Abstract
The incidence of blast injuries has been rising globally, particularly affecting the lungs due to their vulnerability. Primary blast lung injury (PBLI) is associated with high morbidity and mortality rates, while early diagnostic methods are limited. With advancements in medical technology, and portable handheld ultrasound devices, the efficacy of ultrasound in detecting occult lung injuries early remains unclear. This study evaluates the effectiveness of immediate lung ultrasound in diagnosing PBLI. The study involved 25 healthy male Bama mini-pigs subjected to BST-I-type biological shock wave tubes. The pigs were randomly assigned to non-injured and injured groups with driving pressures of 4.0 MPa, 4.5 MPa, and 4.8 MPa. Four PBLI models were created: no injury, minor, moderate, serious and severe. Immediate lung ultrasound following the BLUE-PLUS protocol and arterial blood gas analysis were conducted pre-injury and 0.5 h, 3 h, 6 h, 12 h, and 24 h post-injury, respectively. The study analyzed lung ultrasound score differences and their correlations with lung function parameters, using ROC analysis to determine early diagnostic standards and mortality prediction efficacy. The study found that in cases of moderate and severe PBLI, lung ultrasound scores and AaDO2 significantly increased at 0.5 h post-injury, while PaO2 decreased. There was good consistency between left and right lung ultrasound results at all times. Lung ultrasound scores were significantly correlated with PaO2 and AaDO2 but not with PaCO2. The scores accurately predicted injury severity at various time points within 24 h post-injury, and the 0.5 h lung ultrasound score predicted 24 h mortality with 95.8% efficiency. PBLI exhibits hidden severity, necessitating improved early diagnostics. Immediate lung ultrasound provides effective differentiation for moderate and severe PBLI at multiple time points within 24 h post-injury, is easy to implement, and offers effective mortality risk prediction as early as 0.5 h post-injury. These findings underscore lung ultrasound’s significant clinical application value in pre-hospital early treatment settings for PBLI.
Similar content being viewed by others
Introduction
Blast injuries are common in military conflicts, terrorist attacks, and industrial accidents, leading to large numbers of casualties and significant medical resource strain1,2. Enhancing the triage rate of blast injury patients is crucial for improving rescue success rates3,4.
Among the blast injuries, PBLI is found commonly, doing great harm to life5. PBLI can be difficult to detect outside of the hospital, and failure to confirm the diagnosis promptly often leads to serious consequences6. Given the critical “golden hour” within 1 h post-injury for saving lives and reducing disability, and early treatment within approximately 3 h, followed by specialized treatment after 6 h, a reliable method is urgently needed to assess the severity and evolution of injuries at various time points7,8,9. Immediate lung ultrasound has been widely used in in-hospital trauma diagnosis and treatment, especially the BLUE-PLUS protocol in acute lung injury10,11. However, its application in lung examination remains unclear in the battlefield trauma care guidelines12,13,14.
The emergence of handheld ultrasound has increased the accessibility of immediate ultrasound examinations. Previous studies by our team have demonstrated that lung ultrasound can be used early in PBLI, but the protocols are complex, the timeliness and standards for ultrasound assessment are not clearly defined15,16.
Therefore, we used the BLUE-PLUS ten-zone protocol commonly applied in critical care to collect lung ultrasound images at different time points within 24 h post-injury in pigs, aiming to clarify the clinical application value of handheld ultrasound in PBLI.
Methods
Animal preparation and model establishment
Animals
Twenty-five healthy male Bama miniature pigs, aged 4–6 months and weighing 25.28 ± 2.95 kg, were provided by the Experimental Animal Center of the Army Medical University. The experimental protocol was approved by the Experimental Ethics Committee of the Army Medical University (AMUWEC20223478). The animals were fasted for 12 h and water-restricted for 6 h before the experiment. They were acclimated to the laboratory conditions, maintained at 25 ± 0.5 °C and 60 ± 5% humidity. Throughout the experimental procedures, all animals were kept under analgesia and sedation. The surgical site was shaved and depilated using a depilatory cream, followed by alcohol cleaning. After preparation, the animals were stabilized for 30 min, during which baseline parameters were recorded.
Induction of anesthesia
Anesthesia was induced by intramuscular injection of ketamine hydrochloride at a dose of 4 mg/kg (Zhejiang Jiuxu, China) and intravenous administration of propofol at a dose of 5 mg/kg (Corden Pharma S.P.A, UK) via a 24G venous catheter in the left auricular vein (Jingma, China). The pigs were positioned supine, and the tongue was secured with a tongue clamp to ensure spontaneous breathing. Local anesthesia was performed by subcutaneous injection of 0.5% lidocaine at the surgical site.
Maintenance of analgesia and sedation
Continuous analgesia and sedation were maintained through intravenous infusion in the left auricular vein with propofol at 3.2-6 mg/kg/h, sufentanil citrate at 0.4–0.65 µg/kg/h (Renfu, China), and ketamine hydrochloride at 0.4–0.65 mg/kg/h. The depth of sedation and analgesia was monitored using the bispectral index (Bispectral Index, BIS), and the pain was assessed using the Critical-Care Pain Observation Tool (CPOT). The infusion rates were adjusted to maintain a BIS between 65 and 80 and a CPOT between 1 and 2 score. Perform local subcutaneous anesthesia with lidocaine in the local area before invasive procedures.
Induction of injury
The animals were placed in the experimental section of a BST-I type biological shock wave tube (developed by the Third Affiliated Hospital of Army Medical University, China), with a supporting frame positioning the animal’s left side facing the shock wave source and the end sealed (Fig. 1A). Based on prior research experience, we applied driving pressures of 4.8 MPa, 4.5 MPa, and 4.0 MPa to induce injury. Post-injury targeted analgesia and sedation were continued, and data were collected for 24 h.
Lung ultrasound examination
A handheld ultrasound device (HuaSheng Medical, China) was used following the BLUE-PLUS protocol, which employs a ten-zone method for both lungs (Fig. 1B,C)17. One video clip (over 10 s) and one image were collected for each zone in both lungs. These collections were repeated at six time points: pre-injury, 0.5 h, 3 h, 6 h, 12 h, and 24 h post-PBLI. The lung ultrasound examination was scored using the cLUSS (chest lung ultrasound score) criteria: 0 points: A-line or ≤ 2 B-lines; 1 point: ≥3 B-lines; 2 points: coalescent B-lines; 3 points: tissue-like pattern (Supplementary Table 1)18.
Blood gas analysis
A 1 ml sample was drawn from the femoral artery catheter and analyzed using a portable blood gas analyzer (Abbott, USA) to measure partial pressures of oxygen (PaO2) and carbon dioxide (PaCO2). Samples were collected at the same time points as the ultrasound examinations. The alveolar-arterial oxygen gradient (AaDO2) was calculated using the formula: AaDO2 = [(PB - PH2O) × FiO2 - PACO2 / R] - PaO2.
Injury assessment of blast lung injury
Animals that died within 24 h post-injury were immediately autopsied, while those surviving more than 24 h were euthanized with an overdose of anesthesia and then autopsied. Gross anatomical characteristics such as lung bleeding, tears, and the percentage of the affected area were recorded and compared with the ultrasound results obtained at the closest time point to the autopsy. Injury severity was assessed according to the PSSLBI criteria (Supplementary Table 2)19. Tissue samples were taken for hematoxylin and eosin (HE) staining and observed under a light microscope for pathological examination. Tissue samples: Partial lung tissue in the superior, middle and inferior lobes dorsal side of the right lung, and part of the lung tissue at the junction of the upper and lower lobes and upper and lower lobes on the dorsal side of the left lung. Ten different microscopic images were taken for pathological scoring in each section, and the final average was the pathological score of this lung (Supplementary Table 3).
Statistical analysis
Values are expressed as mean ± standard error of the mean (SEM). Two-way ANOVA with repeated measures and the Friedman test were used to compare normally and non-normally distributed parameters across different time points and injury levels, with Bonferroni correction applied. Linear regression and Bland-Altman analysis were employed to determine the correlation between left and right lung ultrasound scores. Spearman’s rank correlation coefficient (r) was used to evaluate the relationship between any two parameters. ROC curve analysis assessed predictive efficiency, with the optimal cutoff value determined by the maximum Youden index. Data were processed using SPSS 27.0 software, with P < 0.05 indicating statistical significance. GraphPad Prism 8.0 software (software available at https://www.graphpad.com/scientific-software/prism/www.graphpad.com/scientific-software/prism/).
Results
Establishment of animal models with different degrees of blast lung injury
According to the PSSLBI criteria, there were 9 cases of serious and severe PBLI, with mortality rates of 44.44% within 6 h and 55.56% within 24 h. 6 animals had moderate injuries, 5 had minor injuries, and 5 served as the control group, all surviving beyond 24 h.
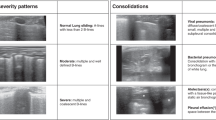
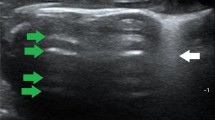
Comparison of lung ultrasound images with gross anatomical and pathological results at corresponding time points revealed the following: Normal lungs showed A-lines and a maximum of 2 B-lines on ultrasound, with normal gross anatomy and pathology. Minor PBLI presented with ≥ 3 well-spaced B-lines on ultrasound, scattered punctate lesions (0.5 cm x 0.5 cm) or occasional (≤ 2) patchy hemorrhages (1.5 cm x 1.5 cm) on gross examination, and pathological findings of interstitial edema and inflammatory cells, with some alveoli ruptured and fused. Moderate PBLI showed coalescent B-lines on ultrasound, scattered punctate lesions (1.5 cm x 1.5 cm) or occasional (≤ 2) patchy hemorrhages (3 cm x 3 cm) on gross examination, and pathological findings of disorganized alveolar structure, with approximately 50% interstitial edema and inflammatory cells. Severe and above PBLI exhibited a tissue-like pattern on ultrasound, with gross examination showing significantly distended and glossy lungs, diffuse large patchy hemorrhages, and pathological findings of > 50% interstitial edema and inflammatory cells, loss of alveolar structure, and numerous red blood cells (Fig. 2 and Supplementary Figs. 1–3).
Correspondence between lung pathology, gross anatomy, and ultrasound images. Ultrasound. White arrows: A lines. Red arrows: B lines. Continuous-line arrows: coalescent B lines. Oval: tissue-like pattern. Gross anatomical manifestations of PBLI in varying degrees. Yellow circle: Partial lung tissue of HE staining. HE staining of lung tissue (200×). Yellow arrows point to inflammatory cells. Green arrows point to red blood cells. Blue arrows point to pulmonary interstitial edema. Black arrows point to alveolar rupture.
Dynamic comparison of differences in lung function indicators between injury grades
Timeliness of lung function
There were significant differences in the lung function parameters (PaO2, PaCO2, and AaDO2) across different severities of blast lung injury (Fig. 3).
Changes in Lung Function Parameters in PBLI. (A) PO2 (F = 8.893, p < 0.001) showed statistically significant differences between injury grades, with PO2 levels decreasing in minor PBLI at 0.5 h (p < 0.001) and 12 h (p = 0.011); in moderate PBLI, PO2 decreased at 0.5 h (p < 0.001), 3 h (p = 0.008), 6 h (p = 0.001), 12 h (p = 0.004), and 24 h (p = 0.017); in severe and serious PBLI PO2 decreased at 0.5 h (p < 0.001), 3 h (p = 0.006), 6 h (p < 0.001), 12 h (p < 0.001), and 24 h (p = 0.001) . (B) PCO2 (F = 1.997, p = 0.026) showed that in minor PBLI PCO2 decreased at 0.5 h (p = 0.001) and 6 h (p = 0.011), in moderate PBLI PCO2 decreased at 0.5 h (p < 0.001), 3 h (p = 0.006), 6 h (p = 0.001), 12 h (p < 0.001), and 24 h (p = 0.001), in severe and serious PBLI PCO2 increased at 0.5 h (p < 0.001), while it decreased at 3 h (p = 0.008), 6 h (p = 0.001), 12 h (p = 0.004), and 24hs (p = 0.017). (C) AaDO2 (F = 17.107, p < 0.001) showed that in minor PBLI AaDO2 decreased at 0.5 h (p < 0.001), 3 h (p = 0.009), 6 h (p < 0.001), and 12 h (p = 0.005), in moderate PBLI AaDO2 decreased at 0.5 h (p < 0.001), 3 h (p < 0.001), 6 h (p < 0.001), 12 h (p < 0.001), and 24 h (p < 0.001),in severe and serious PBLI AaDO2 decreased at 0.5 h (p < 0.001), 3 h (p = 0.001), 6 h (p < 0.001), 12 h (p < 0.001), and 24 h (p < 0.001). *p < 0.05, †p < 0.01, $p < 0.001. Comparisons were made using RM ANOVA between groups and within groups at each time point post-injury vs. pre-injury.
Timeliness of lung ultrasound scores
The lung ultrasound scores showed significant differences across different severities of blast injuries. Lung ultrasound scores increased significantly at 0.5 h post-injury for minor, moderate, serious and severe PBLI (Fig. 4).
Trends in Lung Ultrasound Scores across Different Injury Severities within 24 h Post-Injury. (A) cLUSS (F = 40.154, p < 0.001) showed a significant increase in minor PBLI at 0.5 h (p = 0.000), 3 h (p < 0.001), 6 h (p = 0.002), and 12 h (p = 0.006). Moderate PBLI revealed elevated cLUSS scores at 0.5 h (p < 0.001), 3 h (p < 0.001), 6 h (p < 0.001), 12 h (p < 0.001), and 24 h (p < 0.001). Serious and severe PBLI also showed increased cLUSS scores at 0.5 h (p < 0.001), 3 h (p < 0.001), 6 h (p < 0.001), 12 h (p < 0.001), and 24 h (p < 0.001). (B) R-cLUSS (F = 3.267, p = 0.005) demonstrated a rise in minor PBLI at 0.5 h (p = 0.009), 3 h (p < 0.001), 6 h (p = 0.002), and 12 h (p < 0.001). Moderate PBLI exhibited increased ultrasonographic scores at 0.5 h (p < 0.001), 3 h (p < 0.001), 6 h (p < 0.001), 12 h (p < 0.001), and 24 h (p < 0.001) post-injury. Serious and severe PBLI also displayed elevated ultrasonographic scores at 0.5 h (p < 0.001), 3 h (p < 0.001), 6 h (p < 0.001), 12 h (p < 0.001), and 24 h (p < 0.001) post-injury. (C) L-cLUSS (F = 19.503, p < 0.001) indicated an increase in minor PBLI at 0.5 h (p = 0.048) and 3 h (p = 0.012) post-injury compared to pre-injury levels. Moderate PBLI demonstrated higher ultrasonographic scores at 3 h (p = 0.006), 6 h (p = 0.003), 12 h (p = 0.003), and 24 h (p < 0.001) post-injury. Serious and severe PBLI also showed elevated ultrasonographic scores at 0.5 h (p < 0.002), 3 h (p < 0.001), 6 h (p < 0.001), 12 h (p < 0.001), and 24 h (p < 0.001) post-injury. *p < 0.05, †p < 0.01, $p < 0.001. Comparisons were made using RM ANOVA between groups at each time point post-injury vs. pre-injury (Friedman test).
Comparison of lung function indices with lung ultrasound findings
Linear analysis within 24 h post-PBLI showed no statistically significant difference between ultrasound scores and PaCO2 (p > 0.05). However, a negative correlation was observed between ultrasound scores and PaO2 (p < 0.05, Fig. 5) and a positive correlation between ultrasound scores and AaDO2 (p < 0.05, Fig. 6).
The effectiveness of lung ultrasound findings in predicting the degree of injury at each time point
There was a linear correlation between the ultrasound scores of the left and right lungs (Fig. 7).
Consistency Analysis of Bilateral Lung Ultrasound Scores. (A & B) Linear regression analyses of the gradient between R-cLUSS and L-cLUSS after 0.5 h, A Bland–Altman analysis showed the mean bias was 2.625 ± 2.871. (C & D) Linear regression analyses of the gradient between R-cLUSS and L-cLUSS after 3 h, A Bland–Altman analysis showed the mean bias was 2.429 ± 2.925. (E & F) Linear regression analyses of the gradient between R-cLUSS and L-cLUSS after 6 h, A Bland–Altman analysis showed the mean bias was 2.667 ± 3.12. (G & H) Linear regression analyses of the gradient between R-cLUSS and L-cLUSS after 12 h, A Bland–Altman analysis showed the mean bias was 1.95 ± 2.481. (L & M) Linear regression analyses of the gradient between R-cLUSS and L-cLUSS after 24 h, A Bland–Altman analysis showed the mean bias was 3.1 ± 2.989. *Solid line represents linear regression between cLUSS with the AaDO2. Dash lines indicate 95% confidence intervals.
Predictive efficiency of lung ultrasound for injury severity and mortality
In moderate and severe PBLI, the chest lung ultrasound score (cLUSS) demonstrated good efficiency in recognizing injury severity within 24 h post-injury (p < 0.05). Furthermore, at 0.5 h post-injury, cLUSS showed good predictive efficiency for 24-hour mortality (AUC = 0.958, p < 0.05) (Fig. 8, Supplementary Table 4).
Predictive Efficiency Analysis of Lung Ultrasound. (A) Predictive efficiency of lung ultrasound within 24 h for moderate PBLI. (B) Predictive efficiency of lung ultrasound within 24 h for serious and sever PBLI. (C) Predictive efficiency of lung ultrasound at 0.5 h post-PBLI for predicting 24-hour mortality.
Discussion
The incidence of blast injuries from military conflicts and daily industrial accidents has been increasing in recent years. Handheld ultrasound devices have potentially replaced some traditional clinical monitoring functions. The application of ultrasound in lung diseases is gaining increasing attention20,21,22. PBLI is defined as acute lung injury occurring within 12 h, and its pathophysiological evolution was studied using handheld ultrasound to capture lung changes within 24 h4. However, it is challenging to conduct human trials on pulmonary blast injuries. Pigs are similar to humans in anatomical size and structure, immunology, and physiology, and thus preferable to rodent models for translational and clinical research applications. Our study is the first to clarify the association between handheld lung ultrasound images using the BLUE-PLUS protocol, gross anatomical and pathological findings within 24 h post-PBLI.
Further analysis revealed the correlation between lung ultrasound and lung function parameters at various time points post-injury (0.5 h, 3 h, 6 h, 12 h, and 24 h). Lung ultrasound demonstrated a certain level of diagnostic capability for moderate and above PBLI within 24 h, providing an effective method for evaluating lung function. This offers a simple, easy-to-implement, and effective tool for assessing PBLI early.
Immediate critical lung ultrasound is commonly used in clinical settings to evaluate ARDS with satisfactory results7,23,24. Blood gas analysis is the most frequently used clinical method for rapid lung function assessment, with PaO2 commonly used to determine lung function and PaCO2 to evaluate ventilation function. Together, these can assess the type of respiratory failure, and AaDO2 can be calculated to evaluate lung diffusion function25,26,27. However, it requires invasive procedures and specialized equipment, which demand stringent environmental conditions28,29. In contrast, a non-invasive method, handheld ultrasound devices with simplified operating protocols are more suitable for early diagnostic scenarios and real-time monitoring30,31. The BLUE-PLUS ten-zone method allows for the observation of ventral lung lobe injuries and pneumothorax at the upper BLUE point, lower BLUE point, and PLAPS point; pleural effusion and acute lung consolidation at the PLAPS point and diaphragm point; and dorsal lung lobe injuries at the posterior blue point8,32,33,34. Our results indicate a correlation between ten-zone lung ultrasound scores and traditional lung function parameters. Unlike traditional vital signs or laboratory test parameters, portable handheld ultrasound is convenient, with fewer situational restrictions and easy inter-individual operational transfer35,36. Compared to X-ray and CT imaging parameters, it poses no radiation hazards, is easily implemented pre-hospital and on-site, and may potentially alter conventional diagnostic methods31,37,38,39. Meanwhile, previous studies have shown that lung ultrasound is highly sensitive to chest injuries40.
Anatomical data is considered the gold standard for lung evaluation, but it requires high technical skill and is difficult to perform at the bedside in real time. Lung ultrasound allows real-time, non-invasive, radiation-free, portable, and dynamic assessment. It evaluates lung lesions from an imaging and pathophysiological perspective, reflecting the lung’s air-fluid distribution and pathophysiological phenotype41,42,43,44. Our study found that blast-induced lung injuries were uneven, and even if gross anatomy appeared normal, pathological examination often revealed varying degrees of alveolar structure damage and interstitial inflammatory cell infiltration. Ultrasound examination detected abnormal lung structure damage45,46. Comparing the ultrasound scores of both lungs showed good consistency, indicating that single-side lung ultrasound could be used in emergencies to assess PBLI severity, enabling early intervention and treatment.
Lung ultrasound is a common clinical tool for monitoring lung injuries, guiding treatment, and predicting mortality risk. Although these parameters’ changes and predictive efficacy are well-documented in PBLI, they have not been widely applied for early triage and treatment of this type of acute lung injury28,31,47,48,49. Lung ultrasound has a sensitivity of 98% and a specificity of 88% for diagnosing acute pulmonary edema, outperforming chest X-rays for diagnosing interstitial syndrome49. Our study found that in moderate, serious and severe PBLI, PaO2 decreased immediately post-injury while AaDO2 increased. This indicates pulmonary hemorrhage, interstitial edema, and impaired pulmonary diffusion function post-blast injury, consistent with anatomical and pathological findings of pulmonary hemorrhage, interstitial edema, inflammatory exudation, and alveolar structure damage. Comparison between lung ultrasound and conventional lung function parameters revealed a good correlation between ultrasound scores and PaO2 and AaDO2 at various time points, suggesting a strong association with pulmonary diffusion function. However, there may be some limitations in evaluating ventilation function. This demonstrates that lung ultrasound can guide respiratory function treatment strategies post-injury.
Previously, ultrasound examination was considered less effective for air-filled cavities. However, as research has progressed, more lung ultrasound protocols have been developed for lung assessment. The compact and convenient nature of handheld ultrasound devices has led to widespread use in pre-hospital settings and even on-site post-injury evaluations50. It can be performed and analyzed anytime, anywhere. Our study proves lung ultrasound can be used not only for assessing PBLI severity but also for injury grading and mortality risk prediction51. Handheld ultrasound has been currently applied in the field of critical care. During the early on-site treatment stage of PBLI, these operations are usually carried out by military medical personnel, who may not all be familiar with the procedures and operations of lung ultrasound examination. Therefore, it is necessary to disseminate knowledge about handheld ultrasound (including the BLUE-PLUS Protocol, obtaining a complete examination within a specified time, requirements for image acquisition, etc.) to all personnel involved in the early injury assessment and treatment of PBLI. However, in high-stress environments, how to complete the examination quickly and accurately requires repeated training at ordinary times. The recent development of wearable ultrasound technology may provide an intelligent triage strategy for real-time monitoring of such lung trauma in the future52.
Our study has several limitations. Firstly, the small sample size could have resulted in a type 1 error, to minimize errors associated with a limited number of animals, both blank control groups and self-comparison before and after treatment were established. This approach enables the determination of the effectiveness of the injury-inducing methods in the experiment. It also helps to exclude the interference of non-injury factors (such as the experimental environment, anesthesia, etc.). The comparison solely before and after injury may be affected by the variability of the samples themselves, while the blank control can enhance the statistical reliability of the experimental results. Additionally, it can provide a range of data under normal physiological conditions, facilitating a more comprehensive interpretation of the results. Besides, considering the impact of the subjective actions of ultrasound operators on experimental quality, we designed a single-blind study. We first collected data, then performed dissections to assess the overall condition and severity of lung injuries. The personnel were selected based on having passed ultrasound training assessments and demonstrated proficiency. Ten seconds of ultrasound images were captured at each point and a standardized scoring system was applied. Strict standards were enforced to minimize variability. The primary focus of the design and implementation of the study was on exploring the handheld ultrasound capabilities and applications of the imaging technology under investigation. The inclusion of such comparisons might have potentially altered the conclusions and the direction of the research.
Conclusion
The BLUE-PLUS scoring system consistently correlates with pathological scores, lung function, and mortality rates. Ultrasound can accurately detect the extent of lung damage caused by shock waves. Our research method provides a new strategy for monitoring blast-induced ARDS using ultrasound, especially with the potential for real-time assessment through future wearable ultrasound technology.
Data availability
Data is provided within the manuscript and supplementary information files.
Abbreviations
- PaO2 :
-
Arterial partial pressure of oxygen
- PaCO2 :
-
Arterial partial pressure of carbon dioxide
- SaO2 :
-
Arterial oxygen saturation
- LAC:
-
Lactate
- PcvO2 :
-
Central venous partial pressure of oxygen
- PcvCO2 :
-
Central venous partial pressure of carbon dioxide
- cLUSS:
-
Coalescence lung ultrasound score
- AaDO2 :
-
Alveolar to arterial oxygen tension difference
- PB:
-
Atmospheric pressure, approximately equal to 760 mmHg
- PH2O:
-
Is the water vapor pressure in the alveoli, with a value of 6.25 kPa (47 mmHg) at 37°CR is the respiratory quotient, typically assumed to be 0.8
- PACO2 :
-
Is the alveolar CO2 partial pressure, which can be calculated from PaCO2
References
Depalma, R. G. et al. Blast injuries. N. Engl. J. Med. 352(13), 1335–1342 (2005).
Wolf, S. J. et al. Blast injuries. Lancet (London, England) 374(9687), 405–415 (2009).
Scott, T. E. et al. Primary Blast Lung Injury: The UK Military Experience. Mil. Med. 185(5–6), e568–e572 (2020).
Scott, T. E. et al. Primary blast lung injury—a review. Br. J. Anaesth. 118(3), 311–316 (2017).
Li, J., Zhang, J., Shi, M., et al. Crosstalk between Inflammation and Hemorrhage/Coagulation Disorders in Primary Blast Lung Injury. Biomolecules, 2023, 13(2).
Li, N., Geng, C., Hou, S., et al. Damage-Associated Molecular Patterns and Their Signaling Pathways in Primary Blast Lung Injury: New Research Progress and Future Directions. International journal of molecular sciences, 2020, 21(17).
Hu, W. et al. The “Golden Hour” and field triage pattern for road trauma patients. J. Safety Res. 75, 57–66 (2020).
Kotwal, R. S., Champion, H. R. & Gross, K. R. A military-specific injury scoring system to aid in understanding the golden hour-reply. JAMA Surg. 151(5), 491–492 (2016).
Medrano, N. W. et al. Access to trauma center care: a statewide system-based approach. The Journal of Trauma and Acute Care Surgery 95(2), 242–248 (2023).
Kornblith, A. E. et al. Development of a consensus-based definition of focused assessment with sonography for trauma in children. JAMA Netw. Open 5(3), e222922 (2022).
Manson, W. C. et al. Focused assessment with sonography in trauma (FAST) for the regional anesthesiologist and pain specialist. Reg. Anesth. Pain Med. 44(5), 540–548 (2019).
Xue, Y. Q., Wu, C. S., Zhang, H. C., et al. Value of lung ultrasound score for evaluation of blast lung injury in goats. Chinese Journal of Traumatology = Zhonghua chuang shang za zhi, 2020, 23(1): 38–44.
Mongodi, S. et al. Quantitative lung ultrasound: Technical aspects and clinical applications. Anesthesiology 134(6), 949–965 (2021).
Smit, M. R., Mayo, P. H., Mongodi, S. Lung ultrasound for diagnosis and management of ARDS. Intensive care medicine, 2024.
Peng, Q. Y. et al. Lung ultrasound score based on the BLUE-plus protocol is associated with the outcomes and oxygenation indices of intensive care unit patients. Journal of clinical ultrasound: JCU 49(7), 704–714 (2021).
Van der Weide, L. et al. Prehospital ultrasound in the management of trauma patients: Systematic review of the literature. Injury 50(12), 2167–2175 (2019).
Lichtenstein, D. A. BLUE-Protocol and FALLS-Protocol. Chest 147(6), 1659–1670 (2015).
Mongodi, S., Bouhemad, B., Orlando, A., et al. Modified lung ultrasound score for assessing and monitoring pulmonary aeration. Ultraschall in der Medizin (Stuttgart, Germany: 1980), 2017, 38(5): 530–7.
Jihong, Z. Trauma Scoreology 1st edn. (Science Press, 2018).
Dietrich, C. F., Görg, C., Horn, R., et al. Ultrasound of the lung. Ultraschall in der Medizin (Stuttgart, Germany: 1980), 2023, 44(6): 582–99.
Koenig, S. et al. Lung ultrasound scanning for respiratory failure in acutely Ill patients: A review. Chest 158(6), 2511–2516 (2020).
Díaz-Gómez, J. L., Mayo, P. H. & Koenig, S. J. Point-of-care ultrasonography. The New England journal of medicine 385(17), 1593–1602 (2021).
Smith, M. J. et al. Point-of-care lung ultrasound in patients with COVID-19—a narrative review. Anaesthesia 75(8), 1096–1104 (2020).
Raimondi, F., Migliaro, F., Corsini, I., et al. Lung ultrasound score progress in neonatal respiratory distress syndrome. Pediatrics, 2021, 147(4).
Howe, C. A. et al. Validation of a noninvasive assessment of pulmonary gas exchange during exercise in hypoxia. Chest 158(4), 1644–1650 (2020).
Schwartz, J. C. et al. Alveolar to arterial gas exchange during constant-load exercise in healthy active men and women. Journal of sports sciences 39(9), 961–968 (2021).
West, J. B. et al. Measuring the efficiency of pulmonary gas exchange using expired gas instead of arterial blood: comparing the “ideal” Po(2) of Riley with end-tidal Po(2). American journal of physiology Lung cellular and molecular physiology 319(2), L289–L293 (2020).
Reske, A. W. et al. Bedside estimation of nonaerated lung tissue using blood gas analysis. Critical care medicine 41(3), 732–743 (2013).
Carlino, M. V. et al. Arterial blood gas analysis utility in predicting lung injury in blunt chest trauma. Respiratory physiology & neurobiology 274, 103363 (2020).
Leblanc, D. et al. Early lung ultrasonography predicts the occurrence of acute respiratory distress syndrome in blunt trauma patients. Intensive care medicine 40(10), 1468–1474 (2014).
Chan, K. K., Joo, D. A., Mcrae, A. D., et al. Chest ultrasonography versus supine chest radiography for diagnosis of pneumothorax in trauma patients in the emergency department. The Cochrane database of systematic reviews, 2020, 7(7): Cd013031.
Bekgoz, B. et al. BLUE protocol ultrasonography in Emergency Department patients presenting with acute dyspnea. The American journal of emergency medicine 37(11), 2020–2027 (2019).
Buessler, A. et al. Accuracy of several lung ultrasound methods for the diagnosis of acute heart failure in the ED: A multicenter prospective study. Chest 157(1), 99–110 (2020).
Brat, R. et al. Lung ultrasonography score to evaluate oxygenation and surfactant need in neonates treated with continuous positive airway pressure. JAMA pediatrics 169(8), e151797 (2015).
Ding, C. et al. Establishment and evaluation of an in vitro blast lung injury model using alveolar epithelial cells. Frontiers in public health 10, 994670 (2022).
April, M. D. et al. Vital sign thresholds predictive of death in the combat setting. The American journal of emergency medicine 44, 423–427 (2021).
Lull, R. J. et al. Radionuclide imaging in the assessment of lung injury. Seminars in nuclear medicine 10(3), 302–310 (1980).
Mcdonald Johnston, A. & Ballard, M. Primary blast lung injury. American Journal of Respiratory and Critical Care Medicine 191(12), 1462–1463 (2015).
Singleton, J. A. et al. Primary blast lung injury prevalence and fatal injuries from explosions: insights from postmortem computed tomographic analysis of 121 improvised explosive device fatalities. The journal of trauma and acute care surgery 75(2 Suppl 2), S269–S274 (2013).
Stengel, D., Leisterer, J., Ferrada, P., et al. Point-of-care ultrasonography for diagnosing thoracoabdominal injuries in patients with blunt trauma. The Cochrane database of systematic reviews, 2018, 12(12): Cd012669.
Sultan, S. R. Association between lung ultrasound patterns and pneumonia. Ultrasound quarterly 38(3), 246–249 (2022).
Lichtenstein, D. A. & Mezière, G. A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 134(1), 117–125 (2008).
Peng, Q. Y., Wang, X. T. & Zhang, L. N. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive care medicine 46(5), 849–850 (2020).
Chiumello, D. et al. Assessment of lung aeration and recruitment by CT scan and ultrasound in acute respiratory distress syndrome patients. Critical care medicine 46(11), 1761–1768 (2018).
Schwarz, S. Pulmonary sonography—neonatal diagnosis part 1. Ultraschall in der Medizin (Stuttgart, Germany: 1980), 2023, 44(1): 14–35.
Brogi, E. et al. Thoracic ultrasound for pleural effusion in the intensive care unit: a narrative review from diagnosis to treatment. Critical care (London, England) 21(1), 325 (2017).
Sayed, M. S. et al. The validity of quantifying pulmonary contusion extent by lung ultrasound score for predicting ARDS in blunt thoracic trauma. Critical care research and practice 2022, 3124966 (2022).
Mayo, P. H. et al. American college of chest physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest 135(4), 1050–1060 (2009).
Kraaijenbrink, B. V. C. et al. Defining basic (lung) ultrasound skills: not so basic after all?. Intensive care medicine 48(5), 628–629 (2022).
Zieleskiewicz, L. et al. Bedside POCUS during ward emergencies is associated with improved diagnosis and outcome: an observational, prospective, controlled study. Critical care (London, England) 25(1), 34 (2021).
Fröhlich, E., Beller, K., Muller, R., et al. Point of care ultrasound in geriatric patients: Prospective evaluation of a portable handheld ultrasound device. Ultraschall in der Medizin (Stuttgart, Germany: 1980), 2020, 41(3): 308–16.
Van Neer, P. et al. Flexible large-area ultrasound arrays for medical applications made using embossed polymer structures. Nature communications 15(1), 2802 (2024).
Acknowledgements
We would like to thank Yue Shen, Xinan Lai from Daping Hospital, Army Medical University, for manuscript discussions throughout the process.
Funding
This work was supported by the Key specialty of the army’s clinical focus.
Author information
Authors and Affiliations
Contributions
Shifeng Shao: original draft, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation. Zhengbin Wu: Writing—review & editing, Methodology, Investigation, Data curation. Jun Liu: Writing—review & editing, Methodology, Investigation, Data curation. Zhikang Liao: Writing—review & editing, Methodology, Investigation, Data curation. Yuan Yao: Writing—review & editing, Methodology, Investigation, Data curation. Liang Zhang: Writing—review & editing, Software, Methodology, Formal analysis. Yaoli Wang: Writing—review & editing, Supervision, Funding acquisition, Formal analysis, Conceptualization. Hui Zhao: Writing—review & editing, Supervision, Resources, Project administration, Methodology, Formal analysis, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
All experimental procedures were conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals established by the Animal Ethics Committee of the Army Medical University (AMUWEC20223478) and complied with Directive 2010/63/EU of the European Parliament. The animal production license number is SCXK (Chongqing) 2017-0002, and the animal use license number is SYXK (Chongqing) 2017-0002. The care and handling of the animals were conducted in strict accordance with the guidelines outlined in the “Guide for the Care and Use of Laboratory Animals“(https://www.nature.com/srep/journal-policies/editorial-policies#experimental-subjects). All surgeries were performed with ketamine hydrochloride, propofol and sufentanil citrate anesthesia, and all efforts were made to minimize suffering. All methods were performed in accordance with the relevant guidelines and regulations. The study was carried out in compliance with the ARRIVE guidelines (https://arriveguidelines.org).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shao, S., Wu, Z., Liu, J. et al. Evaluating the effectiveness of handheld ultrasound in primary blast lung injury: a comprehensive study. Sci Rep 15, 2358 (2025). https://doi.org/10.1038/s41598-025-86928-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-86928-6