Abstract
Hypertension, a major global health issue and leading cause of death, is often under-assessed by traditional metrics like Body Mass Index which fail to capture comprehensive cardiovascular risks associated with obesity. The Cardiometabolic Index (CMI), which evaluates abdominal obesity and dyslipidemia, offers a more accurate assessment of visceral fat and metabolic dysfunction. In this study, we analyzed data from 45,250 participants from the National Health and Nutrition Examination Survey spanning 1999 to 2020. Using multivariable logistic regression, we explored the association between CMI and hypertension, employing Restricted Cubic Spline analysis to assess non-linear relationships and two-piecewise linear regression to identify threshold effects. Subgroup analyses confirmed the consistency of our findings across various demographic and clinical characteristics. Findings confirmed that hypertensive participants exhibited significantly higher CMI levels (median 0.46 vs. 0.73), with adjusted logistic regression showing a notable association between increased CMI and hypertension prevalence (OR 1.30, 95% CI 1.25–1.35, P < 0.01), characterized by a nonlinear L-shaped curve with a critical threshold identified at a CMI value of 1.37. Subgroup analysis revealed a more pronounced impact of CMI on hypertension in females. These results underscore CMI’s potential to enhance cardiovascular risk assessment across diverse U.S. populations.
Similar content being viewed by others
Introduction
Hypertension is a major global health concern, affecting approximately 1.28 billion individuals worldwide and representing a significant challenge for public health systems1. As the leading cause of mortality globally, hypertension exerts profound impacts on vital organs, including the heart, brain, and kidneys, while also inflicting substantial distress on patients and their families2,3,4. In the United States (U.S.), nearly half of the adult population (48.1%, roughly 119.9 million people) suffers from high blood pressure5. The financial implications are staggering, with the total economic burden in the U.S. projected to rise by $130.4 billion from 2010 to 2030, encompassing both direct healthcare costs and indirect losses such as reduced productivity6. Therefore, early detection and effective management of risk factors for hypertension are essential to prevent its progression.
Given the significant role of obesity as both a precursor and a risk factor for hypertension, it is vital to explore the intricate relationship between adiposity and cardiovascular risks7,8,9. Traditional measures such as Body Mass Index (BMI) are widely used to assess cardiovascular risks associated with obesity. However, BMI does not effectively reflect the actual distribution of body fat or distinguish between fat and lean mass increases10,11. The Cardiometabolic Index (CMI), introduced by Japanese researcher Wakabayashi in 2015, is calculated from the waist-to-height ratio (WHtR) and the triglyceride-to-HDL cholesterol ratio (TG/HDL-C)11. This index, by integrating markers of abdominal obesity and dyslipidemia, has been validated as a robust and independent discriminator of clinical adiposity, offering a more precise correlation with metabolic abnormalities compared to conventional anthropometric indicators13,14. Studies focusing on CMI have confirmed its utility in identifying a spectrum of obesity-related and cardiovascular conditions, including diabetes, nonalcoholic fatty liver disease, kidney disease, atherosclerosis, and ischemic stroke10,12,15,16,17,18. Although a previous study has established a positive correlation between CMI and hypertension incidence19, this was limited to a rural Chinese cohort, potentially skewing the results due to unique regional dietary and lifestyle factors. Additionally, significant physiological differences in body composition and blood lipid profiles exist between Chinese and American populations20, suggesting that findings from one may not directly apply to the other. Given this context, there is a clear need for research within a diverse U.S. demographic to determine if the association between CMI and hypertension observed in China extends to the U.S.
This study seeks to investigate the relationship between CMI and the prevalence of hypertension among U.S. adults using extensive National Health and Nutrition Examination Survey (NHANES) datasets from 1999 to 2020. By doing so, it aims to bridge the research gap on whether CMI can effectively predict hypertension in the American population, potentially informing targeted prevention strategies and improving clinical outcomes for a condition that impacts millions silently across the globe.
Methods
Study population
The NHANES, conducted annually since 1999 by the U.S. Centers for Disease Control and Prevention (CDC), employs complex, multistage, stratified sampling that disproportionately includes minorities and older adults. Ethical approval for NHANES was obtained from the National Center for Health Statistics Ethics Review Board. Data collection methods, including household questionnaires, telephone interviews, and medical examinations, ensured participants’ informed consent. More details on the survey’s methodology are available on the CDC website (https://www.cdc.gov/nchs/nhanes/index.htm).
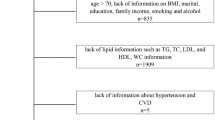
Our study utilizes NHANES data from 1999 to 2020, focusing on a representative sample of the U.S. civilian, non-institutionalized population. The initial participant pool comprised 116,876 individuals who provided informed consent. Using stringent inclusion criteria, we excluded those under 20 years (52,563), pregnant women (1,635), individuals with a history of cancer, and those with incomplete data on hypertension diagnosis (72), CMI (8,645), or other relevant covariates (2,588). Finally, 45,250 individuals participated in the study (Fig. 1).
Calculation of cardiometabolic index
The CMI was calculated by dividing triglycerides in millimoles per liter by high-density lipoprotein cholesterol in millimoles per liter and then multiplying the result by the ratio of waist circumference in centimeters to height in centimeters. The formula for calculating CMI is presented as follows21:
Based on this index, participants were categorized into four groups according to quartiles. More specifically, the first quartile (Quartile 1) served as the reference, including Quartile 1 (CMI ≤ 0.33), Quartile 2 (0.33 < CMI ≤ 0.60), Quartile 3 (0.60 < CMI ≤ 1.10), and Quartile 4 (CMI > 1.10).
Blood pressure measurement and definition of hypertension
Blood pressure was measured during two separate sessions: at home by a trained research assistant and at a mobile examination clinic by a skilled clinician, with three readings taken at each session. Participants were instructed to rest quietly for five minutes before measurements were taken. To calculate the average blood pressure, the protocol specifically excluded the first reading from each session to eliminate the initial measurement variability. If only one reading was obtained, that reading was used as the average. For diastolic pressure, any reading recorded as zero was excluded from the average calculation. If all diastolic readings were zero, the average diastolic pressure was recorded as zero. The average of the second and third measurements from each session was then used to establish systolic and diastolic blood pressures.
Hypertension was diagnosed based on the average blood pressure readings meeting any of the following criteria: a systolic blood pressure of 130 mm Hg or higher, a diastolic blood pressure of 80 mm Hg or higher, a self-reported history of hypertension diagnosed by a physician, or current use of medication to lower blood pressure22. The threshold of 130/80 mmHg for diagnosing hypertension aligns with the standards set by the American Heart Association23.
Definition of covariates
The covariates were selected based on previous literature and substantive reasoning that suggested their potential association with both CMI levels and hypertension prevalence. Standardized questionnaires were utilized to gather information on various factors, including age, sex, race, BMI, waist circumference, height, systolic blood pressure (SBP), diastole blood pressure (DBP), educational level, marital status, smoking status, diabetes mellitus history, cardiovascular disease (CVD) history, and chronic kidney disease (CKD)history. Race was categorized into five groups: Non-Hispanic White, Non-Hispanic Black, Mexican American, Other Hispanic, and Other. Marital status was categorized into three groups: never married, married/ living with partner, and widowed/divorced/separated. Education was stratified into three levels: less than high school, high school or equivalent, and more than high school. Participants were classified as never smokers, former smokers, or current smokers based on their responses to questions about smoking at least 100 cigarettes during their lifetime and whether they were currently smoking. CVD was assessed by a set of self-reported questionnaires. Diabetes mellitus was defined based on any of the following criteria: self-reported diagnosis, use of insulin or hypoglycemic medications, hemoglobin A1c (HbA1c) levels of 6.5% or higher, fasting blood glucose (FBG) levels of 7.0 mmol/L or higher, or a 2-hour postprandial glucose (2hPG) level of 11.1 mmol/L or higher24. Prediabetes was identified by HbA1c levels between 5.7% and 6.4%, FBG levels between 5.6 mmol/L and 6.9 mmol/L, or 2hPG levels between 7.8 mmol/L and 11.0 mmol/L24. CVD was diagnosed based on participant reports of a physician’s confirmation of coronary heart disease, congestive heart failure, heart attack, stroke, or angina25. CKD is defined as persistent kidney abnormalities for at least three months, indicated by an eGFR < 60 mL/min/1.73 m² or a urine albumin-to-creatinine ratio ≥ 30 mg/g26. The eGFR is calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation based on serum creatinine27, while urinary albumin and creatinine levels are measured via solid-phase fluorescence immunoassay and enzymatic methods, respectively. Detailed information on specimen collection, processing, quality assurance, and monitoring are described in the section of the biospecimen program in NHANES.
Statistical analysis
Following NHANES analysis guidelines, data from 1999 to 2020 were integrated for this study. To account for the varying survey cycles, we constructed combined sample weights by utilizing the 4-year weights for the 1999–2002 period and 2-year weights for each subsequent survey cycle. These adjustments ensure that the analysis appropriately reflects the complex, stratified sampling design of NHANES and maintains representativeness across the entire study period.
Our analysis incorporated the complex sampling design of the NHANES, adhering to the guidelines for utilizing NHANES data. Descriptive statistics were presented based on the normality of continuous variables, which was assessed using histograms (Supplementary Fig. 1). Continuous variables with normal distributions were expressed as weighted means ± standard deviations (SD), while non-normally distributed variables were reported as weighted medians (95% confidence intervals, CI). Categorical variables were presented as weighted percentages (95% CI). Depending on the data distribution, continuous variables were analyzed using t-tests or Mann-Whitney U tests, and categorical variables were analyzed using weighted Chi-square tests. P-values were weighted to account for the survey design.
We examined the association between CMI levels and hypertension prevalence using both univariate and multivariate logistic regression. For multivariate logistic regression, initial models were unadjusted, while subsequent models accounted for various confounders. Model I adjusted for age, sex, and race, while Model II included additional variables such as age, sex, race, marital status, educational level, smoking status, TC, and history of diabetes mellitus, CVD, and CKD.
A dose-response relationship was explored using restricted cubic spline (RCS) analysis to assess the non-linear associations and potential thresholds. The RCS model included 3 nodes, chosen based on recommended practices to ensure sufficient flexibility while avoiding overfitting25,28. The model’s fit was compared through a log-likelihood ratio test between a single linear model and a segmented regression model. The inflection point of the segmented model was identified using a recursive method.
Subgroup analyses were conducted based on key demographic and health attributes, including age, sex, race, marital status, educational level, smoking status, diabetes mellitus, CVD, and CKD. Interaction effects were evaluated using the likelihood ratio test to determine statistical significance (P-interaction).
To ensure the robustness of the results, sensitivity analyses were conducted using data processed through multiple imputation for missing values. The mice package in R was employed to perform multiple imputation, generating five imputed datasets. The results of multivariate logistic regression analyses of the association between CMI and hypertension were then reevaluated using these imputed datasets, confirming the stability of the findings.
All analyses were performed using R statistical software (http://www.R-project.org), with statistical significance set at a two-sided P-value of less than 0.05.
Results
Characteristics of participants
This study enrolled 45,250 participants, representing 169,174,501 non-institutionalized adults (20 years of age and older) in the U.S., with 50.01% male and 66.94% identifying as non-Hispanic white. The median age was 45 years (interquartile [IQR]: 32–57), and the median CMI was 0.58 (IQR: 0.32–1.08). Among the participants, 53.16% (n = 24,053) were diagnosed with hypertension. Characteristics stratified by hypertension status are detailed in Table 1.
Compared to participants without hypertension, those with the condition had higher levels of mean CMI (0.73 ± 0.01 vs. 1.10 ± 0.01), mean age (39.31 ± 0.18vs. 52.32 ± 0.19), BMI (27.20 ± 0.07 vs. 30.47 ± 0.08 kg/m²), waist circumference (93.41 ± 0.18 vs. 103.59 ± 0.19), height (168.81 ± 0.10 vs. 169.08 ± 0.11), SBP (111.72 ± 0.11 mmHg vs. 132.07 ± 0.18 mmHg), DBP (67.21 ± 0.12 mmHg vs. 76.61 ± 0.18 mmHg), TC (4.93 ± 0.01 vs. 5.18 ± 0.01), and TG (1.46 ± 0.01 vs. 1.92 ± 0.02), while had lower level of HDL-C (1.39 ± 0.01 vs. 1.34 ± 0.00) (P < 0.05). Those with hypertension were more likely to be male, less educated, of non-Hispanic white or black race, and were often former smokers, while were less likely to be never married (P < 0.05). They also had a higher prevalence of diabetes mellitus, CVD, and CKD (P < 0.05).
Association between CMI and hypertension
In exploring the link between CMI and the incidence of hypertension, our univariate logistic regression revealed a significant association, with the details elucidated in Supplementary Table 2. Multivariate logistic regression analysis was also conducted to evaluate the association between the CMI and the risk of hypertension, as detailed in Table 2. The analysis demonstrated a positive correlation between CMI and hypertension across various models. When treated as a continuous variable, each unit increase in CMI was associated with a significant increase in the odds of developing hypertension. Specifically, the odds ratios (OR) increased by 47% in the crude model, 41% in Model I, and 30% in Model II, each adjustment demonstrating a statistically significant trend (P < 0.01). CMI was also analyzed as a categorical variable based on quartiles. The risk of hypertension escalated with higher CMI quartiles. In the fully adjusted model (Model II), participants in the highest quartile (Quartile 4) were at a greater risk compared to those in lower quartiles, with ORs of 2.71 for Quartile 4, 1.98 for Quartile 3, 1.46 for Quartile 2, and 1 (reference) for Quartile 1, indicating a significant trend across quartiles (P for trend < 0.01). The crude model adjusted for no covariates, Model I adjusted for age, sex, and race, and Model II incorporated comprehensive adjustments for age, sex, race, marital status, educational level, smoking status, TC, and history of diabetes mellitus, CVD, and CKD. Each successive model refinement highlighted the robust association between elevated CMI levels and the increased likelihood of hypertension.
Detection of dose–response relationship between CMI and prevalence of hypertension
An RCS regression analysis was performed to examine the dose-response relationship between CMI and the odds of hypertension. The analysis revealed a non-linear L-shaped curve after adjusting for age, sex, race, marital status, educational level, smoking status, TC, and history of diabetes mellitus, CVD, and CKD (Fig. 2). This analysis indicated a significant non-linearity in the relationship (P for non-linearity < 0.01). Further analysis employing a two-piecewise linear regression model identified a critical threshold in the CMI effect on hypertension odd. As shown in Table 3, a CMI value of 1.37 marks this threshold. Below this threshold (CMI < 1.37), the odds of hypertension prevalence increased by 137% for each unit increase in CMI (OR = 2.27, 95% CI: 2.14–2.40, P < 0.01). Above this threshold (CMI > 1.37), the association plateaued, and the adjusted OR was 1.01 (95% CI: 0.98–1.03, P = 0.57), suggesting no significant relationship. In addition, RCS analyses between CMI and SBP, as well as CMI and DBP, exhibited non-linear L-shaped trends that closely resembled the dose-response relationship observed between CMI and hypertension (Supplementary Fig. 2).
Association between CMI and hypertension performed by restricted cubic spline analysis. Red lines represent the odds ratio, and red transparent areas represent the 95% confidence intervals. The results are weighted based on the survey. OR odds ratio, CI confidence interval, CMI cardiometabolic index.
Subgroup analysis
We conducted detailed subgroup analyses stratified by age, sex, race, marital status, educational level, smoking status, diabetes mellitus, CVD and CKD to investigate the association between CMI levels and hypertension, alongside performing interaction tests (Fig. 3). The analyses demonstrated a consistent and positive association between higher CMI values and the prevalence of hypertension across most subgroups, with these associations being statistically significant. Notably, the effect of CMI on hypertension was significantly moderated by sex, indicating a distinct interaction (P for interaction < 0.01). In contrast, other variables did not significantly alter the relationship between CMI and hypertension (P for interaction > 0.05), underscoring the robustness of CMI as a predictor of hypertension across diverse demographic and clinical profiles.
Association between CMI and hypertension in various subgroups. Results are expressed as multivariable-adjusted odds ratio after controlling covariates that include age, sex, race, marital status, educational level, smoking status, TC, and history of diabetes mellitus, CVD, and CKD, except for the variable used in each specific subgroup analysis, which was not adjusted for in its own analysis. The results are weighted based on the survey. OR odds ratio, CI confidence interval, Ref reference, CMI cardiometabolic index, TC total cholesterol, CVD cardiovascular disease, CKD chronic kidney disease.
Sensitivity analysis
Sensitivity analysis using multiple imputed data reaffirmed the positive association between CMI and hypertension, consistent with the findings from the weighted logistic regression. This relationship was evident across both non-adjusted and adjusted models. In the fully adjusted Model II, each unit increase in CMI was linked to a 30% higher likelihood of developing hypertension. Furthermore, the odds of hypertension escalated with higher CMI quartiles, with ORs of 1.33 (95% CI: 1.24–1.42) for Q2, 1.76 (95% CI: 1.63–1.91) for Q3, and 2.44 (95% CI: 2.27–2.63) for Q4 (Supplementary Table 4). These findings underscore the robustness and reliability of our results derived from the sampling-weighted logistic regression analysis.
Discussion
To the best of our knowledge, this cross-sectional study is the first to investigate the association between CMI and hypertension within the U.S. population. Utilizing data from 45,250 participants from the NHANES, we analyzed the association between CMI levels and the prevalence of hypertension. Our results indicate that individuals with hypertension exhibited significantly higher CMI levels compared to their non-hypertensive counterparts. Additionally, an increase in CMI was associated with a higher likelihood of developing hypertension, evident in both continuous and categorical analyses of CMI. The relationship between CMI levels and hypertension was characterized by a nonlinear, L-shaped curve, with a critical inflection point identified at a CMI value of 1.37. Subgroup analyses further validate the consistent and significant association across various demographic and clinical subgroups, with sex showing a notable interaction effect, suggesting variations in CMI’s impact on hypertension between male and female.
CMI, which assesses visceral fat and metabolic dysfunction, has demonstrated its efficacy in predicting cardiovascular diseases and diabetes12,16,17. Our study confirms that higher CMI values are significantly correlated with an increased prevalence of hypertension, corroborating previous findings from the Chinese population19. Extending this validation to the U.S. population underscores CMI’s broad applicability, reinforcing its value as a predictive tool for hypertension across diverse demographic groups. This positive relationship between CMI and hypertension can be explained through various physiological mechanisms, primarily the effects of visceral adiposity and associated metabolic dysfunctions. Firstly, visceral fat, unlike subcutaneous fat, is metabolically active and secretes a range of adipokines and inflammatory cytokines such as adiponectin, leptin, interleukin-6, and tumor necrosis factor-α29,30. These compounds contribute to systemic inflammation, insulin resistance, heightened sympathetic nerve activity, and activation of the renin-angiotensin-aldosterone system (RAAS)31,32,33,34,35. Such changes compromise endothelial function and promote sodium and water reabsorption in the tubules, culminating in increased blood pressure. Moreover, the accumulation of fat around the kidneys can compress renal structures, impairing renal blood flow and enhancing sodium retention36. This disruption stimulates compensatory mechanisms that elevate blood pressure, a process that is intensified by increased sympathetic and RAAS activity, further deteriorating renal function, and escalating arterial pressure37. Additionally, visceral adiposity as quantified by CMI is closely linked to dyslipidemia, characterized by elevated triglycerides and reduced HDL cholesterol. This lipid imbalance impairs endothelial function, diminishing nitric oxide production and baroreflex sensitivity, which disrupts blood pressure regulation38,39. It also reduces the distensibility of large elastic arteries, weakening the windkessel effect40, and subsequently raising systolic blood pressure. These interconnected pathways underscore the multifaceted role of CMI as a significant predictor of hypertension, highlighting its potential for targeted interventions to mitigate hypertension risk in varied populations.
To our knowledge, the previous study among the Chinese population had not investigated the potential curvilinear relationship between CMI and hypertension19. This study is the first to examine the nonlinear association. After adjusting for confounding factors, our analysis revealed an L-shaped nonlinear curve describing the relationship between CMI and hypertension. We identified a threshold CMI value of 1.37. Below this threshold, there was a significant increase in the odds of hypertension. Above this threshold, the trend for hypertension odds leveled off, showing no significant association. Interestingly, a similar L-shaped nonlinear association has been observed between the Visceral Adiposity Index (VAI) and hypertension, further supporting our findings41. Since both CMI and VAI are measures of visceral fat, this consistency across different adiposity indices strengthens the robustness of the identified relationship between CMI and hypertension. The emergence of this association may be due to the body’s compensatory mechanisms for maintaining blood pressure homeostasis either becoming overwhelmed or reaching their maximum efficiency, highlighting a delicate balance between pathological processes and the body’s compensatory responses.
In addition to the findings above, our results revealed a significant interaction effect of sex on the association between CMI levels and hypertension, indicating that the impact of CMI on hypertension odds is notably more pronounced in females than in males. This disparity may stem from differences in biological characteristics and lifestyle behaviors between the sexes42. Although it might seem counterintuitive, considering that pre-menopausal women are generally thought to be at lower risk for cardiovascular diseases due to estrogen’s protective effects, elevated CMI presents a unique risk factor. This elevation typically indicates increased visceral fat accumulation, leading to metabolic disruptions such as insulin resistance and inflammation31,33,35,39. These metabolic disturbances can significantly diminish the protective cardiovascular effects of estrogen, unexpectedly making premenopausal women more susceptible to hypertension compared to men as CMI rises43. This risk becomes even more pronounced after menopause, when the sharp decrease in estrogen levels reduces its protective roles, such as promoting nitric oxide production, essential for vasodilation and arterial flexibility44, and increases the activity of the RAAS, resulting in greater sodium retention and higher blood pressure45. Furthermore, studies suggest that women tend to experience more severe anxiety symptoms under stress46,47, which can exacerbate the activation of the sympathetic nervous system and elevate cortisol secretion, potentially contributing to increased blood pressure48. Women are also more likely to engage in emotional eating behaviors when stressed, which has been linked to elevated blood pressure49,50. These factors may further explain our findings that the impact of CMI on hypertension odds is more significant in females than in males. While our findings provide insights into the observed sex differences, they highlight the need for further research to explore the underlying mechanisms of this interaction and to investigate the role of lifestyle behaviors and biological factors in shaping the association between CMI and hypertension.
This study is the first to investigate the association between the CMI and hypertension across a large and diverse sample of the U.S. population, utilizing data from 45,250 participants from the NHANES database. However, it is important to note some limitations in our study. The cross-sectional design limits our ability to infer causality, and there may be residual confounding from factors not measured in the dataset. While the findings are relevant for the U.S. population, caution should be exercised when generalizing these results to other populations, which may differ in environmental and health dynamics. Additionally, the reliance on self-reported diagnoses of hypertension could introduce some measurement bias. These points highlight the need for ongoing research, especially longitudinal studies, to further validate the predictive power of CMI and refine hypertension risk assessment strategies.
Conclusion
In conclusion, this study underscores the CMI as a significant predictor of hypertension risk within the U.S. population, using comprehensive data from 45,250 NHANES participants. It uniquely reveals an L-shaped nonlinear relationship between CMI and hypertension, highlighting a critical threshold effect and notable sex-specific differences. These findings pave the way for further research to validate CMI’s utility in clinical settings and suggest its potential inclusion in routine health evaluations to enhance hypertension management across diverse demographic groups.
Data availability
The data utilized in this study are derived from the NHANES: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx, and the data used are publicly available. The corresponding author can be contacted for data.
References
World Health Organization. Hypertension. https://www.who.int/news-room/fact-sheets/detail/hypertension (2023).
Olsen, M. H. et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet 388, 2665–2712. https://doi.org/10.1016/s0140-6736(16)31134-5 (2016).
Mills, K. T. et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 134, 441–450. https://doi.org/10.1161/circulationaha.115.018912 (2016).
Bundy, J. D. et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2, 775–781. https://doi.org/10.1001/jamacardio.2017.1421 (2017).
Centers for Disease Control and Prevention (CDC). Hypertension Cascade: Hypertension Prevalence, Treatment and Control Estimates Among US Adults Aged 18 Years and Older Applying the Criteria From the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html (2023).
Heidenreich, P. A. et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123, 933–944. https://doi.org/10.1161/CIR.0b013e31820a55f5 (2011).
Chandra, A. et al. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J. Am. Coll. Cardiol. 64, 997–1002. https://doi.org/10.1016/j.jacc.2014.05.057 (2014).
DeMarco, V. G., Aroor, A. R. & Sowers, J. R. The pathophysiology of hypertension in patients with obesity. Nat. Rev. Endocrinol. 10, 364–376. https://doi.org/10.1038/nrendo.2014.44 (2014).
Van Gaal, L. F., Mertens, I. L. & De Block, C. E. Mechanisms linking obesity with cardiovascular disease. Nature 444, 875–880. https://doi.org/10.1038/nature05487 (2006).
Gómez-Ambrosi, J. et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int. J. Obes. (Lond.) 36, 286–294. https://doi.org/10.1038/ijo.2011.100 (2012).
Okorodudu, D. O. et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int. J. Obes. (Lond.) 34, 791–799. https://doi.org/10.1038/ijo.2010.5 (2010).
Wakabayashi, I. & Daimon, T. The cardiometabolic index as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin. Chim. Acta 438, 274–278. https://doi.org/10.1016/j.cca.2014.08.042 (2015).
Liu, X. et al. Cardiometabolic index: a new tool for screening the metabolically obese normal weight phenotype. J. Endocrinol. Investig. 44, 1253–1261. https://doi.org/10.1007/s40618-020-01417-z (2021).
Wang, H. et al. Body adiposity index, lipid accumulation product, and cardiometabolic index reveal the contribution of adiposity phenotypes in the risk of hyperuricemia among Chinese rural population. Clin. Rheumatol. 37, 2221–2231. https://doi.org/10.1007/s10067-018-4143-x (2018).
Liu, Y. & Wang, W. Sex-specific contribution of lipid accumulation product and cardiometabolic index in the identification of nonalcoholic fatty liver disease among Chinese adults. Lipids Health Dis. 21, 8. https://doi.org/10.1186/s12944-021-01617-3 (2022).
Wakabayashi, I., Sotoda, Y., Hirooka, S. & Orita, H. Association between cardiometabolic index and atherosclerotic progression in patients with peripheral arterial disease. Clin. Chim. Acta 446, 231–236. https://doi.org/10.1016/j.cca.2015.04.020 (2015).
Wang, H., Chen, Y., Guo, X., Chang, Y. & Sun, Y. Usefulness of cardiometabolic index for the estimation of ischemic stroke risk among general population in rural China. Postgrad. Med. 129, 834–841. https://doi.org/10.1080/00325481.2017.1375714 (2017).
Wang, H. Y. et al. Value of reduced glomerular filtration rate assessment with cardiometabolic index: insights from a population-based Chinese cohort. BMC Nephrol. 19, 294. https://doi.org/10.1186/s12882-018-1098-8 (2018).
Wang, H. et al. Validity of cardiometabolic index, lipid accumulation product, and body adiposity index in predicting the risk of hypertension in Chinese population. Postgrad. Med. 130, 325–333. https://doi.org/10.1080/00325481.2018.1444901 (2018).
Misra, A., Wasir, J. S. & Vikram, N. K. Waist circumference criteria for the diagnosis of abdominal obesity are not applicable uniformly to all populations and ethnic groups. Nutrition 21, 969–976. https://doi.org/10.1016/j.nut.2005.01.007 (2005).
Xu, B. et al. Is systemic inflammation a missing link between cardiometabolic index with mortality? Evidence from a large population-based study. Cardiovasc. Diabetol. 23, 212. https://doi.org/10.1186/s12933-024-02251-w (2024).
Su, Y., Ding, N., Zhou, Y., Yang, G. & Chai, X. The association between bedtime at night and hypertension in adults. Postgrad. Med. 135, 370–378. https://doi.org/10.1080/00325481.2022.2144031 (2023).
Whelton, P. K. et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71, e13–e115. https://doi.org/10.1161/hyp.0000000000000065 (2018).
American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 44, S15–S33. https://doi.org/10.2337/dc21-S002 (2021).
Ainiwaer, A. et al. Association of red cell distribution width (RDW) and the RDW to platelet count ratio with cardiovascular disease among US adults: a cross-sectional study based on the National Health and Nutrition Examination Survey 1999–2020. BMJ Open 13, e068148. https://doi.org/10.1136/bmjopen-2022-068148 (2023).
KDIGO. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 105, S117–S314. https://doi.org/10.1016/j.kint.2023.10.018 (2024).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Harrell, F. E. Regression modeling strategies. In With Applications to Linear Models, Logistic Regression, and Survival Analysis. https://doi.org/10.1007/978-1-4757-3462-1 (Springer, 2001).
Heymsfield, S. B. & Wadden, T. A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 376, 254–266. https://doi.org/10.1056/NEJMra1514009 (2017).
Leggio, M. et al. The relationship between obesity and hypertension: an updated comprehensive overview on vicious twins. Hypertens. Res. 40, 947–963. https://doi.org/10.1038/hr.2017.75 (2017).
Chen, Y. et al. Impact of BMI and waist circumference on epigenome-wide DNA methylation and identification of epigenetic biomarkers in blood: an EWAS in multi-ethnic Asian individuals. Clin. Epigenet. 13, 195. https://doi.org/10.1186/s13148-021-01162-x (2021).
Covassin, N. et al. Experimental weight gain increases ambulatory blood pressure in healthy subjects: implications of visceral fat accumulation. Mayo Clin. Proc. 93, 618–626. https://doi.org/10.1016/j.mayocp.2017.12.012 (2018).
Dias, I. B. et al. Relationships between emerging cardiovascular risk factors, z-BMI, waist circumference and body adiposity index (BAI) on adolescents. Clin. Endocrinol. (Oxf.) 79, 667–674. https://doi.org/10.1111/cen.12195 (2013).
Engeli, S. et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension 45, 356–362. https://doi.org/10.1161/01.HYP.0000154361.47683.d3 (2005).
Fox, C. S. et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116, 39–48. https://doi.org/10.1161/circulationaha.106.675355 (2007).
Shariq, O. A. & McKenzie, T. J. Obesity-related hypertension: a review of pathophysiology, management, and the role of metabolic surgery. Gland Surg. 9, 80–93. https://doi.org/10.21037/gs.2019.12.03 (2020).
Healy, V., Thompson, C. & Johns, E. J. The adrenergic regulation of proximal tubular Na⁺/H⁺ exchanger 3 in the rat. Acta Physiol. (Oxf.) 210, 678–689. https://doi.org/10.1111/apha.12181 (2014).
Casino, P. R., Kilcoyne, C. M., Quyyumi, A. A., Hoeg, J. M. & Panza, J. A. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation 88, 2541–2547. https://doi.org/10.1161/01.cir.88.6.2541 (1993).
Piccirillo, G. et al. Influence of aging and other cardiovascular risk factors on baroreflex sensitivity. J. Am. Geriatr. Soc. 49, 1059–1065. https://doi.org/10.1046/j.1532-5415.2001.49209.x (2001).
Wilkinson, I. B. et al. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J. Am. Coll. Cardiol. 39, 1005–1011. https://doi.org/10.1016/s0735-1097(02)01723-0 (2002).
Zhou, H. et al. Association of visceral adiposity index with hypertension (NHANES 2003–2018). Front. Cardiovasc. Med. 11, 1341229. https://doi.org/10.3389/fcvm.2024.1341229 (2024).
Kadier, K. et al. Maintaining ideal cardiovascular health is associated with higher serum anti-aging protein klotho in the middle-aged and older populations. J. Nutr. Health Aging 28, 100224. https://doi.org/10.1016/j.jnha.2024.100224 (2024).
Powell-Wiley, T. M. et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 143, e984–e1010. https://doi.org/10.1161/cir.0000000000000973 (2021).
Sabbatini, A. R. & Kararigas, G. Estrogen-related mechanisms in sex differences of hypertension and target organ damage. Biol. Sex. Differ. 11, 31. https://doi.org/10.1186/s13293-020-00306-7 (2020).
Visniauskas, B. et al. Estrogen-mediated mechanisms in hypertension and other cardiovascular diseases. J. Hum. Hypertens. 37, 609–618. https://doi.org/10.1038/s41371-022-00771-0 (2023).
McLean, C. P., Asnaani, A., Litz, B. T. & Hofmann, S. G. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 45, 1027–1035. https://doi.org/10.1016/j.jpsychires.2011.03.006 (2011).
Arcand, M., Bilodeau-Houle, A., Juster, R. P. & Marin, M. F. Sex and gender role differences on stress, depression, and anxiety symptoms in response to the COVID-19 pandemic over time. Front. Psychol. 14, 1166154. https://doi.org/10.3389/fpsyg.2023.1166154 (2023).
Fontes, M. A. P. et al. Neurogenic background for emotional stress-associated hypertension. Curr. Hypertens. Rep. 25, 107–116. https://doi.org/10.1007/s11906-023-01235-7 (2023).
Wu, Y. K., Pacchioni, T. G., Gehi, A. K., Fitzgerald, K. E. & Tailor, D. V. Emotional eating and cardiovascular risk factors in the police force: the Carolina blue project. Int. J. Environ. Res. Public. Health 21, 332. https://doi.org/10.3390/ijerph21030332 (2024).
Chao, A. et al. Examining the mediating roles of binge eating and emotional eating in the relationships between stress and metabolic abnormalities. J. Behav. Med. 39, 320–332. https://doi.org/10.1007/s10865-015-9699-1 (2016).
Acknowledgements
Both the study participants and the NHANES researchers are deserving of our appreciation. Jing Zhang (Shanghai Tongren Hospital, China), thank you for your work on the NHANES database. His outstanding contributions to the “nhanesR” package and website make it easy for us to access the NHANES database.
Funding
This study was supported by Natural Science Foundation of Hunan Province (2023JJ40867), Hunan Health and Family Planning Commission Project (B202310007057), Natural Science Foundation of Changsha (kq2208330), Key Research and Development Program of Hunan Province (2019SK2022), and Key Project of Hunan Provincial Science and Technology Innovation (2020SK1014-2).
Author information
Authors and Affiliations
Contributions
T.G. chose the topic. Y.Z. and G.Y. provided methodological support. T.G. completed the subsequent data analysis and article writing. L.S. and X.C. provided guidance and assistance throughout the process. All authors revised the manuscript for important intellectual content participated in the decision to submit the manuscript for publication, and approved the final submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The ethics review board of the National Center for Health Statistics approved all NHANES protocols, and written informed consents were obtained from all participants or their proxies. All the experiment protocol for involving humans was in accordance to guidelines of national/international/institutional or Declaration of Helsinki in the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, T., Zhou, Y., Yang, G. et al. Association between cardiometabolic index and hypertension among US adults from NHANES 1999–2020. Sci Rep 15, 4007 (2025). https://doi.org/10.1038/s41598-025-87029-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87029-0
Keywords
This article is cited by
-
The association between cardiometabolic index and hypertension-diabetes comorbidity among adults aged 45 and older: evidence from two national databases
BMC Endocrine Disorders (2026)
-
Association between cardiometabolic index and self-reported symptoms of obstructive sleep apnea: a cross-sectional study
Scientific Reports (2025)
-
The association of nutritional and inflammatory status with cardiovascular and all-cause mortality risk among US patients with metabolic syndrome
Scientific Reports (2025)