Abstract
Epithelial-mesenchymal transition (EMT) is designated as one of the prime causes of chemoresistance in many cancers. In our previous study we established that cisplatin resistance in ovarian cancer (OC) is associated with EMT using sensitive OV90 cells and its resistant counterparts OV90CisR1 and OV90CisR2. In this study, we revealed through RNAseq analysis that ITGA1 can play essential part in EMT mediated cisplatin resistance in OC. We found large number of EMT related terms predominant in the top gene ontologies (GO). We also found Extracellular matrix (ECM) and actin cytoskeleton genes highly altered in the resistant cells. This was further confirmed by the protein-protein interaction (PPI) analysis where we identified that the core ECM components e.g., collagen, fibronectin, metalloproteases and integrins possessed most interactions. The pathway analysis revealed the Wnt signaling as the leading pathway. Since integrins have significant interaction with Wnt signaling, we focused our study on integrins among which, ITGA1, ITGA6, ITGA11 and ITGAV were primarily altered. We validated our results by western blotting and found that ITGA1 was highly expressed in resistant cells. Additionally, the high ABCA5 (efflux transporter) expression in resistant cells also supports the EMT proposition. The western blotting also revealed high β-catenin expression in resistant cells confirming the high Wnt signaling activity. Further, we induced xenograft tumors in nude mice. The histopathological analysis confirmed the aggressive nature of resistant tumors and showed the presence of necrotic core which could be implicated to EMT. Finally, the immunohistochemical staining confirmed the high protein expression in resistant tumor.
Similar content being viewed by others
Introduction
OC is one of the most life-threatening gynecological malignancies, ranking among the deadliest cancers worldwide1. Platinum-based chemotherapy is a cornerstone of OC treatment, often eliciting strong initial responses. However, the relapse and the subsequent development of resistance to treatment are common2,3. Despite various proposed mechanisms of resistance, the lack of a comprehensive understanding suggests that fundamental aspects of this process remain unidentified4. In our previous work, we successfully developed two cisplatin resistant cells, OV90CisR1 and OV90CisR2 from the parental OV90 OC cell lines, using two different methods pulse treatment and continuous step-wise incremental exposure5. These approaches model different clinical scenarios, intraperitoneal chemotherapy versus conventional intravenous chemotherapy. The study uncovered key molecular changes in the cells during resistance development, including alterations in DNA repair pathways, antiapoptotic mechanisms, and metastatic potential. Notably, phenotypic changes characteristic of EMT were observed, marked by the downregulation of E-cadherin, a known EMT marker.
EMT is characterized by vast biochemical changes, including changes in the expression of membrane and cytoskeleton proteins, as well as elevated production of ECM components6. EMT enables epithelial cells to lose their polarity and acquire mesenchymal properties, characterized by loss of membrane adhesion proteins (e.g., E-cadherin) and gain of mesenchymal proteins (e.g., N-cadherin, vimentin). These changes lead to poor cell adhesion and enhanced migratory capacity promoting invasiveness. Besides tumor progression, EMT also causes drug resistance7. EMT is believed to cause chemoresistance through various mechanisms e.g., inducing stemness, which can reduce drug influx or increase efflux, and activating anti-apoptotic or suppressing apoptotic pathways that help cells evade programmed cell death8, or even modulation of immune response9.
To unveil the molecular mechanisms leading to the EMT-associated cisplatin resistance we compared two cisplatin resistant cell types, OV90CisR1 and OV90CisR2 with the parental OV90 cells through RNA sequencing analysis. The cells were generated from parental ovarian cancer cell line, OV90 by two different methods, pulse treatment methods, and continuous step-wise incremental exposure, respectively10. Each of these represent two different clinical scenarios, intraperitoneal chemotherapy versus conventional intravenous chemotherapy. RNA sequencing analysis facilitates the comparison of target cell gene expression with the control cells and the identification of significantly up or downregulated genes. This enables us to determine molecular changes in cancer cells, identify altered pathways and reveal critical protein interactions providing a key understanding of the cellular transformation. Using these biostatistical tools we obtained a collection of visual and critical results ranging from heatmaps and pathways to the protein networks to get a deeper knowledge of the underlying biochemical changes further validated by the in vitro and in vivo analyses.
Results
Cell viability
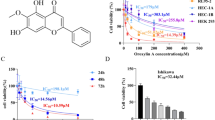
To evaluate the cell response to cisplatin treatment we conducted cell viability test using MTT assay. Cells were treated with cisplatin at concentrations ranging from 0 to 200 µM. OV90CisR1 and OV90CisR2 cells exhibited much higher cell viability than the OV90 cells at equal cisplatin concentrations. The IC50 values of OV90CisR1 and OV90CisR2 were 36.67µM and 38.3µM respectively, which is more than 2-fold higher than that of OV90 cells (17.28 µM) thus depicting the drug resistant nature of these cells. Figure 1 provides the cell viability curves of the three cell types reflecting their IC50 values.
Identifying differentially expressed genes (DEGs)
Principal component analysis (PCA) was performed to visualize the transcriptomic distance among samples. The PCA, which account for 35% of variance along the x-axis (PC1) and 21.3% along the y-axis (PC2), demonstrated a clear distinction between all 3 cell types with OV90CisR1 and OV90CisR2 not only separated from OV90, but also distinctly differentiated from each other (Fig. 2a).
Differential gene expression of OV90CisR1 and OV90CisR2 cells compared to the sensitive parental OV90 cells; (a) PCA analysis plot showing OV90CisR1 and OV90CisR2 cells well separated from OV90 cells, (b) volcano plot of the DEGs for OV90CisR1 and OV90CisR2 cells and (c) list of top 25 DEGs for OV90CisR1 and OV90CisR2 cells.
Differential gene expression (DEG) analysis was then performed. DEGs were defined as fold change (FC) > 2 compared to control and a BH-adjusted P-value < 0.05. A total of 1094 significant DEGs were identified in OV90CisR1 and 447 in OV90CisR2, relative to the parental OV90 cells. Among these DEGs, there were 608 upregulated and 486 downregulated genes in OV90CisR1, and 316 upregulated and 131 downregulated genes in OV90CisR2 cells (Fig. 2b).
A bar plot of the top 25 ranked upregulated and downregulated genes was obtained among which 11 upregulated and 2 downregulated genes were shared between OV90CisR1 and OV90CisR2 cells (Fig. 2c).
RNAseq data reveals mesenchymal phenotype is predominant in resistant cells
We performed GO analysis to identify the relevant terms associated with the DEGs. The dot plots show GO terms associated with biological process (BP), molecular function (MF) and cellular components (CC) (Fig. 3). A key observation in these dot plots is the presence of organ development and ECM-related GO terms. Among the top 25 GO terms of major importance that appear in the list of biological processes are “embryonic organ development”, “muscle tissue development”, “mesenchyme development”, “renal system development”, “kidney development”. Others are “heart development”, “respiratory tube development”, “lung development” and etc. This is interesting as it shows substantial changes in the resistant cells resembling embryonic development which is comparable to EMT in cancer cells. A detailed network showing the major GO terms and the linked genes is provided in the supplementary information (Fig. S1).
We further carried out gene enrichment analysis on some specific terms like cancer cell proliferation. The GO-gene network plot of significantly enriched GO terms related to cell growth is shown in Fig. 4. We found significant enrichment of the proliferation gene sets in OV90CisR1 and OV90CisR2 compared to OV90. Although there were differences in the enrichment between the two resistant cell types, the overall proliferation in the resistant cells was significantly higher than the parental OV90 cells. A heatmap depicting the precise differences in the proliferation of the resistant and the parental cells is provided in Fig. S2. Gene expression in OV90CisR1 includes terms related to the proliferation of mesenchymal cells and immune cells while in OV90CisR2, terms related to proliferation of epithelial cells and stem cells are prominent.
These results together with our previous findings present strong evidence of the involvement of EMT in the resistance.
ECM and actin reorganization is high in resistant cells
The heatmap pertaining to significant DEGs depicted clear differences in the gene expression of the sensitive and the resistant cells in most regions. However, the gene expression of the two resistant cell lines has a significant distinction with each other as well (Fig. 5). The common region in the heatmap where both types of resistant cells have similar DEGs is cluster 1. Some of the significant top GOs include “actin filament organization”, “regulation of actin filament-based process”, “regulation of supramolecular fiber organization”, and “regulation of actin filament organization”, etc. The ranking of these terms at the top shows that there is significant actin reorganization which may alter the cytoskeletal properties of the cells and may affect the actin dependent cellular functions e.g., cell adhesion, motility etc.
However, the other clusters show that ECM related terms are highly prevalent in the top GO terms. These terms include “extracellular structure organization”, “extracellular matrix organization”, “external encapsulating structure organization”, and “collagen metabolic process”. The other top important GO terms include “actin filament organization”, regulation of actin filament-based process, “regulation of actin filament organization”, and “regulation of actin cytoskeleton organization”.
Additionally, the molecular functions related GOs include terms that are related to transmembrane proteins which may in turn indicate a relation with the regulation of ECM. Among the top 25 upregulated GO terms are “channel activity”, “active transmembrane transporter activity”, “ATPase-coupled transmembrane transporter activity”, “ABC type transporter activity”, etc. Others include “extracellular matrix structural constituent”, and “extracellular matrix structural constituent conferring compression resistance”. Among the downregulated GO terms only “actin binding” and “actin filament binding” appeared in the list (Fig. 3b).
Also, the upregulated GO terms related to the cellular components include “collagen-containing extracellular matrix”, “endoplasmic reticulum lumen”, “synaptic membrane”, “transporter complex”, “transmembrane transporter complex”, etc. The downregulated GO terms include “sarcolemma”, “T-tubule”, “actin cytoskeleton, “membrane raft”, “membrane microdomain”, etc. (Fig. 3c).
According to these observations, the ECM and actin cytoskeleton seem to be largely altered in OV90CisR1 and OV90CisR2 cells. Major cellular and extracellular components like actin and collagen respectively, may be affected.
Pathway analysis
Pathway analysis was performed using Pathview version 1.40.0. which showed the high activity of Wnt signaling pathway (Fig. 6). The top GO terms include “canonical Wnt signaling pathway”, “Wnt signaling pathway, planar cell polarity pathway”, “Wnt signaling pathway”, “regulation of Wnt signaling pathway”, “canonical Wnt signaling pathway” etc. This suggests that Wnt signaling pathway has some role in resistance. Other important pathway designated in the results is the notch signaling pathway including terms like “notch signaling pathway” and “regulation of notch signaling pathway”, however, the Wnt signaling pathway is more prominent. This is quite relevant because Wnt signaling has major effects on the actin cytoskeleton reorganization and can affect cell adhesion and motility10.
Protein-protein interaction analysis
We used the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database to analyze and visualize protein-protein interactions (PPI). We obtained a PPI network to identify important proteins or protein interactions related to cancer (Fig. 7). Some of the important proteins showing strong interactions include those associated with integrin superfamily (ITGA), collagen superfamily, metalloproteases superfamily (ADAM’s and MMPs) and mesenchymal markers (e.g., CD34, CD24). We observed proteins like ITGA1, ITGA6, ITGA11 and ITGAV having strong interactions with collagen proteins including COL3A1, COL4A1, COL7A1, COL11A1. The interaction of fibronectin protein (FN1) was also observed to be high. Among the proteins of ADAMs superfamily are ADAMTS2, ADAMTS7, ADAMTSL1, and ADAMTSL4. Among these, integrins have most significant role in Wnt signaling, especially ITGA1. Since β-catenin is also the most important protein in Wnt signaling, we evaluated both, ITGA1 and β-catenin expression by western blotting.
Western blotting
We performed western blotting assay on ITGA1, and β-catenin for validation of the RNAseq results (Fig. 8). Both of these proteins were consistently overexpressed in the resistant cell lines, OV90CisR1 and OV90CisR2, compared to the parental OV90. This further suggests a possible interplay between the integrins, activation of Wnt signaling and the development of resistance. A detailed understanding of this relationship is a matter of further investigation. Given that the mesenchymal phenotype is associated with dramatic changes in membrane permeability, which greatly contribute to chemoresistance, we also evaluated the efflux transporter, ABCA5. This gene showed high differential expression in the original data in the resistant cell lines which also coincided with our in vitro assay. For more information the original immunoblots are included in a supplementary file (Fig. S3a to S3d).
Validation of proteins predicted by RNA seq. data through western blotting; (a) western blot images showing expression levels of ITGA1, β-catenin and ABCA5 in OV90 (Lane I), OV90CisR1 (Lane II) and OV90CisR2 (Lane III) cells, (b) Quantitative assessment of the protein expression obtained from western blotting using ImageJ software. The original blots are presented in Supplementary Fig. S3a to S3d.
Tumor proliferation
Figure 9 depicts the proliferation of tumor in mice over the period of 4 weeks. Figure 9a and b show the excised tumor tissues from the mice at the end of the experiment. The tumor proliferation in mice is depicted in terms of increase in the tumor volume (mm3) against time in days (Fig. 9c). Tumor was palpable after the first week of the cell implantation in both cases. The tumor growth varied between the mice in each group, however, the mean tumor growth in OV90CisR2 group was much higher than OV90, although the differences were not statistically significant. Consistent with the tumor size results, the weight of the excised tumors was also observed to be greater in OV90CisR2 compared to OV90 group but not statistically significant (Fig. 9d).
Histopathology
The immunohistochemical analysis showed marked upregulation of ITGA1, β-catenin and ABCA5 in all OV90CisR2 tumor samples. This further confirms the high activity of integrins in the resistant tumor which may be coupled with high activity of Wnt signaling pathway given the β-catenin activity is also high (Fig. 10). The high β-catenin activity is also evident form the positive nuclear staining in resistant OV90CisR2 tumors (indicated by the arrows) in contrast to the OV90 tumors where there was negative staining. It is well known that upon Wnt signaling, β-catenin translocates to nucleus to regulate the gene expression11. In aggressive tumors, β-catenin localization to nucleus is large, yielding a distinguished nuclear staining during IHC assay12,13,14. In fact, nuclear staining of β-catenin (by IHC) is a characteristic feature of aggressive tumors which is well known and reported extensively in literature13,15,16. These observations strongly correlate with our RNAseq results.
Immunohistochemical staining in OV90 and OV90CisR2 tumors showing high expression of (a) ITGA1, (b) β-catenin and (c) ABCA5 in OV90CisR2 tumors at different magnifications (x2, x10, x40). The panels I and II represent OV90 and OV90CisR2 respectively. The OV90CisR2 tumor sections staining for ITGA1 and ABCA5 can be seen as more intense compared to OV90. Black arrows demonstrate positive β-catenin nuclear staining of OV90CisR2 cells.
We also performed H&E staining on one tumor sample per mouse in both OV90 and OV90CisR2 groups. The representative images are shown in Fig. 11a and b. The necrotic areas were observed in all H&E-stained slides of OV90CisR2 tumors while in OV90 samples these were completely absent except that a very small necrotic area was present in one sample (Fig. S4). The quantification was performed by measuring the %age of necrotic area in each sample in comparison to the non-necrotic area using ImageJ software. The final results are presented as bar plots of “% necrotic area” (± SD) against each group (i.e., OV90 and OV90CisR2). The graph clearly shows a significantly higher necrosis in OV90CisR2 group potentially linked to the induction of EMT creating a microenvironment that promotes metastasis. This is consistent with our previous results which revealed the metastatic nature of the resistant OV90CisR2 cells.
Necrotic regions in xenograft tumors. Representative images of H&E-stained samples from (a) OV90 and (b) OV90CisR2 tumors. The presence of necrotic regions in OV90CisR2 tumor depicts its aggressive nature which can lead to metastasis; (c) Graph providing the quantitative assessment of the necrotic areas in OV90 and OV90CisR2 tumor samples (p < 0.05).
Discussion
EMT is a well-known mechanism contributing to drug resistance in cancer. In our previous study, we identified the involvement of EMT in the development of drug resistance in OC cells. To further explore the underlying mechanisms driving EMT and identify the key genes involved, we performed RNA sequencing analysis on the same OC cells in this study. Our analysis revealed the prominence of terms related to mesenchymal development, organ development, ECM organization and actin cytoskeleton regulation. We also observed increased activity in epithelial, mesenchymal, and stem cell proliferation. As we narrowed down our analysis, we gained deeper insights into the molecular events and identified significant activity in the Wnt signaling pathway. Using STRING data, we mapped protein-protein interactions, uncovering several noteworthy findings, which are discussed in detail in the subsequent paragraphs.
Actin cytoskeleton and ECM play fundamental roles in maintaining cellular integrity by providing both internal and external support17,18. Besides serving as a structural framework, ECM and actin cytoskeleton regulate crucial cellular processes such as adhesion, migration, and proliferation19,20. These structures are constantly remodeled to support various physiological processes, including embryonic development, morphogenesis, cell differentiation, as well as pathological conditions like tissue regeneration and cancer. In such contexts, the ECM and actin cytoskeleton undergo extensive reorganization, often accompanied by changes in ECM composition to facilitate tissue remodeling. In cancer, this reorganization is closely associated with EMT, a process marked by significant ECM remodeling, primarily involving collagen deposition, which increases tumor stiffness. Collagen stiffness, a marker for poor survival in some cancers e.g., breast and OC, has been implicated as an initiator of EMT21,22. There is a strong correlation between collagen expression and various carcinomas, including lung, liver, bladder, and OC23. Exposure of cancer cells to collagen in vitro can induce EMT, altering their morphology and attachment properties through complex interplay of fibers and proteins24. During EMT, cells undergo dramatic biochemical changes, losing cell polarity and adopting a mesenchymal phenotype characterized by enhanced stemness and migration ability. This is relevant to our findings, as mesenchymal and organ development terms prominently appeared in our GO analysis. This phenotypic transformation can also change membrane transport properties, leading to increased drug efflux - one of the key mechanisms by which EMT induces drug resistance. Our data supports this proposition, as GO analysis revealed elevated expression of efflux transporters (ABCA5, ABCD2). To further investigate the molecular basis of these observations, we performed pathway and PPI analyses.
Pathway analysis revealed increased activity in the Wnt signaling, which is closely associated with the induction of EMT. Wnt signaling can promote EMT through several mechanisms: (i) by directly targeting genes (SNAIL, SLUG, and TWIST), which are linked with EMT, via the translocation of β-catenin into the nucleus, (ii) by enhancing the stem cell characteristics of the cancer cells25; (iii) by activating other signaling pathways such as Notch or TGF-β signaling. To gain further insight into the downstream molecular interactions, we conducted a PPI analysis.
While focusing on the largest connected component of the network, we observed interactions among key protein super-families, including collagen (COL3A1, COL4A1, COL7A1, COL11A1), fibronectin (FN1), and metalloproteases (ADAMTS2, ADAMTS7, ADAMTSL1, and ADAMTSL4), all of which support the hypothesis of ECM reorganization. Collagen, fibronectin and metalloproteases are major ECM components that were highly altered in the OV90CisR1 or OV90CisR2 cells, demonstrating substantial ECM remodeling. Beyond collagen and fibronectin, the ADAMs proteases significantly influence the tumor microenvironment and cancer development26,27. ADAMTS2, in particular, is essential for cleaving procollagen to collagen, enabling the formation of functional collagen helices. Dysregulation of the myosin proteins (MYL12A, MYL12B, and MYLK), which regulate the actin cytoskeleton, supports the notion of actin reorganization in these cells. Most importantly, we observed the strong interactions with integrins (ITGA1, ITGA6, ITGA11 and ITGAV), which is significant as integrins serve as the primary connection points between the ECM and actin cytoskeleton28,29,30. Integrins not only physically link the ECM to the actin cytoskeleton but also play a crucial role in cell signaling, transmitting molecular cues in and out of the cell31,32. The altered expression of integrins in cancer is well documented, with both upregulation and downregulation observed across different types of cancer, although most integrins are generally highly expressed33,34,35. Through interactions with major ECM components like collagen and fibronectin, integrins can mediate mechanotransduction36,37, a process by which external physical stimuli initiate biochemical changes within the cell38,39,40,41. In cancer cells, this process can activate Wnt signaling through FAK signaling, which ultimately promotes the nuclear translocation of β-catenin and modulating RNA polymerase activity42. Integrins have been shown to activate Wnt signaling even in the absence of Wnt ligands, leading to increased expression of key components such as β-catenin43. β-catenin activation then leads to the induction of EMT44. This is consistent with our previous findings, where we demonstrated downregulation of E-cadherin in OV90CisR1 and OV90CisR2 cells, concluding that it served as a marker of EMT. Loss of E-cadherin is a key step in EMT initiation, as E-cadherin normally forms complexes with β-catenin to mediate cell to cell adhesion45,46. The loss of E-cadherin at the cell junctions increases β-catenin availability, leading to enhanced nuclear translocation and further driving EMT.
The altered expression of membrane transporters, particularly ABC transporters such as ABCA5, and ABCD2 can be linked to EMT, contributing to increased drug efflux and ultimately leading to drug resistance.
We validated these findings through both in vitro and in vivo experiments, which were consistent with the RNA sequencing data. Western blotting assays confirmed elevated expression of ITGA1, β-catenin and ABCA5 in the resistant cells (Fig. 7). Furthermore, immunohistochemical staining of resistant tumors samples (OV90CisR2) also revealed high ITGA1 expression.
Materials and methods
Cell lines
Cisplatin sensitive OC cell lines, OV90 and its chemo-resistant counterparts, OV90CisR1 and OV90CisR2 were used in the study. OV90 cells were purchased from Korea BioTech Co., Ltd. (Seoul, Korea). The cells were grown with or without cisplatin in MCDB 105 media containing 15% fetal bovine serum (FBS) and 1% penicillin–streptomycin obtained from Sigma-Aldrich, St. Louis, USA. Anti-ITGA1 and anti-β-catenin antibodies were purchased from Cell Signaling Technology while anti-ABCA5 and anti-β-actin were purchased from Novus Biologicals and Santa Cruz Biotechnology respectively. For immunohistochemical staining we used Aminoethyl carbazole (AEC) Chromogen/Substrate Bulk Kit from ScyTek and for H&E staining we used Meyer’s hematoxylin from Muto Pure Chemicals and eosin from Bioworld.
Resistant cell lines generation
OV90CisR1 and OV90CisR2 were generated from OV90 cells, by two different methods described previously, pulse treatment and continuous step-wise incremental exposure methods respectively. The detailed description of the resistant cell line generation can be found in our previous publication5. Briefly, OV90CisR1 cells were obtained by the pulse treatment method while OV90CisR2 cells were obtained by step-wise incremental method. OV90CisR1 cells were generated by pulse treatment wherein the cells were treated with a constant 100 µM dose for a relatively short period multiple times (e.g., 2 h). OV90CisR2 cells were generated by continuously exposing cisplatin with small increments starting from 10 to 100 µM. The cells were exposed to each dose several times (e.g., 10 times) before moving to the next dose.
RNA quantitation and data analysis
Ribonucleic acid (RNA) from the cells was isolated by Trizol reagent and subsequently quantified using ND-2000 Spectrophotometer (Thermo Inc., DE, USA). Prior to quantification, the quality assessment of the RNA was performed using an Agilent 2100 bioanalyzer. To prepare the RNA sequencing library we used QuantSeq 3’ mRNASeq Library Prep Kit from Lexogen, Inc. The resulting double-stranded library underwent purification with magnetic beads followed by amplification and purification again. For high-throughput sequencing, we employed the NextSeq 550 platform from Illumina, Inc. conducting single-end 75 sequencing.
Raw sequence reads produced by the sequencer were cleaned using FastQC version 0.12.0. The cleaned reads were aligned on the human reference genome (GRCh38.p13) and quantified using R subread version 2.16.047.
Identification and analysis of differentially expressed genes (DEGs)
DEGs were identified using DESeq2 version 1.40.2 with the Benjamini-Hochberg (BH)-adjusted P-value < 0.05 and |Log2 fold change (FC)| > 2 threshold for significance48. GO terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment was accessed using clusterProfiler version 4.9.049. The GO terms include three categories i.e., biological process (BP), cellular components (CC), and molecular functions (MF) for both up- and down-regulated genes. The GO terms and KEGG pathways were regarded as significant when the BH-adjusted P-value was less than 0.05. The KEGG pathway map colored by Log2 FC of DEGs was generated using Pathview version 1.40.050. Enrichment scores of each sample on gene sets and cell markers were calculated using GSVA version 1.48.351. Protein-protein interaction (PPI) analysis was performed using STRINGdb version 2.12.152 and igraph version 1.6.053. Volcano, bar, box, dot, and network plots and heatmaps were generated using ggplot2 (version 3.4.4) and Complex-Heatmap (version 2.16.0). All statistical analysis and visualizations were performed under R (version 4.3.2) and RStudio environment.
MTT assay
MTT assay was performed as per the protocol mentioned elsewhere54. Briefly, 5 × 103 cells in 100 µl MCDB 105 media were seeded in each well of 96 well plate and incubated at 37 °C and 5% CO2 until at least 80% confluency was achieved. The cells were treated with various cisplatin concentrations (200, 100, 50 ,25, 12.5, 6.25, 3.125 and 0 µM) and incubated for 24 h. After proper washing, the cells were subsequently treated with 100 µl of 1:1 MTT reagent (5%)/media and incubated for 4 h. The resulting formazan crystals were appropriately dissolved in 100 µl of DMSO and analyzed for absorbance at 570 nm in a plate reader (Multiskan SkyHigh, Thermo Fisher Scientific).
Western blotting
The cell lysates from OV90, OV90CisR1 and OV90CisR2 were run on an SDS-PAGE gel (6% for ITGA1 and ABCA5 and 8% for β-actin and β-catenin) and transferred to a PVDF membrane by wet and semi-dry methods for 6% and 8% gels respectively. This was followed by blocking with 5% skimmed milk, and overnight incubation with primary antibodies against ITGA1, beta-catenin, ABCA5, and beta-actin (loading control) at 4 °C. After incubation with HRP-conjugated secondary antibodies, proteins were detected by imaging on autoradiography films using enhanced chemiluminescence (ECL) substrate.
In vivo xenograft study
Xenograft tumors were grown in nude mice to determine the protein expression in sensitive and resistant tumors. The experiments were approved by the Animal Experiment Ethics Committee of Jeonbuk National University Hospital (JBUH-IACUC-2023-7). All the experiments were carried out in accordance with the relevant guidelines and regulations. We selected OV90CisR2 cells for in vivo studies, given the cells were generated by intermittent treatment method which produces more robust resistant cells55. 6 mice were divided in 2 groups of 3 mice each, representing sensitive (OV90) and resistant (OV90CisR2) tumors. Mice were anesthetized using 0.1 ml ketamine intraperitoneal injections. OV90 or OV90CisR2 tumor cells were injected subcutaneously into the right and the left flanks of the nude mice. Each flank received 3.3 × 106 cells. After injecting the cancer cells into mice, the tumor growth was monitored and as soon as the tumor was palpable, the tumor volume was recorded after every 3 days using a vernier caliper. The mice were sacrificed after 4 weeks and the tumor was excised and preserved in formalin.
Histopathological staining
The tumor tissues were subjected to dehydration using graded alcohols and finally xylene, embedded in paraffin blocks and cut into 3 μm sections using a microtome machine. The sections were mounted onto charged slides using a water bath maintained at 40–45 °C.
Prior to staining, the tissue sections were deparaffinized and rehydrated using xylene and graded alcohols followed by 3D water. The sections were either subsequently subjected to Hematoxylin and eosin stain (H&E) or immunohistochemical staining. For H&E, the tissue sections were transiently treated with hematoxylin (7 dips) and immediately rinsed with tap water. This was followed by eosin staining likewise and again rinsing with tap water. Finally, the slides were scanned and the images were saved.
During immunohistochemical staining, the tissue sections, after rehydration were subjected to antigen retrieval using EDTA buffer, pH 9 or citrate buffer, pH 6 as per the manufacturers’ protocol for each antibody. After blocking, the tissue sections were incubated with primary antibodies followed by Horseradish Peroxidase (HRP)-conjugated secondary antibodies. Color intensity was visualized using AEC chromogen. Finally, the tissue sections were counterstained with hematoxylin, fixed with an aqueous mounting solution and the images were saved using a scanner.
Data availability
The datasets generated during the current study are available in the Gene Expression Omnibus (GEO) repository, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE267752. The accession number is GSE267752.
References
Momenimovahed, Z., Tiznobaik, A., Taheri, S. & Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Womens Health. 11, 287–299 (2019).
Xie, W. et al. Ovarian cancer: Epigenetics, drug resistance, and progression. Cancer Cell. Int. 21, 1–16 (2021).
Pokhriyal, R., Hariprasad, R., Kumar, L. & Hariprasad, G. Chemotherapy resistance in advanced ovarian cancer patients. Biomark. Cancer. 11, 1179299X19860815 (2019).
Norouzi-Barough, L. et al. Molecular mechanisms of drug resistance in ovarian cancer. J. Cell. Physiol. 233, 4546–4562 (2018).
Bahar, E., Kim, J. Y., Kim, H. S. & Yoon, H. Establishment of acquired cisplatin resistance in ovarian cancer cell lines characterized by enriched metastatic properties with increased twist expression. Int. J. Mol. Sci. 21, 7613 (2020).
Kalluri, R. & Weinberg, R. A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009).
Ashrafizadeh, M. et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed. Pharmacother. 141, 111824 (2021).
De Rivas, L. Cancer drug resistance induced by EMT: Novel therapeutic strategies. Arch. Toxicol. 95, 2279–2297 (2021).
Terry, S. et al. New insights into the role of EMT in tumor immune escape. Mol. Oncol. 11, 824–846 (2017).
Tejeda-Muñoz, N. et al. Canonical wnt signaling induces focal adhesion and integrin beta-1 endocytosis. Iscience 25, 104123 (2022).
Herencia, C. et al. Nuclear translocation of β-catenin during mesenchymal stem cells differentiation into hepatocytes is associated with a tumoral phenotype. PLoS ONE. 7, e34656 (2012).
Compton, M. L. et al. SALL-4 and beta-catenin expression in sinonasal teratocarcinosarcoma. Head Neck Pathol. 16, 229–235 (2022).
Ng, T. L. et al. Nuclear beta-catenin in mesenchymal tumors. Mod. Pathol. 18, 68–74 (2005).
Donmez, H. G., Demirezen, S. & Beksac, M. S. The relationship between beta-catenin and apoptosis: A cytological and immunocytochemical examination. Tissue Cell. 48, 160–167 (2016).
Rajabi, P., Heydarpoor, M., Maghsoudi, A., Mohaghegh, F. & Mobarakeh, M. D. The study for diagnostic value of β-catenin immunohistochemistry marker in distinction of aggressive and non-aggressive basal cell carcinoma. Iran. J. Pathol. 14, 52 (2018).
Yamada, Y. et al. A comparison of the usefulness of nuclear beta-catenin in the diagnosis of desmoid‐type fibromatosis among commonly used anti‐beta‐catenin antibodies. Pathol. Int. 71, 392–399 (2021).
Olson, M. F. & Sahai, E. The actin cytoskeleton in cancer cell motility. Clin. Exp. Metastasis. 26, 273–287 (2009).
Brayford, S., Schevzov, G., Vos, J. & Gunning, P. The role of the actin cytoskeleton in cancer and its potential use as a therapeutic target in The Cytoskeleton in Health and Disease (ed. Schatten, H.) Cytoskeleton Health Disease, 373–391 (Springer, 2015).
Juliano, R. L. & Haskill, S. Signal transduction from the extracellular matrix. J. Cell. Biol. 120, 577–585 (1993).
Sever, R. & Brugge, J. S. Signal transduction in cancer. Cold Spring Harb Perspect. Med. 5, a006098 (2015).
Mieulet, V. et al. Stiffness increases with myofibroblast content and collagen density in mesenchymal high grade serous ovarian cancer. Sci. Rep. 11, 4219 (2021).
Deng, Y., Chakraborty, P., Jolly, M. K. & Levine, H. A theoretical approach to coupling the epithelial-mesenchymal transition (EMT) to extracellular matrix (ECM) stiffness via LOXL2. Cancers (Basel). 13, 1609 (2021).
Tzanakakis, G. et al. Role of the extracellular matrix in cancer-associated epithelial to mesenchymal transition phenomenon. Dev. Dyn. 247, 368–381 (2018).
Kirkland, S. Type I collagen inhibits differentiation and promotes a stem cell-like phenotype in human colorectal carcinoma cells. Br. J. Cancer. 101, 320–326 (2009).
Kim, W. K. et al. β-catenin activation down-regulates cell-cell junction-related genes and induces epithelial-to-mesenchymal transition in colorectal cancers. Sci. Rep. 9, 18440 (2019).
Cal, S. & López-Otín, C. ADAMTS proteases and cancer. Matrix Biol. 44, 77–85 (2015).
Bridges, L. C. & Bowditch, R. D. ADAM-Integrin interactions: Potential integrin regulated ectodomain shedding activity. Curr. Pharm. Des. 11, 837–847 (2005).
Bachmann, M., Kukkurainen, S., Hytönen, V. P. & Wehrle-Haller, B. Cell adhesion by integrins. Physiol. Rev. 99, 1655–1699 (2019).
Defilippi, P. et al. Actin cytoskeleton organization in response to integrin-mediated adhesion. Microsc Res. Tech. 47, 67–78 (1999).
Simon, K. O. & Burridge, K. in Integrins 49–78 (Elsevier, 1994).
Moreno-Layseca, P. & Streuli, C. H. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 34, 144–153 (2014).
Humphries, J. D., Chastney, M. R., Askari, J. A. & Humphries, M. J. Signal transduction via integrin adhesion complexes. Curr. Opin. Cell. Biol. 56, 14–21 (2019).
Zhu, T. et al. The prognostic value of ITGA and ITGB superfamily members in patients with high grade serous ovarian cancer. Cancer Cell. Int. 20, 1–9 (2020).
Dhaliwal, D. & Shepherd, T. G. Molecular and cellular mechanisms controlling integrin-mediated cell adhesion and tumor progression in ovarian cancer metastasis: A review. Clin. Exp. Metastasis. 39, 291–301 (2022).
J Mizejewski, G. Role of integrins in cancer: Survey of expression patterns. Proc. Soc. Exp. Biol. Med. 222, 124–138 (1999).
Gharibi, A. et al. ITGA1 is a pre-malignant biomarker that promotes therapy resistance and metastatic potential in pancreatic cancer. Sci. Rep. 7, 10060 (2017).
Ando, T. et al. Integrin α11 in non–small cell lung cancer is associated with tumor progression and postoperative recurrence. Cancer Sci. 111, 200–208 (2020).
Sun, Z., Guo, S. S. & Fässler, R. Integrin-mediated mechanotransduction. J. Cell. Biol. 215, 445–456 (2016).
Isomursu, A., Lerche, M., Taskinen, M. E., Ivaska, J. & Peuhu, E. Integrin signaling and mechanotransduction in regulation of somatic stem cells. Exp. Cell. Res. 378, 217–225 (2019).
Miyamoto, S., KATHZ, B. Z., Lafrenie, R. M. & Yamada, K. M. Fibronectin and integrins in cell adhesion, signaling, and morphogenesis. Ann. N Y Acad. Sci. 857, 119–129 (1998).
Hou, J., Yan, D., Liu, Y., Huang, P. & Cui, H. The roles of integrin α5β1 in human cancer. Onco Targets Ther. 13, 13329–13344 (2020).
Wei, S. C. et al. Matrix stiffness drives epithelial–mesenchymal transition and tumour metastasis through a TWIST1–G3BP2 mechanotransduction pathway. Nat. Cell. Biol. 17, 678–688 (2015).
Du, J. et al. Extracellular matrix stiffness dictates wnt expression through integrin pathway. Sci. Rep. 6, 20395 (2016).
Noronha, C. et al. Cadherin expression and EMT: A focus on gliomas. Biomed 9, 1328 (2021).
Wijnhoven, B., Dinjens, W. & Pignatelli, M. E-cadherin—catenin cell—cell adhesion complex and human cancer. Br. J. Surg. 87, 992–1005 (2000).
Van Roy, F. & Berx, G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 65, 3756–3788 (2008).
Liao, Y., Smyth, G. K. & Shi, W. The subread aligner: Fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 41, e108–e108 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 1–21 (2014).
Wu, T. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2 (2021).
Luo, W. & Brouwer, C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 29, 1830–1831 (2013).
Hänzelmann, S., Castelo, R. & Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 14, 1–15 (2013).
Szklarczyk, D. et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 49, D605–D612 (2021).
Csardi, G. & Nepusz, T. The igraph software. Complex. syst. 1695, 1–9 (2006).
Van Meerloo, J., Kaspers, G. J. & Cloos, J. Cell sensitivity assays: The MTT assay in Cancer cell. Culture: Methods Protocols, (ed. Cree, I.) 237–245 (Humana Press, 2011).
Yan, X. D., Li, M., Yuan, Y., Mao, N. & Pan, L. Y. Biological comparison of ovarian cancer resistant cell lines to cisplatin and taxol by two different administrations. Oncol. Rep. 17, 1163–1169 (2007).
Acknowledgements
This work was supported in part by the Gyeongsang National University Fund for Professors on Sabbatical Leave, 2023 and by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (Ministry of Science and ICT-2022R1A2C1009939 and 2022R1C1C1008833).
Author information
Authors and Affiliations
Contributions
T.U.W.: Experiments, data analysis, literature survey, data interpretation, manuscript writing, prepared figures. H.I.K.: Data analysis, software, figures. G.H.L.: Review, feedback. Y.J.L.: Review, feedbackH.J.C.: Supervision, guidance. J.Y.K.: Literature survey, data interpretation, critical review and editing. H.Y.: Supervision, guidance, data interpretation, critical review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wani, T.U., Kim, HY., Lee, GH. et al. Mechanistic insights into epithelial-mesenchymal transition mediated cisplatin resistance in ovarian cancer. Sci Rep 15, 3053 (2025). https://doi.org/10.1038/s41598-025-87388-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87388-8