Abstract
This study aimed to explore whether ultra-early indicators can predict the severity of acute hypertriglyceridemic pancreatitis (HTGP) and guide clinical decisions. This retrospective study analyzed data from HTGP patients who were categorized into mild acute pancreatitis (MAP) and moderately severe/severe acute pancreatitis (MSAP/SAP) groups based on their final clinical outcomes. Ultra-early indicators (serum calcium, triglyceride [TG], interleukin-6 [IL-6], D-dimer, hemoglobin A1c [HbA1c], arterial lactate) were measured within 6 h of admission. Among 110 patients, 56 had MAP and 54 had MSAP/SAP. Within 6 h of admission, TG, IL-6, D-dimer, HbA1c, and arterial lactate levels were significantly higher in the MSAP/SAP group, while serum calcium was lower. Multivariable logistic regression and receiver operator characteristic curve identified IL-6, D-dimer, and serum calcium as independent risk factors and ultra-early predictors of HTGP severity. Patients with MSAP/SAP who received blood purification within 24 h had a shorter hospital stay compared to those treated later. IL-6, D-dimer, and serum calcium are promising biomarkers for early prediction of HTGP severity. Early blood purification within 24 h reduces complications and hospital stay in MSAP/SAP patients, while traditional treatments remain effective for MAP patients, potentially reducing medical costs.
Similar content being viewed by others
Introduction
Acute pancreatitis (AP) is a common acute abdominal disease that requires immediate hospitalization. Epidemiologically, AP has a mortality rate of 5–10%, while severehyperlipidemic acute pancreatitis(HTGP) accounts for a mortality rate of 36–50%1,2,3. HTGP is attributed to recent dietary and lifestylechanges, surpassing alcoholic pancreatitis as the second leading cause of AP in China4. The incidence of HTGP increases yearly. Unlike other types of AP, patients with HTGPexhibit severe clinical symptoms, which are occasionallyrecurrent and associated with a poor prognosis5,6.
Hypertriglyceridemia is the primary risk factor for HTGP7. The consequences of elevated triglycerides are severe regardless of their locationin human body. For example, in the heart, they can lead to coronary heart disease and myocardial infarction, while in brain, they may result in stroke8,9. High triglyceridelevel in the fundus of the eye can cause impaired vision, blindness, and renal failure. The presence of blood in the limbs is also attributed to high triglycerides. Consequently, poor blood flow causes necrosis. Triglycerides are broken down into a large amount of free fatty acids that exceed the binding capacity of albumin, causing cell membrane toxicity through lipid peroxidation. This mechanism consequently damages acinar cells and capillary endothelial cells10. Moreover, hypertriglyceridemia is associated with the hypercoagulable state of blood and induces pancreatic microcirculation disturbance11.
Reduction in blood lipid level is the key treatment for hyperlipidemic pancreatitis. Integration of targeted lipidemiasuppression with general therapy, such as fasting and administration of low molecular weight heparin and insulin, can reduce blood lipid and recuperation among non-severe patients. Drug therapy alone in severe patients is unlikely to rapidly reduce blood lipid levels. Early application of blood purification therapy has been widely adopted to rapidly reduce blood lipid levels among patients with severe pancreatitis. Therefore, identifying high-risk patients at the early stages of the disease is crucial since it can help clinicians formulate an effective management approach or refer the diagnosed patients to expert care for advanced clinical prognosis.
Risk stratification and severity prediction methods at the early stage of AP have been developed for decades, including some clinical scoring systems and laboratory parameters. Previous studies identified several indicators that could predict the severity of AP, including D dimer12, serum calcium13, IL-614,15, arterial lactate16, C-reactive protein(CRP), red cell distribution width (RDW)17,18, modified CT severity index (MCTSI)19, andserumtriglyceride(TG)5, among other indicators. However, these indicators are frequently detected within 24–48 h post-admission. In most cases, patients with severe pancreatitis do not enjoy the best opportunity for blood purification therapy, which prolongs their length of hospital stay and increases medical expenses. Early identification of a severe form of pancreatitis is among the major challenges for its management.
Numerous studies have investigated the differences in clinical characteristics between HTGP and non-HTGP16,20. Only a few studies have assessed the ultra-early risk factors for HTGP. Herein, we collected blood samples and tested the six indicators within 6 h of admission. Data from patients with HTGP obtained within 6 h of admission were analyzed to characterize their early risk factors for HTGP and provide novel approaches for its prevention and treatment. Early evaluation of HTGP is key in determining the immediate use of blood purification and drug lipid-lowering therapies. Consequently, drug lipid reduction therapy can ameliorate the conditions of patients and save medical expenses when blood purification therapy is not needed. Therefore, this study aimed to identify ultra-early indicators in the prediction of HTGP severity, and assess the effect of early blood purification therapy in patients with HTGP.
Methods
Study design and population
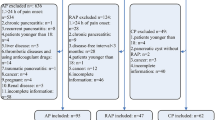
This retrospective study was conducted in accordance with local and national laws and the Declaration of Helsinki.We obtained data from 110 patients with HTGP during admission at the Second Affiliated Hospital, Fujian Medical University (Quanzhou, China) between January 2017 and February 2020. Complete case data for the participants were retrospectively analyzed. The present study was approved by the Ethics Committee of the Second Affiliated Hospital, Fujian Medical University (Approval number: 2021 − 215). The informed consent was waived by the Ethics Committee because of the retrospective nature of this study. The personalinformation of the patients was kept confidential, and the principle of confidentiality was observed.
The inclusion criteria included the following: (i) Patients who met the diagnostic criteria for AP. The AP was diagnosed based on two of the following factors (determined ≥ 3 times): abdominal pain, increased serum amylase and/or lipase, and abdominal imaging examination in line with imaging changes typical for AP. (ii) The serum TG level of ≥ 11.3 mmol/L at the onset of the disease21. However, TG levels between 5.65 and 11.3 mmol/L were accepted except for AP caused by other factors such as cholelithiasis and alcoholism. (iii) All patients underwent abdominal enhanced computed tomography (CT) imaging within 72 h of admission. Patients with alcoholic AP, post-ERCP pancreatitis, chronic pancreatitis, and chronic renal dysfunction were excluded22,23. The classification of AP was based on the latest 2012 revision of the Atlanta classification: (i) MAP: met the diagnostic criteria of AP and is not associated with organ failure and local or systemic complications; (ii) MSAP: associated with transient organ failure (recovered within 48 h) and local or systemic complications; (iii) SAP: with persistent organ failure (> 48 h) and a modified Marshall score of ≥ 2. Initially, the enrolled patients received targeted lipidemia-lowering and general therapies, which include fasting, low molecular weight heparin and insulin to reduce blood lipid, gastrointestinal decompression, fluid resuscitation, nutritional therapy, organ function maintenance, prophylaxis against gram-negative bacilli, and the traditional Chinese medicine approach, receiving raw rhubarb to restore gastrointestinal tract dynamics and treat pancreatitis. Blood purification therapies, including plasma exchange and hemofiltration, were conducted in patients diagnosed with a severe tendency on admission. According to the final clinical outcomes, patients with HTGP were divided into mild acute pancreatitis (MAP)and moderately severe acute pancreatitis/severe acute pancreatitis (MSAP/SAP) groups. Patients demographic and clinical data were collected, and their ultra-early indicators (serum calcium, TG, IL-6, D-dimer, HbAc1, and arterial lactate) levels were measured within 6 h of admission (“ultra-early” refers to the time after the patient’s admission, rather than being calculated from the onset of the disease). Inaddition, preventive antibiotics were not routinely used in all patients. Instead, they were administered based on clinical indications, such as signs of infection or necrosis.
Data collection and definitions
We collected baseline information about the patients, including age, sex, body mass index (BMI), medical history, admission date, and length of hospital stay.Additionally, on admission, vital signs data were collected, while necessary laboratory tests, radiological data, and clinical outcomes were determined after hospital stay. Within 6 h of admission, the following laboratory parameters were determined: TG, IL-6, D-dimer, HbAc1 (HbA1c was measured to assess the long-term glycemic control of patients, as chronic hyperglycemia can influence the severity and prognosis of acute pancreatitis. While the higher frequency of diabetes in the MSAP/SAP group is expected, evaluating HbA1c provided quantitative data on glucose metabolism disorders, which may contribute to disease severity), and arterial lactate levels. Enhanced CT was performed to examine the extent of necrotic tissue and the fluid locus. The modified Marshall score was used to evaluate the severity of AP. The decision toearly blood purification therapy within 24 h after admission was based on: the decision to initiate blood purification within the first 24 h was based on: (1) Clinical severity of symptoms: Including persistent abdominal pain, signs of systemic inflammation, and hemodynamic instability. (2) Laboratory measurements: Extremely elevated triglyceride levels (TG istent abdominal pain, signs of systemic inflammation, and hemodynamic instability Marshall sco 3) Imaging findings: Early signs of pancreatic necrosis or severe inflammation on abdominal CT scans. In addition, an external data (n = 110) from our hospital set were collected retrospectively for validation.
Statistical analysis
IBM Statistical Package for the Social Sciences software (version 20.0; Chicago, USA) was used for statistical analysis. The results are presented as a percentage (%) or mean ± standard deviation. Comparisons were performed using the Student’s t-test, and the Mann–Whitney U test was used for comparison of the two groups of independent samples. Categorical data are presented as n (%) prevalence. The χ2-test or Fisher’s exact test was usedto analyze between-group differences.Multivariablelogistic regression analysis was performed to identify the independent risk factors for HTGP severity. Demographic and clinical characteristics withstatistically significant (all P < 0.05) in the univariable logistic regression analysis were included as confoungers into the multivariable logistic regressionfor adjustment. The area under the receiver operating characteristic (ROC) curve (AUC) was determined to evaluate the performance of the predictive model. The performance of the predictive models were validated by the external data. Given the range of 0–1 of AUC, a variable with > 0.7 was considered useful, whereas an AUC value of 0.8–0.9 denoted excellent diagnostic accuracy. Chi-square test was applied to estimate the length of hospital stay and complications in the MSAP/SAP group post-plasma exchange within or > 24 h. A two-sided P < 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of the study population
Of the 110 patients with HTGP, 56 were classified with MAP and 54 with MSAP/SAP. Though the age of onset in the MAP group was higher (44.3 ± 4.1 versus 33.6 ± 4.9 years), their incidence of type 2 diabetes mellitus disease was lower (32 versus 43 patients) compared with the MSAP/SAP group (P = 0.028). The sex of patients between the groups was not statistically different (P > 0.05). The MAP group included more patients with a lower BMI than the MSAP/SAP group (P = 0.042; Table 1).
The MSAP/SAP group exhibited significantly higher levels of triglyceride (17.10 ± 5.06 versus 13.94 ± 2.37 mmol/L), IL-6 (32.61 ± 5.09 versus 25.26 ± 4.29 pg/mL), CRP (42.51 ± 14.21 versus 37.42 ± 15.99 mg/L), HbA1c (6.7 ± 0.6% versus 6.2 ± 0.6%), and arterial lactate (1.82 ± 0.45 versus 1.47 ± 0.36 mmol/L) than the MAP group (Table 2). However, serum calcium was significantly lower in the MSAP/SAP group than in the MAP group (2.01 ± 0.13 versus 2.29 ± 0.21 mmol/L). The CRP was not statistically significant between the MAP and MSAP/SAP patients.
Performance of several serumindicatorsin the prediction of the severity of HTGP
Figure 1 depicts the sensitivity, specificity, and AUC results in the prediction of HTGP severity. Some parameters were highly accurate in the prediction of HTGP severity. In the prediction of MSAP/SAP, IL-6 (≥ 27.4 pg/mL) exhibited the highest accuracy with 87% sensitivity, 73% specificity, and 0.86 AUC. In the prediction of HTGP severity, D-dimer (≥ 2.65 mg/L) exhibited 63% sensitivity, 94% specificity, and 0.82 AUC. Arterial lactate (≥ 1.69 mmol/L) exhibited 57% sensitivity, 79% specificity, and 0.73 AUC. Serum calcium (< 2.14 mmol/L) exhibited 72% sensitivity, 70% specificity, and 0.77 AUC (Table 3).
Independent prognostic factors in the MSAP/SAP group at admission
To further evaluate the relationship between admission indicators and MSAP/SAP, we constructed a multivariable logistic regression analysis model consisting of four parameters (IL-6, D-dimer, arterial lactate, and serum calcium) within 6 h of admission, and adjusted for age, concomitant type 2 diabetes mellitus, and BMI which were statistically significant (all P < 0.05) in the univariablelogistic regression analysis. The multivariable logistic regression model identified IL-6, D-dimer, and serum calcium as independent risk factors for MSAP/SAP. Table 4 presents the odds ratio (OR), respectively. D-dimer (≥ 2.65 mg/L), IL-6 (≥ 27.4 pg/mL), or serum calcium (< 2.14 mmol/L) significantly increased the risk of HTGP transformation to severe. As a result, combining the three independent risk factors in the prediction of HTGP severity further improved the prediction accuracy with a 0.88 AUC (Fig. 2). Moreover, the ROC analysis for combining the three independent risk factors based on the external data (n = 110) yield a 0.89 AUC (Fig. 3).
The effect of early blood purification treatment onMSAP/SAP patients
The clinical and demographic characteristics (all P > 0.05) between patients who received blood purification treatment and those did not were comparable (Table 5). Early blood purification therapy within 24 h after admission could shorten the length of hospital stayinpatients with HTGP with severe tendency. Among the 50 patients with SAP with higher-level clinical indicators (D-dimer ≥ 2.35 mg/L or IL-6 ≥ 27.4 pg/mL) at admission, 28 received early blood purification therapy within 24 h, whereas the 22 were delayed. Patients who received early blood purification therapy exhibited a shorter length of hospital stay with fewer complications than those whose treatment was delayed (Table 6). For patients with MAP, blood purification treatment could not shorten length of hospital stay and significantlyincrease medical expenses. However, the traditional lipid-lowering treatment scheme could yield a better therapeutic effect.
Complications
Local complications, such as pancreatic necrosis (19, 17%), pseudocyst formation (10, 9%), and peripancreatic fluid collections (24, 22%) were obseverd. Systemic complications, including systemic inflammatory response syndrome (SIRS) (38, 35%), organ failure (respiratory, renal, circulatory) (32, 30%), and infectious (6, 5%) were obseverd.
Discussion
This study identified IL-6, D-dimer, and serum calcium as ultra-early biomarkers that can predict the severity of acute hypertriglyceridemic pancreatitis (HTGP) within 6 h of admission, facilitating early intervention. Early blood purification therapy within 24 h of admission for patients with moderate to severe HTGP significantly reduces hospital stay and complications, while traditional treatments remain effective and cost-efficient for milder cases.
The incidence of HTGP has been increasing and is frequently associated with more severe clinical processes. HTGP is mainly reported in young people, especially those who are obese, alcoholic, or diabetic. Hypertriglyceridemia is the leading risk factor for HTGP. Previous studies reported that patients with HTGP are susceptible to persistent organ failure, and the incidence of complications and mortality are significantly higher than those of AP due to other causes. Therefore, an immediate decrease in serum triglyceride level to < 5.65 mmol/L in the early stage of the disease interrupts the vicious cycle between triglyceride and inflammation, lowering the disease severity and improving the prognosis. Heparin and insulin have a synergistic effect in reducing serum triglyceride. The synergistic effect of heparin and insulin on HTGP has been clinically recognized and is adopted as a first-line treatment for severe HTGP24,25.Blood purification, including plasma exchange and hemofiltration, can be used in HTGP treatment. A recent systematic review reported that serum triglyceride in most patients with HTGP decreases significantly after plasma exchange, followed by improvement of clinical symptoms or laboratory indicators.However, plasma exchange cannot reduce patient mortality26. Additionally, blood purification is not superior regarding clinical outcomes and costs. There are some research deviations in these conclusions. For example, the patients in the HTGP group were not graded for severity, which cannot reflect the advantages of blood purification therapy for severe patients and whether blood purification therapy is necessary for non-severe patients. However, the time of plasma exchange might be the critical point. If severe patients with HTGP can receive plasma exchange promptly, they may have a good prognosis27. The primary drugs for HTG are fenofibrate, gemfibrozil and other fibrates, niacin, statins, and omega-3 fatty acids. Niacin is restricted due to its multiple side effects28. In a randomized, anonymized controlled trial, the dual treatment of omega-3 fatty acids and fenofibrate reduced the median TG concentration by 60.8%,while fenofibrate monotherapy alone reduced it by 53.8%. However, these two treatments were not statistically significant29. The role of statins is controversial. For instance, statins mainly act on hyperlipidemia characterized by elevated cholesterol levels; however, some studies reported that they have a protective effect, while others reported they can cause pancreatitis30. Fibrates are the recommended first-line drug treatment in clinical practice.
Our study identified IL-6, D-dimer, and serum calcium as independent risk factors associated with the severity of HTGP. These findings are supported by current scientific evidence. Several studies have highlighted the role of IL-6 in the pathogenesis of acute pancreatitis. IL-6 is a pro-inflammatory cytokine that is elevated in the early stages of HTGP and is associated with systemic inflammatory response syndrome (SIRS) and organ dysfunction31,32. Similarly, D-dimer, a marker of fibrin degradation, is elevated in patients with severe acute pancreatitis and indicates the presence of microvascular thrombosis and coagulation activation33,34. Low serum calcium levels have been consistently associated with increased severity and poor outcomes in acute pancreatitis, likely due to the role of calcium in cell signaling and inflammation35,36. Our results align withtheseprevious studies confirming that IL-6, D-dimer, and serum calcium are valuable ultra-early indicators for predicting the severity of HTGP. These biomarkers can facilitate early diagnosis and prompt intervention, potentially improving patient outcomes.
Specifically, we found that during the ultra-early stage of HTGP, there are indicators that can better predict the severity of pancreatitis. This is key for immediately evaluating the disease progression and actively adopting targeted treatment measures. Consequently, effective intervention can be performed in the early stage of HTGP to achieve the goal of timely control of disease development. We found that ultra-early indicators of IL-6 and D-dimer may be useful biomarkers in assessing AP severity in patients with HTGP, which facilitates the prompt diagnosis of patients with HTGP with severe tendencies using these indicators. A previous study reported that HTG is often accompanied by leukocyte chemoattractant protein-1, malondialdehyde, nitric oxide, catalase, and other indicators of oxidative stress37. Additionally, previous studies reported that for patients with LPL or Apo-C2 gene defects and repeated HTG-AP38,39, the administration of antioxidant therapy has a clear clinical effect in AP prevention and suggests that oxidative stress may be an essential mechanism for HTG to induce AP37. Given these approaches, early intervention may be deemed appropriate as they facilitate patient rehabilitation.
Then, we found that for patients diagnosed with MSAP/SAP, blood purification therapy within 24 h of admission can shorten their length of hospital stay. This demonstrates that early blood purification therapy can reduce blood lipid levels and eliminate inflammatory factors that inhibit pancreatitis progression and are conducive to disease recovery. Considering the high medical cost of blood purification therapy and the potential risk of blood-borne infection, our research found that for patients diagnosed with MSAP/SAP, the conventional treatment scheme can result in a good therapeutic effect, whereas the blood purification method will shorten their length of hospital stay. Therefore, early assessment after admission is essential and can determine the preliminary outcome of the disease through indicative indicators. Immediate blood purification therapy should be implemented in patients with severe manifestations. However, cheap traditional treatment schemes can be adopted for patients without severe manifestationsto enhance shorter length of hospital stay. It is worth noting that, in our patient cohort, preventive antibiotics were not routinely used in all patients. Instead, they were administered based on clinical indications, such as signs of infection or necrosis.
In addition, we observed that the average age of patients in the mild acute pancreatitis (MAP) group was higher, while the prevalence of type 2 diabetes mellitus (T2DM) was higher in the moderately severe to severe acute pancreatitis (MSAP/SAP) group. These differences may impact our study results. However, elderly patients may have poorer disease tolerance due to decreased physiological functions and an increase in comorbidities, potentially leading to more severe disease progression and unfavorable prognoses. However, in our study, it was observed that the MAP group had a higher average age than the MSAP/SAP group. To assess the impact of age on the severity of acute pancreatitis, we included age as a potential confounding factor in our multivariable logistic regression analysis. After adjustment, IL-6, D-dimer, and serum calcium remained independent predictors of the severity of hypertriglyceridemic pancreatitis (HTGP). This indicates that even considering the influence of age, these indicators still have significant predictive value. Besides, T2DM has been widely recognized as an important factor affecting the prognosis of acute pancreatitis40. A hyperglycemic state can exacerbate pancreatic inflammatory responses, increase oxidative stress levels, and lead to more severe pancreatic injury41. In our study, the prevalence of T2DM in the MSAP-SAP group was significantly higher than in the MAP group. To control for the interference of T2DM on the study results, we also included T2DM in the multivariable analysis for adjustment. The results showed that after adjusting for T2DM, IL-6, D-dimer, and serum calcium were still closely related to the severity of HTGP and continued to serve as independent predictors. Therefore, by controlling for potential confounding factors such as age and T2DM, we enhanced the reliability of our study results.
This study has some limitations. Firstly, this was a retrospective study that is prone to selection bias. To minimize the possibility of selection bias, we adopted strict inclusion criteria and expanded the sample size. Seceondly, this study included patitents in a single-center, which would limiting the generalizability of our findings. Thirdly, the relatively small sample size, which may affect the statistical power and the reliability of the findings. Fourthly, this study might have potential selection bias, such asage appears to be higher in the mild group, due to the retrospective nature of the study. Finally, this study lack of long-term follow-up data, preventing assessment of long-term outcomes and recurrence rates. Despite these limitations, this study provides effective information on treatment strategies for HTGP.
Conclusion
Our results indicate that early detection of IL-6, D-dimer, and blood calcium concentration may predict the onset of pancreatitis after admission of patients with HTGP. Therefore, early detection facilitates the implementation of different effective treatment schemes in the early stage of HTGP, accelerating the recovery of patients with pancreatitis and reducing medical expenses.
Data availability
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Abbreviations
- HTGP:
-
Hypertriyceridemic pancreatitis
- MAP:
-
Mild acute pancreatitis
- TG:
-
Triglyceride
- IL-6:
-
Interleukin-6
- HbAc1:
-
Hemoglobin A1c
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- SPSS:
-
Statistical Package for Social Sciences
- MCP-1:
-
Chemoattractant protein-1
- MDA:
-
Malondialdehyde
- NO:
-
Nitric oxide
References
Banks, P. A. et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 62, 102–111 (2013).
Forsmark, C. E., Vege, S. S. & Wilcox, C. M. Acute pancreatitis. N. Engl. J. Med. 375, 1972–1981 (2016).
Li, J. et al. Interleukin-6 is better than C-reactive protein for the prediction of infected pancreatic necrosis and mortality in patients with acute pancreatitis. Front. Cell. Infect. Microbiol. 12. (2022).
Zhu, Y. et al. A study on the etiology, severity, and mortality of 3260 patients with acute pancreatitis according to the revised Atlanta classification in Jiangxi, China over an 8-Year period. Pancreas 46, 504–509 (2017).
Nawaz, H. et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am. J. Gastroenterol. 110, 1497–1503 (2015).
Sue, L. Y. et al. Effect of serum triglycerides on clinical outcomes in Acute Pancreatitis: findings from a Regional Integrated Health Care System. Pancreas 46, 874–879 (2017).
Kiss, L. et al. Mechanisms Linking Hypertriglyceridaemia to Acute Pancreatitis e13916 (Acta physiologica, 2023).
Bhatt, D. L. et al. Cardiovascular risk reduction with Icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380, 11–22 (2019).
Nicholls, S. J. et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. Jama 324, 2268–2280 (2020).
Havel, R. J. Pathogenesis, differentiation and management of hypertriglyceridemia. Adv. Intern. Med. 15, 117–154 (1969).
Valdivielso, P., Ramírez-Bueno, A. & Ewald, N. Current knowledge of hypertriglyceridemic pancreatitis. Eur. J. Intern. Med. 25, 689–694 (2014).
Wan, J. et al. Serum D-dimer levels at admission for prediction of outcomes in acute pancreatitis. BMC Gastroenterol. 19, 67 (2019).
Peng, T. et al. Serum calcium as an indicator of persistent organ failure in acute pancreatitis. Am. J. Emerg. Med. 35, 978–982 (2017).
Li, N., Wang, B. M., Cai, S. & Liu, P. L. The role of serum high mobility group box 1 and interleukin-6 levels in acute pancreatitis: a meta-analysis. J. Cell. Biochem. 119, 616–624 (2018).
Kolber, W. et al. Does the automatic measurement of interleukin 6 allow for prediction of complications during the first 48 h of acute pancreatitis? Int. J. Mol. Sci. 19 (2018).
Shu, W. et al. Initially elevated arterial lactate as an independent predictor of poor outcomes in severe acute pancreatitis. BMC Gastroenterol. 20, 116 (2020).
Yao, J. & Lv, G. Association between red cell distribution width and acute pancreatitis: a cross-sectional study. BMJ Open 4, e004721 (2014).
Zhang, F. X., Li, Z. L., Zhang, Z. D. & Ma, X. C. Prognostic value of red blood cell distribution width for severe acute pancreatitis. World J. Gastroenterol. 25, 4739–4748 (2019).
Yang, L. et al. Comparison of BISAP, Ranson, MCTSI, and APACHE II in predicting severity and prognoses of hyperlipidemic acute pancreatitis in Chinese patients. Gastroenterol. Res. Pract. 2016, 1834256 (2016).
Cao, X. et al. Early predictors of hyperlipidemic acute pancreatitis. Exp. Ther. Med. 16, 4232–4238 (2018).
Kyriakidis, A. V. et al. Management of acute severe hyperlipidemic pancreatitis. Digestion 73, 259–264 (2006).
Cameron, J. L., Crisler, C., Margolis, S., DeMeester, T. R. & Zuidema, G. D. Acute pancreatitis with hyperlipemia. Surgery 70, 53–61 (1971).
Fortson, M. R., Freedman, S. N. & Webster, P. D. 3 Clinical assessment of hyperlipidemic pancreatitis. Am. J. Gastroenterol. 90, 2134–2139 (1995).
Scherer, J., Singh, V. P., Pitchumoni, C. S. & Yadav, D. Issues in hypertriglyceridemic pancreatitis: an update. J. Clin. Gastroenterol. 48, 195–203 (2014).
Lu, X. S. et al. Effect of lower-molecular weight heparin in the prevention of pancreatic encephalopathy in the patient with severe acute pancreatitis. Pancreas 39, 516–519 (2010).
Carr, R. A., Rejowski, B. J., Cote, G. A., Pitt, H. A. & Zyromski, N. J. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology?. Pancreatol. Official J. Int. Associ. Pancreatol. (IAP) [et al]. 16 469–476. (2016).
Joglekar, K., Brannick, B., Kadaria, D. & Sodhi, A. Therapeutic plasmapheresis for hypertriglyceridemia-associated acute pancreatitis: case series and review of the literature. Therapeutic Adv. Endocrinol. Metab. 8, 59–65 (2017).
Titcomb, T. J. et al. rd, Eating pattern and nutritional risks among people with multiple sclerosis following a modified paleolithic diet. Nutrients 12. (2020).
Oscarsson, J. et al. Effects of free omega-3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non-alcoholic fatty liver disease: a double-blind, randomized, placebo-controlled study. J. Clin. Lipidol. 12, 1390–1403e1394 (2018).
Badalov, N. et al. Drug-induced acute pancreatitis: an evidence-based review. Clin. Gastroenterol. Hepatol. Official Clin. Pract. J. Am. Gastroenterol. Assoc. 5, 648–661 (2007).
Chen, G. et al. Nanoparticles Fueled by Enzyme for the Treatment of Hyperlipidemic Acute Pancreatitis (ACS biomaterials science & engineering, 2024).
Wang, X. et al. Baicalein alleviates pyroptosis and inflammation in hyperlipidemic pancreatitis by inhibiting NLRP3/Caspase-1 pathway through the miR-192-5p/TXNIP axis. Int. Immunopharmacol. 101, 108315 (2021).
Lin, Y. et al. Development and validation of a nomogram for predicting the severity of the first episode of hyperlipidemic acute pancreatitis. J. Inflamm. Res. 17, 3211–3223 (2024).
Zhou, W. et al. Analysis of the clinical profile and treatment efficiency of hyperlipidemic acute pancreatitis. Lipids Health Dis. 23, 70 (2024).
Lu, J. et al. Penta-therapy for severe acute hyperlipidemic pancreatitis. Am. J. Emerg. Med. 36, 1789–1795 (2018).
Ji, F. et al. A nomogram to predict the occurrence of pseudocyst in patients with acute pancreatitis. Pancreatol. Off. J. Int. Assoc. Pancreatol. (IAP) [et al] 24, 863–869 (2024).
Guo, Y. Y., Li, H. X., Zhang, Y. & He, W. H. Hypertriglyceridemia-induced acute pancreatitis: progress on disease mechanisms and treatment modalities. Discov. Med. 27, 101–109 (2019).
Baass, A., Paquette, M., Bernard, S. & Hegele, R. A. Familial chylomicronemia syndrome: an under-recognized cause of severe hypertriglyceridaemia. J. Intern. Med. 287, 340–348 (2020).
Surendran, R. P. et al. Mutations in LPL, APOC2, APOA5, GPIHBP1 and LMF1 in patients with severe hypertriglyceridaemia. J. Intern. Med. 272, 185–196 (2012).
Cozma, M. A. et al. Implications of type 2 diabetes mellitus in patients with acute cholangitis: a systematic review of current literature. Healthcare. (Basel) 10 (11), 2196 (2022).
Lisco, G. et al. Hyperglycemia-induced immune system disorders in diabetes mellitus and the concept of hyperglycemic memory of innate immune cells: a perspective. Endocr. Metab. Immune Disord. Drug Targets 22 (4), 367–370 (2022).
Acknowledgements
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Author information
Authors and Affiliations
Contributions
JW and TYF conceived the study. YZL, XTT, ZLR, CWL, and XPP participated in the study design. JW collected the data. YZL, XTT and ZLR performed the statistical analyses. JW and CWL drafted the manuscript. XPP edited and checked the manuscript. The authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
The informed consent was waived by the Ethics Committee of the Second Affiliated Hospital, Fujian Medical University(Approval number:2021 − 215) because of the retrospective nature of this study.
Ethics approval and consent to participate
The present study was approved by the ethical review committee of the Second Affiliated Hospital, Fujian Medical University (Quanzhou, China). (Approval number: 2021 − 215).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, J., Liang, Y., Tang, X. et al. Ultra-early indicators of acute hypertriglyceridemic pancreatitis may influence treatment decision-making. Sci Rep 15, 1572 (2025). https://doi.org/10.1038/s41598-025-85847-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-85847-w