Abstract
This study aimed to develop a Least Absolute Shrinkage and Selection Operator (LASSO) logistic regression (LR) model using quantitative imaging features from Shear Wave Elastography (SWE) and Contrast-Enhanced Ultrasound (CEUS) to assess the malignancy risk of BI-RADS 4 breast lesions (BLs). The features predictive of malignancy in the LASSO analysis were used to construct a nomogram. Female patients (n = 111) with BI-RADS 4 BLs detected via routine ultrasound at Ma’anshan People’s Hospital underwent SWE, CEUS, and histopathological examinations were enrolled in this study. The histopathological results served as the gold standard. A time-intensity curve (TIC) was used to analyze the peak intensity (PI), area under the curve (AUC), and other CEUS parameters. The Young’s modulus was used for the SWE analysis. Bootstrap sampling was used to validate the nomogram. The performance of the model was evaluated using calibration curves, receiver operator characteristics curve (ROC) analysis, and decision curve analysis (DCA). The histopathological analysis revealed 35 malignant and 76 benign BLs. The multivariate LR analysis identified PI (odds ratio [OR] = 5.788, p < 0.05), AUC (OR = 6.920, p < 0.05), and SWE_Max (OR = 10.802, p < 0.05) as predictive of malignancy. The nomogram based on these features demonstrated an AUC of 0.875 (95% CI 0.805–0.945), sensitivity of 88.6%, specificity of 68.4%, good calibration, and excellent clinical utility. The nomogram could be used to improve the classification of BI-RADS 4 BLs and hence reduce the need for invasive biopsies to confirm malignancy.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most common cancer in women worldwide1, The incidence of BC has increased significantly in young females globally. Early detection and accurate diagnosis of BC can facilitate the delivery of timely interventions and improve the prognosis of BC patients. Mammography is often used to screen for BC in Western countries. However, compared to Western women, Asian women tend to have denser breasts. Mammography is less effective in diagnosing BC in dense breasts because the similar radiopaque appearance of dense tissue and potential malignancies can obscure abnormalities. Moreover, mammography does not provide any quantitative information on the lesion stiffness and vasculature that could be used to characterize BLs. Therefore, the Chinese Society of Imaging and Radiation Oncology recommends the use of breast ultrasound to screen for breast malignancies2. The American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) is used to assess the risk of malignancy of breast lesions (BLs). This classification is based on the morphological and structural appearance of the BLs on ultrasound3. The BI-RADS score ranges from 1 to 5, with BI-RADS 4 indicating a potentially malignant BL. Despite its high sensitivity, the BI-RADS scoring system has low specificity in predicting the degree of malignancy of BLs classified as BI-RADS 44. As a result, an invasive biopsy is still recommended for patients with BI-RADS 4 to confirm the presence of malignancy5.
To facilitate the distinction between benign and malignant BI-RADS 4 BLs, new ultrasound techniques, such as real-time Shear Wave Elastography (SWE) and Contrast-Enhanced Ultrasound (CEUS), are being employed to evaluate the stiffness and level of vascularization within BLs. SWE determines tissue stiffness by analyzing the speed of shear ultrasound waves generated within the breast tissue6. Malignant BLs exhibit a desmoplastic reaction characterized by increased collagen deposition, extracellular matrix remodeling, and the activation of cancer-associated fibroblasts, which contribute to tumor stiffness and progression7. As a result, compared with benign BLs, malignant BLs tend to have higher shear values8,9. In addition, malignant BLs tend to be more vascular than benign BLs. As a result, malignant BLs demonstrate rapid, uneven, and intense contrast enhancement patterns on CEUS. In addition, time-intensity curves (TIC) analysis could be used to quantify changes in the contrast enhancement within BLs over time. Various TIC metrics such as peak intensity (PI), arrival slope (AS), and area under the curve (AUC), could be used to characterize the perfusion characteristics of BLs. As a result, compared to conventional ultrasound, SWE and CEUS provide better objective measurable diagnostic indicators that could be used to characterize BLs, particularly BI-RADS 4 BLs.
In this study, we aimed to develop a nomogram to predict the risk of malignancy of BI-RADS 4 BLs to enhance the diagnostic efficacy of the standard BI-RADS system, and ultimately reduce the biopsy rate for BI-RADS 4 BLs. To achieve this aim a Least Absolute Shrinkage and Selection Operator (LASSO) regression model was used to extract predictive quantitative imaging features from SWE and CEUS. The predictive features were then used to construct a simple nomogram that could be used to predict the risk of malignancy of BI-RADS 4 BLs. The predictive accuracy of the nomogram was compared with a histopathological ground truth.
Materials and methods
Patient selection
This retrospective study included female patients diagnosed with BI-RADS 4 BLs between January 2020 to September 2023 at the Ma’anshan People’s Hospital (China). Patients were included in the study if they underwent conventional ultrasound, SWE, and CEUS, and had a single BL confirmed as benign or malignant by needle biopsy or surgery. Patients who did not provide written informed consent were excluded from the study. In addition, patients with multiple BLs, and those who received treatment for a previous breast malignancy were also excluded from the study.
Ethical considerations
This study was approved by the Medical Ethics Review Committee of the Ma’anshan People’s Hospital (Review number: 2022-001 001). A full explanation of the research process was provided to all the patients. Written informed consent was obtained from all patients who were willing to participate.
Imaging procedure
Conventional ultrasound and SWE examination
Patients were examined in the supine position with their arms raised using the Aixplorer ultrasound system (Supersonic, Imagine, France) for B-mode ultrasound assessment of the breasts. A probe with a frequency range of 4 to 15 MHz was used to perform the scans in both the transverse and longitudinal planes starting from the periphery of the breast to the nipple. Lower frequencies (around 4 MHz) were used to optimize penetration for deeper tissues, while higher frequencies (12 MHz or above) were employed to achieve better resolution of superficial structures, such as breast tissue. The visual analysis of the BL involved an assessment of the risk of malignancy based on the margin, orientation, shape, internal echo pattern, posterior characteristics, vascularity, and presence of calcifications as outlined in the ACR BI-RADS fifth edition10. The maximum size of the BL was also measured. Subsequently, the SWE images were acquired in real-time by positioning the transducer covered with ultrasound gel on the breast without applying pressure to minimize motion artifacts. We selected a 2-mm sampling box (Q-box) as the region of interest (ROI) for further analysis. The Q-box was placed within the lesion, and real-time stiffness values were displayed on the screen as the box was moved. Measurements were taken at the region with the highest stiffness, which could be located at the center, edge, or other areas of the lesion. The average SWE (SWE_Mean), minimum SWE (SWE_Min), and maximum SWE (SWE_Max) values (in kPa) were measured four times: twice along the transverse plane and twice along the maximum perpendicular plane of the lesion. All measurements were acquired from the hardest part of the lesion, as this region provides the best correlation with pathological characteristics. In addition, stiffness measurements of the surrounding adipose tissue were acquired using a 2-mm ROI. The elasticity modulus (EM) ratio was calculated by dividing the average SWE stiffness of the lesion by that of the surrounding tissue.
Contrast-enhanced ultrasound (CEUS) examination
The Chinese Mindray Resona 7 color Doppler ultrasound scanner and a multifrequency probe (3–9 MHz) were used for the CEUS examination. The images were acquired by an ultrasound doctor with 18 years of experience. The microbubble contrast agent SonoVue® (Bracco, Italy) was used to acquire the CEUS. Prior to the examination, 5.0 mL of 0.9% sodium chloride solution was mixed with the contrast agent and shaken vigorously to form a microbubble suspension. The largest cross-section of the lesion was selected on the B-mode ultrasound and used for real-time harmonic imaging analysis. Once the image was stabilized (i.e., the lesion was clearly visible with no significant motion artifacts), 4.8 mL of the SonoVue® suspension was rapidly injected via the median cubital vein, and the timer was started simultaneously. During the contrast-enhanced phase, the ultrasound transducer was used to capture real-time images of the lesion and surrounding tissue only (not the whole breast). These images were used to assess the movement of the contrast agent within and around the lesion and to identify features suggestive of malignancy such as wash-in, wash-out, hyperenhancement, and tissue perfusion. The contrast-enhanced region was identified, and ROI was drawn in the area with the most prominent enhancement. The built-in ultrasound software was employed to generate a TIC. Several TIC metrics were calculated to evaluate the flow within the contrast-enhanced lesion;
-
(i)
Ascending Slope (AS): The rate at which contrast agent intensity increases within the lesion. Malignant lesions typically show a steeper slope, indicating rapid enhancement.
-
(ii)
Arrival Time (AT): The time it takes for the contrast agent to first appear in the lesion. This parameter reflects the blood flow speed with the lesion. Malignant lesions usually have a shorter AT due to a richer blood supply.
-
(iii)
Peak Intensity (PI): Indicates the maximum contrast enhancement intensity within the lesion. PI is correlated with the volume of blood reaching the lesion. Malignant lesions generally show higher PI, caused by increased blood volume reaching the lesion.
-
(iv)
Area Under the Curve (AUC): Quantifies the overall contrast agent uptake across the lesion. Higher AUC values are often seen in malignant lesions, suggesting richer blood flow.
-
(v)
Time to Peak (TTP): The time required to reach the maximum contrast enhancement intensity. Malignant lesions typically exhibit a shorter TTP, signifying faster enhancement.
-
(vi)
Baseline Intensity (BI): Measures the background signal before contrast injection, and is typically used as a reference for comparison.
-
(vii)
Half-Time to Peak (DT/2): The time it takes for the signal to decay to half of its peak value. This value reflects the duration of blood perfusion. Malignant lesions often have a longer half-time, indicating more sustained perfusion.
-
(viii)
Descending Slope (DS): Indicates the rate at which the contrast agent clears from the lesion. Malignant lesions tend to have a slower descending slope, suggesting impaired clearance.
Histopathological examination
All BI-RADS 4 BLs identified on the standard ultrasound were biopsied or surgically treated. A histopathological examination was performed to identify the benign and malignant lesions.
Statistical analysis
The power analysis available on the PASS version 15 software was used to calculate the sample size. For this analysis, we used LASSO regression as the primary feature selection method and selected a minimum effect size of 0.25, a significance level (α) of 0.05, and a statistical power of 0.8. Based on these parameters, the minimum required sample size for reliable feature selection and model development was determined to be 102 cases. To mitigate potential issues such as data loss or bias, we recruited a slightly larger sample of 111 patients.
The normally distributed continuous variables were expressed as the mean ± standard deviation and analyzed by independent sample t-test. The non-normally distributed variables were expressed as the median and interquartile ranges [P25, P75] and analyzed by the Mann–Whitney test. Lastly, the categorical variables were expressed as counts (%) and analyzed by the chi-square test.
The univariate and LASSO logistic regression (LR) analyses were employed to screen for SWE and CEUS quantitative imaging features predictive of malignancy. The impact of age on the prediction of breast malignancy was also evaluated. The glmnet package available on the R software was used for this analysis. The optimal lambda value (λ = 0.012) was determined through 10-fold cross-validation, which minimized the mean squared error (MSE). Features with non-zero regression coefficients under this parameter were retained for further analysis and subsequently used to construct the nomogram. The LASSO regression method was selected since it effectively mitigates multicollinearity, ensuring that only the most relevant variables are retained for malignant risk assessment. Subsequently, the selected variables were subjected to a stepwise bivariate multivariate LR analysis, and the statistically significant variables were used to construct the predictive nomogram. The calibration of the model was assessed by using 1000 bootstrap samples. The discriminatory efficacy of the model was determined by calculating the AUC values of the receiver operating characteristic (ROC) curves. The sensitivity, specificity, and accuracy of the model were also measured. Additionally, the clinical utility of the model was evaluated by conducting a decision curve analysis (DCA) to quantify its net benefit within the threshold probability range. Data processing and analysis were performed using the R software (version 4.3.1). The nomogram and calibration curves were generated using the “rms” package, the ROC curves were generated using the “pROC” and “ggplot2” packages, and the DCA was conducted using the “ggDCA” package. A two-sided p-value below 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 111 female patients aged between 26 and 75 years (n = 111; 48 ± 12.62 y) were recruited in this study. All patients had a single BL. Among the 111 BLs evaluated 32% (n = 35) were malignant and 68% (n = 76) were benign. The maximum diameter of the BLs ranged between 3.80 mm and 50.00 mm (18.38 ± 9.87 mm). The most common histology for the malignant BLs was invasive ductal carcinoma of no special type (NST) (n = 27), followed by ductal carcinoma in situ (DCIS) (n = 3), invasive micropapillary carcinoma with invasive ductal carcinoma NST (n = 2), mucinous carcinoma (n = 1), invasive ductal carcinoma NST with partially high-grade cribriform DCIS (n = 1), and intraductal papillary carcinoma (partially invasive, mucinous carcinoma) (n = 1). The most common benign histological subtype identified in our sample was breast fibroadenoma (n = 42), followed by adenosis with fibroadenoma formation (n = 31), lobulated tumor grade I with adenosis and cyst formation (n = 1), localized granulomatous inflammation (n = 1), and fibroadenomatous nodules with calcification and chronic inflammation (n = 1). Table 1 provides the detailed characteristics of the benign and malignant BLs. Compared to the benign BLs, the malignant BLs had significantly higher SWE parameters, AUC, and PI.
ROC analysis of the continuous variables
As shown in Table 2, only the SWE_Mean, SWE_Max, SWE_Min, PI, and AUC values differed significantly between the benign and malignant BLs (p < 0.001). The SWE_Max had the highest predictive ability (AUC 0.757), followed by SWE_Min, SWE_Mean, PI, and AUC.
Univariate LR analysis
Table 3 illustrates the univariate LR analysis evaluating the impact of the patient’s age and various SWE and CEUS parameters on the risk of malignancy. Age, SWE_Mean, SWE_Max, SWE_Min, and EM ratio were identified as predictive indicators of malignancy. The univariate LR analysis showed that compared to the benign BLs, the malignant BLs had significantly higher age, SWE_Mean, SWE_Max, SWE_Min, PI, and AUC values (p < 0.001). Among these significant variables, SWE_Min above 40.45 exhibited the highest predictive value for malignancy with an odds ratio (OR) of 10.094. No significant differences in the AT, TTP, and AS parameters were noted between the benign and malignant BLs.
Predictors of malignancy according to the LASSO regression analysis
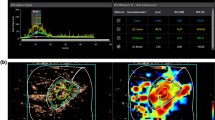
To mitigate multicollinearity issues among all features, LASSO regression analysis was used to further refine the coefficients based on the results of univariate analysis. SWE_Max, PI, and AUC had non-zero coefficients and were therefore included in the multivariate LR regression analysis (Table 4; Fig. 1a, b).
Plot illustrating the feature selection process using the LASSO regression analysis. (a) Variability in coefficient characteristics of the parameters identified as risk factors using LASSO regression analysis. The horizontal axis represents the log(lambda) values, where lambda is the regularization parameter. As lambda increases, the degree of shrinkage increases, resulting in more coefficients being reduced to zero. The vertical axis shows the coefficients of the features included in the model. Each of the colored lines corresponds to a different feature. (b) Selection process of optimal parameters in the LASSO regression model through cross-validation methods. The point where the dashed vertical line intersects the curves indicates the optimal lambda value, selected through 10-fold cross-validation.
Multivariable binary LR of selected variables
The SWE_MAX, AUC, and PI were identified as significant predictors of malignancy in the stepwise bidirectional multivariable LR analysis (Table 4). SWE_MAX greater than 46.80 kPa showed the strongest association with the risk of malignant tumors, with an OR of 10.802 (95% CI: 3.677–36.901, p < 0.001). A higher SWE_MAX indicates higher lesion stiffness which is a key characteristic of malignant tumors. Similarly, an AUC greater than 1750.05 (OR: 6.920, 95% CI: 2.286–23.155, p = 0.001) and a PI greater than 22.41 (OR: 5.788, 95% CI: 1.857–19.847, p = 0.003) were associated with increased risk of malignancy. A higher AUC and PI indicate an increased perfusion rate and higher contrast enhancement within the lesion. These features are typically associated with malignant tumors.
Development and verification of predictive nomogram
The predictive nomogram was constructed based on the significant predictors of breast malignancy identified in the multivariate LR analysis (Fig. 2a). The nomogram based on these features exhibited an AUC, sensitivity, and specificity of 0.875 (95% CI: 0.805–0.945), 0.886, and 0.684, respectively. Additionally, the nomogram exhibited good calibration and clinical utility, as indicated by the calibration and DCA curves (Fig. 2b–d).
(a) Nomogram for predicting breast malignancy. The horizontal lines represent the SWE or CEUS variables predictive of malignancy. According to the variable value, a score is assigned by drawing a vertical line to intersect the points line. The total number of points obtained from the three variables is then used to determine the risk score. (b) Nomogram calibration curve illustrating the consistency between the predicted risk of breast malignancy and actual outcomes. (c) Nomogram ROC (d) Nomogram DCA. The x-axis represents the threshold probability for malignancy, and the y-axis represents the net benefit. The red line represents the nomogram, while the blue line represents “treat all,” and the yellow curve represents “treat none.” According to the DCA curve, the nomogram offers greater net benefit across a threshold probability range between 0 and 1.0.
Visual analysis of BLs
Figure 3a–j highlight the differences between benign and malignant BLs on conventional ultrasound, SWE, and CEUS images. These images show how the addition of SWE and CEUS could be used to facilitate the discrimination between benign and malignant BI-RADS 4 BLs identified on conventional ultrasound.
(a–e) Breast lesion in a 39-year-old female. (a) Conventional ultrasound showing a solid hypoechoic irregular mass with angular margins measuring approximately 21 mm × 21 mm × 9 mm. The lesion has a parallel orientation. No calcifications and no change in posterior acoustic features were noted. The lesion was categorized as BI-RADS 4 A. (b) SWE_Max: 26.2 kPa. (c) CEUS demonstrates no enlargement of the lesion post-contrast administration. (d) PI: 13.88, AUC: 0.996. When incorporated into the nomogram model, the risk of malignancy was less than 10%. (e) Histopathological examination (HE) staining; (original magnification 40×) confirmed the presence of fibroadenoma with adenosis in the breast tissue. (f–j) Breast lesion in a 47-year-old female. (f) Conventional ultrasound showing a solid hypoechoic mass irregular mass with angular margins measuring approximately 18 × 15 × 9 mm. The lesion has a parallel orientation, no calcifications, and no change in the posterior acoustic features. The lesion was categorized as BI-RADS 4 A. (g) SWE_Max: 61.7 kPa. (h) CEUS shows enhancement. (i) PI: 27.88, AUC: 1901.76. When incorporated into the nomogram model, the risk of malignancy of the breast lesion was more than 90%. (j) Histopathological examination (original magnification 40×) confirmed intraductal carcinoma.
Discussion
The BI-RADS classification system based on traditional ultrasound scanning has achieved satisfactory results in discriminating between benign and malignant BLs. However, BI-RADS 4 BLs can vary widely in their risk of malignancy (2–95%). Class 4 BLs are categorized into 3 categories; 4 A, 4B, and 4 C with corresponding malignancy risks ranging from 2 to 10%, 10 to 50%, and 50 to 95%, respectively. Therefore, a biopsy is generally recommended for patients with BI-RADS 4 BLs11. Therefore new imaging biomarkers are required to discern between benign and malignant BI-RADS 4 BLs12. SWE and CEUS can provide additional information on tumor texture and vascularity13,14. Malignant BLs often exhibit irregular, inhomogeneous, and rapid enhancement patterns on CEUS due to abnormal blood vessel formation, while benign BLs typically show more uniform, slower, and less intense enhancement15,16,17. SWE technology quantitatively assesses tissue stiffness18. Malignant BLs typically exhibit higher collagen content19, resulting in harder tissue texture. However, the visual assessment of texture and contrast enhancement of ultrasound images remains subjective. In order to overcome this problem, several quantitative metrics have been proposed to quantify the blood flow and stiffness of BLs on CEUS and SWE images20,21. Nevertheless, there is still no consensus on the optimal parameters that could be used to measure the degree of malignancy. Therefore, we developed a nomogram based on SWE and CEUS parameters to facilitate the prediction of malignancy of BI-RADS 4 BLs and thus reduce the number of unnecessary biopsies.
The assessment of BLs based solely on BI-RADS often leads to misdiagnosis. The use of either CEUS or SWE parameters in combination with BI-RADS can improve the distinction between certain benign and malignant BLs. For instance, fibroadenomas often lack clear boundaries and tend to have irregular shapes22. As a result, they are often classified as BI-RADS 4 A. However, fibroadenomas tend to have lower AUC values on CEUS23, and lower SWE parameters than malignant BLs. Similarly, ductal carcinoma in situ (DCIS), confined within ducts tends to have a regular shape and therefore these BLs are often classified as non-malignant on the BI-RADS scoring system24. Nevertheless, DCIS tends to have higher SWE stiffness values and higher AUC values on CEUS25. Granulomatous lobular mastitis (GLM) often presents with malignant features such as uneven local glandular echoes, unclear boundaries, irregular morphology, and diffuse spreading and is frequently categorized as BI-RADS 4 or higher26. However, its stiffness is markedly lower than that observed in malignant BLs27. Inflammatory BLs exhibit abundant neovascularization, inflammatory cell infiltration, and varying degrees of necrosis and malignant features on CEUS such as rapid enhancement, enlarged areas, and filling defects. However, inflammatory BLs tend to have very low SWE values thus confirming the benign nature of these BLs. These findings highlight the need to integrate SWE and CEUS parameters with the BI-RADS systems to distinguish between benign and malignant BLs and thus reduce the need for unessential invasive biopsies. In line with the literature, our CEUS analysis showed that malignant BLs tend to have elevated AUC and PI levels compared with benign BLs. In addition, we identified SWE_Max above 46.8 kPa as a good indicator of malignancy, exhibiting an AUC, sensitivity, and specificity of 0.778, 68.6%, and 88.2%, respectively. Using the cases in Fig. 3 as an example, both Fig. 3a and f are BI-RADS 4ABLs, but their SWE_Max values differ significantly, and the pathological results are different. When SWE_Max is below 46.8 kPa (Fig. 3b), the pathology is fibroadenoma with adenosis(Fig. 3e); when SWE_Max is above 46.8 kPa (Fig. 3g), the pathology is intraductal carcinoma(Fig. 3j). Therefore, SWE_Max above 46.8 kPa is a key parameter for distinguishing between benign and malignant BI-RADS 4 BLs. BLs below this threshold can be considered for follow-up observation, while BLs above this threshold are recommended for early intervention or surgical treatment. Although several studies have shown that both the SWE and CEUS parameters could be employed to assess the risk of malignancy in BLs, few studies have evaluated the impact of using both parameters for the assessment of BI-RADS 4 BLs28. Therefore based on the results of the multivariate regression analysis we developed a predictive nomogram incorporating both SWE and CEUS parameters. In our study, the assessment of BI-RADS 4 BLs using the standard ultrasound BI-RADS systems results in an AUC of 0.729 (95% CI: 0.638–0.820 p < 0.001), sensitivity of 51.4%, specificity of 92.1%, positive predictive value of 79.3%, and negative predictive value of 75.0%. These findings show that while the BI-RADS system demonstrates high specificity, its sensitivity remains limited. Compared with the standard BI-RADS system, the nomogram proposed in this study, achieved a higher AUC (0.875 (95% CI: 0.805–0.945) and sensitivity (88.6%) but lower specificity (68.4%). Our nomogram also had a good positive predictive value (81.7%), and a negative predictive value (79.8%). The results indicate that our nomogram can better balance sensitivity with specificity when evaluating BI-RADS 4 BLs, and could therefore reduce the need for unnecessary diagnostic interventions. The DCA also confirmed that the nomogram exhibited good clinical utility in discriminating between benign and malignant BLs, offering valuable support for clinical decision-making.
This study has several limitations that must be acknowledged. The sample size for this study was small and obtained from a single institution. Moreover, the small sample size and selective inclusion of patients who underwent SWE and CEUS with pathological confirmation may have introduced selection bias, leading to the underrepresentation of certain breast lesion subtypes that can be both benign and malignant such as intraductal papillomas and phyllodes tumors. Therefore, larger multicenter studies are necessary to improve the generalizability and clinical applicability of the nomogram. External validation is also necessary to enhance the diagnostic efficiency of the model and its widespread adoption clinically. Although the nomogram can provide a good estimate of the risk of malignancy, pathological examination is still recommended to obtain a definitive diagnosis, particularly for highly uncertain cases. This study primarily focused on the application of SWE and CEUS. However, other emerging imaging techniques such as MRI and contrast-enhanced mammography could also be used to assess the risk of malignancy of breast lesions. It is important to note that all imaging techniques have advantages and limitations. While MRI provides detailed information on breast lesions, its high cost and longer exam times may limit its widespread use. On the other hand, contrast-enhanced mammography also offers additional dynamic blood flow data that could be used to predict the risk of malignancy in BI-RADS 4BLs. However, mammography makes use of harmful ionizing radiation to acquire the images. In addition, although mammography offers better resolution and is more effective at detecting microcalcifications, it can be less sensitive in identifying BLs in women with dense breasts, as dense tissue may obscure tumors. As a result, contrast-enhanced mammography requires further validation, particularly in dense breasts. Moreover, due to data limitations, we could not compare the performance of our model in relation to other existing ultrasound models. Therefore, more research is recommended to compare the efficacy of our model in distinguishing between benign and malignant lesions in relation to other existing models based on ultrasound, contrast-enhanced mammography, and MRI.
Conclusion
The SWE_MAX, AUC, and PI were identified as significant predictors of malignancy for BLs classified as BI-RADS 4. Our nomogram, incorporating these parameters, could be used to improve the characterization of BI-RADS 4 BLs. The integration of this model in clinical practice could reduce the need for additional invasive biopsies to confirm the presence of malignancy. However, further research is required to validate the accuracy of this nomogram.
Data availability
All data generated or analysed during this study are included in this article.
Abbreviations
- ACR:
-
American College of Radiology
- AS:
-
Ascending slope
- AT:
-
Arrival time
- AUC:
-
Area under the curve
- BC:
-
Breast cancer
- BI:
-
Baseline intensity
- BI-RADS:
-
Breast imaging report and data system
- BLs:
-
Breast lesions
- CEUS:
-
Contrast-enhanced ultrasound
- DCA:
-
Decision curve analysis
- DCIS:
-
Ductal carcinoma in situ
- DS:
-
Descending slope
- DT/2:
-
Half peak time
- EM:
-
Elasticity modulus
- LASSO:
-
Least absolute shrinkage and selection operator
- LR:
-
Logistic regression
- NST:
-
No special type
- OR:
-
Odds ratio
- PI:
-
Peak intensity
- ROC:
-
Receiver operating characteristic curves
- ROI:
-
Region of interest
- SWE:
-
Shear wave elastography
- SWE_max:
-
Maximum value of shear wave elastography
- SWE_mean:
-
Mean value of shear wave elastography
- SWE_min:
-
Minimum value of shear wave elastography
- TTP:
-
Time to peak
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Magny, S. J., Shikhman, R. & Keppke, A. L. inStatPearls (StatPearls Publishing, 2024).
Ding, R. et al. Breast cancer screening and early diagnosis in Chinese women. Cancer Biol. Med. 19, 450–467. https://doi.org/10.20892/j.issn.2095-3941.2021.0676 (2022).
Li, Q. et al. Meta-analysis: Contrast-enhanced ultrasound versus conventional ultrasound for differentiation of benign and malignant breast lesions. Ultrasound Med. Biol. 44, 919–929. https://doi.org/10.1016/j.ultrasmedbio.2018.01.022 (2018).
Cortadellas, T. et al. Estimation of tumor size in breast cancer comparing clinical examination, mammography, ultrasound and MRI-correlation with the pathological analysis of the surgical specimen. Gland Surg. 6, 330–335. https://doi.org/10.21037/gs.2017.03.09 (2017).
Chen, J. et al. Multi-parameter ultrasonography-based predictive model for breast cancer diagnosis. Front. Oncol. 12, 1027784. https://doi.org/10.3389/fonc.2022.1027784 (2022).
Youk, J. H., Gweon, H. M. & Son, E. J. Shear-wave elastography in breast ultrasonography: The state of the art. Ultrasonography 36, 300–309. https://doi.org/10.14366/usg.17024 (2017).
Barr, R. G., Engel, A., Kim, S., Tran, P. & De Silvestri, A. Improved breast 2D SWE algorithm to eliminate false-negative cases. Invest. Radiol. 58, 703–709. https://doi.org/10.1097/rli.0000000000000972 (2023).
Wang, C. & Che, Y. A ultrasonic nomogram of quantitative parameters for diagnosing breast cancer. Sci. Rep. 13, 12340. https://doi.org/10.1038/s41598-023-39686-2 (2023).
Rao, A. A., Feneis, J., Lalonde, C. & Ojeda-Fournier, H. A. Pictorial review of changes in the BI-RADS Fifth Edition. Radiographics 36, 623–639. https://doi.org/10.1148/rg.2016150178 (2016).
Wan, C. F. et al. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: correlation with prognostic factors. Radiology 262, 450–459. https://doi.org/10.1148/radiol.11110789 (2012).
Tang, Y., Liang, M., Tao, L., Deng, M. & Li, T. Machine learning-based diagnostic evaluation of shear-wave elastography in BI-RADS category 4 breast cancer screening: A multicenter, retrospective study. Quant. Imaging Med. Surg. 12, 1223–1234. https://doi.org/10.21037/qims-21-341 (2022).
Jia, C. et al. Value of an expanded range of lesions on contrast-enhanced ultrasound for the diagnosis of hypervascular breast masses. Gland Surg. 12, 824–833. https://doi.org/10.21037/gs-23-165 (2023).
Ren, T. et al. The diagnostic significance of the BI-RADS classification combined with automated breast volume scanner and shear wave elastography for breast lesions. J. Ultrasound Med. 42, 1459–1469. https://doi.org/10.1002/jum.16154 (2023).
Weng, H. et al. A diagnostic model for breast lesions with enlarged enhancement extent on contrast-enhanced ultrasound improves malignancy prediction. Ultrasound Med. Biol. 49, 1535–1543. https://doi.org/10.1016/j.ultrasmedbio.2023.02.016 (2023).
Yuan, Y. et al. Clinical value of contrast-enhanced ultrasound in breast cancer diagnosis. Comput. Math. Methods Med. 2022, 2017026. https://doi.org/10.1155/2022/2017026 (2022).
Yan, M., Peng, C., He, D., Xu, D. & Yang, C. A Nomogram for enhancing the diagnostic effectiveness of solid breast BI-RADS 3–5 masses to determine malignancy based on imaging aspects of conventional ultrasonography and contrast-enhanced Ultrasound. Clin. Breast Cancer 23, 693–703. https://doi.org/10.1016/j.clbc.2023.06.002 (2023).
Sigrist, R. M. S., Liau, J., Kaffas, A. E., Chammas, M. C. & Willmann, J. K. Ultrasound elastography: Review of techniques and clinical applications. Theranostics 7, 1303–1329. https://doi.org/10.7150/thno.18650 (2017).
Zhou, B. Y. et al. Decoding the molecular subtypes of breast cancer seen on multimodal ultrasound images using an assembled convolutional neural network model: A prospective and multicentre study. EBioMedicine 74, 103684. https://doi.org/10.1016/j.ebiom.2021.103684 (2021).
Xue, Y., Yao, S., Li, X. & Zhang, H. Value of shear wave elastography in discriminating malignant and benign breast lesions: A meta-analysis. Med. (Baltim). 96, e7412. https://doi.org/10.1097/md.0000000000007412 (2017).
Pu, H. et al. The efficacy of added shear wave elastography (SWE) in breast screening for women with inconsistent mammography and conventional ultrasounds (US). Clin. Hemorheol. Microcirc. 71, 83–94. https://doi.org/10.3233/ch-180398 (2019).
Salati, S. A. Breast fibroadenomas: A review in the light of current literature. Pol. Przegl Chir. 93, 40–48. https://doi.org/10.5604/01.3001.0014.5676 (2020).
Jung, E. M., Jung, F., Stroszczynski, C. & Wiesinger, I. Quantification of dynamic contrast-enhanced ultrasound (CEUS) in non-cystic breast lesions using external perfusion software. Sci. Rep. 11, 17677. https://doi.org/10.1038/s41598-021-96137-6 (2021).
Liu, W. et al. Ultrasound characteristics of sclerosing adenosis mimicking breast carcinoma. Breast Cancer Res. Treat. 181, 127–134. https://doi.org/10.1007/s10549-020-05609-2 (2020).
Wang, B. et al. The diagnostic value of contrast-enhanced ultrasonography in breast ductal abnormalities. Cancer Imaging 23, 25. https://doi.org/10.1186/s40644-023-00539-w (2023).
Yin, L. et al. Differentiation between granulomatous lobular mastitis and breast cancer using quantitative parameters on contrast-enhanced Ultrasound. Front. Oncol. 12, 876487. https://doi.org/10.3389/fonc.2022.876487 (2022).
Toprak, N. et al. Does ARFI elastography complement B-mode ultrasonography in the radiological diagnosis of idiopathic granulomatous mastitis and invasive ductal carcinoma? Acta Radiol. 63, 28–34. https://doi.org/10.1177/0284185120983568 (2022).
Lu, J. et al. Diagnostic value of contrast-enhanced ultrasonography with sonovue in the differentiation of benign and malignant breast lesions: A meta-analysis. Technol. Cancer Res. Treat. 19, 1533033820971583. https://doi.org/10.1177/1533033820971583 (2020).
Acknowledgements
We would like to thank TopEdit (www.topeditsci.com) for the English language editing of this manuscript.
Funding
This study was supported by the Ma’anshan People’s Hospital Youth Scientific Research Fund(No. YQ-2022-1).
Author information
Authors and Affiliations
Contributions
TT. R. and LF. Y. carried out the literature search, and designed and wrote the manuscript. TT. R. and X.L. conceived of the project, and participated in its design and coordination, and helped to draft the manuscript. ZZ. G. and WB. C.are responsible for figures processing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Medical Ethics Review Committee of Ma’anshan People’s Hospital (Review number: 2022-001 001). All methods were carried out in accordance with relevant guidelines and regulations.
Informed consent
A full explanation of the research process was provided to all patients and written informed consent was obtained from all patients willing to participate in this study.
Tables.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ren, T., Gao, Z., Yang, L. et al. Development of a nomogram for predicting malignancy in BI-RADS 4 breast lesions using contrast-enhanced ultrasound and shear wave elastography parameters. Sci Rep 15, 1356 (2025). https://doi.org/10.1038/s41598-025-85862-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85862-x