Abstract
A high-calorie diet and lack of exercise are the most important risk factors contributing to metabolic dysfunction-associated steatotic liver disease (MASLD) initiation and progression. The precise molecular mechanisms of mitochondrial function alteration during MASLD development remain to be fully elucidated. In this study, a total of 60 male C57BL/6J mice were maintained on a normal or amylin liver NASH (AMLN) diet for 6 or 10 weeks. Some of the mice were then subjected to voluntary wheel running, while the other mice were fed a normal or AMLN diet until 14 and 18 weeks. The results showed that hepatic lipid deposition and the PERK-eIF2α-ATF4 pathway were significantly increased with prolonged duration of AMLN diet. However, expression of mitochondrial unfolded protein response (UPRmt) genes and mitokine FGF21 secretion were significantly enhanced in the 14-week AMLN diet mice, but were markedly reduced with the excessive lipid deposition induced by longer AMLN diet. Additionally, the exercise intervention acts as a regulator to optimize UPRmt signal transduction and to enhance mitochondrial homeostasis by improving mitochondrial function, reversing the UPRmt activation pattern, and increasing FGF21 secretion, which plays a pivotal role in delaying the occurrence and development of MASLD.
Similar content being viewed by others
Introduction
The manifestations of metabolic dysfunction-associated steatotic liver disease (MASLD) are becoming a growing challenge for public health. MASLD progression is caused by an imbalance between lipid acquisition and lipid disposal due to the overwhelmed metabolic capacity in the liver. This progression of MASLD is currently explained by the “multiple-hit” hypothesis, which proposes that multiple concurrent resultants, such as insulin resistance, oxidative stress, intracellular stress response and mitochondrial dysfunction, eventually contribute to liver injury and MASLD progression1,2. Hepatocytes have abundant ER, which is crucial in regulating calcium homeostasis and lipid metabolism in cells. Many genetic or dietary models of fatty liver in recent studies have demonstrated that free fatty acid (FFA) overload and lipotoxicity are critical factors that lead to ER homeostasis disequilibrium in the liver3. ER stress can induce multiple effects by activating its downstream pathways, including the unfolded protein response (UPR), apoptotic4 integrated stress response (ISR)5, and inflammatory pathways6, contributing to the progression from initial steatosis to non-alcoholic steatohepatitis (NASH).
Mitochondrial dysfunction is closely connected with the onset and progression of MASLD, and the use of mitochondria as a target for MASLD therapy has been gaining traction based on rodent models and human studies7. With increased lipid deposition in the liver, hepatic metabolism might shift to protect hepatocytes from the lipid burden at the initial stage. For example, studies have shown that hepatic metabolic adaptation and mitochondrial flexibility at the early stages of MASLD development, such as increased mitochondrial fatty acid oxidation (FAO), enhanced the tricarboxylic acid (TCA) cycle, and raised oxidative phosphorylation (OXPHOS)8. However, at the advanced stages of MASLD development, the imbalance between mitochondrial FAO and the electron transport chain (ETC) will cause reactive oxygen species (ROS) overproduction, which not only threatens the OXPHOS machinery but also other mitochondrial proteins, lipids, and mtDNA9. In response to increased ROS production during MASLD development, unfolded or misfolded proteins can aggregate in the mitochondrial matrix, leading to the initiation of the mitochondrial unfolded protein response (UPRmt). The UPRmt is an important mechanism for maintaining mitochondrial proteostasis during stress. Under mitochondrial stress, UPRmt process is initiated by inducing nuclear gene-encoded proteins transcription, such as chaperones heat shock protein 10 (HSP10) and heat shock protein 60 (HSP60), proteases caseinolytic protease (ClpP) and Lon peptidase 1 (LONP1) to maintain mitochondrial proteostasis. Indeed, increasing studies have documented that the UPRmt exerts a protective effect in liver metabolic related diseases10,11, while an abnormal UPRmt triggers a series of aging-related diseases. Interestingly, evidence shows that the increased expression of UPRmt related proteases and chaperone proteins is accompanied by elevated expression of mitokines such as fibroblast growth factor 21 (FGF21) and GDF1512. FGF21 is a protein that is highly synthesized in the liver and was the first cytokine reported in mammalian systems, and is also described as a mitochondrial cytokine or “mitokine”. Abundance of evidences have confirmed that circulating FGF21 content is implicated in the pathobiology of MASLD13,14,15, and abnormal FGF21 signaling is a major pathological changes in the development and progression of MASLD16. Indeed, evidences have reported that FGF21 expression can be stimulated by the ER stress response via the PKR-like ER kinase (PERK)/eukaryotic initiation factor 2α (eIF2α)/activating transcription factor 4 (ATF4) axis17. In addition, some of the previously described that UPRmt and UPRER have interactive effects under homeostasis disruption, and PERK-ATF4 axis is the core hub collaborating UPRER and UPRmt, hence regulating proteostasis by promoting ER and mitochondrial interactivities and improving their functions18.
Exercise-induced mitochondrial adaptation not only favorable for improving muscle health, but also plays a vital role in mitochondrial dysfunction related metabolic alterations associated with liver diseases. Although the main mechanisms of exercise effect on MASLD treatment include the reduction in intrahepatic fat content, attenuation of inflammation and oxidative stress, maintenance of ER homeostasis, and improvement of insulin signaling and mitochondrial function19,20, the underlying molecular mechanism are not completely elucidated. Acute and chronic forms of physical exercise was shown to modulate mitochondrial metabolism and hepatic redox state21. In particular, exercise intervention can improve the antioxidant defense system capacity, regulate the PERK-eIF2α-ATF4 pathway, inhibit hepatocyte apoptosis and ameliorate hepatic lipid deposition22. Moreover, novel exercise-inducible cytokines are hypothesized to play a vital role in lipid metabolism such as myokines, hepatokines, and adipokines. Some researches in human and animal models have demonstrated the mechanisms for ameliorating MASLD by detecting FGF21 during exercise23,24. Others also suggested that FGF21 may play a crucial role in exercise to improve MASLD25, and serum FGF21 is largely secreted from the liver during aerobic exercise26. Thus, to further elucidate the specific mechanisms underlying the advantage of exercise intervention during different stages of MASLD progression, It would be worth investigating how exercise regulates the activation levels of UPRmt and hepatocyte FGF21 secretion levels in response to the PERK-ATF4 axis at different stages of MASLD development.
In this study, it highlighted the effect of exercise on protecting against Amylin Liver NASH (AMLN) diet-induced lipid accumulation in hepatocytes during MASLD progression in mice. According to results, we first identified the different levels of UPRmt at different stages of MASLD development, and found that the regulatory effects of 8 weeks voluntary exercise on UPRmt vary according to the degree of MASLD progression. Exercise intervention exhibited a regulator role to optimize UPRmt signal transduction and to enhance mitochondrial homeostasis, which plays a pivotal role in delaying the occurrence and development of MASLD. Furthermore, the variation tendency for FGF21 secretion and the UPRmt activation during MASLD progression is alike, which may be a novel potential strategy for diagnosing the developmental stages of MASLD.
Results
Exercise protects against AMLN diet-induced body weight gain and dyslipidemia during MASLD progression
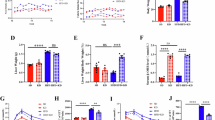
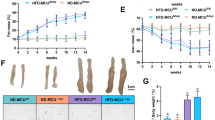
During the experiment, animals fed with an AMLN diet exhibited a greater increase in body weight than those fed with a ND diet, and the effect of different dietary on mice body weight showed a significant difference from the 10th week (Fig. 1a). Considering the effect of exercise, results revealed that mice in AMLN14 + E8 and AMLN18 + E8 groups gained less weight than AMLN diet mice (P < 0.05, Fig. 1b–d). On this basis, this study further examined the liver index, revealing that the mice in the AMLN diet group displayed an increased liver index compared to mice in the ND group, whereas the higher liver index level in the AMLN18 group was improved by the exercise intervention (P < 0.01, Fig. 1e).
Effect of AMLN diet and exercise on the mice body weight, liver index, and serum lipid levels. (a) Mice body weight in different weeks. (b) Representative time course curves of 14-week mice body weight. (c) Representative time course curves of 18-week mice body weight. (d) Mice body weight at the time of execution. (e) Liver index, liver weight (g)/body weight (100g). (f–i) Serum CHOL, TG, LDL-C and HDL-C concentrations. (j) ALT and AST levels in serum. All data are shown as means ± SEM, n = 6–8. * P < 0.05, ** P < 0.01, effect of diet; # P < 0.05, ## P < 0.01, effect of exercise. n = 6 is the number used for serum CHOL, TG, LDL-C and HDL-C analyses, and n = 8 is the number used for body weight, liver index.
Next, the serum biochemistry profile from different groups at 14 and 18 weeks were characterized. As shown in Fig. 1f and h, 14-week AMLN treatment prominently increased the serum levels of CHOL and LDL-C compared to ND mice. In addition, compared to ND18 mice, the levels of CHOL, TG, LDL-C, and HDL-C in the mice of 18-week AMLN group were markedly raised (P < 0.05, Fig. 1f–i). It exists significant differences in the serum concentrations of CHOL, TG, and LDL-C when compared between the AMLN18 and AMLN14 groups. Notably, the 18-week AMLN diet-induced dyslipidemia mice showed significant improvement after 8 weeks of exercise intervention, with significant attenuation of serum CHOL, TG, and LDL-C levels (P < 0.01, Fig. 1f–h). In addition, the AMLN diet effect on liver injury was further evaluated, and found that 18-week AMLN diet contribute to liver damage as shown by significantly increased ALT and AST levels, and significantly higher than AMLN14 group. Similarly, 8-week exercise intervention was effective in suppressing liver injury induced by chronic AMLN diet (P < 0.05, Fig. 1j).
Exercise alleviates AMLN diet-induced hepatic lipid accumulation during MASLD progression
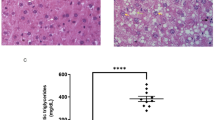
To evaluate the biochemical evidence of liver lipid deposition, the liver tissue morphology and liver histological characteristics were quantified. From the liver tissue, it was observed that the AMLN diet resulted in a fatty color change and increased liver volume (Fig. 2a). Liver sections of ND group mice exhibited normal hepatic cells each with prominent nucleus, well-arranged in plates or cords, and a branch of hepatic artery along the centrilobular vein. However, numerous lipid droplets and disordered liver structures appeared in the hepatocytes of 14- and 18-week AMLN diet mice (Fig. 2b). Moreover, histological examination was used to indicated that 14- and 18-week AMLN diet significantly increased the score of hepatic steatosis, inflammation, locular ballooning, and MASLD activity score. Furthermore, lipid accumulation between the AMLN14 and AMLN18 groups appeared a significant difference. Compare to the AMLN14 and AMLN18 groups, the lipid content was significantly declined in AMLN14 + E8 and AMLN18 + E8 groups (P < 0.05, Fig. 2d–g), suggesting that the longer the AMLN diet feeding duration, the more significant the lipid deposition, and that exercise can positively regulate AMLN dietary-induced lipid accumulation in different MASLD developmental stages.
Effect of AMLN diet and exercise on liver lipid deposition in mice. (a) Representative liver morphology. (b) H&E staining of mice liver tissue (scale bar = 20 μm). (c) Oil Red O staining of mice liver tissue (scale bar = 20 μm). (d–g) The analysis of MASLD activity score. (h) Liver lipid droplet area (%) as assessed by Image J. (i) Hepatic triglyceride content. All data are shown as means ± SEM, n = 6. * P < 0.05, ** P < 0.01, effect of diet; # P < 0.05, ## P < 0.01, effect of exercise. n = 6 is the number used for H&E, oil red O staining and TG analyses.
To further measure the intrahepatic fat content, the hepatic lipid droplets were evaluated by Oil Red O staining. The areas of lipid droplets (LDs) in the 14-week and 18-week AMLN groups were increased significantly compared to those in the ND group, and mice in the AMLN18 group developed greater intrahepatic fat content than mice in the AMLN14 group (P < 0.05, Fig. 2c, h). Consistent with the above findings, AMLN diet mice indicated that the number and area of hepatic LDs were significantly decreased after exercise intervention, suggesting that exercise prevented aberrant LD deposition during MASLD development (P < 0.05, Fig. 2h). Liver TG content measurements also showed the same trend with AMLN dietary and exercise intervention (P < 0.05, Fig. 2i).
Exercise attenuates AMLN diet-induced hepatic PERK-eIF2α-ATF4 signaling activation especially at the later stages of MASLD progression
To further illuminate the mechanism by which different degrees of lipid deposition affect ER stress, especially the PERK-eIF2α-ATF4 signaling arm. Expression of GRP78 and its downstream target genes were next examined. As shown in Fig. 3a–d, 14- and 18-week AMLN diet mice were noted to have significantly increased GRP78 expression, as detected by Immunohistochemistry and western blotting compared to normal diet mice, suggesting the activation of ER stress. Present results also revealed the beneficial effects of exercise on alleviating ER stress, as demonstrated by decreased GRP78 expression in mice from the AMLN18 + E8 group compared to AMLN18 group (P < 0.05, Fig. 3b,d). Additionally, the GRP78 downstream pathways, including PERK, eIF2α, ATF4, and CHOP expressions were observed after AMLN diet with or without exercise intervention. The western blotting results indicated that the PERK and eIF2α phosphorylation levels were highly increased by AMLN diet feeding, both at 14 and 18 weeks, where the longer the AMLN feeding period, the higher the level of pathway activation (P < 0.05, Fig. 3e,f). Furthermore, compared to ND diet mice, ATF4 and CHOP expression levels in the 14- and 18-week AMLN diet mice were also significantly increased associated with the upstream signaling PERK-eIF2α activation (P < 0.05, Fig. 3g,h). Notably, this AMLN-induced ER stress activation after 18 weeks was mitigated when mice were exercised for 8 weeks, as evidenced by decreased GRP78 and PERK-eIF2α-ATF4-CHOP pathway expression in AMLN18 + E8 group mice.
Effect of AMLN diet and exercise on PERK-eIF2α-ATF4 signaling pathway during MASLD development. (a) Representative images of hepatic GRP78 IHC staining (scale bar = 20 μm). (b) GRP78 staining quantification in > 5 fields per animal using Image J. (c) Western blot results for GRP78, PERK, p-PERK, eIF2α, p-eIF2α, ATF4, CHOP and GAPDH. (d–h) Western blotting quantification of relative protein expression using Image J. All data are shown as means ± SEM, n = 6. * P < 0.05, ** P < 0.01, effect of diet; # P < 0.05, ## P < 0.01, effect of exercise. n = 6 is the number used for GRP78 staining and western blotting analyses.
Exercise restores AMLN diet-induced mitochondrial dysfunction and UPRmt impairment at the later stages of MASLD development
To study the effect of AMLN and exercise on mitochondrial function, this study examined mitochondrial morphology by using electron microscopy. The results showed that hepatocytes from AMLN diet mice exhibited accumulation of large lipid droplets, as well as mitochondrial swelling and cristae fragmentation, particularly in the AMLN18 group. These striking features of damaged mitochondria were significantly improved and exhibited normalized mitochondrial structure after an 8-week voluntary wheel running intervention in both AMLN14 + E8 and AMLN18 + E8 groups (Fig. 4a). Next, mitochondrial function indicators were tested, including mitochondrial respiration function, membrane potential, and mitochondrial respiratory chain complexes subunits. As shown in Fig. 4b and c, compared to ND mice, 14- and 18-week AMLN diet mice exhibited a trend of decreased mitochondrial state III oxygen consumption and a significant decrease in mitochondrial RCR (P < 0.05). Moreover, after 18 week, the expression of mitochondrial respiratory chain complex subunits in AMLN diet mice tended to decline (Fig. 4d,e). Similar results were appeared to mitochondrial membrane potential (P < 0.05, Fig. 4f), showing that 18 weeks of AMLN feeding significantly increased the proportion of mitochondria with lower membrane potential. In contrast to the negative effect of AMLN diet on mitochondrial function, exercise intervention was able to protect the mitochondria of hepatocytes in AMLN diet mice to some extent, especially the effect on mitochondrial respiratory function and OXPHOS protein expression (P < 0.05, Fig. 4c,d).
Effect of AMLN diet and exercise on mitochondrial function and UPRmt marker gene expression. (a) Representative TEM images of mitochondrial morphology (scale bars = 1 μm). The red arrows represents the area of mitochondrial damage caused by the AMLN diet. (b) Mitochondrial oxygen consumption was examined at 30 ℃ by treating mitochondria with glutamate without ADP(state IV) or with ADP (state III). (c) Mitochondrial RCR: state III/state IV. (d) Western blot results for the upper five enzyme complexes of the electron transport chain (ETC) and VDAC. (e) Western blotting quantification of the five ETC complexes expression using Image J. (f) Mitochondrial membrane potential. (g) Western blot results for LONP1, mtHSP70, mtHSP60, ClpP and VDAC. (h–k) Western blotting quantification of relative protein expression using Image J. All data are shown as means ± SEM, n = 6. * P < 0.05, ** P < 0.01, effect of diet; # P < 0.05, ## P < 0.01, effect of exercise. n = 6 is the number used for mitochondrial oxygen consumption, mitochondrial RCR, mitochondrial membrane potential and western blotting analyses.
Given that exercise rescued the lipid accumulation-associated liver phenotype and improved mitochondrial function, hepatic UPRmt activation in this experimental model was further investigated. As shown in Fig. 4g–k, western blot revealed that hepatic UPRmt components expressions, including that of LONP1, HSP70, and HSP60 in the mitochondrial matrix were significantly elevated in 14-week AMLN diet mice when compared to the 14-week ND mice. Interestingly, compared to the ND18 group, mice on an AMLN diet for 18 weeks exhibited a sharp decline in UPRmt genes contents and ClpP protein expression, highlighting a disconnect between hepatic lipid deposition and UPRmt activation, which may be due to the longer AMLN diet-induced impaired mitochondrial function associated with the more serious degree of liver damage. In addition, the 8-week wheel running intervention significantly reversed the suppression of mtHSP70 (P < 0.01, Fig. 4g) and mtHSP60 (P < 0.01, Fig. 4h) expressions, and tended to increase the mitochondrial chaperone proteins and proteases in AMLN18 + E8 group mice.
Positive correlation between FGF21 secretion and the UPRmt activation level during AMLN diet and exercise intervention
Circulating FGF21 is highly correlated with mitochondrial function27. Further analysis of serum and hepatic FGF21 levels revealed that the serum FGF21 levels were markedly higher in the 14-week AMLN diet group than in the ND14 group, whereas these levels were dramatically reduced in the 18-week AMLN diet group (P < 0.05, Fig. 5a,b). Thus, an apparent difference in serum and hepatic FGF21 level was observed between the 14- and 18-week AMLN groups (Fig. 5a,b), which agreed with previous UPRmt-related data. Next, to determine whether serum or hepatic FGF21 content is correlated with UPRmt activation level during MASLD progression, this study performed linear regression analysis between the circulating FGF21 level and the value of UPRmt protein expression in all samples. As shown in Fig. 5c–f, there were positive correlations between the serum FGF21 level and UPRmt proteins contents, including LONP1, mtHSP70, mtHSP60, and ClpP, suggesting a positive correlation between FGF21 secretion and the UPRmt activation level.
The FGF21 expression was closely correlated with UPRmt genes expression level. (a) Serum FGF21 concentrations. (b) Western blot results for hepatic FGF21 expression. (c–f) Correlation tests were performed between the UPRmt genes expression level and serum FGF21. All data are shown as means ± SEM, n = 6. * P < 0.05, ** P < 0.01, effect of diet; # P < 0.05, effect of exercise. n = 6 is the number used for serum FGF21 concentrations, correlation tests and western blotting analyses.
Discussion
Overnutrition and physical inactivity are precipitating factors that cannot be ignored in the occurrence of metabolic complications such as insulin resistance, type2 diabetes mellitus (T2DM), cardiovascular disease (CVD), and MASLD. MASLD is a progressive disease, and excessive consumption of fat and/or sugar, particularly fructose, can cause hepatic steatosis and dyslipidemia. Amylin Liver NASH (AMLN) diet is the preferred diet to induce many clinically relevant characteristics of NASH. Different periods of AMLN diet will result in different levels of lipid deposition in liver tissue, with consequent changes in oxidative and ER stress levels, as well as mitochondrial function28. Interestingly, a recent study indicated that mitochondria function in liver tissue are highly plastic in diet-induced metabolic alteration29. Exercise has also been confirmed to have significant effects on improving hepatic lipid metabolism, mitochondrial function, and the oxidative stress level. Zou et al. recently showed that moderate-intensity exercise induced a greater improvement in antioxidant ability and reduced stress status via regulation of ER stress pathway-related proteins30. Therefore, this study first to compare the changes concerning lipid deposition, ER stress signaling pathway, UPRmt, and mitokine secretion after 14 and 18 weeks of AMLN diet intervention, which are two time points during the progression of MASLD. On this basis, this study further explored the role of an 8-week voluntary wheel running intervention in regulating MASLD occurrence and progression, focusing mainly on the molecular mechanism involved in the PERK-eIF2α-ATF4 aix and UPRmt. The relationship between UPRmt levels and FGF21 secretion was also investigated.
The AMLN diet is a common dietary formula that induces liver damage in a manner similar to that observed in the human liver diseases. Trevaskis et al. reported that animals had AMLN diet for 12 weeks demonstrated increased body weight and liver fat content without fibrosis, but showed progression toward fibrosis when fed for a chronic 30-week period31. Here, mice were fed an AMLN diet for 14 and 18 weeks to establish a model of hepatic steatosis and prefibrosis and found that hepatic lipid deposition occurred significantly in both time periods. Moreover, these changes were associated with abnormal blood lipid levels in the 18-week AMLN diet animals, but not in the AMLN14 group. As the circulating fatty acids uptake is the major way for liver to acquire lipids32, the absence of dyslipidemia in the 14-week AMLN diet mice may be a result of the increased fatty acid intake and FA storage as TGs in the liver, which normalize serum lipid levels. However, this regulatory mechanisms were disappeared in the 18-week AMLN diet animals. These data suggest that prolonged administration of an AMLN diet not only leads to an abnormal increase in blood lipids but also induces massive macro- and microsteatosis. Although the mechanisms by which exercise reduces liver fat are still largely unknown, the underlying mechanism involves β-oxidation33, lipogenesis, and lipid export. An improvement in insulin resistance with exercise intervention is thought to reduce the uptake of circulating FFAs to the liver34. Therefore, exercises can be helpful in orchestrating the lipid synthesis, export35, and their use as energy substrates. The results showed that liver lipid deposition in AMLN-fed animals at two stages improved significantly after an 8-week voluntary wheel running exercise intervention. However, this study failed to observe significantly decreased blood lipid levels in the early stages mediated by exercise, which may be due to the relatively lower blood lipid levels in the early stages than in the late.
To further understand the alleviated AMLN diet-induced lipid deposition displayed by exercise intervention and with a focus on mitochondria-related metabolic mechanisms, the PERK-eIF2α-ATF4 signaling pathway was first examined, which is ER stress branch pathway and has been shown to be closely related to hepatic lipogenesis and steatosis regulation36. The activation of this ER stress or UPR mainly relies on the luminal chaperone GRP78 activation. In terms of its downstream genes, a study showed that decreased hepatic lipogenesis and steatosis in HFD fed mice is accompanied by long-term dephosphorylation of PERK downstream: the elongation initiation factor eIF2α37. In this study, the PERK-eIF2α-ATF4 signaling pathway was significantly awakened in both 14- and 18-week AMLN diet mice compared to the ND diet mice, which indicated that abnormal lipid accumulation often coincides with perturbed ER proteostasis in hepatocytes. The more severe the hepatic lipid deposition, the more obvious the PERK-eIF2α-ATF4 signaling pathway. Importantly, after 8 weeks of exercise intervention, the significant elevation of GRP78 and its downstream target gene expression was apparently improved in AMLN diet mice. These results indicate that this improvement induced by exercise in the ER stress signaling pathway is a beneficial adaptive mechanism. However, although ER stress responses were markedly activated by AMLN diets fed for different duration in this experiment and attenuated after exercise intervention, the underlying mechanisms may not be consistent. One clinical research showed that there is a variable degrees of ER stress activation in the patients with MASLD38. Prolonged phosphorylation of eIF2α via activated PERK could specifically increase the downstream effectors ATF4 and CHOP, which enable the cell restore proteostasis28. Conversely, several studies have shown that ER eIF2a phosphorylated downstream elements, which lead to the severe oxidative stress by down regulating NFE2-related factor 2 (Nrf2) and depleting glutathione (GSH), eventually leading to apoptosis39,40,41. It has also been shown eIF2α phosphorylation is a key molecular event in mammalian UPRmt activation42. Under stress conditions, the phosphorylation of eIF2α triggers ATF4 activation, induces CHOP expression, and promotes UPRmt activation. Elevated transcript levels of ATF4 and CHOP in AMLN diet mice may be related to mitochondrial stress. Thus, next step was to investigate whether mitochondrial function and the UPRmt molecular pathways were altered under different duration of AMLN diet, and the influence mechanism of exercise on it.
The UPRmt intimately associated with mitochondrial quality control system and plays an important role on protein homeostasis by stabilizing mitochondrial function against several pathologies. A recent study showed that the hepatic mitochondrial is closely related to the pathogenesis of MASLD43. Results in this study demonstrated that mitochondrial membrane potential was significantly reduced in both 14- and 18-week AMLN diet mice, whereas mitochondrial respiration function, detected by the RCR, and OXPHOS genes expression were markedly decreased only in the 18-week AMLN diet group. These data suggest that mitochondria in excessive lipid deposition hepatocytes display decreased expression of respiratory chain component proteins. The reason why mitochondrial RCR was only decresed at 18 weeks but not 14 weeks probably due to 14-week induced mitochondria membrane potential decline is not yet severe enough to affect mitochondrial RCR. In terms of UPRmt, the expression of its downstream effectors (LONP1, mtHSP70, and mtHSP60) were elevated in 14 weeks AMLN diet mice in the presence of UPRmt compared to those fed an AMLN diet for 18 weeks in the absence of UPRmt. This suggests that UPRmt activation was triggered at the early stages of MASLD progression and was impaired during higher-grade hepatic lipid deposition. It is speculated that the impairment in UPRmt associated with excessive lipid deposition could be due to imbalanced redox homeostasis or the heavily activated PERK-eIF2α-ATF4 pathway, which causes mitochondria to lose their ability to repair themselves. Since the endoplasmic reticulum is an important site for lipid synthesis and prone to stress response, while mitochondria is the core site for lipid oxidation, which may alleviate further lipid deposition through compensatory reaction at the initial stage of lipid deposition44. Another possible explanation could be that dysfunctional mitochondrial protein import due to the prolonged AMLN diet induced the overall decline in mitochondrial function, which further impeded the process of transporting nuclear genome-encoded mitochondrial chaperone or protease to the mitochondrial matrix45. Taken together, these data point out the possibility that UPRmt may have dual effects: the 14-week AMLN diet associated with slight stress can initiate UPRmt and restore mitochondrial proteostasis, conversely, chronic UPRmt activation may be harmful to cell survival. Moreover, it is worth noting that, the decreased expression of some UPRmt components in 18-week AMLN diet mice, such as Hsp60, Hsp70, ClpP and LONP1 were restored with exercise intervention, as shown in AMLN18 + E8 group animals. These results highlighted the role of exercise in relieving mitochondrial stress and improving the UPRmt process, which coordinated to ensure mitochondrial function and viability. Future study is needed to probe the deep regulation mechanisms of UPRmt induction after exercise intervention.
Mitochondria have been considered to crosstalk with other distant tissues through serum cytokines non-cell autonomously46. This study next focused on FGF21, a mitokine, mediated by the UPRmt-related eIF2α-ATF4-CHOP ER stress response axis, and is also a well-known exercise-induced hepatokine that stimulates lipolysis and fatty acid oxidation and suppresses lipogenesis in the liver47,48. Myung-Shik Lee’s group demonstrated that ATF4-dependent FGF21 induction was accompanied by impaired mitochondrial function, which contribute to suppress the diet-induced hepatic steatosis in animals49. The present study found that compared to ND mice, the FGF21 levels in serum and liver tissue were significantly enhanced after 14-week AMLN diet intervention, but the content was markedly reduced in 18-week AMLN diet mice, which is consistent with the previous UPRmt alteration. This observation suggests the secretion of FGF21 is more closely related to the eIF2α-ATF4 activity regulated by mitochondrial stress. Furthermore, it found a positive correlation between UPRmt-related gene expression levels, including LONP1, HSP60, HSP70, and ClpP, and hepatic FGF21 levels as well as serum FGF21 content, suggesting that FGF21 is secreted by cells experiencing mitochondrial stress. Different from linear correlation, the relationship between mitochondrial perturbations-mediated UPRmt and hepatic lipid accumulation demonstrated a mitohormetic response, which suggests that mild mitochondrial stress initiates a diverse set of retrograde stress responses from mitonuclear interaction, whereas higher doses of stress can have harmful effects on cellular function50,51. Notably, this study also demonstrated that the decrease in circulating and hepatic FGF21 associated with excessive lipid accumulation was improved in response to exercise intervention. Based on the evidence presented above, this result may be due to the effect of exercise on regulating PERK-eIF2α-ATF4 or the UPRmt-related eIF2α/ATF4 pathway, both of which induce adaptive responses by triggering mitochondrial to nuclear communications, thereby recovering mitochondrial UPR and enabling mitochondrial self-healing. In line with the concept of mitohormesis, exercise-mediated FGF21 secretion may be largely dependent on mitohormesis effect. Under normal conditions, long-term exercise can enhance cellular adaptations to an extent by triggering mitochondrial stress and mitonuclear communication. Moreover, in a state of severe lipid deposition in liver cells, exercise intervention could induce the mitohormetic response by improving the levels of oxidative stress and mitochondrial function, enhancing mitochondrial chaperones and proteases expression, and secreting mitokines.
Conclusion
In summary, this study has demonstrated alterations in the PERK-eIF2α-ATF4 signaling pathway, mitochondrial UPR protein expression, and mitokine secretion during different developmental stages of MASLD pathogenesis. Different from the linear correlation between lipid deposition and the PERK branch of ER stress in the liver, the changes in reduced mitochondrial function, downregulated UPRmt component expression, and decreased FGF21 secretion were found at the later stages of fatty liver development, indicating that severe lipid deposition leads to impaired UPRmt through overactivating the eIF2α/ATF4 pathway, resulting in mitochondria losing their self-repair ability and miscommunication with the nucleus. Importantly, exercise intervention rescued the 18-week AMLN diet-induced hepatic phenotype and reversed mitochondrial function, UPRmt activation pattern, and FGF21 secretion (Fig. 6). These results highlight the pivotal role of exercise in regulating the UPRmt and its related mitokine secretion to delay MASLD progression. Moreover, this study not only suggests that serum FGF21 content may be an important indicator for clinical diagnosis of the occurrence and development of MASLD, but also confirms the significance of implementing exercise interventions as the primary prevention of MASLD in clinical practice.
Possible mechanism of AMLN diet and exercise on mitochondrial homeostasis during MASLD development. Under normal circumstances, the liver is ruddy in color and the mitochondria function properly. However, alterations were found in PERK-eIF2α-ATF4 signaling pathway, mitochondrial UPR gene expression, and mitokine secretion at different stages of MASLD pathogenesis development. (A) Early stage of MASLD development: a 14-week AMLN diet induced hepatic steatosis and ER stress. This mild mitochondrial stress initiated a diverse set of retrograde stress responses, including mitonuclear communication, UPRmt, and FGF21 secretion, which contribute to maintaining mitochondrial function, enabling mitochondrial self-healing, and alleviating liver damage. (B) Late stage of MASLD development: an 18-week AMLN diet aggravated the accumulation of hepatic lipids. Severe lipid deposition led to disconnect communication between mitochondria and nuclear, impaired UPRmt through overactivating the eIF2α/ATF4 pathway, then resulted in mitochondria losing their self-repair ability. Moreover, exercise intervention significantly improved the imbalanced mitochondrial homeostasis due to chronic AMLN diet, which plays a pivotal role in reversing the progression of MASLD. Red arrows: increase effect; Black arrows: decreased effect.
Materials and methods
Animals
Sixty male eight-week-old C57BL/6J mice were purchased from Jiangsu Gempharmatech Co., Ltd, Nanjing, China and were individually housed in cages in the Jiangsu Academy of Agricultural Sciences at an immobile temperature and humidity environment with freely access to food and water. Sixty C57BL/6J mice were divided equally into two groups, feed with normal diet (ND), contains 14.9% fat, 22.7% protein, and 62.4% carbohydrate (XIETONG.ORGANISM, 1,010,084, Nanjing, China) and Amylin Liver NASH (AMLN) diet, contains fat (40%), fructose (20%) , and cholesterol (2%) (Research Diets, #D09100301, USA)52. After 6 and 10 weeks of AMLN diet feeding, half of the mice were divided into voluntary wheel running and sedentary groups with AMLN diet (Fig. 7). All the methods in this experiment were approved by the Research Ethics Committee of the Nanjing Sport Institute, and all experiments were performed in accordance with the Animal Ethics and Welfare Committee of Nanjing Sport Institute (Approval No. GZRDW-2020-03). In addition, all experiments in our study were performed in accordance with ARRIVE guidelines. After 18 weeks, the mice were anesthetized with 10% pentobarbital sodium (Sigma, USA) at a dose of 0.1 mL/10 g. and sacrificed to collect retro-orbital blood, liver tissues for subsequent analysis.
Training protocol
Mice in the exercise intervention group were housed in cages equipped with a 14-cm-diameter voluntary running wheel. The number of runners’ revolutions were recorded by using a magnetic counter, and then converted into distance (kilometers) run per day according to a conversion formula. In this study, mice exercised an average of 6–11 km per day, reaching the standard of voluntary wheel running53.
Biochemical measurements in serum
After intraperitoneal injection of 10% barbiturate sodium, blood was obtained from the mouse ocular vein. Blood samples were centrifuged in 4 °C, 5000 g for 10 min after standing on ice for 30 min before. The content of serum aspartate aminotransferase (AST), and alanine aminotransferase (ALT), cholesterol (CHOL), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) were detected according to the prescriptive protocol.
Serum FGF-21 concentration was tested by mouse FGF-21 Elisa kit (Bio-techne China Co., Ltd, MF2100) following the manufacture’s instructions.
Histological staining
3 mm cubes of liver tissues were collected after fixing with paraformaldehyde. Next, embedded tissue were cut into 4-μm slices before deparaffinizing and staining with Oil Red O (SIGMA-ALDRICH, #SHBL1039) or H&E (Biosharp, BL702A). Finally, the pathological morphology of the liver were observed by microscope (Zeiss, Axio Imager A2) at a magnification of 400x. For each sample, five visual fields were randomly selected. The Oil Red O-stained area was quantified using ImageJ software. The data is represented by the Oil Red O-stained area as a proportion of the total area.
Immunohistochemical analysis
Dewaxed liver sections were applied to 3% H2O2 treatment for 10 min at room temperature and washed with phosphate buffer (PBS). Then, the tissue slices were treated with citrate buffer at 95 °C, and blocked. The samples were refrigerated overnight at 4 °C by adding GRP78 (Bioworld). Next, slices were added HRP-labeled anti-rabbit IgG (Boster Biological Technology) and incubated at 37 °C. Diaminobenzidine (DAB) was performed for 10 min at room temperature, counterstaining was performed using hematoxylin, 1% alcohol hydrochloric acid was differentiated and neutral gum was used for sealing. Finally, the pathological morphology of the liver were observed by microscope (Zeiss, Axio Imager A2) and quantified by using Image J. For each sample, five visual fields were randomly selected. The area of the GRP78 was quantified using ImageJ software. The data are presented as the ratio of the total GRP78 area to the overall fields area.
MASLD activity score evaluation
The morphological changes in the liver tissue were assessed using the MASLD Activity Score (NAS) standard54. The NAS system is a histological scoring system used to evaluate the severity of metabolic associated fatty liver disease (MASLD) based on three key histological features. Steatosis is graded based on the percentage of hepatocytes affected: > 66% (3), 33%–66% (2), 5%–33% (1), and < 5% (0). Lobular inflammation is graded based on the number of foci of inflammatory cells per 200 × field: > 4 foci (3), 2–4 foci (2), < 2 foci (1), and none (0). Hepatocellular ballooning, which represents ballooned degeneration of hepatocytes, is graded as prominent (2), few (1), or none (0). For each sample, five visual fields were randomly selected. This comprehensive scoring system allows for a detailed assessment of the histological features of MASLD, providing a standardized approach to evaluate disease severity and progression.
Determination of the hepatic TG level
Liver TG was detected with Triglyceride (TG) Content Assay kit (Boxbio, AKFA003M). TG was extracted from live tissue with normal heptane/dimethylcarbinol (1:1), evaporated. Then the liver TG concentration was determined and calculated according to the instructions.
Electron microscopy
Animals were perfused with physiological sodium and 4% paraformaldehyde for pre-fixation before transmission electron microscopy (TEM). Livers from C57BL/6 J mice were harvested, and liver samples were cut into 1 mm cubes and fixed in 2.5% glutaraldehyde. Then samples were treated with 1% osmium tetroxide, dehydrated in alcohols, embedded in araldite resin. Tissues were cut into 1 μm slices, stained with methylene blue and so on. The images were collected by JEM-1400 electron microscope.
Mitochondrial isolation
Fresh liver tissue were minced and homogenized, then isolated by differential centrifugation, as described previously55. Samples were resuspended in Buffer I (70 mM sucrose, 210 mM mannitol, 1 mM ethylene glycol tetraacetic acid (EGTA) 5 mM HEPES, 0.5% BSA, pH 7.4). After multi-step centrifugation, the pellet was resuspended in Buffer II, which include 210 mM mannitol, 70 mM sucrose, 10 mM MgCl2, 5 mM K2HPO4, 10 mM MOPS, 1 mM EGTA, pH 7.4, and the mitochondrial samples were obtained.
Mitochondrial oxygen consumption
Clarke-type electrode (Oxytherm, Hansatech, UK) was used for measuring mitochondrial oxygen consumption. Respiration buffer includes 20 mmol/L Tris–HCl, 125 mmol/L KCl, and 0.1 mmol/L EGTA, pH 7.4 and a volume of 75 ul mitochondrial sample were added to the reaction chamber, and determination of the proportion of dissolved oxygen consumption in fresh mitochondria with adding the presence of glutamate and ADP (state III respiration) or glutamate (state IV respiration). The ratio of state III to state IV is defined as the mitochondrial respiratory control rate (RCR). The oxygen consumption was valued by normalizing the oxygen consumption rate (nmole/s) to the total protein content (mg). The data were collected and analyzed by using O2 view software (Hansatech, UK).
Measurement of mitochondrial membrane potential
Liver mitochondrial membrane potential were determined by using enhanced mitochondrial membrane potential assay kit (Beyotime, C2003S). The JC-1 working solution was configured as indicated, and the fluorescence intensity was detected by flow cytometry (ACEA NovoCyteTM, USA) (excitation spectra at 490 and 525 nm and emission spectra at 530 and 590 nm). The phycoerythrin (PE) channel was set to detect JC-1 aggregates with orange-red fluorescence, and the fluoresceine isothiocyanate (FITC) channel was set to detect JC-1 monomers with green fluorescence. Data were collected and analyzed using Novo ExpressTM software (ACEA NovoCyteTM, USA).
Immunoblotting
Whole tissue proteins and mitochondrial proteins were extracted from liver tissue, then were quantified by using BCA detection kit (Epizyme, ZJ102). SDS-PAGE gels were used to separate proteins by electrophoresing under certain conditions, and transferred to a PVDF membrane (MERCK, ISEQ00010). After the membranes were washed with TBST and blocked with milk or BSA, the primary antibodies (Proteintech: ATF4, LONP1, mtHSP60, VDAC, and GAPDH; Affinity: p-PERK, eIF2α, p-eIF2α, and GRP78; Bioworld: PERK; SANTA: mtHSP70; absin: ClpP; bioss: FGF-21) were added to incubate overnight. The next day, the membrane was incubated with secondary antibodies: anti-rabbit IgG H&L (HRP) (Bioworld) and goat anti-mouse IgG H&L (HRP) (Cell Signaling), after washing with TBST. The blots were visualized by ECL system and were analyzed by using the Image Lab detection system (5200Multi, Tanon).
Statistical analyses
The statistical analysis were carried out using two-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparison test (with AMLN diet and exercise intervention as factors) and t tests with GraphPad Prism 5.01 software (San Diego, CA, USA). The significant differences were considered at P < 0.05. The results are presented as the mean ± standard error of means (SEM).
Data availability
All data generated or analyzed during this study are included in this published article.
References
Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908–922 (2018).
Takaki, A., Kawai, D. & Yamamoto, K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int. J. Mol. Sci. 14, 20704–20728 (2013).
Ajoolabady, A. et al. Endoplasmic reticulum stress in liver diseases. Hepatology. 77, 619–639 (2023).
Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 287, 664–666 (2000).
Marciniak, S. J., Chambers, J. E. & Ron, D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Discov. 21, 115–140 (2022).
Willy, J. A., Young, S. K., Stevens, J. L., Masuoka, H. C. & Wek, R. C. CHOP links endoplasmic reticulum stress to NF-κB activation in the pathogenesis of nonalcoholic steatohepatitis. Mol. Biol. Cell. 26, 2190–2204 (2015).
Mansouri, A., Gattolliat, C. H. & Asselah, T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 155, 629–647 (2018).
Verma, A. K., Sharma, A., Subramaniyam, N. & Gandhi, C. R. Augmenter of liver regeneration: Mitochondrial function and steatohepatitis. J. Hepatol. 77, 1410–1421 (2022).
Dikalov, S., Panov, A. & Dikalova, A. Critical role of mitochondrial fatty acid metabolism in normal cell function and pathological conditions. Int. J. Mol. Sci. 25, 6498 (2024).
Yi, H. S. Implications of mitochondrial unfolded protein response and mitokines: A perspective on fatty liver diseases. Endocrinol. Metab. (Seoul). 34, 39–46 (2019).
Kleinridders, A. et al. Leptin regulation of Hsp60 impacts hypothalamic insulin signaling. J. Clin. Investig. 123, 4667–4680 (2013).
Lee, H. Y., Nga, H. T., Tian, J. & Yi, H. S. Mitochondrial metabolic signatures in hepatocellular carcinoma. Cells. 10, 1901 (2021).
He, L. et al. Diagnostic value of CK-18, FGF-21, and related biomarker panel in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Biomed. Res. Int. 2017, 9729107 (2017).
Tucker, B., Li, H., Long, X., Rye, K. A. & Ong, K. L. Fibroblast growth factor 21 in non-alcoholic fatty liver disease. Metabolism. 101, 153994 (2019).
Liu, J., Xu, Y., Hu, Y. & Wang, G. The role of fibroblast growth factor 21 in the pathogenesis of non-alcoholic fatty liver disease and implications for therapy. Metabolism. 64, 380–390 (2015).
Tillman, E. J. & Rolph, T. FGF21: An emerging therapeutic target for non-alcoholic steatohepatitis and related metabolic diseases. Front. Endocrinol (Lausanne). 11, 601290 (2020).
Kim, S. H. et al. Fibroblast growth factor 21 participates in adaptation to endoplasmic reticulum stress and attenuates obesity-induced hepatic metabolic stress. Diabetologia. 58, 809–818 (2015).
Liu, X. et al. Endoplasmic reticulum stress sensor protein kinase R-like endoplasmic reticulum kinase (PERK) protects against pressure overload-induced heart failure and lung remodeling. Hypertension 64, 738–744 (2014).
Guo, R., Liong, E. C., So, K. F., Fung, M. L. & Tipoe, G. L. Beneficial mechanisms of aerobic exercise on hepatic lipid metabolism in non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 14, 139–144 (2015).
Passos, E., Ascensão, A., Martins, M. J. & Magalhães, J. Endoplasmic reticulum stress response in non-alcoholic steatohepatitis: The possible role of physical exercise. Metabolism. 64, 780–792 (2015).
Ascensão, A. et al. Modulation of hepatic redox status and mitochondrial metabolism by exercise: Therapeutic strategy for liver diseases. Mitochondrion. 13, 862–870 (2013).
Zhang, Y. et al. Exercise and metformin intervention prevents lipotoxicity-induced hepatocyte apoptosis by alleviating oxidative and ER stress and activating the AMPK/Nrf2/HO-1 signaling pathway in db/db Mice. Oxid. Med. Cell Longev. 2022, 2297268 (2022).
Gao, Y. et al. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 36, 101635 (2020).
Tian, H. et al. Fibroblast growth factors for nonalcoholic fatty liver disease: Opportunities and challenges. Int. J. Mol. Sci. 26, 4583 (2023).
Wang, Z., Sun, T., Yu, J., Li, S., Gong, L. & Zhang, Y. FGF21: A sharp weapon in the process of exercise to improve NAFLD. Front. Biosci. (Landmark Ed). 28, 351 (2023).
Li, X. H. et al. Aerobic exercise regulates FGF21 and NLRP3 inflammasome-mediated pyroptosis and inhibits atherosclerosis in mice. PLoS ONE. 17, e0273527 (2022).
Jena, J., García-Peña, L. M. & Pereira, R. O. The roles of FGF21 and GDF15 in mediating the mitochondrial integrated stress response. Front. Endocrinol. (Lausanne). 14, 1264530(2023).
Carneros, D., López-Lluch, G. & Bustos, M. Physiopathology of lifestyle interventions in non-alcoholic fatty liver disease (NAFLD). Nutrients. 12, 3472 (2020).
Chen, Z., Tian, R., She, Z., Cai, J. & Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease [published correction appears in free radic biol med. Free Radic. Biol. Med. 152, 116–141 (2020).
Zou, Y. & Qi, Z. Understanding the role of exercise in nonalcoholic fatty liver disease: ERS-linked molecular pathways. Mediators Inflamm. 2020, 6412916 (2020).
Trevaskis, J. L. et al. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G762–G772 (2012).
Clapper, J. R. et al. Diet-induced mouse model of fatty liver disease and nonalcoholic steatohepatitis reflecting clinical disease progression and methods of assessment. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G483–G495 (2013).
Ipsen, D. H., Lykkesfeldt, J. & Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 75, 3313–3327 (2018).
van der Windt, D. J. et al. the effects of physical exercise on fatty liver disease. Gene Exp. 18, 89–101 (2018).
Ferré, P. & Foufelle, F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 12(Suppl 2), 83–92 (2010).
Lebeaupin, C. et al. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 69, 927–947 (2018).
Melber, A. & Haynes, C. M. UPRmt regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res. 28, 281–295 (2018).
Puri, P. et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134, 568–576 (2008).
Ashraf, N. U. & Sheikh, T. A. Endoplasmic reticulum stress and oxidative stress in the pathogenesis of non-alcoholic fatty liver disease. Free Radic. Res. 49, 1405–1418 (2015).
Fuchs, C. D., Claudel, T., Scharnagl, H., Stojakovic, T. & Trauner, M. FXR controls CHOP expression in steatohepatitis. FEBS Lett. 591, 3360–3368 (2017).
Song, B., Scheuner, D., Ron, D., Pennathur, S. & Kaufman, R. J. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Investig. 118, 3378–3389 (2008).
Derisbourg, M. J., Hartman, M. D. & Denzel, M. S. Perspective: Modulating the integrated stress response to slow aging and ameliorate age-related pathology. Nat. Aging 1, 760–768 (2021).
Fromenty, B. & Roden, M. Mitochondrial alterations in fatty liver diseases. J. Hepatol. 78, 415–429 (2023).
Dong, J. et al. Mic19 depletion impairs endoplasmic reticulum-mitochondrial contacts and mitochondrial lipid metabolism and triggers liver disease. Nat. Commun. 15, 168 (2024).
Di Ciaula, A. Nonalcoholic fatty liver disease (NAFLD). Mitochondria as players and targets of therapies? Int. J. Mol. Sci. 20, 5375 (2021).
Mottis, A., Herzig, S. & Auwerx, J. Mitocellular communication: Shaping health and disease. Science 366, 827–832 (2019).
Flippo, K. H. & Potthoff, M. J. Metabolic messengers: FGF21. Nat. Metab. 3, 309–317 (2021).
Lewis, J. E., Ebling, F. J. P., Samms, R. J. & Tsintzas, K. Going back to the biology of FGF21: New insights. Trends Endocrinol. Metab. 30, 491–504 (2019).
Kim, K. H. et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 19, 83–92 (2013).
Cheng, Y. W., Liu, J. & Finkel, T. Mitohormesis. Cell Metab. 35, 1872–1886 (2023).
Bárcena, C., Mayoral, P. & Quirós, P. M. Mitohormesis, an antiaging paradigm. Int. Rev. Cell. Mol. Biol. 340, 35–77 (2018).
Perakakis, N. et al. The selective peroxisome proliferator-activated receptor gamma modulator CHS-131 improves liver histopathology and metabolism in a mouse model of obesity and nonalcoholic steatohepatitis. Hepatol. Commun. 4, 1302–1315 (2020).
Manzanares, G., Brito-da-Silva, G. & Gandra, P. G. Voluntary wheel running: patterns and physiological effects in mice. Braz. J. Med. Biol. Res. 52, e7830 (2018).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Hynes, J., Swiss, R. L. & Will, Y. High-throughput analysis of mitochondrial oxygen consumption. Methods Mol. Biol. 1782, 71–87 (2018).
Acknowledgements
This work was funded by Major Programs for Basic Science (Natural Science) Research in Higher Education Institutions in Jiangsu Province (2020240487), Youth Project of National Natural Science Foundation of China (32000839), Qing Lan Project of Jiangsu Province of China ([2021]11), The National Key R&D Program of China (No. 2020YFC2007002), Graduate Research and Innovation Projects of Jiangsu Province (KYCX22_2247, KYCX23_2363, KYCX23_2380 and KYCX24_2420).
Author information
Authors and Affiliations
Contributions
Y.Z., J.L., and Q.T. conceived and designed this experiment. X.Y., W.S., Y.X., M.X., Y.G., H.X., and W.F .conducted experiments and data collection. X.Y., W.S., Y.X., M.X., and L.Z. analyzed and interpreted the data. X.Y. and W.S. write manuscripts. Y.Z., J.L., and Q.T. substantively revised it. All authors read and have given critical comments and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yuan, X., Sun, W., Xu, Y. et al. Altered mitochondrial unfolded protein response and FGF21 secretion in MASLD progression and the effect of exercise intervention. Sci Rep 15, 3686 (2025). https://doi.org/10.1038/s41598-025-87190-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87190-6