Abstract
The evolution of genetic diversity and population structure of Plasmodium vivax as malaria elimination approaches remains unclear. This study analyzed the genetic variation and molecular epidemiology of P. vivax from Yala Province in southern Thailand, an area in the pre-elimination phase. Seventy P. vivax isolates, collected between 2017 and 2020, were genotyped for domain II of pvdbp and the 42-kDa region of pvmsp1 using amplicon deep sequencing. Data from Yala province were compared to published data from Tak province, where transmission was higher. Key analyses included nucleotide diversity (π), haplotype diversity (Hd), natural selection, recombination rates, and complexity of infection (COI). Genetic diversity in Yala was relatively low (π = 0.008dbp and 0.014msp1; Hd = 0.774dbp and 0.407msp1) compared to Tak (π = 0.012dbp and 0.027msp1; Hd = 0.849dbp and 0.962msp1). In Yala, polyclonal infections were found in 53.7% of pvdbpII and 47.8% of pvmsp142 isolates, with average COI of 1.6 and 1.7. Both genes were under balancing selection. Distinct genetic differences were found between Yala and Tak in pvmsp142, providing a local genotypic profile useful for tracing parasite origins.
Similar content being viewed by others
Introduction
Malaria incidents in Thailand have recently declined due to the government’s intensified control efforts, including vector control and early diagnosis. Thailand is in the pre-elimination campaign, aiming to be malaria-free by 20301,2. Despite this, over twenty thousand malaria cases were reported nationwide in 2023, with transmission highly concentrated in remote and border areas.
Yala province, located in the southernmost part of Thailand bordering Malaysia, is known for its natural scenery, such as tropical forests, mangrove swamps, and national parks. The main economic activity is agriculture, which includes rubber and palm oil production. Yala has been affected by violence and conflict related to separatist movements, which has hindered the effective implementation of public health policies1,3. The geographical characteristics and the livelihood of the people put residents at risk of malaria infection. Historically, Yala has been the second most significant malaria hotspot in Thailand, following Tak province. However, Yala has recently shown a remarkable reduction in malaria cases, decreasing from 5,852 cases in 2016 to only 118 cases in 20234. Assessing the genetic diversity and molecular epidemiology in Yala, therefore, garners the attention for investigating the microevolution associated with the dramatic decline in parasite populations. Such evaluations also aid in monitoring parasite movement, enhancing surveillance and control of reintroducing cases, especially in areas in the pre-elimination phase.
Plasmodium vivax in Thailand exhibits high levels of genetic diversity, as shown by studies on various molecular markers such as multidrug-resistant gene 1 (mdr1), merozoite surface proteins family (msp), circumsporozoite surface protein (csp), and microsatellite markers5,6,7,8,9. These studies have revealed a diverse pool of P. vivax strains in Thailand. Protein antigens, such as merozoite surface protein 1 (pvmsp1) and Duffy binding antigen (pvdbp), play significant roles in the erythrocytic invasion of P. vivax and display high polymorphisms due to host immune exposure10,11,12,13. The carboxyl-terminus of the pvmsp1 42 kDa region (pvmsp142) and the amino-terminal cysteine-rich region on pvdbp domain II (pvdbpII) are considered attractive targets for vaccine-mediated immunity, although they naturally present highly polymorphic patterns. However, there are limited studies on the genetic polymorphisms of these two antigens in Thailand.
We previously conducted a study in Tak province, the region with the highest malaria endemicity and sustained high transmission intensity in the country14. However, the data obtained were insufficient to represent the whole country. This study aims to extend the recently published genetic diversity profiles and investigate the molecular epidemiology of P. vivax in areas with lower transmission. We collected samples in Yala province, which has the second highest transmission intensity in the country but is currently in the malaria elimination phase, as indicated by the decreasing number of cases and lower annual incidence. Yala serves as another ideal study site in Thailand for investigating parasite diversity and genotypic patterns, given its notably lower incidence rates compared to Tak province. This contrast allows for a comparative analysis of parasite diversity between regions with differing transmission intensities, providing valuable insights into the relationship between genetic diversity and malaria transmission. This approach can offer a more comprehensive understanding of the disease burden, in addition to traditional epidemiological parameters like prevalence and incidence. Investigating the genetic diversity of parasites from Yala will help assess how transmission intensity influences parasite evolution and inform more effective malaria control strategies.
Results
Nucleotide polymorphisms and amino acid mutations of southern Thailand pvdbp II and pvmsp1 42
All 70 samples collected in the years 2017 (n = 28), 2018 (n = 27), 2019 (n = 14), and 2020 (n = 1) from Yala province, southern Thailand, were successfully amplified and sequenced. The sequencing data included 395 bp of the pvdbpII covering nucleotide positions 1,045–1,439 and 341 bp of the pvmsp142 covering nucleotide positions 4,241–4,581. The study identified a total of 11 mutations in pvdbpII, of which 4 were singleton variable sites, where mutations occured in only one individual among the sequences, and 7 were parsimony informative sites, with at least two different nucleotides each appearing in at least two sequences. Out of 11 polymorphisms, 1 was a synonymous change, whereas the remaining 10 were nonsynonymous changes. pvmsp142 revealed a higher number of polymorphisms compared to the pvdbpII, with a total 28 mutations identified. Out of these, 4 were singleton variable sites and 24 were parsimony informative sites. The analysis showed that 2 of the mutations were synonymous changes, while the remaining 26 were nonsynonymous.
Protein mutation derived from nonsynonymous substitution has been observed to manifest in distinct patterns across parasites from southern and northwestern Thailand. Specifically, ten out of a total of 131 amino acids of pvdbpII displayed mutation from that differed to the P01 reference strain (which was collected in West Papua, Indonesia). Eight out of these 10 mutation points were found in both southern and northwestern Thailand isolates, albeit at different proportions. In pvdbpII, S363T (37.1%) and K402S (35.7%) were the most frequently observed mutations, followed by E340K (31.4%), T359R (28.6%), K341N (28.6%), and K402T (8.6%). In contrast, N330D, A360E, N372K, and W392R were found at considerably lower frequencies of 1.4% (Table 1). Moreover, a single position was dimorphic, displaying both K402S and K402T mutations. Regarding the 113-amino acid pvmsp142, it was observed that 23 amino acid changes occurred frequently. To illustrate, 25 out of 27 point mutations were found to occur in both southern and northwestern Thailand isolates. The most frequently observed mutations were K1449M (100%), D1484A (97.1%), L1451V (95.7%), E1491D and L1493I (92.9%), N1487K, Q1489E, and A1504T (91.4%), followed by N1490D and E1492K (90%), A1507T and K1508Q (88.6%), A1500S and F1509S (87.1%), E1499D (85.7%), T1488A, K1495T, E1497G, and T1510M (84.3%), and N1498S and N1501T (82.9%). In contrast, T1510I was found to occur at a low frequency of 14.3%, whereas A1500T (7.1%), F1509L (2.9%), A1477E, Q1478E, and T1503A (all 1.4%) were observed to occur at even lower frequencies. Notably, six positions exhibited dimorphisms, namely A1477T/E, T1488A/K, Q1489E/H, A1500S/T, F1509S/L, and T1510I/M (Table 1). The nucleotide sequences of pvdbpII and pvmsp142 were translated into amino acid sequences and analyzed using BepiPred to predict linear B cell epitopes. Eight residues or peptides within the internal stretch of the pvdbpII sequence (TDMEGIGYSKV, E, G, GEKAQQHRKQWWNE, VKKRLKGNFIWICKINVAVNIEP, T, DGKINYTDKKVCKVP) and six residues of pvmsp142 (IKDPYKLLDLEKKKKLIGSY, DK, LA, AYYNKMEEL, T, KVEDDINTQNEELKKIENEANKTAEKAKFTAKKAELEKYLPFL) were identified as potential B-cell epitopes. Analysis revealed that 3 of the 10 mutations in pvdbpII (E340K, K341N, and N372K) were located on B-cell epitopes (Fig. 1a). The majority of mutations in pvmsp142, specifically 22 of the 27 mutations (K1449M, L1451V, D1484A, N1487K, T1488A, Q1489E, N1490D, E1491D, E1492K, L1493I, K1495T, E1497G, N1498S, E1499D, A1500S/T, N1501T, T1503A, A1504T, A1507T, K1508Q, F1509S/L, and T1501I/M), were located on B-cell epitopes (Fig. 1b). This suggests an adaptive response of this parasite antigen to evade immune recognition.
Based on the crystal structure of (A) pvdbpII (PDB ID: 3RRC)15 and (B) the predicted pvmsp142 (AlphaFold ID: F1AQZ6), the locations of nonsynonymous changes in each amino acid are highlighted in red and the predicted B-cell epitopes are marked in yellow. Amino acid changes localizing on the predicted B-cell epitopes are denoted in red labels.
The comparison of genetic diversity and natural selection in pvdbp II and pvmsp1 42 among isolates in southern and northwestern Thailand
Overall, 26 and 57 unique haplotypes of pvdbpII and pvmsp142 were detected across 140 isolates from southern (Yala) and northwestern (Tak) Thailand. Both genes showed lower genetic diversity in Yala compared to Tak, as reflected by all diversity measures (Table 2). In Yala, pvdbpII had a nucleotide diversity (π) of 0.008, haplotype diversity (Hd) of 0.774, 11 mutations (η), 11 segregating sites (S), and an average of 3.257 nucleotide differences (k). For pvmsp142, the values were π = 0.014, Hd = 0.407, η = 28, S = 28, and k = 4.868. In contrast, Tak exhibited higher diversity with pvdbpII showing π = 0.012, Hd = 0.849, η = 17, S = 17, and k = 4.782, while pvmsp142 had π = 0.027, Hd = 0.962, η = 42, S = 42, and k = 9.139. These results consistently indicate reduced genetic diversity in Yala for both genes.
pvdbpII and pvmsp142 were subject to balancing selection within the overall dataset, as determined by a range of tests that indicate natural selection, such as nucleotide substitution rates, Tajima’s D, and Fu and Li’s D* and F*. The results of the overall analysis demonstrated that pvmsp142 displayed a significantly positive value across all tests, thus suggesting a substantial level of adaptive fitness in the parasite with respect to this gene. However, when analyzing the data solely from Yala province, signs of excess of low-frequency variants in the population were observed by negative values, albeit without statistical significance (Table 3).
Complexity of infection
The within-host genetic diversity was assessed using 67 isolates, as 3 additional isolates fell below the detection threshold of the DADA2 pipeline (Table 4). Among 67 isolates being analyzed, 53.7% and 47.8% of samples harbored polyclonal infections for pvdbpII and pvmsp142, respectively. On average, each patient had 1.6 cocirculating variants for pvdbpII and 1.7 for pvmsp142. Remarkably, some isolates contained up to 4 variants of pvdbpII and 6 variants of pvmsp142. Technical limitations disallowed the estimation of the complexity of infection (COI) in the Tak data set.
Haplotypic and phylogenetic relationship
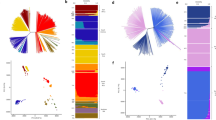
The genetic relatedness of P. vivax parasites in southern and northwestern Thailand populations was investigated using haplotype network and phylogenetic analysis. For pvdbpII, a haplotype network was constructed from 140 sequences representing 26 unique haplotypes (Fig. 2a). Blue and red circles represented haplotypes found in Yala and Tak isolates, respectively. Haplotypes were separated by one to two mutation steps, and 46.1% of haplotypes appeared in only one isolate. Haplotype D1 was predominant, with 32 genetically identical isolates. Haplotype D1, D2, and D9 were present in Yala and Tak in ratios of 27:5, 11:3, and 1:15 for Yala and Tak, respectively. In contrast, the haplotype network for pvmsp142 exhibited a greater level of diversity, consisting of 56 haplotypes derived from 140 sequences (Fig. 2b). A large proportion of these haplotypes, specifically 77.2% (44 out of 57), were present only in one isolate. The most predominant haplotype, M1, was identified with 54 genetically identical isolates and was only present in Yala. The haplotypes were separated by a range of one to twelve mutational steps. The haplotypes derived from pvmsp142 in Yala and Tak isolates were distinctly separated, and no shared haplotypes were observed.
Haplotype networks were constructed using the median-joining algorithm to depict the relationships among 26 haplotypes (D1-D26) of pvdbpII (A) and 56 haplotypes (M1-M56) of pvmsp142 (B) from 140 Thai isolates (70 from Yala and 70 from Tak). Each circle represents a haplotype, with its size indicating the frequency of occurrence. The proximity of circles reflects the degree of genetic relatedness between haplotypes, with the number of nucleotide-base changes marked by hatch marks.
The phylogenetic relationship was analyzed using 140 sequences from 70 isolates each from southern (Yala) and northwestern (Tak) Thailand. Yala isolates are represented by blue circles, while those from Tak isolates are denoted by red circles (Fig. 3). The analysis revealed clustering of parasite isolates based on geographic locations. Similar to the haplotype network, the phylogenetic tree for pvmsp142 showed a clear separation between parasites from Yala and Tak provinces, suggesting that pvmsp142 could serve as a potential molecular marker for identifying the geographic origins of P. vivax infections.
Phylogenetic analysis depicting the relationships among 140 nucleotide sequences of pvdbpII (A) and pvmsp142 (B), with 70 sequences from southern Thailand (Yala) and 70 from northwestern Thailand (Tak). Isolates from Yala are represented by blue circles, those from Tak by red circles, and the P. vivax P01 reference is indicated by a black box.
Recombination and linkage disequilibrium
The minimum number of recombination events (Rm) between adjacent polymorphic sites in pvmsp142 and pvdbpII was investigated and compared. Overall, the results presented in Table 2 indicate that Rm values were higher in pvmsp142 (Rm = 13) compared to pvdbpII (Rm = 5). Furthermore, both gene fragments exhibited linkage disequilibrium, as evidenced by the decline in R2 with increasing nucleotide distance. The regression analysis revealed that the R2 values for pvdbpII decreased gradually with nucleotide distance, and the regression line was represented by the equation Y = 0.3492–1.2428X (Fig. 4a). Whereas the R2 values for pvmsp142 decreased sharply to zero, and the regression line had a lower slope, represented by the equation Y = 0.4874–2.2201X, suggesting that genetic diversity was contributed by intragenic recombination within both genes, but the recombination rates were significantly higher in pvmsp142 (Fig. 4b).
Discussion
The results in this study shows variation in the genetic diversity of P. vivax across two endemic provinces of Thailand experiencing different transmission intensities. pvmsp142 showed higher diversity than pvdbpII through the overall dataset. Based on these two markers, the genetic diversity of P. vivax was lower in Yala compared to Tak province, reflecting lower transmission intensity. Lower transmission of P. vivax in Yala is possibly caused by the developed infrastructure and high percentage of urbanization in Malaysia, which may restrict the spread of malaria. In contrast, Tak province is a remote region adjacent to Myanmar, characterized by ecological conditions, population mobility, socioeconomic status, and prevention and control measures that favour higher transmission rates of malaria compared to the southern region of Thailand and Peninsular Malaysia.
The higher degree of genetic diversity observed in pvmsp142 compared to pvdbpII can be attributed to a combination of factors. For instance, pvmsp142 faces stronger immune pressure and shows higher antigenic variation, as indicated by an excess of nonsynonymous mutations and a greater number of amino acid changes. This pressure drives the evolution of multiple strains, leading to more allelic forms. The localization of MSP1 on the merozoite surface may subject it to stronger selective pressure and, consequently, higher diversity. While, DBP appears to be under functional constraint, resulting in lower diversity.
Six of the 10 nonsynonymous mutations identified in pvdbpII, including N330D, K341N, S353T, T359R, N372K, and W392R, were previously discovered in Brazil16, Sudan17, and China18. It was observed that residues N372K and W392R play a role in antigenic drift and contribute to the development of resistance to inhibitory antibodies10. In the present study, we identified fixed mutations A360E and K402T in Yala, occurring at frequencies of 1.4% and 8.6%, respectively, but these mutations were not detected in Tak. Conversely, mutations N330D, N372K, and W392R were more frequently observed in Tak but were rare in Yala. Furthermore, a number of mutations, including I322T, K326E, G339D, H345R, I374M, and I379L, were identified only in Tak but were not present in Yala, reflecting region-specific protein mutations in the P. vivax populations in different area of Thailand over pvdbpII marker. On the other hand, the absence of shared haplotype across regions suggests that mutations in pvmsp142 have remained highly specific to each study site, likely due to limited gene flow and population structure. The small population size in Yala may also contribute to the persistence of certain mutations, which remain private to this location.
The haplotype network and phylogenetic analysis of pvmsp142 (and to a lesser extent pvdbpII) revealed a distinct separation between parasite populations in Yala and Tak provinces, underscoring pvmsp142 as a potential marker for identifying the origin of P. vivax in Thailand. However, additional genetic markers are needed for a more accurate determination of the parasite’s origin. These two study sites are over 1,700 km apart, and exhibit distinct ecological and social characteristics. Along with the environmental factors, variation in the Anopheles species between the southern region19 and the northwestern region of Thailand20 may also contribute to this genetic differentiation.
In order to generate new recombinant haplotypes during meiotic reproduction, polyclonal infection is a prerequisite21,22. The current study found a high degree of recombination. The pvmsp142 displays a higher minimum number of Rm compared to pvdbpII. This higher diversity of pvmsp142 can be attributed to its localization on the parasite surface, where it directly elicits natural immune responses, leading to antigenic drift and high antigenic diversity. In Brazil, a large proportion (90–95%) of patients infected with malaria have been shown to produce antibodies against pvmsp123,24. On the other hand, antibodies against pvdbp are detected in a smaller proportion (44.5%) of primary infected individuals, as pvdbp is rapidly secreted during reticulocyte invasion, leading to a short-lasting antibody response24. The genotyping of P. vivax isolates from our previous study in northwestern Thailand was carried out using Illumina platform-based BT-Seq (Celemics, Seoul, South Korea). However, the analysis of multiclonal infections was constrained by missing primer regions in the data from this platform. As a result, this current study focused on isolates from Yala to determine COI. Consequently, the COI reported here may not be representative of the entire country. Nonetheless, as expectated, the COI of 1.7 for pvmsp142 in this study was significantly lower than the 3.8 reported in a similar analysis of Cambodian isolates collected between 2010 and 2011, which came from a setting with much higher transmission intensity16.
In summary, there was a significant association between transmission intensity and parasite diversity within Thailand. In regions nearing pre-elimination, such as Yala, relatively low genetic diversity was observed compared to the highly endemic areas like Tak. However, despite the reduced transmission intensity in Yala. Haplotype network and phylogenetic analyses revealed distinct genetic signatures in pvmsp142 between the Yala and Tak regions, suggesting a unique local genotypic profile. This finding indicates that pvmsp142 could potentially serve as a molecular marker to trace the geographic origin of Plasmodium vivax parasites, aiding in elimination and control efforts.
Materials and methods
Study site and ethical consideration
Blood samples were collected from patients who attended the malaria clinic in Bannang Sata District, Yala province, a Thailand-Malaysia border province approximately 1,070 km away from Bangkok (Fig. 5). The annual case numbers of vivax malaria were 2,839 in 2017, 1,412 in 2018, 1,193 in 2019, 881 in 2020, 164 in 2021, and 75 in 2022, respectively (Fig. 5). P. vivax genomic DNA was isolated from whole blood samples and the species confirmed by nested qPCR targeting 18S rRNA genes25. Written informed consent/assent was obtained from all patients, including children and their parents. Utilizing of these parasite DNA samples and data materials in this study was acted under the approval of the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok (approval number MUTM 2018-016-06 and MUTM 2021-067-01). All methods were performed in accordance with the relevant guidelines and regulations.
Study sites. Samples were collected from Yala province, the region with the highest malaria endemicity in southern Thailand. The accompanying table shows the average malaria incidence (per 1,000 population) in both Yala and Tak Provinces during the years of sample collection. A line chart demonstrates the decline in P. vivax incidence in Yala over the past decade. Data were sourced from the Malaria Online System, Department of Disease Control, Ministry of Public Health, Thailand (https://malaria.ddc.moph.go.th/malariar10; accessed on 17 September 2024). The map was created using QGIS v3.40.2 software (http://www.qgis.org).
Amplification of pvdbp II and pvmsp1 42
Seventy P. vivax genomic DNA samples were collected from Yala province between 2017 and 2020. The pvdbpII (Fig. 6a) and pvmsp142 (Fig. 6b) subunits were amplified using Illumina Nextera-style tag sequences with overhang P5- and P7-tag primers (indicated in lowercase in Supplementary Material: S2). The PCR mixture was prepared with 10 μM of forward and reverse primers, 2X Phusion High-Fidelity Master Mix with HF Buffer (Thermo Fisher Scientific, USA), and 5 μl of genomic DNA, resulting in a final PCR volume of 50 μl. The first-round PCR conditions were as follows: pre-denaturation at 98 °C for 2 min, followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 57.8 °C for pvdbpII and 59 °C for pvmsp142 for 1 min each, and extension at 72 °C for 1 min. A post-extension step was conducted at 72 °C for 10 min. The PCR products were then purified using the GeneapHlow™ Gel/PCR Kit (Geneaid, Taiwan) according to the manufacturer’s protocol.
Schematic diagram of (A) the Duffy binding protein and (B) the merozoite surface protein-1. The black regions indicate the targeted sites of domain II (pvdbpII) and the 42-kDa region (pvmsp142) selected for amplicon sequencing, with numbers indicating the amino acid boundaries. Shown are the crystal structure of the pvdbpII dimer (PDB ID: 3RRC)15 and the predicted structure of pvmsp142 (AlphaFold ID: F1AQZ6). The target amplicons contain a 395-bp variable region of pvdbpII and a 341-bp variable region of pvmsp142, respectively.
DNA library preparation and sequencing
Purified PCR amplicons of both genes containing overhang sequence were pooled and performed the second-round PCR to prepare the DNA library using Phusion High-Fidelity Master Mix with 5X HF Buffer (New England Biolabs, MA) and 10 μM of P5 index 1 and P7 index 2 from Nextera XT Index Kit v2 Set A (Illumina, CA). The PCR reaction was executed under the conditions as follows: pre-denaturation at 98 °C for 30 s, followed by 12 cycles of denaturation at 98 °C for 10 s, annealing at 60 °C for 20 s, and extension at 72 °C for 20 s. Post extension was carried out at 72 °C for 10 min. The PCR products with barcode indexes were purified with 1.8X AMPure XP beads (Beckman Coulter, CA), and the expected amplicon size to be sequenced was determined using Agilent High Sensitivity D1000 ScreenTape Assay on the 4150 TapeStation (Agilent Technologies, CA) according to manufacturer’s instruction. DNA concentration of individual samples was measured by DenoVix QFX Fluorometer (DeNovix, DE) and normalized to 4 nM with the appropriate volume of 10 mM Tris–HCl pH 8.5. Equal concentrations of each library were pooled and denatured by 0.2 N NaOH, then diluted to a final concentration of 8 pM with hybridization HT1 buffer (Illumina, CA). Sequencing was performed on an Illumina MiSeq platform in pair-end mode using MiSeq v2 Reagent Kit 500 Cycles PE (Illumina, CA) together with 5% spike-in PhiX Control v3 (Illumina, CA).
Additional sequence retrieval
Homologous DNA sequences from parasite populations of our previous study in Tak province, northwestern Thailand, were retrieved from the NCBI GenBank to compare genetic diversity with isolates from Yala province, southern Thailand. The sequences for pvdbpII (n = 70, accession numbers PP706549–PP706618) and pvmsp142 (n = 70, accession numbers PP706479–PP706548) were included in the analysis.
Data analysis
The quality of forward (R1) and reverse (R2) reads of individual samples were examined using FastQC26. Reads with poor quality were trimmed off using Trim Galore27. Only reads with a quality score above 30 were mapped with BWA28, generating a binary alignment map (BAM) file. The BAM file was sorted according to the chromosomal order with samtools sort, then converted to variant call format (VCF) using bcftools mpileup29. Single nucleotide polymorphisms (SNPs) were identified against the P01 reference sequence using bcftools call29, and variants that did not meet the quality score and read depth criterion (QUAL < 30, DP < 20) were filtered. Only VCF files with quality-passed variants were then used to generate the consensus sequence by bcftools consensus29 and stored in FASTA format for further analysis.
Population genetics measures the degree of genetic diversity, such as number of segregating sites (S), number of mutations (η), nucleotide diversity (π), average number of nucleotide differences (k), number of haplotypes (H), haplotype diversity (Hd), and their corresponding standard deviation (SD) were computed using DnaSP v5.1 software30. Departing from neutrality of evolution was examined by Tajima’s D31, Fu and Li’s D* and F* test32, and nucleotide substitution rates using MEGA v10.2.6 software33.
The computation of the minimum number of recombination events (Rm) and linkage disequilibrium (LD) were carried out using R2 in relation to nucleotide distance, utilizing DnaSP v5.1 software. LD was evaluated to determine the level of meiotic recombination in gene fragments of isolates from southern Thailand at various polymorphic sites. The haplotypic relationship of P. vivax between the southern and northwestern Thailand populations was investigated by constructing a haplotype network with the Median Joining algorithm through PopART v1.7 software34. Each circle in the network represents a haplotype, with the size of the circle indicating the frequency of the haplotype. The closeness between the circles illustrates the level of genetic relatedness of each haplotype, and the number of nucleotide-base changes is shown as a number of hatch marks. The phylogenetic analysis of P. vivax from the two regions of Thailand was conducted using the Maximum Likelihood method with Tamura-Nei model through MEGA v10.2.6 software33. In the resulting tree, each dot represents an isolate: red dots denote isolates from the northwestern region, blue dots denote isolates from the southern region, and the P01 reference is marked by a black box. Linear B-cell epitopes were predicted from pvdbpII and pvmsp142 using BepiPred with a default threshold score of 0.5 (http://tools.iedb.org/main/bcell/)35. Additionally, the positions of nonsynonymous changes on the predicted B-cell epitopes were visualized on the structures of pvdbpII and pvmsp142 (PDB ID: 3RRC and AlphaFold ID: F1AQZ6, respectively) using PyMOL software v2.5.2. The COI was determined using the Diversive Amplicon Denoising Algorithms (DADA2), an R package widely used for high-resolution sequence inference in Illumina-based amplicon sequencing data36. COI was defined as the highest number of distinct haplotypes observed in each sample for either amplicon. For haplotype detection, we targeted a minimum haplotype coverage of 100 reads and set a detection threshold of 1% for the limit of detection.
Data availability
The sequence data generated and analyzed during the current study have been deposited in the GenBank repository, under accession numbers PQ436089-PQ436158 for pvdbpII and PQ436159-PQ436228 for pvmsp142.
References
DDC. Guide to malaria elimination for Thailand’s local administrative organizations and the health network. (Bureau of Vector Borne Diseases, Department of Disease Control, Ministry of Public Health, Thailand, 2019).
Sudathip, P. et al. Assessing Thailand’s 1-3-7 surveillance strategy in accelerating malaria elimination. Malar. J. 21, 222. https://doi.org/10.1186/s12936-022-04229-z (2022).
Jitpiromsri, S. The deep South of Thailand: 15 years in fields of open conflict, violence and peace narratives. Asian Int. Stud. Rev. 20, 79–108 (2019).
DDC. Guide to malaria elimination for Thailand’s local administrative organizations and health network. (Bureau of Vector Borne Diseases, Department of Disease Control, Ministry of Public Health, Thailand, 2023).
Cui, L. et al. Genetic diversity and multiple infections of Plasmodium vivax malaria in Western Thailand. Am. J. Trop Med. Hyg. 68, 613–619. https://doi.org/10.4269/ajtmh.2003.68.613 (2003).
Kittichai, V., Nguitragool, W., Ngassa Mbenda, H. G., Sattabongkot, J. & Cui, L. Genetic diversity of the Plasmodium vivax multidrug resistance 1 gene in Thai parasite populations. Infect. Genet. Evol. 64, 168–177. https://doi.org/10.1016/j.meegid.2018.06.027 (2018).
Maneerattanasak, S. et al. Genetic diversity among Plasmodium vivax isolates along the Thai-Myanmar border of Thailand. Malar. J. 15, 75. https://doi.org/10.1186/s12936-016-1136-6 (2016).
Mataradchakul, T. et al. Plasmodium vivax rhomboid-like protease 1 gene diversity in Thailand. Exp. Parasitol. 181, 1–6. https://doi.org/10.1016/j.exppara.2017.06.007 (2017).
Kittichai, V., Koepfli, C., Nguitragool, W., Sattabongkot, J. & Cui, L. Substantial population structure of Plasmodium vivax in Thailand facilitates identification of the sources of residual transmission. PLoS Negl. Trop. Dis. 11, e0005930. https://doi.org/10.1371/journal.pntd.0005930 (2017).
VanBuskirk, K. M. et al. Antigenic drift in the ligand domain of Plasmodium vivax duffy binding protein confers resistance to inhibitory antibodies. J. Infect. Dis. 190, 1556–1562. https://doi.org/10.1086/424852 (2004).
Singh, S. K., Hora, R., Belrhali, H., Chitnis, C. E. & Sharma, A. Structural basis for Duffy recognition by the malaria parasite Duffy-binding-like domain. Nature 439, 741–744. https://doi.org/10.1038/nature04443 (2006).
Bastos, M. S. et al. Antigenic polymorphism and naturally acquired antibodies to Plasmodium vivax merozoite surface protein 1 in rural Amazonians. Clin. Vaccine Immunol. 14, 1249–1259. https://doi.org/10.1128/CVI.00243-07 (2007).
Zeyrek, F. Y. et al. Analysis of naturally acquired antibody responses to the 19-kd C-terminal region of merozoite surface protein-1 of Plasmodium vivax from individuals in Sanliurfa, Turkey. Am. J. Trop. Med. Hyg. 78, 729–732 (2008).
Tapaopong, P. et al. Genetic diversity and molecular evolution of Plasmodium vivax Duffy Binding Protein and Merozoite Surface Protein-1 in northwestern Thailand. Infect. Genet. Evol. 113, 105467. https://doi.org/10.1016/j.meegid.2023.105467 (2023).
Batchelor, J. D., Zahm, J. A. & Tolia, N. H. Dimerization of Plasmodium vivax DBP is induced upon receptor binding and drives recognition of DARC. Nat. Struct. Mol. Biol. 18, 908–914. https://doi.org/10.1038/nsmb.2088 (2011).
Almeida-de-Oliveira, N. K. et al. Extensive genetic diversity of Plasmodium vivax dbp-II in Rio de Janeiro Atlantic Forest and Brazilian Amazon Basin: Evidence of positive selection. Malar. J. 19, 81. https://doi.org/10.1186/s12936-020-03159-y (2020).
Hoque, M. R. et al. Diversity pattern of Duffy binding protein sequence among Duffy-negatives and Duffy-positives in Sudan. Malar. J. 17, 297. https://doi.org/10.1186/s12936-018-2425-z (2018).
Shi, T. Q. et al. Genetic diversity and natural selection of plasmodium vivax duffy binding protein-II from China-Myanmar border of Yunnan Province, China. Front. Microbiol. 12, 758061. https://doi.org/10.3389/fmicb.2021.758061 (2021).
Wamaket, N. et al. Anopheles bionomics in a malaria endemic area of southern Thailand. Parasit. Vectors 14, 378. https://doi.org/10.1186/s13071-021-04870-8 (2021).
Tananchai, C., Manguin, S., Bangs, M. J. & Chareonviriyaphap, T. Malaria vectors and species complexes in thailand: Implications for vector control. Trends Parasitol. 35, 544–558. https://doi.org/10.1016/j.pt.2019.04.013 (2019).
Dias, S., Longacre, S., Escalante, A. A. & Udagama-Randeniya, P. V. Genetic diversity and recombination at the C-terminal fragment of the merozoite surface protein-1 of Plasmodium vivax (PvMSP-1) in Sri Lanka. Infect. Genet. Evol. 11, 145–156. https://doi.org/10.1016/j.meegid.2010.09.007 (2011).
Cole-Tobian, J. & King, C. L. Diversity and natural selection in Plasmodium vivax Duffy binding protein gene. Mol. Biochem. Parasitol. 127, 121–132. https://doi.org/10.1016/s0166-6851(02)00327-4 (2003).
Oliveira, T. R., Fernandez-Becerra, C., Jimenez, M. C., Del Portillo, H. A. & Soares, I. S. Evaluation of the acquired immune responses to Plasmodium vivax VIR variant antigens in individuals living in malaria-endemic areas of Brazil. Malar. J. 5, 83. https://doi.org/10.1186/1475-2875-5-83 (2006).
Barbedo, M. B. et al. Comparative recognition by human IgG antibodies of recombinant proteins representing three asexual erythrocytic stage vaccine candidates of Plasmodium vivax. Mem. Inst. Oswaldo Cruz 102, 335–339. https://doi.org/10.1590/s0074-02762007005000040 (2007).
Wampfler, R. et al. Strategies for detection of Plasmodium species gametocytes. PLoS One 8, e76316. https://doi.org/10.1371/journal.pone.0076316 (2013).
Andrews, S. (Babraham Bioinformatics, Babraham Institute, Cambridge, United Kingdom, 2010).
Krueger, F. Trim Galore!: A wrapper around Cutadapt and FastQC to consistently apply adapter and quality trimming to FastQ files, with extra functionality for RRBS data. Babraham Institute (2015).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27, 2987–2993 (2011).
Rozas, J. DNA sequence polymorphism analysis using DnaSP. Bioinformatics for DNA sequence analysis, 337–350 (2009).
Tajima, F. The amount of DNA polymorphism maintained in a finite population when the neutral mutation rate varies among sites. Genetics 143, 1457–1465 (1996).
Fu, Y.-X. & Li, W.-H. Statistical tests of neutrality of mutations. Genetics 133, 693–709 (1993).
Stecher, G., Tamura, K. & Kumar, S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 37, 1237–1239 (2020).
Leigh, J. W. & Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116 (2015).
Larsen, J. E., Lund, O. & Nielsen, M. Improved method for predicting linear B-cell epitopes. Immunome Res. 2, 2. https://doi.org/10.1186/1745-7580-2-2 (2006).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Acknowledgements
This research project is supported by the National Institute for Allergy and Infectious Diseases, The National Institute of Health (U19 AI089672 and U19 AI181583) and Mahidol University (Fundamental Fund: fiscal year 2023 by National Science Research and Innovation Fund (NSRF)).
Author information
Authors and Affiliations
Contributions
PT: designed the study, performed NGS experiment, analyzed data, wrote original draft, reviewed and edited manuscript; GS: analyzed data reviewed and edited manuscript; AH: analyzed data reviewed and edited manuscript; WR: performed NGS experiment; CS: provided and processed DNA samples; KM: provided and processed DNA samples; KP: provided and processed DNA samples; AK: analyzed data; PK: analyzed data; CK: reviewed and edited manuscript; LC: acquired funding, reviewed and edited manuscript; JS: acquired funding and provided resources; WN; designed the study, acquired funding, reviewed and edited manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tapaopong, P., da Silva, G., Holzschuh, A. et al. Molecular epidemiology and genetic diversity of disappearing Plasmodium vivax in southern Thailand. Sci Rep 15, 2620 (2025). https://doi.org/10.1038/s41598-025-86578-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-86578-8