BP15 possessed a circular double-stranded DNA of 59,584 bp with a G + C content of 46.7% and lacked tRNA genes, virulence genes, and lysogeny genes. These findings highlight the promising potential of phage BP15 as a biocontrol agent against Vibrio parahaemolyticus in Taiwan.
Similar content being viewed by others
Introduction
The bacterial species belonging to the genus Vibrio pose a serious threat to both humans and marine animals, causing diseases such as vibriosis, typically characterized by symptoms such as diarrhea, abdominal cramping, nausea, vomiting, fever, and chills. Given the rise of global cases of vibriosis attributed to climate change, there is a growing need for a deeper understanding of Vibrio epidemiology and human transmission1. Among the over 20 species of Vibrio contributing to vibriosis, including V. parahaemolyticus, V. harveyi, V. alginolyticus, V. campbelli, V. penaeicida, V. splendidus, V. fluvialis, and V. tubiashii, V. parahaemolyticus is the most significant2, causing an estimated 80,000 illnesses and 100 deaths in the United States every year (Centers for Disease Control and Prevention, CDC). Additionally, according to the Taiwan Food and Drug Administration (TFDA), the incidence of food poisoning caused by Vibrio parahaemolyticus ranks first in Taiwan.
Viral diseases are the most severe and widespread infectious diseases in aquatic crustaceans, encompassing various conditions such as white spot syndrome, Taura syndrome, and yellow head disease. However, among the major bacterial diseases in crustaceans, vibriosis stands out as one of the most severe in aquaculture. It is estimated that approximately 60% of disease losses in shrimp aquaculture are caused by viral pathogens, with bacterial pathogens accounting for approximately 20% 3. Vibriosis, a bacterial disease, is responsible for mortality in cultured shrimp worldwide4,5. Infections caused by pathogenic or opportunistic Vibrio bacteria can be devastating, particularly during the larval production stage of crustaceans, as reported in several countries6,7,8,9. V. harveyi and V. parahaemolyticus are important bacterial pathogens of penaeid shrimp that have been linked to mass mortalities. These diseases can significantly impact the aquaculture industry, resulting in substantial economic losses. Therefore, vibriosis poses a threat to meeting the growing food demand and global food security.
In shrimp aquaculture, the use of antibiotics for the control of Vibrio bacteria has led to the emergence of antimicrobial resistance (AMR) in Vibrio species, with antimicrobial-resistant Vibrio reportedly causing mortalities in Penaeus monodon shrimp larvae and Litopenaeus vannamei shrimp10,11. Some report indicted antimicrobial susceptibility pattern was determined in shrimp aquaculture, many strains of Vibrio isolated from the L. vannamei shrimp and identified phenotypically. A high antibiotic-resistance index was observed, with phenotypic profiles including monoresistance, cross-resistance to β-lactams, and multiple resistance, particularly in China, Thailand, Vietnam, India, Malaysia, and Brazil12,13. Due to the emergence of AMR strains, the use of antibiotics in food production has come under stricter scientific and public scrutiny14.
In the past, farmers from Taiwan and other countries worldwide relied excessively on drugs and antibiotics, with AMR bacteria being often detected in the environment15,16. However, in recent years, farmers (both in agriculture and aquaculture) have gradually embraced green agriculture. Among the various alternatives to antibiotics proposed to date, phage therapy may offer a better option for controlling diseases of crops and aquatic organisms. Bacteriophages (phages), which are viruses that kill bacteria, are being actively considered as biocontrol agents in various research and development areas17,18,19,20,21,22. In aquaculture, the application of various phages has been successful in treating many diseases, including lactococcosis, bacterial hemorrhagic ascites disease, vibriosis, luminescent vibriosis, hemorrhagic septicemia, severe epizootics skin ulceration syndrome (SUS), massive mortality of Pacific oyster larvae, septicemia, edwardsiellosis, enteric septicemia, rainbow trout fry syndrome (RTFS), bacterial coldwater disease (CWD), columnaris disease, and furunculosis. Pathogenic bacteria that have been successfully treated via phage therapy include Vibrio sp., Aeromonas sp., Flavobacterium sp., Edwardsiella sp., Pseudomonas plecoglossicida strain PTH-9802, and Lactococcus garvieae23,24,25,26. In recent years, many phages with potent antibacterial activity have been isolated from natural environments. Among these phages, BA3, CA8, VP882, pVp1, ϕVP-1, Vpms1, A3S, VpKK5, VPp1, and VVP1 have been proven effective against V. parahaemolyticus24,27,28,29,30. However, these phages may not work globally due to their high specificity.
Here, we isolated and characterized a novel phage specifically infecting Taiwanese V. parahaemolyticus strains. We also evaluated the potential efficacy of the isolated phage used infection dynamics, and conventional phage study. For application as biocontrol agents, we further analyze the complete genomic characterization, antibiotic-resistance genes (ARGs), and virulence genes of phage by whole genome sequencing31,32. This report will further expand our knowledge about V. parahaemolyticus phages and their future applications as novel therapeutic or biocontrol agents.
Results
Morphology and biological characteristics of phage BP15
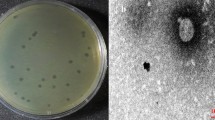
Phage BP15 was isolated from an oyster sample collected from Taisi Township in Yunlin County, Taiwan. When propagated on its host strain, BCRC12959, BP15 formed plaques on the bacterial lawn with a clear round morphology and a well-defined boundary (Fig. 1a). The plaque size was approximately 2–5 mm in diameter after overnight infection at 30 °C. Transmission electron microscopy (TEM) revealed that BP15 had an icosahedral head measuring 69.11 ± 5.38 nm in length and 65.40 ± 6.89 nm in width, with a non-contractile sheathed tail measuring 139.81 ± 14.79 nm (n = 20) (Fig. 1b). Based on the recommendation of the Bacterial and Archaeal Viruses Subcommittee (BAVS) of the International Committee on Taxonomy of Viruses (ICTV), phage BP15 was classified as a member of the family Siphoviridae within the order Caudovirales. This phage was designated as vB_VpaS_BP15, following the naming conventions recommended by Kropinski et al.33,34. BP15 could be released from burst pores in V. parahaemolyticus BCRC12959 (Fig. 1c). The results of the spot inoculation test indicated that phage BP15 only lysed the test strains of V. parahaemolyticus which were isolated from aquaculture fields in Taiwan. Conversely, phage BP15 did not lyse other strains of V. parahaemolyticus from other countries. Thus, phage BP15 exhibited a narrow lytic spectrum, specifically infecting only Taiwanese strains of V. parahaemolyticus among the tested species (Table 1).
Morphology of phage BP15. (a) Plaque morphology of BP15 after incubation with V. parahaemolyticus BCRC12959 on a double-layer agar plate after overnight culture. Smaller size plaque was approximately 2 mm (red arrow) and the larger was 5 mm in diameter (black arrow). Scale bar equals to 1 cm. (b) Transmission electron micrographs of BP15 with tail. Morphology of phage BP15 as determined by Negative staining and TEM. BP15 had an icosahedral head measuring 69.11 ± 5.38 nm in length and 65.40 ± 6.89 nm in width, with a non-contractile sheathed tail measuring 139.81 ± 14.79 nm (n = 20). Scale bar equals to 200 nm. (c) Release of phage BP15 from the BCRC12959 cell (black arrow). Scale bar equals to 200 nm.
To gain further insights into the biological properties of phage BP15, we conducted a one-step growth curve, a phage tolerance test, and measured the phage adsorption rate. The results of our phage adsorption assay revealed that more than 80% of the phage propagated within the cell during the latent period, which lasted for at least 30 min (Fig. 2a-b). The latent period represents the minimum time required from phage adsorption to lysis of BCRC12959, leading to the release of progeny virions. The burst size for phage BP15, defined as the average number of progeny virions liberated by one infected BCRC12959 cell at the completion of a growth cycle, was estimated at 120 virions plaque-forming units (PFU) per cell (Fig. 2b). It is worth noting that short latent periods (< 30 min) and larger burst sizes (> 100 PFU per cell) have been observed for other Siphoviridae phages infecting V. parahaemolyticus in Table 2.
Biological properties of phage BP15 against V. parahaemolyticus BCRC12959. (a) Adsorption velocity, (b) one-step growth curve, (c) phage viability in different temperatures, (d) phage viability in different pH, (e) phage viability in different concentrations of chloroform. All values are reported as averages from triplicate experiments (n = 3). The error bars represent the 95% confidence interval of the data set. Statistically significant differences were determined by unpaired Student t test (* p < 0.05 and ** p < 0.005).
Furthermore, phage BP15 exhibited high tolerance to a broad range of pH values, temperatures, and chloroform exposure (Fig. 2c-e). Our thermal stability study revealed that the phage remained active at temperatures ranging from 4 to 50 °C, while phages pre-treated at temperatures above 60 °C significantly lost their infectivity (Fig. 2c). As illustrated in Fig. 2d, the phage demonstrated optimal infectivity rates within a pH range of 6–8, with infectivity completely lost at pH 2 and 12 (Fig. 2d). Notably, phage BP15 displayed insensitivity to chloroform (Fig. 2e), indicating the absence of lipids in the viral capsid.
Infection dynamics of phage BP15
Given that the bacterial inactivation efficiency of phages is a crucial property for potential candidates in phage therapy, this study examined the lytic activity of phage BP15. As depicted in Fig. 3, the OD600 values of the culture containing BCRC12959 alone (bacterial control, MOI 0) steadily increased from approximately 0.04 to 2.25 within 330 min. In contrast, the OD600 values of cultures containing both phage BP15 and BCRC12959 at different MOIs gradually rose during the initial hours and then notably declined, indicating the lysis of BCRC12959 by phage BP15. The mortality curves demonstrated that BP15 was highly effective against BCRC12959 at MOIs ranging from 0.01 to 10 tested, with even minimal quantities of phage BP15 (MOI 0.001) still causing growth retention (Fig. 3). This study reported phage BP15 has highly effective inactivation in the prevention and control of V. parahaemolyticus in a short time. Therefore, the result shows a good application potential in the treatment of pathogens.
Infection dynamics of phage BP15 at various multiplicities of infection (MOIs) against V. parahaemolyticus BCRC12959. Five different MOIs (0.001, 0.01, 0.1, 1, and 10) were used for infecting BCRC12959. The green line represents a standard growth curve of BCRC12959 in solution (i.e., bacterial control). All values are reported as averages from triplicate experiments (n = 3). The error bars represent the 95% confidence interval of the data set.
Characterization of phage BP15 genome
Whole-genome sequencing revealed that the phage BP15 genome consisted of a 59,584-bp circular double-stranded DNA with a G + C content of 46.7%. The genome of phage BP15 contained 66 putative open reading frames (ORFs) located on both the forward and reverse strands, with an average length of 670 bp and sizes ranging from 147 to 2,442 nucleotides. The majority of ORFs in the genome started with an ATG start codon (80.3%), with only 12 starting with GTG and 1 starting with TTG. No transfer RNA (tRNA) gene, virulence genes, or lysogeny genes (e.g., integrase) were detected. Among the 66 putative ORFs in the BP15 genome, 34 were assigned to hypothetical proteins, and 16, 12, 4, and 2 were predicted to encode proteins associated with DNA replication and modification, structural proteins and packaging proteins, lysis functions, and additional modules, respectively (Fig. 4) (Table S1) (Fig. S2).
Phylogenetic and comparative genomic analysis of phage BP15
Phylogenetic trees of phage BP15 were constructed using the whole genome (Fig. 5a) and targeting single gene analysis of the DNA polymerase A gene (Fig. S1a), terminase large subunit protein (Fig. S1b), and major capsid protein (Fig. S1c). The results from the whole genome phylogenetic tree indicated that phage BP15 is closely related to pVco-14 (NCBI Accession No: MW114771), AQKL99 (NCBI Accession No: MT795651), tm (NCBI Accession No: KX198614), CHI (NCBI Accession No: ON457559), and ALK (NCBI Accession No: ON457558). Both the phylogenetic tree of the genome and intergenomic similarity heatmaps revealed that phage BP15 is a new member of the Queuovirinae subfamily but remains unclassified at the genus level in the ICTV database. Only a few members of the Queuovirinae subfamily have been classified into the Nonagvirus, Seuratvirus, Amoyvirus, and Nipunavirus genera, while many vibriophages remain unclassified. The similarity between phage BP15 and pVco-14 was as high as 89.5%, with the similarity between phage BP15 and AQKL99, CHI, ALK, and tm being also high, ranging from 63.7 to 73.2%. The other phages included in the genomic comparisons were very distant from these five strains, with a similarity of only 10.0% (Fig. 5b). The result was performed using EasyFig with phage BP15 and its closely related vibriophages, showing high homology among phages BP15, pVco-14, AQKL99, CHI, tm, and ALK. Phage BP15 harbored DNA replication, modification, structural, packaging, and host lysis modules, as did pVco-14, AQKL99, tm, CHI, and ALK. No differences were observed in the arrangement of functional modules among them, and the nonhomologous regions were putative hypothetical proteins (Fig. 5c). The genome of phage BP15 was predicted to encode one lysozyme (orf 54), one holin (orf 55), and two glycosyl hydrolase (orf 11 and 12), which were similar to pVco-14 but no other phages. The results of phylogenetic trees targeting single genes showed that phage BP15 is closely related to AQKL99, CHI, and ALK but more distantly related to pVco-14 (Fig. S1).
Genomic and phylogenetic analysis of phage BP15. (a) Phylogenetic tree based on Genome-BLAST Distance Phylogeny (GBDP) of phage BP15 and 37 related members of the Queuovirinae subfamily from the ICTV database. (b) VIRIDIC heatmap based on intergenomic similarities amongst viral genomes, with the color scale indicating similarity percentages. (c) Genome comparison of phage BP15 and closely related members using Easyfig. CoDing sequence (CDS) are shown as arrows to indicate the direction of transcription and are mentioned in the bottom legend in accordance with their predicted function. The percentage of sequence similarity is shown as the intensity of the gray to black color.
Discussion
The use of phage as therapeutic agents in aquatic animal health management has gained renewed interest due to the AMR pathogens and safety issues related to antibiotic residues in food products. In the present study, a vibriophage, named vB_VpaS_BP15 (BP15), was isolated from the oyster. Phage BP15 produced clear plaques on double-layer agar plate seeded with bacterial host. The morphology of phage BP15 resembled those of PG28, Vpkk5, pVp-1, SHOU24, and Mar10, which belong to the Siphoviridae family (Table 2) with icosahedral heads, and sheathed tails. Other studies on phages infecting V. parahaemolyticus have reported different morphologies35,36,37,38,39, with some even identifying different species within taxonomic groups. These phages were isolated from various countries, including Taiwan, China, Malaysia, South Korea, and Mexico. Different phages can be isolated from any environment where their host is found. However, factors such as the enrichment type and incubation conditions can affect the rate of phage recovery in these samples40,41,42.
Phage BP15 showed in vitro lytic activity against the test V. parahaemolyticus isolated from Taiwan’s aquaculture field (Table 1). Maybe the local strains are closely related in the phylogenetic tree and are similar to the outer membrane protein that mediates phage infection43,44,45,46. According to the adsorption velocity and one-step growth curve study (Fig. 2a-b), phage BP15 displayed an adsorption time (~ 30 min), a short latency period (~ 30 min), and a burst size (~ 120 PFUs per cell) under standard protocol. The larger burst sizes and shorter latent periods than half of the phages infecting V. parahaemolyticus (Table 2). For obligately lytic phages, there is a trade-off between burst size and latency. This trade-off arises because the release of phage progeny by infected bacteria coincides with the destruction of the machinery needed to produce more phage progeny47. Therefore, phages with short latent periods at the expense of their burst size might be considered reproductive specialists48. The powerful lytic activity over a short period may be attributed to the short latency of phage BP15. Phage BP15 demonstrated high tolerance to a wide range of pH and temperatures (Fig. 2c-d), with all biological properties resembling those of other phages25,35,49,50. The observed insensitivity of phage BP15 to chloroform (Fig. 2e) aligns with the outcomes of previous reports that lipids are rare among phages, occurring in less than 4% of isolates51.
As shown in Fig. 3, the OD600 values of the culture containing BCRC12959 alone (bacterial control) increased continuously from about 0.03 to 2.20 within 330 min. In contrast, the OD600 values at different MOIs increased gradually during the first 3 h and then decreased remarkably, indicating that BCRC12959 was lysed by phage BP15. Although completely eradicating BCRC12959 is difficult, phage BP15 could continuously stabilize and suppress the host bacterial population, even at an MOI of only 0.001. Bacteria can resist phage infection through various mechanisms. The initial step of phage infection involves adsorption to cellular receptors on the bacterial surface. Some bacterial strains have developed mechanisms to prevent this crucial process, such as receptor blockade, extracellular matrix production, and the production of competitive inhibitors. Following adsorption, the second step is the injection of phage DNA into the host cell. However, this process can be hindered by various bacterial defense mechanisms. For instance, superinfection exclusion (Sie) prevents the injection of phage DNA by already infected cells. Additionally, the bacterial restriction modification system can destroy incoming phage DNA. Furthermore, bacteria possess adaptive immunity mechanisms, such as the clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated genes (CRISPR-Cas) systems, which constitute the ultimate defense against incoming foreign DNA52,53,54,55. Initial phage-induced lysis of a bacterial population is followed by bacterial regrowth, driven by the selection of phage-resistant subpopulations56. The antagonistic coevolution between bacterial hosts and infecting phages is considered a significant driving force in the ecological and evolutionary processes of microbial communities.
The genome analysis of phage BP15 indicated a genome size of 59.58 kb with G + C content of 46.7% (Fig. 4) (Table 2). The whole genome features of phage BP15 were compared to those of other vibriophages previously reported to be active on V. parahaemolyticus clade bacteria. The genome size of phage BP15 was similar to that of ALK, CHI, AL-2, BA3, and CA8, but smaller than the jumbo vibriophages VP-1 and Vpkk5. The G + C content of phage BP15 was comparable to that of the previously reported vibriophages (ranging from 39.71 to 53.1%). Phage BP15 also exhibited fewer ORFs with predicted functions compared to AL-2 in the Queuovirinae subfamily. Jumbo-sized vibriophage phi-pp2, with a genome size larger than 200 kb, had 383 ORFs (Table 2). Phage BP15 harbored DNA replication, modification, structural, packaging, and host lysis modules, as the same with pVco-14, AQKL99, tm, CHI, and ALK (Fig. 5). The host lysis modules could encode an endolysin that produced at the later stage of phage replication and would be able to hydrolyze the bacterial peptidoglycan layer of the host cell wall from within, leading lysis of the host bacterium and release newly assembled phage particles57. Phage BP15 genome did not contain tRNA genes, ARGs, integrase, or bacterial virulence genes, making it a suitable candidate for in vivo phage applications to control vibriosis in shrimp aquaculture.
Bacteriophages, which are viruses that infect bacteria, are among the most ubiquitous and diverse entities in the biosphere. There is growing evidence of their significant roles in shaping the structure of various microbiomes. The taxonomic classification of bacteriophages has undergone recent changes. Assigning phages to different taxonomic groups following their discovery is a fundamental step, and the BAVS classifies phages based on various properties, including the molecular composition of the genome (ss/ds, DNA, or RNA), morphology, capsid structure, and host range58. Recently, the development of sequencing technologies and comparative genomic analyses has highlighted the need for a better taxonomic classification with wider acceptance59,60,61. However, due to the lack of a standard and automated virus classification pipeline, the taxonomic characterization of new viruses significantly lags behind sequencing efforts. In August 2022, the ICTV updated the phage classification system and removed the Podoviridae, Myoviridae, and Siphoviridae families62. These changes can significantly affect family classification performance. At the time of writing this article, 7 orders, 63 families, 109 subfamilies, 1360 genera, and 4079 species have been recognized within the class Caudoviricetes. The Queuovirinae subfamily includes four genera (Amoyvirus, Nipunovirus, Nonagvirus, and Seurotvirus), as well as other unclassified phages. Phage BP15 is closely related to pVco-14, AQKL99, tm, CHI, and ALK, which belong to the Queuovirinae subfamily according to the phylogenetic trees and comparative genomic analyses results (Fig. 5 and Fig. S1).
Conclusion
In this study, we isolated and characterized a novel lytic phage, vB_VpaS_BP15, targeting V. parahaemolyticus, a pathogen affecting both humans and marine animals in Taiwan. Phage BP15 belongs to the subfamily Queuovirinae (previous taxonomy is family Siphoviridae). BP15 exhibited a narrow host spectrum, high lytic activity, large burst size, and a short latent period. The circular double-stranded DNA of phage BP15 spans 59,584 base pairs with a G + C content of 46.7% and lacks tRNA genes, virulence genes, and lysogeny genes. These biological and genetic properties suggest the potential of phage BP15 as a biocontrol agent against vibriosis induced by V. parahaemolyticus. However, further experiments are necessary to fully explore its potential as a phage therapy agent. These experiments should investigate aspects such as animal models, administration convenience, dosage, timing, product stability, shelf life, drug compatibility, side effects, attractiveness, and palatability18,22,63,64,65. These follow-up studies will provide valuable insights into the practical application of phage BP15 in combating vibriosis caused by V. parahaemolyticus in the aquaculture industry.
Materials and methods
Bacterial strains and growth condition
In this study, a total of 19 Vibrio sp. were tested. Among these, 12 V. parahaemolyticus strains from Taiwan and seven other Vibrio sp. were generously provided by Dr. Chu-Fang Lo from the International Center for the Scientific Development of Shrimp Aquaculture, National Cheng Kung University (NCKU), Taiwan (Table 1). All strains were cultivated in Luria-Bertani (LB) broth with 3% NaCl at 30 °C in a shaker at 200 rpm. Stock cultures were preserved in LB with 3% NaCl containing 30% (v/v) glycerol at − 80 °C.
Identification of bacterial strains
The bacteria were streaked onto thiosulfate-citrate-bile salts-sucrose agar (TCBS) plates (BD Co. Ltd, Germany) and then incubated overnight at 30°C. Colonies displaying blue to green centers on TCBS plates were identified using PCR assays and the Sanger sequencing method (Seeing Co. Ltd, Taiwan). The PCR assay targeted the 16S rDNA gene. A 1200 bp DNA fragment of the 16S rDNA gene was amplified using gene-specific primers (UNI-L: 5’-AGA GTT TGA TCA TGG CTC AG-3’ UNI-R: 5’-GTG TGA CGG GCG GTG TGT AC-3’) as described by Kuhnert et al. (1996)66. The amplification process involved an initial denaturation at 95 °C for 12 min, followed by 35 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 1.5 min, with a final extension at 72 °C for 10 min. Subsequently, an aliquot of the PCR products was analyzed via electrophoresis on a 1.5% agarose gel.
Phage isolation, purification and preparation
The oysters used for phage isolation were collected from Taisi Township in Yunlin County, Taiwan. The tissues were homogenized in SM buffer containing 10 mM MgSO4, 50 mM Tris-HCl, 1% gelatin, and 100 mM NaCl (pH 7.5). After homogenization, the mixture was centrifuged at 8,000 rpm for 5 min, after which the suspension was filtered through a 0.22 μm membrane (Millipore, Massachusetts, USA) to eliminate bacteria and large particles. Subsequently, the samples were introduced into an exponentially growing host bacterial culture to facilitate plaque formation using the double-layer agar method. Individual clear plaques were collected and suspended in SM buffer. After three rounds of purification, well-separated plaques were collected and stored in SM buffer at 4 °C.
To obtain a high-titer phage suspension, a purified phage plaque was inoculated into an exponentially growing host bacterial culture in phage medium (LB-3% NaCl, 10 mM MgSO4, and 1 mM CaCl2) and amplified overnight. Afterward, the mixture was centrifuged at 8,000 rpm for 20 min. The resulting suspension was filtered through a 0.22 μm membrane to eliminate cell fragments. The filtrate was then precipitated with L2 buffer (containing 30% w/v polyethylene glycol 8,000 and 3 M NaCl) at 4 °C overnight. Subsequently, phage pellets were obtained via centrifugation (at 13,000 × g for 30 min at 4 °C) and resuspended in SM buffer. The phage particles were stored at 4 °C in SM buffer for up to a month or at − 80 °C in 30% (v/v) glycerol.
Transmission electron microscopy
The morphology of the phage was characterized using transmission electron microscopy (TEM). A drop of phage suspension was applied onto the surface of a Formvar-coated EM grid and allowed to adsorb for 1–3 min. The grid was then blotted with filter paper and stained with 1% uranyl acetate for 1 min. After removing the excess solution, the sample was dried in the dark and subsequently observed under a transmission electron microscope (TEM, HT7700, Hitachi, Tokyo, Japan) operating at an acceleration voltage of 75 kV, equipped with a charge-coupled device (CCD) camera.
Determination of the host range
The host range of phage BP15 was determined using the spot assay. In brief, a total of 19 Vibrio strains isolated from Taiwan, Thailand, Vietnam, the United States, and China were tested to characterize the lytic host range of the phage. These bacterial strains were cultured on LB-3% NaCl plates, and single colonies were collected and subsequently incubated in LB with 3% NaCl medium at 30 °C in a shaker operating at 200 rpm. A volume of 0.1 mL from each culture was mixed with 5 mL of 0.6% molten top agar and promptly poured onto a basal agar plate. Once the cell lawn solidified, 0.01 mL of phage lysate was spotted onto each bacterial lawn and incubated at 30 °C overnight. The spots were then evaluated for the phage’s infection ability toward the bacterial host. Each test was conducted in triplicate.
Conventional phage study
Several experiments were conducted to investigate the biological characteristics of the phage. The following experiments were all conducted at a phage concentration of ~ 1.6 × 106 PFU mL− 1. To assess temperature stability, 0.1 mL of phage lysate was incubated for 1 h at various temperatures (4, 20, 30, 40, 50, 60, and 70 °C). Similarly, to assess pH stability, 0.1 mL of phage lysate was mixed with 4.9 mL of SM buffer across a pH range of 2 to 12 (adjusted using NaOH or HCl) and maintained at 30 °C for 1 h. Regarding salinity stability, 0.1 mL of phage lysate was mixed with 4.9 mL of SM buffer over a salinity range of 0–5% and incubated at 30 °C for 1 h. For chloroform stability, 0.1 mL of phage lysate was mixed with 4.9 mL of SM buffer over a range of chloroform concentrations from 0 to 10% at 30 °C for 1 h. Subsequently, the mixtures were centrifuged at 12,000 rpm for 2 min, and the phages were obtained from the upper suspension. Phage titers were determined using the double-layer agar method. All experiments were conducted in triplicate.
The adsorption constant of phage BP15 were determined as described by Thammatinna et al.67 with minor modifications. The following experiments were all conducted at a phage concentration of ~ 2.0 × 105 PFU mL-1. A culture of the BCRC12959 strain (OD600 ~ 0.4) was infected with phage BP15 particles at a multiplicity of infection (MOI) of 0.01 and then incubated at 30 °C. At each time point (0, 5, 10, 15, 20, 25, and 30 min), 0.2 mL of the sample was collected and centrifuged at 13,000 rpm for 2 min at 4 °C. The resulting supernatant was harvested and diluted 10-fold in SM buffer, after which the number of free phages was determined using the double-layer agar method. All experiments were conducted in triplicate.
To study the infectivity and replication ability of the phage, a one-step growth curve analysis was conducted following the method outlined by Thammatinna et al.67 and Kropinski68 with minor modifications. The following experiments were all conducted at a phage concentration of ~ 1.0 × 104 PFU mL-1. Initially, BCRC12959 was infected at an MOI of 0.01 and incubated at 30 °C for 20 min. Subsequently, the cell suspension was centrifuged at 13,000 rpm for 2 min at 4°C, and the resulting pellet was resuspended in 50 mL of phage medium. The mixture was then incubated with vigorous shaking at 200 rpm and 30 °C for 80 min. During this shaking period, samples were taken every 15 min and titred using the double-layer agar method. All experiments were conducted in triplicate. The burst size was calculated as the ratio of the final count of liberated phage particles to the initial count of infected bacterial cells (phage titer at 0 min − phage titer at 0 min with chloroform).
To assess the efficiency of bacterial inactivation, an infection dynamic study was conducted for the phage against V. parahaemolyticus BCRC12959 at different MOIs, and bacterial densities were monitored by measuring the OD600 using a microplate reader. Initially, an overnight culture of V. parahaemolyticus BCRC12959 was diluted to a 1:100 ratio and incubated at 30 °C with shaking until reaching an OD600 of 0.1 to 0.3. Afterward, 1 mM CaCl2 and 10 mM MgSO4 were added to the medium, and the bacterial culture was divided into six flasks. Flasks 1 to 5 were supplemented with phage suspension at various MOIs (0.001, 0.01, 0.1, 1, and 10), whereas flask 6 was left uninfected and used as a bacterial control. The OD600 of the cultures was monitored in real-time and recorded every 30 min. All experiments were conducted in triplicate.
DNA extraction and phage genome sequencing
Phage genomic DNA was extracted using the phenol-chloroform method69. The phage pellet was resuspended in 0.5 mL of buffer L3 containing 100 mM NaCl, 100 mM Tris-HCl, and 25 mM EDTA. An equal volume of phenol/chloroform/isoamyl alcohol (25:24:1) was then added, and the sample was mixed before centrifugation at 13,000 rpm for 10 min at 4 °C. Next, the supernatant was transferred to a fresh microcentrifuge tube and mixed with an equal volume of isopropanol and 0.05 mL of NaOAc, followed by centrifugation at 13,000 rpm for 5 min at 4 °C. After centrifugation, the pellets were washed three times with ice-cold 75% ethanol. Finally, the pellets were air-dried, dissolved in 0.05 mL of ddH2O, and stored at − 20 °C. The phage genome was sequenced and assembled using the Nanopore sequencing platform and the Velvet software.
Bioinformatic analysis of phage BP15 genome
The genes within the phage BP15 genome were predicted and annotated using SnapGene Viewer (Version 7.1.1). The functional characteristics of the predicted genes were determined using the NCBI BLASTp platform with the non-redundant protein sequences (nr) database (http://www.ncbi.nlm.nih.gov/BLAST), using a score threshold of > 50 and an e-value cutoff of < 1.0 × 10− 3. The presence of tRNA was identified using tRNA scan-SE (http://trna.ucsc.edu/tRNAscan-SE/). Additionally, screening for antibiotic resistance genes was performed using Resfinder 4.1 (http://cge.food.dtu.dk/services/ResFinder/ accessed on 8 November 2023), while the presence of bacterial virulent genes was examined using Virulence Finder 2.0 (http://cge.food.dtu.dk/services/VirulenceFinder/ accessed on 8 November 2023). Circular maps of the annotated genomes were generated using DNAPlotter (v18.1.0)70.
The phylogenetic tree of phage BP15 was constructed based on the large terminase subunit, DNA polymerase, and the major capsid protein, along with the most closely related sequences of these proteins and the complete genomes of the phage (Table S2). All sequences were aligned using the ClustalW program71 with the default parameters in MEGA 7 72. Complete phage genomes were analyzed using the VICTOR web service (https://victor.dsmz.de), which allows for genome-based phylogeny and classification of prokaryotic viruses70,73,74. All pairwise comparisons of the nucleotide sequences were conducted using the Genome-BLAST Distance Phylogeny (GBDP) method75 under settings recommended for prokaryotic viruses73. The resulting intergenomic distances were used to infer a balanced minimum evolution tree with branch support via FASTME including SPR postprocessing76. Branch support was inferred from 100 pseudo-bootstrap replicates each. Trees were rooted at the midpoint77 and visualized with ggtree78. Furthermore, the inter-genomic similarities among complete phage genomes were calculated using the VIRIDIC web tool79 with default BLASTn settings. Finally, the genome comparison between BP15 and related phages was visualized using EasyFig v2.2.2 80.
Data availability
All data generated or analyzed in this study are included in this article and its supplementary information files. Nucleotide sequence of the phage BP15 genome was deposited in the GenBank database with the accession number PP768166.
References
Brauge, T., Mougin, J., Ells, T. & Midelet, G. Sources and contamination routes of seafood with human pathogenic Vibrio spp.: a farm-to-fork approach. Compr. Rev. Food Sci. Food Saf. 23, e13283. https://doi.org/10.1111/1541-4337.13283 (2024).
Bell, A. & Bott, M. Vibriosis:: what you and your patients need to know. Dela J. Public. Health. 7, 14–21. https://doi.org/10.32481/djph.2021.001.005 (2021).
Flegel, T. W. Historic emergence, impact and current status of shrimp pathogens in Asia. J. Invertebr. Pathol. 110, 166–173. https://doi.org/10.1016/j.jip.2012.03.004 (2012). https://doi.org/https://doi.org/
Lavilla-Pitogo, C. R., Leaño, E. M. & Paner, M. G. Mortalities of pond-cultured juvenile shrimp, Penaeus monodon, associated with dominance of luminescent vibrios in the rearing environment. Aquaculture 164, 337–349. https://doi.org/10.1016/S0044-8486(98)00198-7 (1998).
Adams, A. Response of penaeid shrimp to exposure to Vibrio species. Fish Shellfish Immunol. 1, 59–70. https://doi.org/10.1016/S1050-4648(06)80020-3 (1991).
Lavilla-Pitogo, C. R., Baticados, M. C. L. & Cruz-Lacierda, E. R. Pena, L. D. Occurrence of luminous bacterial disease of Penaeus monodon larvae in the Philippines. Aquaculture 91, 1–13. https://doi.org/10.1016/0044-8486(90)90173-K (1990). de la.
Karunasagar, I., Pai, R., Malathi, G. R. & Karunasagar, I. Mass mortality of Penaeus monodon larvae due to antibiotic-resistant Vibrio harveyi infection. Aquaculture 128, 203–209. https://doi.org/10.1016/0044-8486(94)90309-3 (1994).
De Schryver, P., Defoirdt, T. & Sorgeloos, P. Early mortality syndrome outbreaks: a microbial management issue in shrimp farming? PLoS Pathog. 10, e1003919. https://doi.org/10.1371/journal.ppat.1003919 (2014).
de Souza Valente, C. & Wan, A. H. L. Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J. Invertebr Pathol. 181, 107527. https://doi.org/10.1016/j.jip.2020.107527 (2021).
Albuquerque Costa, R., Araújo, R. L., Souza, O. V. & Vieira, R. H. S. d. F. Antibiotic-Resistant Vibrios in Farmed Shrimp. BioMed Research International 505914 (2015). (2015). https://doi.org/10.1155/2015/505914
Nurhafizah, W. W. I. et al. Virulence properties and pathogenicity of multidrug-resistant Vibrio harveyi associated with luminescent vibriosis in Pacific white shrimp, Penaeus vannamei. J. Invertebr. Pathol. 186, 107594. https://doi.org/10.1016/j.jip.2021.107594 (2021).
Cheng, H., Jiang, H., Fang, J. & Zhu, C. Antibiotic Resistance and characteristics of integrons in Escherichia coli isolated from Penaeus vannamei at a freshwater shrimp farm in Zhejiang Province, China. J. Food Prot. 82, 470–478. https://doi.org/10.4315/0362-028x.Jfp-18-444 (2019).
Nadella, R. K. et al. Multi-drug resistance, integron and transposon-mediated gene transfer in heterotrophic bacteria from Penaeus vannamei and its culture environment. Environ. Sci. Pollut Res. Int. 29, 37527–37542. https://doi.org/10.1007/s11356-021-18163-1 (2022).
Preena, P. G., Swaminathan, T. R., Kumar, V. J. R. & Singh, I. S. B. Antimicrobial resistance in aquaculture: a crisis for concern. Biologia 75, 1497–1517. https://doi.org/10.2478/s11756-020-00456-4 (2020).
Tsai, H. C. et al. Multidrug-resistance in methicillin-resistant Staphylococcus aureus (MRSA) isolated from a subtropical river contaminated by nearby livestock industries. Ecotoxicol. Environ. Saf. 200, 110724. https://doi.org/10.1016/j.ecoenv.2020.110724 (2020).
Lin, I. C. et al. Genetic diversity, Antimicrobial Resistance, and Toxigenic Profile of Vibrio vulnificus isolated from aquatic environments in Taiwan. Antibiot. (Basel). 10. https://doi.org/10.3390/antibiotics10050505 (2021). Prevalence.
Skurnik, M. & Strauch, E. Phage therapy: facts and fiction. Int. J. Med. Microbiol. 296, 5–14. https://doi.org/10.1016/j.ijmm.2005.09.002 (2006).
Housby, J. N. & Mann, N. H. Phage therapy. Drug Discov Today. 14, 536–540. https://doi.org/10.1016/j.drudis.2009.03.006 (2009).
Loc-Carrillo, C. & Abedon, S. T. Pros and cons of phage therapy. Bacteriophage 1, 111–114. https://doi.org/10.4161/bact.1.2.14590 (2011).
Międzybrodzki, R. et al. Clinical aspects of phage therapy. Adv. Virus Res. 83, 73–121. https://doi.org/10.1016/b978-0-12-394438-2.00003-7 (2012).
Chan, B. K., Abedon, S. T. & Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 8, 769–783. https://doi.org/10.2217/fmb.13.47 (2013).
Rehman, S., Ali, Z., Khan, M., Bostan, N. & Naseem, S. The dawn of phage therapy. Rev Med Virol 29, e (2041). (2019) https://doi.org/10.1002/rmv.2041
Akmal, M., Rahimi-Midani, A., Hafeez-Ur-Rehman, M., Hussain, A. & Choi, T. J. Isolation, characterization, and application of a bacteriophage infecting the Fish Pathogen Aeromonas hydrophila. Pathogens 9 https://doi.org/10.3390/pathogens9030215 (2020).
Sieiro, C. et al. A hundred years of bacteriophages: can phages replace antibiotics in Agriculture and Aquaculture? Antibiotics 9, 493 (2020).
Matamp, N. & Bhat, S. G. Genome characterization of novel lytic myoviridae bacteriophage ϕVP-1 enhances its applicability against MDR-biofilm-forming Vibrio parahaemolyticus. Arch. Virol. 165, 387–396. https://doi.org/10.1007/s00705-019-04493-6 (2020).
Nikapitiya, C., Chandrarathna, H., Dananjaya, S. H. S., De Zoysa, M. & Lee, J. Isolation and characterization of phage (ETP-1) specific to multidrug resistant pathogenic Edwardsiella tarda and its in vivo biocontrol efficacy in zebrafish (Danio rerio). Biologicals 63, 14–23. https://doi.org/10.1016/j.biologicals.2019.12.006 (2020).
Martínez-Díaz, S. F. & Hipólito-Morales, A. Efficacy of phage therapy to prevent mortality during the vibriosis of brine shrimp. Aquaculture 400–401, 120–124. https://doi.org/10.1016/j.aquaculture.2013.03.007 (2013).
Letchumanan, V. et al. Insights into bacteriophage application in Controlling Vibrio species. Front. Microbiol. 7 https://doi.org/10.3389/fmicb.2016.01114 (2016).
Angulo, C., Loera-Muro, A., Trujillo, E. & Luna-González, A. Control of AHPND by phages: a promising biotechnological approach. Reviews Aquaculture. 11, 989–1004. https://doi.org/10.1111/raq.12275 (2019).
Yang, L. et al. The evaluation of bacteriophage therapy in aquaculture: a systematic review and meta-analysis. Aquaculture 588, 740925. https://doi.org/10.1016/j.aquaculture.2024.740925 (2024). https://doi.org/https://doi.org/
Hyman, P. Phages for phage therapy: isolation, characterization, and host range breadth. Pharmaceuticals (Basel). 12. https://doi.org/10.3390/ph12010035 (2019).
Misol, G. N. Jr., Kokkari, C. & Katharios, P. Biological and genomic characterization of a novel jumbo bacteriophage, vB_VhaM_pir03 with Broad Host Lytic activity against Vibrio harveyi. Pathogens 9 https://doi.org/10.3390/pathogens9121051 (2020).
Kropinski, A. M., Prangishvili, D. & Lavigne, R. Position paper: the creation of a rational scheme for the nomenclature of viruses of Bacteria and Archaea. Environ. Microbiol. 11, 2775–2777. https://doi.org/10.1111/j.1462-2920.2009.01970.x (2009).
Adriaenssens, E. & Brister, J. R. How to name and classify your phage: an Informal Guide. Viruses 9 https://doi.org/10.3390/v9040070 (2017).
Ding, T. et al. Isolation and characterization of Vibrio parahaemolyticus bacteriophage vB_VpaS_PG07. Virus Res. 286, 198080. https://doi.org/10.1016/j.virusres.2020.198080 (2020).
Cao, Y., Zhang, Y., Lan, W. & Sun, X. Characterization of vB_VpaP_MGD2, a newly isolated bacteriophage with biocontrol potential against multidrug-resistant Vibrio parahaemolyticus. Arch. Virol. 166, 413–426. https://doi.org/10.1007/s00705-020-04887-x (2021).
Liang, X. et al. Isolation and characterization of a Lytic Vibrio parahaemolyticus phage vB_VpaP_GHSM17 from sewage samples. Viruses 14 https://doi.org/10.3390/v14081601 (2022).
Orozco-Ochoa, A. K. et al. Characterization and genome analysis of six novel Vibrio parahaemolyticus phages associated with acute hepatopancreatic necrosis disease (AHPND). Virus Res. 323, 198973. https://doi.org/10.1016/j.virusres.2022.198973 (2023).
Hsu, T. K. et al. Isolation and characterization of the novel phage BP14 for lysing Vibrio parahaemolyticus and reducing virulence proteins. Aquaculture 581, 740484. https://doi.org/10.1016/j.aquaculture.2023.740484 (2024).
Alagappan, K., Karuppiah, V. & Deivasigamani, B. Protective effect of phages on experimental V. parahaemolyticus infection and immune response in shrimp (Fabricius, 1798). Aquaculture 453, 86–92. https://doi.org/10.1016/j.aquaculture.2015.11.037 (2016).
Lomelí-Ortega, C. O. et al. Isolation and characterization of vibriophage vB_Vc_SrVc9: an effective agent in preventing Vibrio campbellii infections in brine shrimp nauplii (Artemia franciscana). J. Appl. Microbiol. 131, 36–49. https://doi.org/10.1111/jam.14937 (2021).
Yang, M. et al. Characterization and genome analysis of a novel Vibrio parahaemolyticus phage vB_VpP_DE17. Virus Res. 307, 198580. https://doi.org/10.1016/j.virusres.2021.198580 (2022). https://doi.org/https://doi.org/
Tremblay, D. M. et al. Receptor-binding protein of Lactococcus lactis phages: identification and characterization of the saccharide receptor-binding site. J. Bacteriol. 188, 2400–2410. https://doi.org/10.1128/jb.188.7.2400-2410.2006 (2006).
Hong, J. et al. Identification of host receptor and receptor-binding module of a newly sequenced T5-like phage EPS7. FEMS Microbiol. Lett. 289, 202–209. https://doi.org/10.1111/j.1574-6968.2008.01397.x (2008).
Hu, M., Zhang, H., Gu, D., Ma, Y. & Zhou, X. Identification of a novel bacterial receptor that binds tail tubular proteins and mediates phage infection of Vibrio parahaemolyticus. Emerg. Microbes Infect. 9, 855–867. https://doi.org/10.1080/22221751.2020.1754134 (2020).
Zampara, A. et al. Exploiting phage receptor binding proteins to enable endolysins to kill Gram-negative bacteria. Sci. Rep. 10, 12087. https://doi.org/10.1038/s41598-020-68983-3 (2020).
Abedon, S. T., Hyman, P. & Thomas, C. Experimental examination of bacteriophage latent-period evolution as a response to bacterial availability. Appl. Environ. Microbiol. 69, 7499–7506. https://doi.org/10.1128/aem.69.12.7499-7506.2003 (2003).
Chen, Y. et al. Isolation and characterization of a novel phage belonging to a new genus against Vibrio parahaemolyticus. Virol. J. 20, 81. https://doi.org/10.1186/s12985-023-02036-9 (2023).
González-Gómez, J. P. et al. Genomic and biological characterization of the novel phages vB_VpaP_AL-1 and vB_VpaS_AL-2 infecting Vibrio parahaemolyticus associated with acute hepatopancreatic necrosis disease (AHPND). Virus Res. 312, 198719. https://doi.org/10.1016/j.virusres.2022.198719 (2022).
Kim, H. J. et al. Application of the bacteriophage pVco-14 to prevent Vibrio coralliilyticus infection in Pacific oyster (Crassostrea gigas) larvae. J. Invertebr. Pathol. 167, 107244. https://doi.org/10.1016/j.jip.2019.107244 (2019).
Mäntynen, S., Sundberg, L. R., Oksanen, H. M. & Poranen, M. M. Half a century of research on membrane-containing bacteriophages: bringing New concepts to Modern Virology. Viruses 11 https://doi.org/10.3390/v11010076 (2019).
Labrie, S. J., Samson, J. E. & Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327. https://doi.org/10.1038/nrmicro2315 (2010).
Bull, J. J., Vegge, C. S., Schmerer, M., Chaudhry, W. N. & Levin, B. R. Phenotypic resistance and the dynamics of bacterial escape from Phage Control. PLOS ONE. 9, e94690. https://doi.org/10.1371/journal.pone.0094690 (2014).
Dy, R. L., Richter, C., Salmond, G. P. & Fineran, P. C. Remarkable mechanisms in microbes to resist phage infections. Annu. Rev. Virol. 1, 307–331. https://doi.org/10.1146/annurev-virology-031413-085500 (2014).
Rohde, C. et al. Expert Opinion on three phage therapy related topics: bacterial phage resistance, phage training and Prophages in bacterial production strains. Viruses 10 https://doi.org/10.3390/v10040178 (2018).
Oechslin, F. Resistance Development to bacteriophages occurring during bacteriophage therapy. Viruses 10 https://doi.org/10.3390/v10070351 (2018).
Loessner, M. J. Bacteriophage endolysins — current state of research and applications. Curr. Opin. Microbiol. 8, 480–487. https://doi.org/10.1016/j.mib.2005.06.002 (2005). https://doi.org/https://doi.org/
Dion, M. B., Oechslin, F. & Moineau, S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 18, 125–138. https://doi.org/10.1038/s41579-019-0311-5 (2020).
Turner, D., Kropinski, A. M. & Adriaenssens, E. M. A Roadmap for Genome-based phage taxonomy. Viruses 13 https://doi.org/10.3390/v13030506 (2021).
Aiewsakun, P., Adriaenssens, E. M., Lavigne, R., Kropinski, A. M. & Simmonds, P. Evaluation of the genomic diversity of viruses infecting bacteria, archaea and eukaryotes using a common bioinformatic platform: steps towards a unified taxonomy. J. Gen. Virol. 99, 1331–1343. https://doi.org/10.1099/jgv.0.001110 (2018).
Lefkowitz, E. J. et al. Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 46, D708–d717. https://doi.org/10.1093/nar/gkx932 (2018).
Turner, D. et al. Abolishment of morphology-based taxa and change to binomial species names: 2022 taxonomy update of the ICTV bacterial viruses subcommittee. Arch. Virol. 168, 74. https://doi.org/10.1007/s00705-022-05694-2 (2023).
Ramos-Vivas, J., Superio, J., Galindo-Villegas, J. & Acosta, F. Phage therapy as a focused management strategy in aquaculture. Int. J. Mol. Sci. 22, 10436 (2021).
Liu, R. et al. Bacteriophage therapy in aquaculture: current status and future challenges. Folia Microbiol. (Praha). 67, 573–590. https://doi.org/10.1007/s12223-022-00965-6 (2022).
Rai, S. et al. Perspectives on phage therapy for health management in aquaculture. Aquacult. Int. 32, 1349–1393. https://doi.org/10.1007/s10499-023-01220-6 (2024).
Kuhnert, P., Frey, J., Lang, N. P. & Mayfield, L. Phylogenetic analysis of Prevotella nigrescens, Prevotella intermedia and Porphyromonas gingivalis clinical strains reveals a clear species clustering. Int. J. Syst. Evol. Microbiol. 52, 1391–1395. https://doi.org/10.1099/00207713-52-4-1391 (2002).
Thammatinna, K. et al. A novel vibriophage exhibits inhibitory activity against host protein synthesis machinery. Sci. Rep. 10, 2347. https://doi.org/10.1038/s41598-020-59396-3 (2020).
Kropinski, A. M. Practical advice on the one-step growth curve. Methods Mol. Biol. 1681, 41–47. https://doi.org/10.1007/978-1-4939-7343-9_3 (2018).
Pan, Y. J. et al. Klebsiella phage ΦK64-1 encodes multiple depolymerases for multiple host capsular types. J. Virol. 91 https://doi.org/10.1128/jvi.02457-16 (2017).
Carver, T., Harris, S. R., Berriman, M., Parkhill, J. & McQuillan, J. A. Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 28, 464–469. https://doi.org/10.1093/bioinformatics/btr703 (2011).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. https://doi.org/10.1093/nar/22.22.4673 (1994).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. https://doi.org/10.1093/molbev/msw054 (2016).
Meier-Kolthoff, J. P. & Göker, M. VICTOR: genome-based phylogeny and classification of prokaryotic viruses. Bioinformatics 33, 3396–3404. https://doi.org/10.1093/bioinformatics/btx440 (2017).
Meier-Kolthoff, J. P. et al. Complete genome sequence of DSM 30083(T), the type strain (U5/41(T)) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand. Genomic Sci. 9, 2. https://doi.org/10.1186/1944-3277-9-2 (2014).
Meier-Kolthoff, J. P., Auch, A. F., Klenk, H. P. & Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 14, 60. https://doi.org/10.1186/1471-2105-14-60 (2013).
Lefort, V., Desper, R. & Gascuel, O. FastME 2.0: a Comprehensive, Accurate, and fast Distance-based phylogeny inference program. Mol. Biol. Evol. 32, 2798–2800. https://doi.org/10.1093/molbev/msv150 (2015).
Farris, J. S. Estimating phylogenetic trees from Distance matrices. Am. Nat. 106, 645–668. https://doi.org/10.1086/282802 (1972).
Yu, G. Using ggtree to visualize data on Tree-Like structures. Curr. Protocols Bioinf. 69 (e96). https://doi.org/10.1002/cpbi.96 (2020).
Moraru, C., Varsani, A. & Kropinski, A. M. VIRIDIC-A Novel Tool to calculate the intergenomic similarities of Prokaryote-infecting viruses. Viruses 12 https://doi.org/10.3390/v12111268 (2020).
Sullivan, M. J., Petty, N. K. & Beatson, S. A. Easyfig: a genome comparison visualizer. Bioinformatics 27, 1009–1010. https://doi.org/10.1093/bioinformatics/btr039 (2011).
Ye, Y. et al. Characterization and genomic analysis of Novel Vibrio parahaemolyticus phage vB_VpaP_DE10. Viruses 14 https://doi.org/10.3390/v14081609 (2022).
Tian, F. et al. Characterization and complete genome sequence analysis of a newly isolatedphage against Vibrio parahaemolyticus from sick shrimp in Qingdao, China. PLoS One. 17, e0266683. https://doi.org/10.1371/journal.pone.0266683 (2022).
Lal, T. M., Sano, M. & Ransangan, J. Genome characterization of a novel vibriophage VpKK5 (Siphoviridae) specific to fish pathogenic strain of Vibrio parahaemolyticus. J. Basic. Microbiol. 56, 872–888. https://doi.org/10.1002/jobm.201500611 (2016).
Jun, J. W. et al. Bacteriophage therapy of a Vibrio parahaemolyticus infection caused by a multiple-antibiotic-resistant O3:K6 pandemic clinical strain. J. Infect. Dis. 210, 72–78. https://doi.org/10.1093/infdis/jiu059 (2014).
Yuan, L., Cui, Z., Wang, Y., Guo, X. & Zhao, Y. Complete genome sequence of virulent bacteriophage SHOU24, which infects foodborne pathogenic Vibrio parahaemolyticus. Arch. Virol. 159, 3089–3093. https://doi.org/10.1007/s00705-014-2160-x (2014).
Ramírez-Orozco, M., Serrano-Pinto, V., Ochoa-Álvarez, N., Makarov, R. & Martínez-Díaz, S. F. Genome sequence analysis of the Vibrio parahaemolyticus lytic bacteriophage VPMS1. Arch. Virol. 158, 2409–2413. https://doi.org/10.1007/s00705-013-1726-3 (2013).
Alanis Villa, A., Kropinski, A. M., Abbasifar, R., Abbasifar, A. & Griffiths, M. W. Genome sequence of temperate Vibrio parahaemolyticus bacteriophage vB_VpaS_MAR10. J. Virol. 86, 13851–13852. https://doi.org/10.1128/jvi.02666-12 (2012).
Lin, Y. R. & Lin, C. S. Genome-wide characterization of vibrio phage ϕpp2 with unique arrangements of the mob-like genes. BMC Genom. 13, 224. https://doi.org/10.1186/1471-2164-13-224 (2012).
Acknowledgements
The authors thank the Electron Microscopy Center of Institute of Marine Biology at National Taiwan Ocean University for providing technical assistance. The authors sincerely thank Prof. Chu-Fang Lo from National Cheng Kung University (NCKU) for providing some bacterial strains and technical guidance used in this study.
Funding
This study was financially supported by the Ministry of Science and Technology (MOST 110-2313-B-019-004-MY3). This work was also supported by grants from the Center of Excellence for the Oceans (National Taiwan Ocean University) and the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan, ROC.
Author information
Authors and Affiliations
Contributions
Te-Ken Hsu, tkhsu@innocreatebio.com Conceptualization; Methodology; Validation; Investigation; Data curation; Writing—original draft preparation. Yi-Yin Chen, d99445003@ntu.edu.tw Software; Formal analysis; Validation; Investigation; Data curation; Writing—original draft preparation. Shiao-Wen Li, swli67@nuk.edu.tw Software; Formal analysis; Data curation. Hui-Yu Shih, j940903@gmail.com Methodology; Investigation. Hsin-Yiu Chou, hychou@mail.ntou.edu.tw Funding acquisition. Jeff Chia-Kai Hsu, jeffhsu0423@gmail.com Resources. Han-Ching Wang, wanghc@mail.ncku.edu.tw Resources. Li-Li Chen, joechen@ntou.edu.tw Conceptualization; Funding acquisition; Writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hsu, TK., Chen, YY., Li, SW. et al. Characterization and genome analysis of a novel phage BP15 infecting Vibrio parahaemolyticus. Sci Rep 15, 2801 (2025). https://doi.org/10.1038/s41598-025-85513-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85513-1