Abstract
Genetic alterations play a pivotal role in leukemic clonal transformation, significantly influencing disease pathogenesis and clinical outcomes. Here, we report a novel fusion gene and investigate its pathogenic role in acute lymphoblastic leukemia (ALL). We engineer a transposon transfection system expressing the TOP2B::AFF2 transcript and introduce it into Ba/F3 cells. Functional studies, including proliferation, cell cycle, and apoptosis assays, were conducted to assess the fusion gene’s impact. In vitro assays reveal that the TOP2B::AFF2 fusion significantly enhances Ba/F3 cell proliferation and G1/S phase transition while suppressing differentiation and apoptosis. This study identifies TOP2B::AFF2 as a potential oncogenic driver. However, further validation through in vivo studies are warranted to fully elucidate the fusion gene’s role in leukemogenesis.
Similar content being viewed by others
Introduction
Acute lymphoblastic leukemia (ALL) is the most prevalent malignancy in children, characterized by the aberrant clonal proliferation of lymphoid cells1,2. The genetic subtype of ALL is a critical factor influencing both risk stratification and prognosis3. The International Consensus Classification (ICC), updated in 2022, has identified nine new molecular entities within B-ALL, each exhibiting unique prognostic biological features4. Notably, rearrangements involving DUX4 and NUTM1 are associated with favorable outcomes, while MEF2D rearrangements, MYC rearrangements, and KMT2A-like rearrangements indicate unfavorable prognoses. The ZNF384 subtype presents significant prognostic variability; outcomes are most favorable when fused with EP300 but markedly poorer when associated with TCF3. This evolving understanding underscores the critical importance of precise molecular characterization in guiding treatment strategies and improving patient outcomes in B-ALL.
As our understanding of B-ALL continues to evolve, an increasing number of novel fusion genes have been identified through transcriptome sequencing, which is rapidly becoming a standard diagnostic tool for individual patients5. Utilizing this advanced technology, we have recently identified a novel fusion gene, TOP2B::AFF2, in a pediatric B-ALL patient.
Topoisomerase IIβ (TOP2B) is a key regulator of chromatin topology. The trapping of TOP2B has long been identified as the primary factor leading to DNA damage and genomic rearrangements in leukemia and prostate cancer6. TOP2B-mediated DNA double-strand breaks, if improperly repaired, can result in chromosomal translocations, particularly in genes critical for hematopoiesis, potentially leading to leukemia development7. Recently an investigation have established a strong connection between TOP2B activities and the development of cancer, shedding light on the role of TOP2B in cancer pathogenesis7. Another study revealed that TOP2B plays a crucial role in B cell development. TOP2B-deficient mice exhibit impaired B cell differentiation, with significantly reduced numbers of B cell precursors at various developmental stages in both bone marrow and spleen8. This finding underscores the importance of TOP2B in normal B cell maturation and suggests that its dysfunction could contribute to B cell-related disorders. Indeed, B-ALL, a malignancy of B cell precursors, arises from the transformation and uncontrolled proliferation of these progenitor cells. In B-ALL, the differentiation of B lymphoid progenitors is arrested at various immature stages, leading to their unchecked proliferation and the subsequent replacement of normal haematopoietic cells in the bone marrow and peripheral blood1. The C-terminal region of TOP2B is critical for its function, harboring nuclear localization signals and key sites for post-translational modifications. This domain is particularly important for SUMOylation and ubiquitination, which primarily regulate TOP2B’s stability and activity9,10.
The function of AFF2 (also known as FMR2) remains poorly understood. Research has primarily focused on its role in nervous system disorders, particularly intellectual disabilities, where AFF2 has been shown to control the expression of the C9ORF72 mutant allele containing expanded G4C2 repeats. While AFF2 is hypothesized to influence cell transcription, its potential role in hematological malignancies is largely unexplored11.
Given the crucial role of TOP2B in B cells and its association with leukemia, we hypothesize that the fusion of the AFF2 gene with TOP2B results in the partial loss of TOP2B’s C-terminus. This loss likely affects the essential functions of TOP2B in B cells, potentially contributing to the development of B-ALL.
Studying newly discovered fusion genes in leukemia is crucial for understanding the disease’s pathogenic mechanisms. We propose that this fusion gene promotes cell proliferation and G1/S phase transition, inhibits apoptosis, and suppresses B-cell differentiation-related genes likely due to structural changes in TOP2B. Understanding these mechanisms may reveal new therapeutic targets for hematological malignancies involving this genetic alteration.
Materials and methods
Patients and samples
Bone marrow (BM) samples were collected from B-ALL patients at initial diagnosis. This study was approved by the Medical Ethics Committee of the Children’s Hospital of Chongqing Medical University (Approval Number: 2024.138) and conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained from all participants or their legal guardians.
RNA extraction, library preparation, and sequencing
Total RNA was extracted from cryopreserved bone marrow mononuclear cells (MNCs) using TRIzol (Invitrogen, Waltham, USA). RNA concentration, purity and integrity were measured using a NanoDrop™ 2000UV–Vis Spectrophotometer (ThermoFisher, Waltham, USA) and an Agilent 2100 Bio-analyzer (Agilent, CA, USA). RNA sequencing libraries were prepared according to the KAPA RNA HyperPrep Kit protocol (KAPA biosystems, Roche, USA) and the library quality was assessed using the Bioanalyzer 2100. RNA-seq were performed on a HiSeq X platform with 150 paired-end (PE) by the Kindstar Global Company (Wuhan, China).
Methods employed for detecting the sequence variants were as described in a previous study12. Reverse transcription-polymerase chain reaction (RT-PCR) and Sanger sequencing were used to confirm the TOP2B::AFF2 fusion gene.
Whole exome sequencing
Genomic DNA was extracted according to the manufacturer’s instructions for the Eltbio Mag Blood DNA Extraction Kit (Eltbio, Shanghai, China). A total of 250 ng of genomic DNA from each sample underwent end repair, followed by the addition of an “A” base at the ends. The DNA fragments were subsequently ligated to adapters containing a “T” base. Purification of the ligation products was performed using Hieff NGS® DNA Selection Beads (YEASEN, China), followed by PCR amplification and purification to construct libraries for each subject.
Exome capture was executed utilizing the NadPrep Hybrid Capture Reagents kit (Jiangsu, China) in accordance with the manufacturer’s protocol. Library fragment size and concentration were assessed using the Qseq400 kit (Jiangsu, China). After passing quality checks, libraries were sequenced on the Illumina Novaseq Xplus platform.
Sequencing quality was evaluated using fastp. Clean reads were aligned to the hg19 human reference genome with BWA. Single Nucleotide Variants (SNVs) and InDels were detected using GATK, followed by variant annotation with VEP. An in-house algorithm was employed for variant filtering based on annotation results and read alignment at variant sites, yielding high-quality variants with corresponding annotations from databases such as ClinVar, OMIM, and HGMD. Copy Number Variation (CNV) detection was conducted using CNVkit, with annotations provided by AnnotSV. Pathogenicity interpretations for all detected variants adhered to ACMG guidelines.
Bioinformatics analysis
Protein structures were predicted using the AlphaFold Server powered by AlphaFold3 (https://alphafoldserver.com). Protein sequences are provided in Supplementary Material. Chromosome Xq28 in TOP2B::AFF2 patients and other B-ALL female children were manually inspected using the Integrative Genomics Viewer (IGV). Molecular subtyping of patients’ gene expression profiles was performed using machine learning techniques. This model was developed from a combination of local and publicly available databases. Specifically, we referenced the studies by Gu et al.13 and Schmidt et al.14, utilizing a random forest approach to construct a molecular subtype model for B-ALL based on expression profiles from over 1000 cases, along with corresponding patient data for subsequent analysis.
Plasmid construction
The PiggyBac dual promoter transposon vector (pPBd) and PiggyBac transposase expressing vector (pPBase) were obtained from System Biosciences (CA, USA). The pPBd vector features a multiple cloning site (MCS) downstream of the CMV promoter, with copGFP and puromycin resistance genes under the control of the EF1α promoter. TOP2B::AFF2 cDNA was chemically synthesized by HITRO BioTech (Beijing, China) and subsequently cloned into pPBd with an N-terminal FLAG-tag using the ClonExpress II One Step Cloning Kit (Vazyme, Nanjing, China). The resulting construct was designated pPB-TOP2B::AFF2-copGFPPuro (pPBTA).
Cell culture and transfection
Mouse pro-B cells (Ba/F3) cells were obtained from iCell Bioscience (Shanghai, China). Ba/F3 cells were cultured in RPMI-1640 medium (Gibco, Shanghai, China) supplemented with 10% fetal bovine serum (FBS; Procell, Wuhan, China), 1% penicillin/streptomycin (BIOMIKY, Shanghai, China), and 10 ng/mL recombinant mouse IL-3 (Novoprotein, Jiangsu, China). For all experiments, Ba/F3 cells between passages 5 and 6 were used. Various plasmid vectors were co-transfected into 2 × 10^6 Ba/F3 cells using a 4D-Nucleofector™ X Unit with the SG Cell Line 4D-Nucleofector™ X Kit (Lonza, Germany). Stable clones were established through a 14-day selection process using 2 μg/mL puromycin (Meilunbio, Liaoning, China).
Quantitative real‐time polymerase chain reaction (qPCR)
Total RNA was extracted from Ba/F3 cells transfected with either pPBTA or pPBd constructs. RT-qPCR was used to quantify the expression levels of TOP2B::AFF2 and B cell differentiation-associated genes (Vpreb1, Vpreb3, Dntt, Igll1, Btk, Syk, Pax5, Cd79a), with Gapdh serving as the reference gene. TOP2B::AFF2 PCR products were further analyzed by agarose gel electrophoresis. All primers used in these analyses are listed in Supplementary Material.
Cell proliferation assay
To evaluate cellular proliferation, Ba/F3 cells transfected with either pPBTA or pPBd constructs were seeded in 96-well plates at a density of 5000 cells per well in IL-3-free medium. Parental Ba/F3 cells cultured in IL-3-supplemented medium served as a control. Cell viability was assessed daily for four consecutive days using the Cell Counting Kit-8 (Sigma-Aldrich, Shanghai, China) according to the manufacturer’s instructions. All assays were performed in triplicate.
Detection of apoptosis by flow cytometry
Apoptotic cells were stained using Annexin V-7AAD/APC apoptosis detection kits (KeyGEN, Jiangsu, China) following the manufacturer’s instructions. The stained cells were then analyzed by flow cytometry (BD Biosciences, CA, USA). Apoptotic cells were identified as those exhibiting high fluorescence levels of Annexin V and low levels of 7AAD (lower right quadrant). Each assay was performed in triplicate.
Western blotting (WB)
Ba/F3 cells were lysed using a cell lysis kit (BestBio, Shanghai, China). Proteins (20 μg) were separated by 12.5% SDS-PAGE (Epizyme, Shanghai, China) and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, USA). Membranes were blocked with blocking buffer (Yoche, Shanghai, China) for 5 min at room temperature, then incubated overnight at 4 °C with primary antibodies (all from Proteintech, Wuhan, China; 1:1,000 dilution) against Flag, Bcl2, Bax, PCNA, Caspase3, Cdk4 and CyclinE1. Beta-Tubulin served as an internal control. After washing with TBST (Tris-buffered saline with 0.1% Tween-20), membranes were incubated with HRP-conjugated secondary antibodies (1:5000) for 1 h. Protein bands were visualized using an ECL system.
Results
Clinical data of the patient
A 1-year-old female without family cancer history was admitted due to fever, anemia, and diarrhea (February 2021). Initial blood counts revealed: WBC 3.7 × 10⁹/L, ANC 0.3 × 10⁹/L, platelets 197 × 10⁹/L, hemoglobin 69 g/L, and LDH 254 U/L. Bone marrow aspirate showed 42% peroxidase-negative blast cells (Fig. 1. A). Immunophenotyping identified 49.8% blasts positive for CD19 (100%), cCD79a (93.2%), cCD22 (69.4%), CD10 (95.9%), CD20 (18.1%), CD22 (88.1%), IgM (2.3%), cIgM (4.1%), HLA-DR (100%), CD34 (5.2%), CD38 (71.0%). G-banding analysis showed a normal female karyotype 46, XX (Fig. 1D). Common B-ALL fusion genes were undetected by FISH or molecular studies. To identify genetic alterations, the diagnostic bone marrow sample was subjected to transcriptome analysis. Bioinformatic evaluation revealed a novel, in-frame fusion between TOP2B (3p24) exon 35 and AFF2 (Xp28) exon 9 (Fig. 2A, B). According to the clinical characteristics, BM morphology, and immunophenotype, B-ALL was confirmed. The patient responded favorably to induction therapy, which comprised a combination of daunorubicin, vincristine, asparaginase, and corticosteroids. This regimen was supplemented with triple intrathecal chemotherapy consisting of methotrexate, cytarabine, and corticosteroids. Evaluation on day 46 post-induction indicated remission, with minimal residual disease (MRD) levels below 10⁻4, as detected by flow cytometry (Fig. 1C). Following the completion of treatment, the patient remains in sustained remission as of the most recent follow-up evaluation in November 2023 (Fig. 1B, C), and TOP2B::AFF2 was not detected in the bone marrow sample (Supplementary S4).
Clinical data of the patient. (A) Bone marrow morphology assessed at key timepoints: diagnosis (2021.2.22), day 19 (2021.3.13), day 46 (2021.4.12), and the most recent follow-up (2023.11.23). (B) Trends in blood count parameters (WBC white blood count, ALC absolute lymphocyte count, Hb Hemoglobin, PLT platelet count) following the diagnosis of B-ALL. The horizontal shaded areas indicate age-specific normal reference ranges for each parameter, reflecting the expected values for healthy individuals based on established pediatric reference intervals. (C) Clinical follow-up monitoring bone marrow (BM) blast percentages and measurable residual disease (MRD) using flow cytometry at diagnosis, day 19, day 46, and the most recent follow-up. (D) G-banding analysis of bone marrow cells showing a cytogenetic profile of 46, XX.
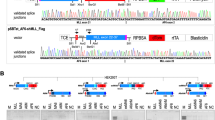
Identification of TOP2B::AFF2 in-frame fusion transcript
The TOP2B::AFF2 fusion was classified as likely pathogenic based on established guidelines for cancer variant interpretation15,16. Our analysis did not detect any reads supporting the presence of the reciprocal AFF2::TOP2B fusion transcript, and we did not identify any other significant mutations. Further details can be found in Supplementary S3. The fusion retains key domains of TOP2B, including the N-terminal ATPase domain, TOPRIM (DNA breakage-rejoining) domain, and a partial C-terminal domain (CTD), along with the CTD of AFF2 (Fig. 2A). The putative fusion protein includes TOP2B (NP_001317629) amino acids 1–1565 (exons 1–35) and AFF2 (NP_002016) amino acids 454–1311 (exons 9–21). RT-PCR amplified a 229 bp junction region from bone marrow MNC RNA. Gel electrophoresis revealed distinct fusion transcripts, and Sanger sequencing confirmed the junction (Fig. 2C).
Bioinformatic analysis
To better understand the structural implications of the fusion, we performed 3D protein structure prediction using AlphaFold server. Figure 3 presents the predicted structures of both TOP2B (Fig. 3A) and TOP2B::AFF2 (Fig. 3B). The TOPRIM domain of the TOP2B::AFF2 structure is hindered by AFF2, which may impact the normal function of TOP2B and affect its protein–protein interaction interfaces17. Given that females have two X chromosomes, one typically inactivated, we investigated whether this rearrangement involved the inactive X chromosome. We hypothesized that the rearrangement could activate the AFF2 gene and its downstream genes on the inactive X, potentially increasing expression levels of X chromosome genes downstream of the fusion site, as both X chromosomes may be expressed simultaneously. However, our analysis found no significant difference in the number of reads mapped to the X chromosome downstream of the TOP2B::AFF2 fusion site compared to other female B-ALL patients. This suggests that the rearrangement either does not involve the inactive X chromosome or has no significant impact on the expression of genes located on it (Fig. 3C). To gain insight into the gene expression profile subtype of patients carrying this fusion gene, we analyzed the transcriptome data using machine learning methods. Our approach was based on models constructed from both local and public databases. The results revealed that the patient exhibited a 41.3% similarity to the Ph-like subtype, while the similarity to other subtypes was consistently below 20% (Fig. 3D).
Structural prediction and genomic analysis of TOP2B::AFF2 fusion protein. (A) Predicted three-dimensional structure of the wild-type TOP2B protein, with the TOPRIM domain highlighted in yellow and indicated by red arrows. (B) Predicted three-dimensional structure of the TOP2B::AFF2 fusion protein, where green represents the TOP2B sequence and purple represents the AFF2 sequence. The TOPRIM domain is also highlighted in yellow and indicated by red arrows, illustrating its retention within the fusion protein. (C) Manual inspection of chromosome Xq28 using integrative genomics viewer (IGV). (D) Cluster analysis for molecular subtyping based on patients’ gene expression profiles.
The effect of TOP2B::AFF2 on acute lymphoblastic leukemia leukemogenesis
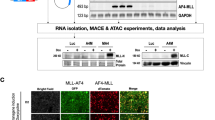
In vitro experiments were conducted using Ba/F3 cells transfected with pPBd and pPBTA. Cell proliferation assays revealed that TOP2B::AFF2 expression enhanced cell proliferation in the presence of IL3 but failed to sustain cell survival in its absence (Fig. 4A). Flow cytometry analysis demonstrated that TOP2B::AFF2 expression significantly mitigated IL3-withdrawal-induced apoptosis at both early and late stages (Fig. 4B,4C). Protein analysis corroborated these findings, showing upregulation of proliferation marker PCNA and anti-apoptotic protein Bcl2, alongside downregulation of pro-apoptotic proteins Bax and Cleaved-Caspase3 in TOP2B::AFF2 overexpressing cells (Fig. 4D). Cell cycle analysis further revealed that TOP2B::AFF2 reduced the proportion of cells in G1 phase while increasing those in S and G2 phases (Fig. 4E). Consistent with these observations, G1/S transition proteins CDK4 and CyclinE1 were upregulated (Fig. 4F), suggesting that TOP2B::AFF2 promotes G1/S transition and cell proliferation. Given TOP2B’s critical role in B cell development, we examined the expression of B cell differentiation genes using RT-qPCR (Fig. 4G). Our analysis revealed a downregulation of differentiation markers across the spectrum in the TOP2B::AFF2 condition, encompassing both early and late-stage genes.
in vitro analysis. (A) Line graphs of the cell proliferation assay (n = 3, ****P < 0.0001). (B) Flow cytometric analysis for the apoptotic ratio of Ba/F3 cells. The early apoptotic cells were marked with positive Annexin V staining (APC-A) and negative 7-AAD staining. (C) Column graph of the early and late apoptosis ratio of Ba/F3 cells (n = 3, ***P < 0.001, *P < 0.05) (D) Western blot analysis of key proteins related to cell proliferation and apoptosis. (E) Representative flow cytometry histograms showing cell cycle distribution in Ba/F3 cells. (F) Western blot analysis of key proteins related to cell cycle. (G) Relative mRNA expression levels of early B cell differentiation markers in Ba/F3 cells. Gene expression was normalized to Gapdh. Data represent the mean ± SD of three independent experiments. The asterisks indicate non-specific bands or protein degradation products. Statistical analyses of all qPCR data and cell cycle assessments were performed using Student’s t-test, while CCK-8 results were analyzed using ANOVA.
Discussion
Genetic alterations play a pivotal role in B-ALL, significantly impacting subtype classification, treatment selection, and prognosis1,18. ALL are typically characterized by deletions of genes involved in B-lymphocyte development and differentiation5. However, in this study, we observed an absence of copy number variations in WES. Additionally, given the negative results from chromosome karyotyping, FISH, and PCR testing for 43 fusion genes or oncogenes associated with various hematological disorders, we opted for whole transcriptome sequencing to further investigate the genetic profile of the disease. This approach ultimately led to the discovery of a novel fusion gene, TOP2B::AFF2, with no other clinically significant mutations detected. This underscoring the potential of whole transcriptome sequencing as a powerful tool in leukemia diagnostics, particularly when conventional genetic analyses yield negative results18,19.
The TOP2B gene is located on chromosome 3p24 and encodes DNA topoisomerase IIβ (TOP2B), a DNA topoisomerase enzyme. Nebral et al. reported a case of acute myeloid leukemia (AML) linked to the NUP98::TOP2B fusion, marking the first identification of TOP2B as a co-partner gene with NUP98 in leukemia, which underscores the important role of TOP2B in malignant transformation20. Recent studies have revealed the critical role of TOP2B in the development of B cells. In a mouse model, TOP2B deficiency impaired B cell development, while its overexpression rescued this impairment8,21. Although the role of the AFF2 gene in leukemogenesis is largely unexplored, it is associated with fusion gene formation, as evidenced by CHD4::AFF2 in neuroendocrine tumors and DEK::AFF2 in head and neck and thoracic tumors. In CHD4::AFF2, breakpoint is located in exon 4, while DEK::AFF2 involves exons 4, 5, 6, and 9 22,23. In our case, breakpoint is in exon 9. Given their similarity to those in other tumors, we hypothesize that AFF2 may also play a significant role in this context.
The CTD of TOP2B plays a crucial role in subcellular localization and post-translational modifications9. Given that the fusion point in our identified TOP2B-AFF2 gene is located within this CTD domain, it is plausible that the fusion disrupts these critical functions of TOP2B, potentially contributing to B-ALL development. To investigate the impact of the fusion gene on B cells, we selected the Ba/F3 cell line as our experimental model. This cell line also serves as a valuable tool for assessing oncogenic transformation potential24,25. We transduced IL-3-dependent Ba/F3 cells with the TOP2B::AFF2 fusion gene and cultured them in the absence of IL-3. Our in vitro analysis indicated that TOP2B::AFF2 was unable to transform Ba/F3 cells, as evidenced by their failure to proliferate independently of IL-3. This result suggests that the TOP2B::AFF2 fusion may not possess strong oncogenic properties on its own.
While the fusion gene did not induce full oncogenic transformation, it nonetheless exerted significant influences on key cellular processes. In the presence of IL-3, the TOP2B::AFF2 fusion enhanced cell proliferation and facilitated the G1/S transition, suggesting a potential acceleration of the cell cycle. Moreover, the fusion gene demonstrated an anti-apoptotic effect by inhibiting cell death induced by IL-3 withdrawal. These findings indicate that the TOP2B::AFF2 fusion gene significantly impacts B-cell functional behavior, potentially contributing to leukemic progression. These results underscore the complex nature of leukemogenesis and highlight the need for further investigation into the molecular mechanisms by which TOP2B::AFF2 alters B-cell behavior. We examined the expression of genes associated with various stages of B-cell maturation. Specifically, we analyzed early development genes (Igll1, Vpreb1, Vpreb3, Dntt) and late development genes (Btk, Syk, Pax5, Cd79a). Our results revealed that the TOP2B::AFF2 fusion gene downregulated the expression of these critical developmental genes. Their reduced expression could compromise the functional maturation and differentiation of B cells, further contributing to leukemogenesis.
In conclusion, while not sufficient for full oncogenic transformation, the TOP2B::AFF2 fusion gene significantly influences B-cell functional behavior, potentially contributing to the leukemic phenotype through enhanced proliferation and survival mechanisms. These findings provide a foundation for further research into the role of this novel fusion gene in B-ALL development and may inform future therapeutic strategies.
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive26 in National Genomics Data Center27, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA-Human: HRA008272) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human.
References
Pagliaro, L. et al. Acute lymphoblastic leukaemia. Nat. Rev. Dis. Primers. 10 (1), 41. https://doi.org/10.1038/s41572-024-00525-x (2024).
Malard, F. & Mohty, M. Acute lymphoblastic leukaemia. Lancet 395 (10230), 1146–1162. https://doi.org/10.1016/s0140-6736(19)33018-1 (2020).
Chang, T. C. et al. Genomic determinants of outcome in acute lymphoblastic leukemia. J. Clin. Oncol. 42 (29), 3491–3503. https://doi.org/10.1200/jco.23.02238 (2024).
Duffield, A. S., Mullighan, C. G. & Borowitz, M. J. International consensus classification of acute lymphoblastic leukemia/lymphoma. Virchows Arch. 482 (1), 11–26. https://doi.org/10.1007/s00428-022-03448-8 (2023).
Brady, S. W. et al. The genomic landscape of pediatric acute lymphoblastic leukemia. Nat. Genet. 54 (9), 1376–1389. https://doi.org/10.1038/s41588-022-01159-z (2022).
Dellino, G. I. et al. Release of paused RNA polymerase II at specific loci favors DNA double-strand-break formation and promotes cancer translocations. Nat. Genet. 51 (6), 1011–1023. https://doi.org/10.1038/s41588-019-0421-z (2019).
Uusküla-Reimand, L. & Wilson, M. D. Untangling the roles of TOP2A and TOP2B in transcription and cancer. Sci. Adv. 8 (44), eadd4920. https://doi.org/10.1126/sciadv.add4920 (2022).
Papapietro, O. & Nejentsev, S. Topoisomerase 2β and DNA topology during B cell development. Front. Immunol. 13, 982870. https://doi.org/10.3389/fimmu.2022.982870 (2022).
Kozuki, T. et al. Roles of the C-terminal domains of topoisomerase IIα and topoisomerase IIβ in regulation of the decatenation checkpoint. Nucleic Acids Res. 45 (10), 5995–6010. https://doi.org/10.1093/nar/gkx325 (2017).
Ma, Y., North, B. J. & Shu, J. Regulation of topoisomerase II stability and activity by ubiquitination and SUMOylation: clinical implications for cancer chemotherapy. Mol. Biol. Rep. 48 (9), 6589–6601. https://doi.org/10.1007/s11033-021-06665-7 (2021).
Yuva-Aydemir, Y., Almeida, S., Krishnan, G., Gendron, T. F. & Gao, F. B. Transcription elongation factor AFF2/FMR2 regulates expression of expanded GGGGCC repeat-containing C9ORF72 allele in ALS/FTD. Nat. Commun. 10 (1), 5466. https://doi.org/10.1038/s41467-019-13477-8 (2019).
Zhang, X. et al. Identification of a novel HOOK3-FGFR1 fusion gene involved in activation of the NF-kappaB pathway. Cancer Cell Int. 22 (1), 40. https://doi.org/10.1186/s12935-022-02451-y (2022).
Gu, Z. et al. PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat. Genet. 51 (2), 296–307. https://doi.org/10.1038/s41588-018-0315-5 (2019).
Schmidt, B. et al. ALLSorts: an RNA-seq subtype classifier for B-cell acute lymphoblastic leukemia. Blood Adv. 6 (14), 4093–4097. https://doi.org/10.1182/bloodadvances.2021005894 (2022).
Li, M. M. et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A joint consensus recommendation of the association for molecular pathology, american society of clinical oncology, and college of american pathologists. J. Mol. Diagn. 19 (1), 4–23. https://doi.org/10.1016/j.jmoldx.2016.10.002 (2017).
Chinese Society of Hematology Chinese Medical Association and Chinese Society of Pathology Chinese Medical Association. Expert consensus on the application of next-generation sequencing in hematological neoplasms. Zhonghua Xue Ye Xue Za Zhi 39 (11), 881–886. https://doi.org/10.3760/cma.j.issn.0253-2727.2018.11.001 (2018).
Pommier, Y., Nussenzweig, A., Takeda, S. & Austin, C. Human topoisomerases and their roles in genome stability and organization. Nat. Rev. Mol. Cell Biol. 23 (6), 407–427. https://doi.org/10.1038/s41580-022-00452-3 (2022).
Hu, Z. et al. Transcriptome sequencing allows comprehensive genomic characterization of pediatric B-acute lymphoblastic leukemia in an academic clinical laboratory. J. Mol. Diagn. 26 (1), 49–60. https://doi.org/10.1016/j.jmoldx.2023.09.013 (2024).
Akkari, Y. M. N. et al. Guiding the global evolution of cytogenetic testing for hematologic malignancies. Blood 139 (15), 2273–2284. https://doi.org/10.1182/blood.2021014309 (2022).
Nebral, K., Schmidt, H. H., Haas, O. A. & Strehl, S. NUP98 is fused to topoisomerase (DNA) IIbeta 180 kDa (TOP2B) in a patient with acute myeloid leukemia with a new t(3;11)(p24;p15). Clin. Cancer Res. 11 (18), 6489–6494. https://doi.org/10.1158/1078-0432.Ccr-05-0150 (2005).
Berger, J. M. & Wang, J. C. Recent developments in DNA topoisomerase II structure and mechanism. Curr. Opin. Struct. Biol. 6 (1), 84–90. https://doi.org/10.1016/s0959-440x(96)80099-6 (1996).
Miller, D. L. et al. Unclassified neuroendocrine tumor with a novel CHD4::AFF2 fusion: expanding the family of AFF2-rearranged head and neck malignancies. Head Neck Pathol. 16 (3), 928–933. https://doi.org/10.1007/s12105-022-01432-x (2022).
Savari, O. et al. First report of thoracic carcinoma with DEK::AFF2 rearrangement: A case report. J. Thorac. Oncol. 17 (8), 1050–1053. https://doi.org/10.1016/j.jtho.2022.05.009 (2022).
Ng, P. K. et al. Systematic functional annotation of somatic mutations in cancer. Cancer Cell. 33 (3), 450-462.e10. https://doi.org/10.1016/j.ccell.2018.01.021 (2018).
Koga, T., Suda, K. & Mitsudomi, T. Utility of the Ba/F3 cell system for exploring on-target mechanisms of resistance to targeted therapies for lung cancer. Cancer Sci. 113 (3), 815–827. https://doi.org/10.1111/cas.15263 (2022).
Chen, T. et al. The genome sequence archive family: Toward explosive data growth and diverse data types. Genom. Proteom. Bioinformat. 19 (4), 578–583. https://doi.org/10.1016/j.gpb.2021.08.001 (2021).
Database Resources of the National Genomics Data Center. China national center for bioinformation in 2022. Nucleic Acids Res. 50 (D1), D27–D38. https://doi.org/10.1093/nar/gkab951 (2022).
Acknowledgements
We express our gratitude for the contribution made by the patients, their families, pediatricians, and investigators. We would like to express our sincere gratitude to Professor Xujie Zhao from the School of Life Sciences at Southeast University for his invaluable guidance throughout this research.
Funding
This study was supported by the National Clinical Research Center for Child Health /National Children’s Regional Medical Center (Grant No. LY03006).
Author information
Authors and Affiliations
Contributions
TL wrote the initial draft. YW, XZA conceived and designed the study. YX and YJZ collected and analyzed the data of the family. JQL, YQW, LH, JCL, and YXPL contributed to the interpretation of the results. JY supervised the research project. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was reviewed and approved by the Medical Ethics Committee of the Children’s Hospital of Chongqing Medical University with the approval file number 2024.138. All methods were performed in accordance with the Declaration of Helsinki.
Consent statement
Written informed consent was obtained from the patient’s legal guardians (parents) prior to the investigation for the publication of any potentially identifiable images or data included in this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, T., Wang, Y., An, XZ. et al. Identification of a novel TOP2B::AFF2 fusion gene in B-cell acute lymphoblastic leukemia. Sci Rep 15, 3280 (2025). https://doi.org/10.1038/s41598-025-86865-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86865-4