Abstract
Palladium nanoparticles were supported on L-H-functionalized KIT-6 (KIT-6@L-H-Pd) and evaluated using various characterization techniques such as TGA, FT-IR, SEM, XRD, EDS, and BET. KIT-6@L-H-Pd showed excellent catalytic performance as a recyclable nanocatalyst for the oxidation of sulfides to sulfoxides and the amination of aryl halides. This approach offers multiple benefits, including the use of readily available and cost-effective materials, a straightforward procedure, short reaction durations, high yields, and a catalyst that is easy to separate and reuse. Additionally, the catalyst can be recovered and reused multiple times without significant palladium loss or alteration in its activity.
Similar content being viewed by others
Introduction
Homogeneous catalysts are well-known for their remarkable selectivity and catalytic performance1,2,3,4. However, their practical use in industrial settings is hindered by challenges related to product separation and catalyst recovery after the reaction5,6. Additionally, these catalysts risk deactivation, often due to the formation of peroxo and oxo-species, typically produced as dimers7. A promising solution to these challenges lies in immobilizing homogeneous catalysts onto solid supports8,9. Various materials, including carbon nanotubes, polymers, mesoporous structures, boehmite particles, biochar, silica, graphene oxide, and magnetic nanoparticles, have been investigated for this purpose10,11. Among these, mesoporous materials such as KIT-6 have garnered significant attention for their exceptional qualities12. Developed in 2003, KIT-6 is a nanostructured mesoporous silica featuring a symmetrical three-dimensional cubic framework. This configuration provides abundant open spaces to host guest species and has proven useful in diverse applications such as catalysis, ion exchange, surface adsorption, and the production of functional materials13. KIT-6's distinct characteristics such as a high surface area, tunable thick walls, excellent hydrothermal stability, and large pore volume enhance its performance when compared to materials with one-dimensional or two-dimensional channels14. These properties optimize the diffusion of catalysts within its structure10,14,15. In addition, the material’s high specific pore volume is conducive to anchoring large molecules, such as organic ligands and metal complexes, within its hexagonal channels16. The pronounced thermal stability of this nanostructured compound further supports its application in conducting organic reactions at elevated temperatures17,18. As a result, the unique features of KIT-6 establish it as an outstanding substrate for creating heterogeneous catalysts.
Recently, the amination of aryl halides and their derivatives has been utilized in the development of drugs, serving as anti-HIV drug candidates as well as antiviral, antibacterial, and anticancer agents. Additionally, this process has emerged as a potent strategy for synthesizing complex organic compounds, natural products, advanced materials, and biologically active compounds19,20,21. Sulfoxides have garnered significant interest because of their extensive range of biological and pharmacological activities13. Consequently, the selective oxidation of sulfides to sulfoxides represents a crucial transformation within the field of organic chemistry22,23. In recent years, various efficient methodologies utilizing hydrogen peroxide, alongside diverse transition metal catalysts, have been reported for achieving selective sulfide oxidation to sulfoxides24,25.
This report outlines the synthesis and characterization of KIT-6@L-H-Pd, an innovative nanostructured catalyst that exhibits high efficiency, stability, and reusability. It is specifically designed for the oxidation of sulfides to sulfoxides and the amination of aryl halides.
Experimental
Preparation of the KIT-6@L-H-Pd
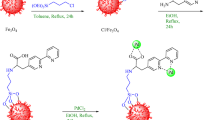
The synthesis of mesoporous KIT-6 material utilized P123 as the structure-directing agent and n-butanol as a co-solvent, conducted in an acidic environment. The process commenced by dissolving 6.0 g of Pluronic P123 in 150 g of distilled water and 8.7 g of a 35% HCl solution. Subsequently, 4.0 g of n-butanol were added to this mixture, which was thoroughly stirred at 35 °C. Next, 10.0 g of TEOS were introduced, and the mixture continued to be stirred for 24 h. After stirring, the suspension was left undisturbed at the same temperature. The solid product obtained was filtered directly, without any washing, and then dried at 60 °C for 12 h. To complete the process, the template was eliminated by calcining the silica at 550 °C for 5 h.
In the third step, 1.6 g of KIT-6 was dispersed in 75 mL of ethanol through sonication for 30 min. Next, 3 mmol of l-histidine was introduced to the reaction mixture and stirred at 90 °C for 24 h. The product was separated using filter paper, washed with ethanol and water, and subsequently dried in an oven at 70 °C, resulting in KIT-6@L–H. To prepare KIT-6@L–H-Pd, the obtained 1 g of KIT-6@L–H was dispersed in 50 mL of ethanol and sonicated for another 30 min. Then, 2.5 mmol of Pd(OAc)2 was added to the mixture, which was stirred under a nitrogen atmosphere with reflux conditions for 24 h. After cooling to 25 °C, the final KIT-6@L–H-Pd nanoparticles were isolated using filter paper, washed with ethanol and distilled water to eliminate residual impurities, and finally dried at 70 °C for 24 h (Fig. 1).
General procedure for the oxidation of sulfides
A mixture of sulfide (1 mmol), H2O2 (0.3 mL), and KIT-6@L-H-Pd (0.01 g) was stirred at room temperature without any solvent for an appropriate amount of time, monitoring the reaction progress with TLC. Upon completion, the catalyst was separated using filter paper, and the product was extracted with ethyl acetate. This ethyl acetate was evaporated to obtain pure sulfoxides.
General procedure for synthesis of anilines
In a typical procedure, a mixture of aryl halide (1 mmol) and ammonium hydroxide (0.003 mol) was prepared, to which 0.03 g of KIT-6 @L-H-Pd catalyst was added. The reaction mixture was then stirred at room temperature for a sufficient period. The progress was monitored through TLC. Upon completion of the reaction, the product was extracted using ethyl acetate. The extracts were dried with Na2SO4, and the solvent was evaporated, yielding the corresponding aniline with excellent results.
Selected NMR data
(Methylsulfinyl)benzene: 1H NMR (DMSO, 400 MHz): δH = 7.23 (m, 2H), 7.11 (d, 3H), 1.89 (s, 3H) ppm. FT-IR (KBr) cm-1:3636, 1671, 1451, 1054, 743.
(Benzylsulfinyl)benzene: 1H NMR (DMSO, 400 MHz): δH = 7.30 (m, 2H), 7.26 (m, 3H), 7.08 (d, 2H), 7.06 (m, 2H), 3.88 (s, 2H) ppm. FT-IR (KBr) cm-1:3176, 1682, 1567, 1452, 1053, 655, 459.
2-(methylsulfinyl) ethan-1-ol :1H NMR (DMSO, 400 MHz): δH = 5.29 (s, 1H), 3.55 (m, 2H), 2.22 (m, 2H), 1.96 (s, 3H) ppm.
2-(phenylsulfinyl)ethan-1-ol:1H NMR (DMSO, 400 MHz): δH = 7.42 (m, 5H), 4.79 (s, 1H), 4.11 (m, 2H), 3.10 (m, 2H), ppm.
Aniline: 1H NMR (DMSO, 400 MHz): δH = 7.03 (m, 5H), 4.75 (s, 2H) ppm. FT-IR (KBr) cm-1:3460, 3353, 1589, 1455, 1271, 1161, 876, 738, 671, 505.
Naphthalen-2-amine: 1H NMR (DMSO, 400 MHz): δH = 7.00 (m, 4H), 6.72 (m, 3H), 4.75 (s, 2H) ppm. FT-IR (KBr) cm-1:3503, 1608, 1254, 1088, 875, 798, 574.
4-Nitroaniline: 1H NMR (DMSO, 400 MHz): δH = 7.44 (m, 2H), 6.21 (m, 2H), 5.18 (s, 2H) ppm. FT-IR (KBr) cm-1:3436, 3299, 2824, 1604, 1461, 1304, 856.
(Ethylsulfinyl)ethane: 1H NMR (DMSO, 400 MHz): δH = 2.20 (m, 2H), 2.04 (m, 2H), 1.04 (m, 6H) ppm.
1-(Butylsulfinyl)butane: 1H NMR (DMSO, 400 MHz): δH = 2.32 (m, 4H), 1.90–2.25 (m, 8H), 0.97 (m, 6H) ppm.
Tetrahydrothiophene 1-oxide: 1H NMR (DMSO, 400 MHz): δH = 2.01 (t, 4H), 1.14 (t, 4H) ppm.
(Sulfinylbis(methylene))dibenzene: 1H NMR (DMSO, 400 MHz): δH = 7.69 (m, 4H), 7.61 (m, 6H), 3.95 (s, 4H) ppm.
2-((Methylsulfinyl)methyl)furan: 1H NMR (DMSO, 400 MHz): δH = 7.23 (s, 1H), 7.61 (s, 2H), 3.67 (m, 2H), 1.96 (s, 3H) ppm.
((Methylsulfinyl)methyl)benzene: 1H NMR (DMSO, 400 MHz): δH = 7.34–7.49 (m, 5H), 4.32 (s, 2H), 2.13 (s, 3H) ppm.
Aniline (Table 4, entry 10): 1H NMR (DMSO, 400 MHz): δH = 6.98–7.01 (m, 5H), 5.37 (s, 2H), ppm.
4-Nitroaniline (Table 4, entry 10): 1H NMR (DMSO, 400 MHz): δH = 7.88 (m, 2H), 7.56 (m, 2H), 5.52 (s, 2H), ppm.
4-Aminophenol: 1H NMR (DMSO, 400 MHz): δH = 9.00 (s, 1H), 7.00 (m, 4H), 4.75 (m, 2H), ppm.
Aniline (Table 4, entry 7): 1H NMR (DMSO, 400 MHz): δH = 6.98 (m, 2H), 6.92 (m, 3H), 5.37 (m, 2H), ppm.
4-Aminobenzonitrile: 1H NMR (DMSO, 400 MHz): δH = 7.50 (m, 2H), 7.08 (s, 2H), 6.05 (m, 2H), ppm.
P-toluidine: 1H NMR (DMSO, 400 MHz): δH = 7.18 (m, 1H), 7.15 (m, 3H), 5.36 (s, 2H), 2.12 (m, 3H), ppm.
4-Methoxyaniline: 1H NMR (DMSO, 400 MHz): δH = 7.33 (m, 2H), 7.18 (m, 2H), 5.36 (s, 2H), 4.33 (m, 3H), ppm.
Results and discussion
Catalyst characterization
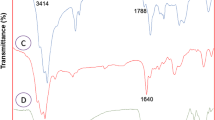
Figure 2 displays the FTIR spectra for KIT-6, KIT-6@L–H, and KIT-6@L–H-Pd materials. In Fig. 2(a), a broad, strong peak centered at 1112 cm⁻1 highlights the siloxane band. Figure 2(b) shows the FTIR spectrum of KIT-6@L–H, featuring distinctive bands around 2780 cm⁻1 that indicate aliphatic carbon-hydrogen stretching vibrations related to alkyl chains. Peaks at 3209 and 3272 cm⁻1 are linked to nitrogen–hydrogen bending vibrations from amine groups, with additional peaks at 1640 cm⁻1 corresponding to elements in the L–H structure. The slight change in band intensity seen in Fig. 2(c) is due to the incorporation of Pd within the internal channels of KIT-6@L–H nanoparticles.
Figure 3 display the small-angle powder X-ray diffraction patterns for both KIT-6 and KIT-6@L-H-Pd. The XRD pattern of the KIT-6 sample displays characteristic peaks corresponding to diffraction at the (100), (110), and (200) planes, which arise from the hexagonal channel arrays inherent to the material (Fig. 3a). The most prominent peak, associated with the (100) plane, confirms the well-ordered mesoporous structure of the silica, as anticipated in the KIT-6 framework. For the KIT-6@L-H-Pd sample, post-synthesis incorporation of the palladium complex within the KIT-6 channels results in diminished peak intensity. This reduction can be attributed to a decreased local order and increased wall thickness of the material retaining the palladium complex (Fig. 3b). Figure 3c illustrates the XRD patterns for KIT-6@L–H-Pd. The broad diffraction peak observed at 2θ = 25 corresponds to amorphous silica. The crystalline structure of the KIT-6 mesoporous silica nanoparticle system remains intact following its functionalization into KIT-6@L–H-Pd (Fig. 3c).
The thermogravimetric analysis (TGA) depicted in Fig. 4 for the mesoporous KIT-6@L-H-Pd organic–inorganic hybrid material demonstrates key thermal behavior. During the initial phase, occurring at temperatures below 200 C, a weight loss of approximately 5.36% is observed. This reduction is likely attributable to the evaporation of residual moisture present in the KIT-6@L-H-Pd composite. In the next step, temperatures ranging from 200 to 700 °C, a further weight loss of 19% is recorded, indicating the thermal stability of the synthesized catalyst. This decline is primarily due to the decomposition of organic components, specifically the L-H-Pd complex associated with the KIT-6 nanoparticles (Fig. 4).
In a separate study, EDX analysis verified that the synthesized KIT-6@L-H-Pd contained elements such as N, Pd, C, Si, S, and O. Figure 5 illustrates that the palladium complex’s attachment to mesoporous silica KIT-6 was confirmed by the detection of N, Pd, O, C, Si, and S elements using dispersive x-ray spectroscopy (EDS) (Fig. 5).
The distribution, size, particle shape, surface morphology, and basic physical properties of KIT-6 @L-H-Pd nanoparticles were assessed using SEM analysis. Figure 6a demonstrates that KIT-6 @L-H-Pd is composed of particles with consistent and uniform shapes. The SEM analysis indicates that the samples exhibit a regular morphology, as shown in Fig. 6a. Figure 6b provides a comparison of SEM spectra for the catalyst after undergoing recycling. As shown, the SEM analysis of KIT-6@L–H-Pd reveals no noticeable changes post-recovery, demonstrating the catalyst’s stability under the applied reaction conditions (Fig. 6).
The ninhydrin test was conducted following established literature to verify the presence of amino groups on KIT-6@L-H-Pd. Upon performing the test, the reaction solution changed to a purple color and exhibited a strong absorbance peak at 580 nm, as shown in Fig. 7. These results confirm the existence of free NH2 groups on KIT-6@L-H-Pd (Fig. 7).
To determine if palladium (Pd) leached from the catalyst during reactions, ICP-OES analysis was conducted to measure its concentration in KIT-6@L-H. The results revealed that after five usage cycles, the palladium content in KIT-6@L-H remained stable at 1.3 × 10−4 mol g−1, closely matching the initial level of the fresh catalyst, recorded at 1.4 × 10−4 mol g−1. These results suggest that palladium leaching is minimal under the reaction conditions employed.
The pore size and surface area distribution of mesoporous silica KIT-6@L-H-Pd were meticulously analyzed using the N2 adsorption–desorption isotherms method, as depicted in Fig. 8. Through this technique, the identified surface area of mesoporous silica KIT-6@L-H-Pd was found to be 17.12 m2/g. Additionally, the pore size distribution and volume were determined via the Barrett-Joyner-Halenda (BJH) method, revealing dimensions of 29.93 nm for pore size and a pore volume of 0.12 cm3/g, respectively (Fig. 8).
Catalytic study
Following the successful synthesis of a series of disulfides, our research shifted focus to the synthesis of sulfoxide compounds employing KIT-6@L-H-Pd as a catalyst alongside hydrogen peroxide. To optimize the reaction conditions, methyl phenyl sulfide was selected as a model substrate. This compound was tested under varying conditions, including different amounts of the catalyst and hydrogen peroxide (H2O2), as well as in the presence and absence of various solvents. The resulting data are detailed in Table 1. Optimal conditions, as illustrated in Table 1, were identified with the use of KIT-6@L-H-Pd (0.01 g) and H2O2 (0.3 mL) as an environmentally friendly oxidant, achieving efficient synthesis under solvent-free conditions at room temperature in a mere 30 min.
After optimizing the reaction conditions, a range of sulfides with different functional groups were successfully transformed into their corresponding sulfoxides with outstanding chemoselectivity, achieving high to excellent yields in a brief timeframe. The mild conditions of the described heterogeneous systems effectively prevented any overoxidation to sulfone during the sulfide oxidation process. The catalytic potential of KIT-6@L-H-Pd was further explored by testing a range of sulfides, including both aliphatic and aromatic types, under optimized conditions using the KIT-6@L-H-Pd catalyst. The reactions consistently produced sulfoxides in a short amount of time, achieving excellent yields. The results are detailed in Table 2.
Previous studies have proposed a plausible mechanism for the oxidation of sulfides catalyzed by KIT-6@L-H-Pd, as illustrated in Fig. 9. Within KIT-6@L-H-Pd, palladium serves as a key component, functioning as a magnetic nanocatalyst that forms the active oxidant complex. This process enables the efficient transfer of oxygen to sulfur, leading to the production of sulfoxide ( Fig. 9).
Table 3 outlines the catalytic efficiency of the KIT-6@L-H-Pd heterogeneous catalyst, assessed through the reaction of aryl halides with aqueous ammonia to synthesize aniline derivatives under varying conditions. Iodobenzene was initially selected as a model nonactivated aryl halide for this purpose, reacting with aqueous ammonia without a base at room temperature using 0.03 g of KIT-6 @L–H-Pd. Various factors were investigated to determine the most effective combination of conditions. This included testing different amounts of catalysts, solvents, and bases, as summarized in Table 3. The findings suggest that increasing the catalyst concentration generally improves product yields, peaking with 0.03 g of KIT-6@L-H-Pd. To establish the best reaction environment, the study examined the effects of solvents like DMF, MtOH, DMSO, EtOH, and H2O, although the reaction did not reach completion within 1.5 h in these media. Consequently, subsequent reactions were carried out under solvent-free conditions. After optimizing the catalyst amount and removing solvents, the influence of different bases on the model reaction was evaluated under these solvent-free conditions to find the most suitable base. A variety of inorganic and organic bases such as Et3N, K2CO3, KOH, and Na2CO3 were tested. According to Table 3, conducting the reaction neat resulted in shorter reaction times and higher yields.
After establishing the optimized reaction conditions, various electron-rich and electron-poor aryl iodides and bromides were reacted with aqueous ammonia to synthesize the corresponding aniline derivatives. A summary of these findings is presented in Table 4.
A potential mechanism for the anthrax reaction is proposed (Fig. 10). The process begins with the oxidative addition of aryl halides to the Pd complex, resulting in the formation of intermediate A. Following this, when intermediate A interacts with ammonia, it gives rise to intermediate B. As illustrated in Fig. 10, during the coupling of ammonia with aryl halides, it is hypothesized that ammonia’s coordination to palladium enhances the proton’s acidity, facilitating HX elimination and leading to the formation of intermediate C. In the final step, reductive elimination takes place, regenerating the original KIT-6@L-H-Pd species and yielding the desired product, as outlined in Fig. 10.
Hot filtration test
To evaluate the catalyst’s characteristics and potential for palladium leaching into the reaction medium, a hot filtration test was performed. At the reaction’s midpoint, with a 65% yield, the KIT-6@L-H-Pd catalyst was separated using filter paper. The remaining reaction mixture (filtrate) was allowed to proceed for an additional 15 min. Only a slight increase in yield (67%) was observed after the catalyst was removed, indicating minimal progress without the catalyst. This test demonstrates that KIT-6@L-H-Pd effectively catalyzes the successful synthesis of the target molecules without any leaching into the reaction medium.
Reusability of catalyst
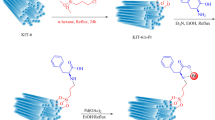
After confirming the versatility of KIT-6@L-H-Pd in the oxidation of sulfides, its recyclability was tested by reapplying the catalyst in the oxidation of methylphenyl sulfide under optimized conditions. Upon completion of the reaction, the catalyst was efficiently retrieved using filter paper. The remaining catalyst was washed with dichloromethane to remove any residual product, followed by decanting the reaction mixture. Once recovered, the catalyst was dried in an oven at 60 °C before being used in the next run. This cycle was repeated five times without any noticeable decrease in activity. The average isolated yield across these runs was 96%, demonstrating the catalyst’s significant practical recyclability on a large scale ( Fig. 11).
Comparison of catalyst
To assess the catalytic performance of KIT-6@L-H-Pd, we examined the oxidation of methylphenyl sulfide using this catalyst and compared the findings with previously reported techniques (Table 5). Interestingly, unlike many other catalysts, KIT-6@L-H-Pd is easy to prepare using inexpensive, readily accessible materials and can be reused up to five cycles without a noticeable drop in efficiency. This catalyst also delivers a shorter reaction time and higher yield compared to alternative methods. Consequently, this innovative catalyst showcases equivalent or even improved features regarding affordability, non-toxicity, stability, and ease of separation.
Conclusions
In summary, we have demonstrated the successful preparation of a palladium complex immobilized on a surface functionalized with l-histidine bonds as ligands. The KIT-6@L–H-Pd catalyst exhibited high catalytic activity in the oxidation of sulfides to sulfoxides and the amination of aryl halides. After each reaction, the nanocatalyst was easily separated using filter paper and could be reused multiple times while maintaining consistent catalytic activity without any noticeable leaching. This approach allows aromatic sulfides and aryl halides, featuring both electron-withdrawing and electron-donating substituents, to display high reactivity, producing the desired oxidation of sulfides to sulfoxides and amination of aryl halides with high yields.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Morales, G. et al. Aldol condensation of furfural and methyl isobutyl ketone over Zr-MOF-808/silica hybrid catalysts. Fuel 339, 127465 (2023).
Vallet-Regí, M., Balas, F. & Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed 46, 7548–7558 (2007).
Zhu, Z., Zhang, W. & Gao, Z. Suzuki-Miyaura carbonylative reaction in the synthesis of biaryl ketones. Prog. Chem. 28, 1626–1633 (2016).
Tahmasbi, B., Moradi, P. & Darabi, M. A new neodymium complex on renewable magnetic biochar nanoparticles as an environmentally friendly, recyclable and efficient nanocatalyst in the homoselective synthesis of tetrazoles. Nanoscale Adv. 6, 1932–1944 (2024).
Liu, C., Yuan, P. & Cui, C. The pore confinement effect of FDU-12 mesochannels on MoS2 active phases and their hydrodesulfurization performance. J. Nanomater. 2016, 5208027 (2016).
Vadia, N. & Rajput, S. Mesoporous material, MCM-41: A new drug carrier. Asian J. Pharmaceut. Clin. Res. 4, 44–53 (2011).
Nikoorazm, M. et al. Synthesis and characterization of a new Schiff-base complex of copper on magnetic MCM-41 nanoparticles as efficient and reusable nanocatalyst in the synthesis of tetrazoles. Polyhedron 244, 116587 (2023).
Pinto, V. H. A. et al. Mn porphyrins immobilized on non-modified and chloropropyl-functionalized mesoporous silica SBA-15 as catalysts for cyclohexane oxidation. Appl. Catal. A Gen. 526, 9–20 (2016).
Hami Dindar, M., Yaftian, M. R., Pilehvari, M. & Rostamnia, S. SBA-15 mesoporous materials decorated with organic ligands: Use as adsorbents for heavy metal ions. J. Iran. Chem. Soc. 12, 561–572 (2015).
Lakshmidevi, J. et al. Pd(5%)-KIT-6, Pd(5%)-SBA-15 and Pd(5%)-SBA-16 catalysts in water extract of pomegranate ash: A case study in heterogenization of Suzuki-Miyaura reaction under external base and ligand free conditions. Sustain. Chem. Pharm. 19, 100371 (2021).
Yang, Y. et al. Facile synthesis of Ag/KIT-6 catalyst via a simple one pot method and application in the CO oxidation. J. Porous Mater. 24, 1661–1665 (2017).
Suba, M., Popa, A., Verdeș, O., Borcănescu, S. & Barvinschi, P. Ni and Ce grafted ordered mesoporous silica KIT-6 for CO2 adsorption. Catalysts 12 (2022).
Nikoorazm, M. et al. Synthesis of a new complex of lanthanum on MCM-41 as an efficient and reusable heterogeneous catalyst for the chemoselective synthesis of sulfoxides and tetrahydrobenzo[b]pyrans. J. Porous Mater. 31, 511–526 (2024).
Villarroel-Rocha, D. et al. Synthesis of micro-mesoporous CPO-27-Mg@KIT-6 composites and their test in CO2adsorption. New J. Chem. 44, 10056–10065 (2020).
Tahmasbi, B., Moradi, P., Mohammadi, F., Abbasi Tyula, Y. & Kikhavani, T. Synthesis of Sm-TADDbBP@MCM-41 as a robust, reusable, and practical nanocatalyst in the homoselective [2 + 3] cycloaddition reaction. Appl. Organomet. Chem. e7791 (2024).
Deshpande, N. et al. Investigating the impact of micropore volume of aminosilica functionalized SBA-15 on catalytic activity for amine-catalyzed reactions. J. Catal. 414, 356–364 (2022).
Karimi, B., Barzegar, H. & Vali, H. Au–Pd bimetallic nanoparticles supported on a high nitrogen-rich ordered mesoporous carbon as an efficient catalyst for room temperature Ullmann coupling of aryl chlorides in aqueous media. Chem. Commun. 54, 7155–7158 (2018).
Deng, M. et al. A facile route of mesoporous TiO2 shell for enhanced arsenic removal. Colloids Surf. A Physicochem. Eng. Asp. 627 (2021).
Mohite, S. B. et al. O-Benzoylhydroxylamines: A versatile electrophilic aminating reagent for transition metal-catalyzed C-N bond-forming reactions. Top. Curr. Chem. 381, 4 (2022).
Murugesan, K. et al. Catalytic reductive aminations using molecular hydrogen for synthesis of different kinds of amines. Chem. Soc. Rev. 49, 6273–6328 (2020).
Dindarloo Inaloo, I., Majnooni, S., Eslahi, H. & Esmaeilpour, M. N-Arylation of (hetero)arylamines using aryl sulfamates and carbamates via C–O bond activation enabled by a reusable and durable nickel(0) catalyst. N. J. Chem. 44, 13266–13278 (2020).
Fakhri, A. & Naghipour, A. Fe3O4@chitosan-bound picolinaldehyde Cu complex as the magnetically reusable nanocatalyst for adjustable oxidation of sulfides. Environ. Prog. Sustain. Energy 37, 1626–1631 (2018).
Morshedtalab, Z. et al. Antibacterial assessment of zinc sulfide nanoparticles against Streptococcus pyogenes and Acinetobacter baumannii. Curr. Top. Med. Chem. 20, 1042–1055 (2020).
Surur, A. S., Schulig, L. & Link, A. Interconnection of sulfides and sulfoxides in medicinal chemistry. Arch. Pharm. 352, 1800248 (2019).
Wang, L., Zhang, Y., Yao, J. & Li, H. Metal-free synthesis of sulfones and sulfoxides through aldehyde-promoted aerobic oxidation of sulfides. Catal. Lett. https://doi.org/10.1007/s10562-021-03706-5 (2021).
Islam, S. M. et al. Selective oxidation of sulfides and oxidative bromination of organic substrates catalyzed by polymer anchored Cu(II) complex. Tetrahedron Lett. 53, 127–131 (2012).
Hussain, S., Talukdar, D., Bharadwaj, S. K. & Chaudhuri, M. K. VO2F(dmpz)2: A new catalyst for selective oxidation of organic sulfides to sulfoxides with H2O2. Tetrahedron Lett. 53, 6512–6515 (2012).
Emad-Abbas, N., Naji, J., Moradi, P. & Kikhavani, T. 3-(Sulfamic acid)-propyltriethoxysilane on biochar nanoparticles as a practical, biocompatible, recyclable and chemoselective nanocatalyst in organic reactions. RSC Adv. 14, 22147–22158 (2024).
Thurow, S. et al. Base-free oxidation of thiols to disulfides using selenium ionic liquid. Tetrahedron Lett. 52, 640–643 (2011).
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the large group Research Project under grant number RGP. 2/315/45
Author information
Authors and Affiliations
Contributions
Nafis Ahmad. Prakash Kanjariya. G.PadmaPriya. Anjan Kumar. Rishabh Thakur. RSK Sharma. Mukesh Kumari. Sharnjeet Kaur. Abhinav Kumar. and Munther Kadheem.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ahmad, N., Kanjariya, P., Priya, G.P. et al. l-Histidine-functionalized KIT-6 with embedded palladium nanoparticles as an efficient heterogeneous catalyst for oxidation of sulfide to sulfoxide and amination of aryl halides. Sci Rep 15, 3071 (2025). https://doi.org/10.1038/s41598-025-86579-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86579-7
Keywords
This article is cited by
-
Condensation polymerization of 2-[(para-tolyl) oxy]-4,6-dichlorotriazine with ethylenediamine and application in metal anchoring
Journal of Polymer Research (2025)