Abstract
In this work, titanium dioxide nanowires were prepared hydrothermally in strong alkaline medium. In parallel, nanostructural biochar was obtained via carbonization of rice husk at relatively high temperature. Then, titanate nanowires were modified with the nanorods of biochar via in-situ and ex-situ approaches in order to determine the best way to produce the nanocomposites with improved properties. Polyvinyl alcohol was used as a commercial matrix to include the superlative nanocomposite obtained and casted as a free-standing nanocomposite film. The synthesized nanowires, nanorods, and their nanocomposites were intensively investigated with transmission electron microscope (TEM), scanning electron microscope (SEM), energy dispersive X-ray (EDX), Fourier transform infrared (FTIR), X-ray diffraction (XRD), and N2 gas sorption. The microscopic images confirmed successful preparation and modification of nanostructures. FTIR showed strong interactions between the surface functional groups of the obtained nanomaterials. XRD exhibited a reduction in the crystallite size upon the treatment step. Also, surface texture analysis of titanate nanowires displayed a significant enhancement, particularly in terms of surface area and total pore volume. These superior properties promote the obtained nanocomposites to be evaluated in the water treatment compared with the pristine. The results confirmed complete removal of methylene blue (20 ppm) from the synthetic wastewater within only 20 min. in dark either by using the nanocomposites as powders or even as films. Kinetics and isotherms indicated that the adsorption process obeyed Langmuir model and follows pseudo-second order. On the other hand, the prepared materials depicted a strong biocidal activity against pathogenic microorganisms. The obtained nanocomposites may open opportunities towards developed adsorbents with superior features and performance for applications in the field of water decontamination.
Similar content being viewed by others
Introduction
There are several uses for dyes, including printing, dyeing, paper, painting, and textiles1. However, because they are poisonous, nonbiodegradable, and carcinogenic substances, dyes are among the most perilous types of water pollution2. Various approaches have been employed to eliminate colors from the effluents. Examples include ozonation, photocatalysis, evaporation, reverse osmosis, ion exchange, filtering, solvent extraction, flotation, and electrochemical oxidation3,4,5,6,7. Moreover, biological approaches are widely utilized for decolorizing and detoxifying wastewater. These methods mostly involve aerobic, anaerobic, or their combination8,9. However, in terms of the cost, the energy consumption, the treated water quality, and the process complexity, most of these techniques have plentiful shortcomings10,11,12. Adsorption is currently one of the most promising mode of treatment because of its ease of use, high capacity, high efficiency, and good regeneration ability13,14. Abundant numbers of materials, namely known as adsorbents, have been synthesized to eliminate cationic and anionic dyes from wastewater14,15,16,17.
Among the well-known adsorbents, titanium dioxide (TiO2) has paid great attention in water treatment applications thanks to its excellent physicochemical properties and low cost18. TiO2 can be fabricated in different nanostructures including nanoparticles, nanotubes, flower-like, and nanorods19,20,21,22. Particularly, titanate nanowires (TNWs) are one-dimensional nanomaterial with unusual lattice structure and properties such as large surface area, unique surface chemistry, and tunable transport features23. TNWs can be obtained utilizing several methods such as electrochemical, thermal oxidation, and hydrothermal24. The latter approach involves boiling titanium dioxide nanoparticles in an alkaline medium under high pressure and temperature in order to grow the nanoparticles into nanowires according to the previously reported mechanism25. This method is simple, inexpensive, and produces ultralong and nano-width wires with a high yield. In the field of water treatment, titanium dioxide nanostructure is generally combined with the wastewater in the dark or even under illumination forming a suspension26. TiO2 can demineralize the wastewater with a high efficiency especially in light via photocatalysis27. Besides, titanium dioxide can act as an adsorbent for a wide range of pollutants including heavy metals, dyes, and microorganisms. In our previous work, TNWs showed excellent performance in lead ions removal28. However, using nano titanium dioxide as fine powders sometimes causes particle aggregation and decreases surface reactive sites29. Furthermore, additional filtration processes are a must to separate the suspended particles30. Moreover, its surface area is not comparable to other adsorbents including carbon-based materials. Therefore, in order to overcome the drawbacks of restricted active sites and avoid particle aggregation along with an easy operational system, TiO2 can be modified and incorporated in a polymer matrix. For instance, hybrid cellulose membranes modified with TNWs coated with iron oxide and copper oxide nanoparticles as filters for bacteria and virus31. Another intensive review presented the recent tends of cellulose included with TiO2 nanocomposite and their potential applications in water treatment32. A very interesting study by Azeroual et al. showed high adsorption of methylene blue and safranin dyes using cryogel beads contain sodium alginate/titanium dioxide nanowire doped with zirconium33.
As commonly known, carbonaceous materials have found strong workability as adsorbents thanks to their intriguing properties. Particularly, biochar (BC) is a solid carbon byproduct obtained during the pyrolysis of carbon-rich biomass at relatively low temperatures34. The physicochemical properties of the produced biochar rely on the source of combusted material35. As a cost-effective adsorbent, BC has unique characteristics in terms of large surface area, plenty of surface functional groups, high porosity, and high adsorption capacity36. Therefore, biochar has emerged as a promising solution to various environmental challenges, in particular, water decontamination36. Several reports have been released discussing the efficiency of different sourced biochar as adsorbents for dyes37,38,39,40. Also, biochar can be obtained in the nanoscale and different morphologies such as nanoparticles and nanorods41,42,43. To improve their properties, people have utilized biochar as a filler for polymer matrices to be eventually employed in the removal of pollutants from the wastewater43,44,45,46,47,48. Incorporation of the nanomaterials in the polymer matrix and formulations as films, gel beads, or fibers could overcome the accompanied challenges of using powder nanomaterials and enhance the efficiency of whole nanocomposite46,49,50. In our previous work, biochar nanorods improved the performance of polyacrylamide hydrogel against the removal of phenol43.
According to the previous discussion and as a trial to overcome the shortcomings of titanate nanowires as an adsorbent by exploiting their unique features, biochar nanorods were used to modify the surface of titanium dioxide nanowires. In this work, TNWs were prepared via hydrothermal method using TiO2 nanoparticles as a precursor in alkaline medium. Then, the obtained sodium titanate nanowires were modified via in-situ and ex-situ approaches with biochar nanorods that produced from carbonization of rice husk. The modified nanowires were incorporated with polyvinyl alcohol matrix so that free-standing nanocomposite films were achieved. The prepared samples were investigated with TEM, SEM, FTIR, XRD, and gas sorption. Also, the characterized samples were evaluated against both of methylene blue removal and microbial growth. Kinetics and adsorption reaction orders were also studied.
Materials and methods
Materials
Titanium dioxide nanoparticles (P25) and sodium hydroxide pellets were purchased from Degussa (USA). Polyvinyl alcohol (PVA, medium molecular weight) was provided from Alfa Aesar (Germany). Rice husk was supplied by Egyptian farmers in Menofiya Governorate. Before using, the collected samples were rinsed well with deionized water. Then, the washed husks were dried in the vacuum oven at ~ 100 °C overnight.
Synthesis of titanium dioxide nanowires (TNWs)
A white suspension was obtained by dispersion of 2 g of titanium dioxide nanoparticles in sodium hydroxide solution according to our earlier work28. Then, the mixture was heated at 240 °C in the well-sealed autoclave. After 72 h., the mixture was filtered out and washed several times with (1.0 M) sodium chloride followed by distilled water. The obtained white precipitate of titanate nanowires was collected and dried overnight at 80 oC under vacuum (Fig. 1).
Synthesis of biochar (BC)
The purified rice husk was charged into an electric furnace and pyrolyzed at 420 °C under nitrogen atmosphere for 60 min43. The sample color was changed into black indicating formation of biochar. After cooling, the sample was collected and kept in a stainless-steel container for further experiment (Fig. 1).
In-situ preparation of titanium dioxide/biochar nanocomposite (ITB)
In this experiment, 10 wt. % of the obtained biochar was added to a dispersed solution of titanium dioxide nanoparticles (2 g) and sodium hydroxide. Then, the mixture was sonicated for 60 min. and left under vigorous stirring overnight in order to acquire a homogenous solution. After that, the grey suspension was transferred into a Teflon tube fixed in a sealed autoclave and placed in an oven at 240 °C for 72 h. under an ambient atmosphere. Once cooling, the autoclave was opened and the ingredients were transferred to a filtration system under suction. The sample was separated and well-washed by distilled water until the pH of filtrate reached normal. Finally, the resulting precipitate of modified titanate nanowires was collected and dried overnight at 80 °C under vacuum (Fig. 1).
Ex-situ preparation of titanium dioxide / biochar nanocomposite (ETB)
The prepared titanium dioxide nanowires were modified with the obtained biochar after successfully synthesizing each individually. Typically, one gram of TNWs was dispersed in 20 mL distilled water for 30 min. Then, a fixed weight of BC (10 wt. %) was added and sonicated for a further 30 min. After that, the mixture was transferred to a magnetic stirrer adjusted at 300 rpm and room temperature. The milky suspension was left for 72 h. in order to achieve strong interaction through the physical mixing. Finally, the resulting mixture was centrifuged, well-washed, and dried in vacuum oven overnight at 80 °C (Fig. 1).
Synthesis of polyvinyl alcohol nanocomposite films (PVATB)
Polyvinyl alcohol was used as a carrier for the prepared titanium dioxide nanowires and its biochar nanocomposites. Three PVA nanocomposite films were prepared, individually, by dispersing 4 wt. % of TNWs, ITB, and ETB, according to polymer weight, in three bottles, each contains 20 mL distilled water in a water bath sonicator for 30 min. Then, each suspension was left under vigorous stirring for 48 h. so that the highest level of dispersion was achieved. A certain weight of PVA (0.5 g) was added to each mixture and heated up 90 °C for 30 min. to dissolve the polymer pellets. Glycerol (50 µL) was also added as a plasticizer. After that, the casting solutions were kept under strong stirring for 24 h. In three glass petri dishes (10 cm), each solution was casted carefully on stand. Next, the dishes were transferred to the oven at 80 °C overnight. Finally, in order to obtain free-standing PVA nanocomposite matrices, each film was peeled off from the petri dish and kept in a sealed package put in a dissector for further usage and to avoid moisture exposure. The codes donated for the obtained films were TNWs@PVA, ITB@PVA, and ETB@PVA assigned to PVA films containing unmodified, in-situ modified, and ex-situ modified titanium dioxide nanowires, respectively. Pure PVA film was also prepared according to the previous protocol in the absence of fillers for comparison (Fig. 1).
Techniques
The chemical structure of the prepared titanium dioxide nanowires and its nanocomposites were identified by Fourier transform infrared spectroscopy (FT-IR, Perkin Elmer).
The alteration in the crystal structure before and after modification with biochar was investigated by X-ray powder diffraction (Bruker D 8 advance target). In this analysis, The Cu Kα was used as a radiation source with a secondly monochromator (λ = 1.5405 Å) at 40 kV and 40 mA. The scanning rate was 0.2o min− 1 for phase identification and line broadening profile analysis, respectively. The change in crystallite size was determined using the Scherrer equation,
where, d is the mean crystalline diameter, λ is the X-ray wavelength, K is the Scherrer constant (0.89), β1/2 is the full width at half maximum (FWHM) of the main diffraction peak of the main crystalline phase, and θ is the diffraction angle.
The surface morphology of the prepared titanium dioxide and its nanocomposites along with PVA films was examined using a transmission electron microscope (TEM, JEOL JEM-1230 with acceleration voltage of about 80 kV) and scanning electron microscope (SEM, JEOL-SEM), respectively. Energy dispersive X-ray (EDX) is a hyphenated unit to SEM instrument was used for elemental analysis. From TEM micrographs and using Image J software, size distribution in terms of wires or rods width was determined and the average values were estimated from histograms. Particularly, an optical microscope (Olympus CX-23 LED binocular research microscope) was also utilized to observe the prepared pristine nanowires at low magnification.
The surface textures were studied for the prepared titanium dioxide nanowires and their biochar nanocomposites with Quantochrome Nova-Touch 4LX automated gas-sorption apparatus (USA). In which, each sample was degassed at 150 °C for 6 h. followed by N2 gas input at 77 K for the adsorption process. The desorption was then carried out after achieving the highest applied gas by decreasing it. The adsorption isotherms were obtained and the surface area (SBET) was calculated according to Brunauer–Emmett–Teller (BET) equation. Moreover, the pore volume and the pore size distribution were estimated from the desorption isotherms according to Barrett, Joyner and Halenda (BJH) method.
The biocidal potential for all prepared samples was investigated against Gram-positive bacteria (Staphylococcus aureus), Gram-negative bacteria (Escherichia coli), and yeast (Candida albican). In the typical procedure with some modifications to the previous report51, certain weight of sample (mg/mL) was incubated under shaking with (50 µL) of bacterial suspension, 0.5 McFarland standards (1.5 × 108 CFU/mL), inoculated to 5 mL of specific medium for each organism in a sterilized test tube at 37 °C for 24 h. The samples were exposed to UV irradiation. The antimicrobial activity of each sample was determined by measuring the optical density (OD) on UV–vis spectrophotometer at a fixed wavelength (600 nm) according to the following equation:
In order to illustrate the ability of the obtained PVA nanocomposite films to remove dyes from synthetic wastewater, a stock of methylene blue dye (MB) of 20 ppm initial concentration was prepared. Utilizing the adsorption mode of treatment, each nanocomposite film compared with pure one was immersed in 30 mL taken from MB’ stock in a 150 mL glass bottle with a plastic cap. Then, the mixtures were stirred at 150 rpm and room temperature in the dark. A sample was withdrawn every 20 min. over 80 min. and the remaining dye was determined by the spectrophotometer (UV-vis JASCO V-730). The removal percentage was finally calculated according to the next equation:
where, Co is the initial dye concentration and Cs is the dye concentration after treatment with the sample at a certain contact time (min.).
Results and discussions
Transmission electron microscope (TEM)
TEM micrographs for [A] Titanium dioxide nanowires (Inset image displays an image under optical microscope), [B] Biochar nanorods, [C] In-situ modified titanium dioxide nanowires (ITB), [D] Ex-situ modified titanium dioxide nanowires (ETB), and [E-F] Width distribution histograms for pristine titanium dioxide nanowires and biochar nanorods, respectively.
TEM micrographs of TiO2 and biochar obtained are shown in Fig. 2. The images showed that the titanium dioxide was formed as rigid nanowires in large numbers with no significant nanoparticle morphology, Fig. 2[A]. This confirmed the successful growth of TiO2 nanoparticles into thin ultra-long nanowires via hydrothermal method. The same observation was previously recorded52.The width of the produced nanowires ranges from 20 to 70 nm. However, the average wire width was determined from the histogram and found equal to 45 nm, Fig. 2[E]. On the other hand, TEM of biochar obtained via carbonization of rice husk exhibited nanorods morphology with some aggregations of irregular shapes which might be attributed to the incomplete conversion of the nanorods, Fig. 2[B]. The corresponding histogram of the obtained nanorods showed average rod width equal ~ 19 nm, Fig. 1[F]. Particularly, micrographs, depicted in Fig. 2[C-D], emphasized the successful modification of titanate nanowires by both suggested approaches, in-situ and ex-situ. The integrity of the nanowires and the nanorods was maintained during the treatment process. Moreover, the biochar nanorods seem interpentrated inside the wires or between their bundles. This might refer to the possible strong interaction between the nanorods and the nanowires. interpenetrated.
Scanning electron microscope (SEM)
Figure 3 depicts the images taken by the scanning electron microscope for titanium dioxide nanowires and their modification. The titanium dioxide micrograph showed bundles of wires in the nanoscale and entangled, Fig. 3[A]. Biochar exhibited aggregates of nanorods (red arrows) can be seen at higher magnification. Additionally, some masses are observed attributed to the formed char, Fig. 3[B]. Upon modification of nanowires, the nanorods (green arrows) were seen interfered and homogenously distributed with titanium nanowires (cyan colour), Fig. 3[C-D]. However, an odd morphology of squared platelets or in other words cubic-like shape (blue triangles) appeared after in-situ modification of TWNs which might be referred to nano silicalite products. This morphology is absent in those modified via ex-situ approach. As known, biochar contains a high amount of silica53 which could be grown into this structure at high pH and under hydrothermal conditions. Several reports discussed the hydrothermal growth of crystalline silica from rice husk54,55,56. These results can be also confirmed by EDX, Fig. 2[E-F], as one can observe the concentration of silicon and sodium elements in the in-situ sample, Fig. 3[E], unlike in the case of ex-situ sample, Fig. 3[F] as a result of arrangement of elements in crystals at high temperature and pH. PVA has a smooth surface without significant cracks confirming the successful casting process, Fig. 3[G]. The inclusion of ex-situ modified titanium dioxide nanowires (ETB) showed well-dispersion and homogeneous distribution for both wires and rods within the matrix emphasizing good interaction between the composition Fig. 3[H].
SEM images for [A] Titanium dioxide nanowires, [B] Biochar nanorods, [C] In-situ modified titanium dioxide nanowires (ITB), [D] ex-situ modified titanium dioxide nanowires (ETB), [E-F] Their EDX spectra, respectively, [G] Top surface of pure PVA film, and [H] Top surface of nanocomposite PVA film (ITB@PVA).
Fourier transform infrared (FTIR)
[A] FTIR spectra for Titanium dioxide nanowires (TNWs), Biochar nanorods (BC), In-situ modified titanium dioxide nanowires (ITB), and ex-situ modified titanium dioxide nanowires (ETB). [B-C] Zooming in FTIR peaks showing more details in the ranges from ~ 1450 to ~ 1800 cm− 1 and from ~ 410 to 520 cm− 1.
FTIR was used to investigate the mode of interaction between TNWs and BC, Fig. 4. However, the spectrum of titanium dioxide nanowires showed a broad band at ~ 3100 cm− 1 revealed to surface hydroxyl groups. Other bands were observed at ~ 838, ~ 517, and ~ 420 cm− 1 assigned to the bending and stretching vibration of Ti–O–Ti bond. These values are close to those previously reported57,58. Meanwhile, biochar displayed characteristic peaks at ~ 3430 and 1625 cm− 1 corresponding to the stretching vibration of -OH groups and C = C, respectively, in agreement with the state of the art43. A low-intensity band appeared at ~ 1730 cm− 1 attributed to the carbonyl group. Indeed, these oxygenated functional groups improved the interaction with the nanowires and the PVA matrix.
Upon modification of titanium dioxide with biochar either by in-situ or by ex-situ, the spectra were changed. The characteristic band of TNWs at 3100 cm− 1 shifted to a higher wavenumber with an increase in the broadness. This might be attributed to the possible interaction through hydrogen bonding between the oxygenated functional groups at both surfaces as schematically (Fig. 5). Moreover, other shifts were observed from 1623 to 1640 cm− 1 and from 410 to ~ 480 cm− 1, Fig. 4[B-C], which might be revealed the probable coordination bond between titanium and π-cloud of the phenyl ring. Ghanem et al. confirmed the same behaviour while modification of ZnO with aromatic hyperbranched polymer59. FTIR confirmed the successful interaction between titanium dioxide nanowires and biochar nanorods by both methods of modification.
X-ray diffraction (XRD)
In order to confirm the interaction between the nanowires and nanorods, X-diffraction was performed, Fig. 6. Particularly, TNWs showed main distinctive θ peaks of sodium titanate (Na2Ti3O7) phase, Fig. 6[Left A], according to (PDF No. 00-059-0666), which is also known as sodium metatitanate in agreement with the published spectrum60,61. Particularly, lines pronounced at 9.5°, 24.9°, 29.4°, 34.3°, 35.0°, 38.4°, 48.3°, and 52.4° reveal the Miller indices of (100), (201), (003), (302), (301), (113), (020), and (411), respectively. BC showed special lines at ~ 25° and ~ 45° assigned to (002) and (101), respectively, related to the graphitic structure confirming the decomposition of cellulosic composition into char, Fig. 6[Right]. Some other peaks can be seen at different theta values related to the metallic residues. These findings are harmonious with previous reports62,63,64. In-situ modification of nanowires, Fig. 6[Left C], exhibited a slight shift in the main peaks with an appearance of new lines at ~ 17.5° and ~ 33° which might be attributed to the phase of sodium silicate crystal, that observed in SEM, in agreement with state of art65. Meanwhile, ex-situ treatment, Fig. 6[Left B], showed more shift for the highest peaks, particularly, that pronounced at ~ 9.5°. This peak moved to a higher value by ~ 0.5°. The other peaks displayed a slight shift. Also, no new peaks were observed in this nanocomposite spectrum which confirmed the potential growth of silicate crystals under hydrothermal conditions at high pH supporting the results of in-situ spectrum and its SEM micrographs. In the same context, the crystallite size was measured for all samples using Sherrer equation66. The obtained values were found equal to 32.84, 23.46, and 18.24 nm for TNWs, ITB, and ETB, respectively. These findings emphasized that the ex-situ treatment provides the lowest crystallite size. Generally, in-situ and ex-situ modification approaches could improve the particle morphology and retard the grain growth of titanate nanowires as stated by Ghanem et al.58. Careful inspection in the XRD spectra, one can observe the absence of biochar main lines in the modified samples which might be attributed to the lower concentration of biochar in the tested sample in addition to the probable interactions in the nanocomposite. The same observation was previously reported in several articles67,68,69.
Surface area
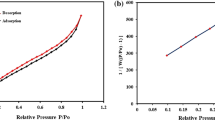
The texture profile of titanate nanowires and their modification was studied with N2 gas adsorption. The obtained isotherms and the pore size distribution are depicted in Fig. 7. As shown, TNWs displayed a uniform adsorption/desorption isotherm of type III without significant hysteresis and with limited gas adsorption. The same observation was recorded previously70. Upon the inclusion of nanorods, the surface texture was significantly altered. The amount of adsorbed gas was increased 6 times and 8 times via insertion of biochar nanorods by in-situ (ITB) and ex-situ (ETB) approaches, respectively. However, both isotherms exhibited.
Type IV of H3 type hysteresis loop in the range P/Po ranges from 0.3 to 0.9 due to capillary condensation by filling up and emptying of mesopore. These results emphasized the presence of a porous structure with increased porosity. Barret–Joyner–Halenda (BJH) method was used to calculate the pore size distribution of the prepared samples as depicted in Fig. 7. All samples presented a mesoporous structure i.e. the pore diameter is the range between 2 and 50 nm. However, the modified samples showed an average pore diameter of 2–5 nm. According to the acquired results, the specific surface area and the other texture parameters were calculated and summarized in Table 1. The calculated surface area of titanate nanowires was increased almost 4- and 6-fold after treatment with the nanorods via in-situ (ITB) and ex-situ (ETB) approaches, respectively. The other texture parameters, including total pore volume and average pore size, were also improved for the same reasons.
Dye adsorption
[A] Methylene blue removal % of modified TiO2 nanowires compared with pristine against contact time, [B] Their corresponding dye absorbance after 20 min., [C-D] Dye absorbance after adsorption over pure PVA and its composite film, respectively, (inset images are the treated water and the nanocomposite film after adsorption at the end of reaction), and [E] Their removal % against contact time.
The prepared titanium dioxide nanowires and their modification products were evaluated in the removal of methylene blue from synthetic wastewater of 20 ppm concentration under normal conditions. It is commonly known that TiO2 is among the strong photocatalysts as it has the ability to decompose organic pollutants under light irradiation71. However, in the present study, titanium dioxide was used as an adsorbent for dye in the dark. As shown in Fig. 8[A], the prepared titanate nanowires have limited adsorption of MB as it recorded MB removal of ~ 60%. Upon modification with biochar nanorods either by in-situ or by ex-situ, the removal % increased up to ~ 80% and ~ 100%, respectively. The characteristic MB peak was mostly disappeared after treatment with ETB adsorbent, Fig. 8[B]. The reason behind this improvement might be attributed to the significant increment in the surface area and the reduction in the crystallite size (cf. “X-ray diffraction (XRD)” section and “Surface area” section)72 i.e. ETB sample showed the highest surface area and lowest crystallite size which in turn enhanced the adsorption of dye with a high rate at the beginning of the reaction. Moreover, it is obvious thatall samples tend to make desorption after 20 min. as the removal % started to decrease by prolonged time i.e. the optimum contact time was only 20 min., Fig. 8[A]. Therefore, ETB sample was selected to be blended with the PVA matrix in order to eventually obtain nanocomposite free-standing film. This film was evaluated also in the removal of MB under the same conditions. However, the pristine matrix was tested at first. PVA can adsorb MB thanks to its surface functional groups that could form strong hydrogen bonding with the dye structure73. The results displayed the maximum removal was ~ 30% after 20 min., Fig. 8[C, E]. Inclusion of only 4 wt% of ETB in the PVA matrix improved the rate of adsorption and led to eliminating all the dye molecules attributed to the synergistic effect, Fig. 8[D-E], i.e. the dye absorbance was not recorded over 40 min. Nonetheless, both films exhibited desorption behaviour by passing the reaction time. Table 2 presents a comparison between some different PVA composites-based adsorbents and the suggested one in terms of the removal efficiency against MB. As depicted, the prepared PVA nanocomposite in this study enjoys the highest removal %.
Kinetics and isotherm
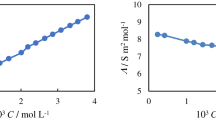
Adsorption isotherm is a most powerful way to understand how the pollutant molecules interact with the adsorbent surface. In this study, Langmuir and Freundlich models were considered for the MB-dye adsorption. Moreover, pseudo-first and pseudo-second order were used to analyze the adsorption kinetics. As shown in Fig. 9, the results suggested that the adsorption of methylene blue on the PVA nanocomposite film obeys Langmuir, monolayer adsorption, and follows the second-order kinetics as the values of the correlation coefficient were 0.996 and 0.999, respectively.
Antimicrobial activity
The biocidal potential of titanate nanowires and their modified forms were investigated in Fig. 10 against three microorganisms i.e. S. aureus as Gram-positive bacteria, E. coli as Gram-negative bacteria, and Candida Albicans as a yeast. The results showed the highest activity against all tested strains. As widely reported, titanium dioxide has strong activity against pathogenic microbes. In a former study, TNWs were examined against the same organisms using the inhibition zone method as a powder sample28. However, in the present study, the investigation was performed utilizing the shake flask method followed by measuring the optical density. Indeed, the obtained results are harmonious with the reported findings. The mode of action for titanium dioxide as a promising antimicrobial agent was intensively investigated in the literature83,84,85. In brief, TiO2 could form reactive oxygen species (ROS) in the medium utilizing hydroxyl surface groups (cf. FTIR section). The produced ROS could react with the microbial cell membrane and penetrate the cell causing DNA destruction. Another reported mechanism was assumed by Cao and his coworkers86. They claimed that the electron clouds located at the surface of the cell membranes of the microbes can be transferred from the bacterial membrane to the titanium dioxide surface because of Schottky barrier effect since TiO2 is a semiconductor that has an electric potential, and the cell membrane can be considered as electron donors. The charge transfer process leads to create holes of positive charge on the cell surface. Also, this causes oxidative stress on the whole cell making the outer cell membrane more porous and eventually death of the cell by the leakage of cytoplasm87. On the other hand, modification of titanate nanowires with biochar nanorods either by in-situ or by ex-situ did not maintain the same activity. However, the ex-situ treatment showed better activity than in-situ one. This might be revealed to the consumption of surface functional groups of TiO2 in the interaction with biochar surface (cf. FTIR section) which might reduce the chances to form ROS and hence the activity decreased. However, the modified samples still exhibited strong biocidal performance since the decrease in activity was not comparable and cannot be considered. As reported, PVA has no action against microbes88. Therefore, people usually incorporate antimicrobial agent in the PVA matrix to impart it the property. In this study, ex-situ modified titanate nanowires were included in this matrix and the biocidal potential was studied. As shown in Fig. 10, the nanocomposite PVA film (NCF) showed good activity i.e. the microbial growth reduction reached 60%. It could be claimed that increasing filler loading % over 4 wt% would enhance its activity.
Conclusions
In this study, titanium dioxide nanowires were prepared in sodium hydroxide solution under pressure using an autoclave at 240 °C for 72 h. Biochar nanorods were obtained via the combustion of rice husk at 400 °C under a nitrogen atmosphere. Then, titanate nanowires were modified with the nanorods of biochar by in-situ and ex-situ approaches. After that, the prepared titanate nanowires and their modifications were incorporated into the polyvinyl alcohol matrix to produce free-standing nanocomposite films. The microscopic images of TEM confirmed the successful preparation of titanium dioxide nanowires and biochar nanorods morphologies of average diameter ~ 45 and ~ 19 nm, respectively. SEM displayed a homogamous distribution of biochar nanorods between the titanate nanowires with the appearance of sodium silicate crystals formed during the in-situ modification step. FTIR showed strong hydrogen bonding and coordination bonds between the surface functional groups of both nanowires and nanorods. XRD exhibited shifting in the main peaks with a significant reduction in the crystallite size by ~ 28 and ~ 44% for in-situ modified and ex-situ modified nanocomposite, respectively. Also, the surface area and the total pore volume increased to a large extent. Thus, the synthesized nanocomposites showed a superior performance in the removal of methylene blue (20 ppm) after 20 min. treatment in the dark. The reaction order was pseudo-second order and follows the Langmuir model. The same activity was also observed for the PVA incorporated with 40 wt% of ex-situ modified nanocomposite. Against yeast along with Gram-positive and Gram-negative bacteria, the obtained nanocomposites showed strong antimicrobial activities close enough to that reported for the unmodified nanowires. Finally, from the study findings, it could be claimed that the ex-situ modified nanocomposite and its PVA film can be selected as the best choice to acte as a developed adsorbent with a high biocidal potential.
Data availability
All data generated or analyzed during this study are included in this article.
References
Hunger, K. Industrial dyes: Chemistry, Properties, Applications (Wiley, 2007).
Mani, S., Chowdhary, P. & Bharagava, R. N. Textile wastewater dyes: Toxicity profile and treatment approaches. Emerging and eco-friendly approaches for waste management 219–244. (2019).
Katheresan, V., Kansedo, J. & Lau, S. Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 6 (4), 4676–4697 (2018).
Hassan, M. M. & Carr, C. M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 209, 201–219 (2018).
Venkatesh, S., Venkatesh, K. & Quaff, A. R. Dye decomposition by combined ozonation and anaerobic treatment: Cost effective technology. J. Appl. Res. Technol. 15 (4), 340–345 (2017).
Chiam, S. L., Pung, S. Y. & Yeoh, F. Y. Recent developments in MnO2-based photocatalysts for organic dye removal: A review. Environ. Sci. Pollut. Res. 27(6): 5759–5778 (2020).
Yi, S., Sun, G. & Dai, F. Removal and separation of mixed ionic dyes by solvent extraction. Text. Res. J. 88 (14), 1641–1649 (2018).
Barragán, B. E., Costa, C. & Marquez, M. C. Biodegradation of azo dyes by bacteria inoculated on solid media. Dyes Pigm. 75 (1), 73–81 (2007).
Frijters, C. et al. Decolorizing and detoxifying textile wastewater, containing both soluble and insoluble dyes, in a full scale combined anaerobic/aerobic system. Water Res. 40 (6), 1249–1257 (2006).
Rame, R., Purwanto, P. & Sudarno, S. Potential of catalytic ozonation in treatment of industrial textile wastewater in Indonesia. Jurnal Riset Teknologi Pencegahan Pencemaran Industri. 11 (1), 1–11 (2020).
Werkneh, A. A. & Rene, E. R. Applications of nanotechnology and biotechnology for sustainable water and wastewater treatment. Water and Wastewater Treatment Technologies 405–430. (2019).
Chen, B. et al. Research advancements in swine wastewater treatment and resource-based safe utilization management technology model construction. Water 16 (5), 661 (2024).
Ibrahim, S. M. et al. Effective single and contest carcinogenic dyes adsorption onto A-zeolite/bacterial cellulose composite membrane: Adsorption isotherms, kinetics, and thermodynamics. J. Environ. Chem. Eng. 10 (6), 108588 (2022).
Yagub, M. T. et al. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 209, 172–184 (2014).
Corda, N. C. & Kini, M. S. A review on adsorption of cationic dyes using activated carbon. in MATEC web of conferences. (EDP Sciences, 2018).
Salleh, M. A. M. et al. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 280 (1–3), 1–13 (2011).
Haque, A. N. M. A. et al. Sustainable adsorbents from plant-derived agricultural wastes for anionic dye removal: A review. Sustainability 14 (17), 11098 (2022).
Hashimoto, K., Irie, H. & Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 44 (12R), 8269 (2005).
Nguyen-Phan, T. D. & Shin, E. W. Morphological effect of TiO2 catalysts on photocatalytic degradation of methylene blue. J. Ind. Eng. Chem. 17 (3), 397–400 (2011).
Zhao, Y. et al. Synthesis and optical properties of TiO2 nanoparticles. Mater. Lett. 61 (1), 79–83 (2007).
Roy, P., Berger, S. & Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 50 (13), 2904–2939 (2011).
Gupta, T., Cho, J. & Prakash, J. Hydrothermal synthesis of TiO2 nanorods: Formation chemistry, growth mechanism, and tailoring of surface properties for photocatalytic activities. Mater. Today Chem. 20, 100428 (2021).
Dilbraiz, M. A. et al. Synthesis of one-dimensional titanium oxide nanowires for polyvinylidene fluoride membrane optimization. Crystals 12 (8), 1164 (2022).
Wang, X. et al. One-dimensional titanium dioxide nanomaterials: Nanowires, nanorods, and nanobelts. Chem. Rev. 114 (19), 9346–9384 (2014).
Wu, D. et al. Sequence of events for the formation of titanate nanotubes, nanofibers, nanowires, and nanobelts. Chem. Mater. 18 (2), 547–553 (2006).
Schneider, J. et al. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 114 (19), 9919–9986 (2014).
Al-Mamun, M. et al. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 7 (5), 103248 (2019).
Saleh, M. G., Badawy, A. A. & Ghanem, A. F. Using of titanate nanowires in removal of lead ions from waste water and its biological activity. Inorg. Chem. Commun. 108, 107508 (2019).
Li, G. et al. Effect of the agglomeration of TiO2 nanoparticles on their photocatalytic performance in the aqueous phase. J. Colloid Interface Sci. 348 (2), 342–347 (2010).
Weimin, X. & Geissen, S. Separation of titanium dioxide from photocatalytically treated water by cross-flow microfiltration. Water Res. 35 (5), 1256–1262 (2001).
Shehab, M. A. et al. Virus and bacterial removal ability of TiO2 nanowire-based self-supported hybrid membranes. Arab. J. Chem. 16 (1), 104388 (2023).
Kamel, S. Recent development of cellulose/TiO2 composite in water treatment. Egypt. J. Chem. 65 (131), 601–612 (2022).
Azeroual, S. et al. A novel approach for adsorption of organic dyes from aqueous solutions using a sodium alginate/titanium dioxide nanowire doped with zirconium cryogel beads. Front. Chem. 12, 1285230 (2024).
Li, Q. et al. Removal of toxic metals from aqueous solution by biochars derived from long-root Eichhornia crassipes. R. Soc. Open Sci.. 5 (10), 180966 (2018).
Kujawska, J. & Wasag, H. Biochar: A low-cost adsorbent of Methylene Blue from aqueous solutions. J. Phys. Conf. Ser. (2021).
Nosratabad, N. A. et al. Exploring nanomaterial-modified biochar for environmental remediation applications. Heliyon. (2024).
Ji, B. et al. Removal of methylene blue from aqueous solutions using biochar derived from a fallen leaf by slow pyrolysis: Behavior and mechanism. J. Environ. Chem. Eng. 7 (3), 103036 (2019).
Kundu, D. et al. Study of methylene blue dye removal using biochar derived from leaf and stem of Lantana camara L. Carbon Res. 3 (1), 22 (2024).
Dwivedi, S. & Dey, S. Review on biochar as an adsorbent material for removal of dyes from waterbodies. Int. J. Environ. Sci. Technol. 20 (8), 9335–9350 (2023).
Paluch, D. et al. Fennel seed biochar: A sustainable approach for methylene blue removal from aqueous solutions. Materials 17 (17), 4350 (2024).
Chausali, N., Saxena, J. & Prasad, R. Nanobiochar and biochar based nanocomposites: Advances and applications. J. Agric. Food Res. 5, 100191 (2021).
Tao, R. et al. Biochar nanoparticles alleviate salt stress in tomato (Solanum lycopersicum) seedlings. Environ. Sci. Nano. 10 (7), 1800–1811 (2023).
Ali, R. et al. Synergistic impact of biochar nanorods on the performance of polyacrylamide matrix: UV-assisted degradation of phenol and biological activity. Egypt. J. Chem. 67 (3), 431–448 (2024).
Kar, A. & Karak, N. Removal of dye using lignin-based biochar/poly (ester amide urethane) nanocomposites from contaminated wastewater. J. Renew. Mater., 12(9). (2024).
Mosaffa, E. et al. Comprehensive analysis of cationic dye removal from synthetic and industrial wastewater using a semi-natural curcumin grafted biochar/poly acrylic acid composite hydrogel. RSC Adv. 14 (11), 7745–7762 (2024).
Das, L. et al. Calcium alginate–bentonite/activated biochar composite beads for removal of dye and biodegradation of dye-loaded composite after use: Synthesis, removal, mathematical modeling and biodegradation kinetics. Environ. Technol. Innov.. 24 101955 (2021).
Das, C. et al. Incorporation of biochar to improve mechanical, thermal and electrical properties of polymer composites. Polymers 13 (16), 2663 (2021).
Bartoli, M. et al. Recent advances in biochar polymer composites. Polymers 14 (12), 2506 (2022).
Darwesh, O. M. et al. Application of microalgal-ZnO-NPs for reusing polyester/cotton blended fabric wastes after modification by cellulases enzymes. Waste Dispos. Sustainable Energy. 5 (4), 471–482 (2023).
El-Gendi, A. et al. Antifouling and antimicrobial polyethersulfone/hyperbranched polyester-amide/Ag composite. RSC Adv. 10 (41), 24169–24175 (2020).
El-Sakhawy, M. et al. Biodegradable carboxymethyl cellulose based material for sustainable/active food packaging application. J. Thermoplast. Compos. Mater. 37 (6), 2035–2050 (2024).
Wei, M. et al. Ultralong single-crystal TiO2-B nanowires: Synthesis and electrochemical measurements. Chem. Phys. Lett. 424 (4–6), 316–320 (2006).
Wang, Y. et al. Environmental effects of silicon within biochar (Sichar) and carbon–silicon coupling mechanisms: A critical review. Environ. Sci. Technol. 53(23): 13570–13582 (2019).
Kongmanklang, C. & Rangsriwatananon, K. Hydrothermal synthesis of high crystalline silicalite from rice husk ash. J. Spectrosc. 2015 (1), 696513 (2015).
Putri, S. E., Anwar, M. & Askar, M. Synthesis of rice husk nanosilica using the hydrothermal method. in IOP Conference Series: Earth and Environmental Science. (IOP Publishing, 2023).
Sawasdee, V. & Pisutpaisal, N. Rice husk ash characterization and utilization as a source of silica material. Chem. Eng. Trans. 93, 79–84 (2022).
Manikandan, K., JafarAhamed, A. & Brahmanandhan, G. Synthesis, structural and optical characterization of TiO2 nanoparticles and its assessment to cytotoxicity activity. J. Environ. Nanotechnol. 6 (3), 94–102 (2017).
Ghanem, A. et al. Photocatalytic activity of hyperbranched polyester/TiO2 nanocomposites. Appl. Catal. A. 472, 191–197 (2014).
Ghanem, A. F. et al. Enhancement the photocatalytic and biological activity of nano-sized ZnO using hyperbranched polyester. J. Inorg. Organomet. Polym Mater. 29, 928–938 (2019).
Bo, A. et al. Mechanical bending properties of sodium titanate (na 2 Ti 3 O 7) nanowires. RSC Adv. 4 (100), 56970–56976 (2014).
Arumugam, M. K. et al. In vitro corrosion and bioactivity performance of surface-treated Ti-20Nb-13Zr alloys for orthopedic applications. Coatings 9 (5), 344 (2019).
Avornyo, V. K. et al. Temperature effects on properties of rice husk biochar and calcinated Burkina phosphate rock. Agriculture 11 (5), 432 (2021).
Zhang, S. & Wang, J. Removal of chlortetracycline from water by immobilized Bacillus subtilis on honeysuckle residue–derived biochar. Water Air Soil Pollut. 232 (6), 236 (2021).
Sarkar, S. & Das, S. K. Removal of cr (VI) and Cu (II) ions from aqueous solution by rice husk ash—column studies. Desalination Water Treat. 57 (43), 20340–20349 (2016).
Pérez-Casas, J. A. et al. Sugarcane bagasse ash as an alternative source of silicon dioxide in sodium silicate synthesis. Materials 16 (18), 6327 (2023).
Cullity, B. Publishing Cos. 102–105. (Addison-Wesley, Reading, 1978).
Parvathiraja, C. et al. Activated carbon-loaded titanium dioxide nanoparticles and their photocatalytic and antibacterial investigations. 12(8) 834. (2022).
Zhang, H. et al. Synthesis and characterization of TiO 2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. 8(60) 34241–34251. (2018).
Gong, Y. et al. Synthesis of highly dispersed and versatile anatase TiO 2 nanocrystals on graphene sheets with enhanced photocatalytic performance for dye degradation. 28 18883–18890. (2017).
Haran, N. H. & Yousif, Q. A. Characterization and Fabrication of nanowires as a photoanode electrode for DSSC. J. Phys. Conf. Ser.. (2019).
Tichapondwa, S. M., Newman, J. & Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth 118 102900 (2020).
Ertuş, E. B., Vakifahmetoglu, C. & Öztürk, A. Enhanced methylene blue removal efficiency of TiO2 embedded porous glass. J. Eur. Ceram. Soc. 41 (2), 1530–1536 (2021).
Ghoshal, D. et al. Methylene blue/PVA composite film for flexible, wide-scale UV–VIS laser cut-off filter. Mater. Res. Express. 6 (7), 075332 (2019).
Merdas, S. M., Al-Graiti, W. & Al-Ameer, A. Using PVA@ WNS composite as adsorbent for methylene blue dye from aqueous solutions. J. Med. Chem. Sci. 5, 1289–1298 (2022).
Radoor, S. et al. Ecofriendly and low-cost bio adsorbent for efficient removal of methylene blue from aqueous solution. Sci. Rep. 12 (1), 20580 (2022).
Jeevitha, P. Elimination of methylene blue using pva and bagasse as potential adsorbents. Int. J. Appl. Eng. Res. 13 (7), 253–257 (2018).
Tan, X. et al. Characterization of cellulose/polyvinyl alcohol/expanded graphite 3D porous foam and adsorption of methylene blue. J. Nat. Fibers. 20 (1), 2190189 (2023).
Umoren, S., Etim, U. & Israel, A. Adsorption of methylene blue from industrial effluent using poly (vinyl alcohol). J. Mater. Environ. Sci. 4 (1), 75–86 (2013).
Yang, X. et al. Adsorption of methylene blue from aqueous solutions by polyvinyl alcohol/graphene oxide composites. J. Nanosci. Nanotechnol. 16 (2), 1775–1782 (2016).
Jaseela, P., Garvasis, J. & Joseph, A. Selective adsorption of methylene blue (MB) dye from aqueous mixture of MB and methyl orange (MO) using mesoporous titania (TiO2)–poly vinyl alcohol (PVA) nanocomposite. J. Mol. Liq. 286, 110908 (2019).
Ng, H. M. & Leo, C. Translucent and adsorptive PVA thin film containing microfibrillated cellulose intercalated with TiO2 nanoparticles for dye removal. Colloids Surf., a. 578, 123590 (2019).
Aljar, M. A. A., Rashdan, S. & Abd El-Fattah, A. Environmentally friendly polyvinyl alcohol – alginate/bentonite nanocomposite hydrogel beads as efficient adsorbents for removal of toxic methylene blue from aqueous solution. (2021).
Younis, A. B. et al. Titanium dioxide nanoparticles: recent progress in antimicrobial applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 15 (3), e1860 (2023).
de Dicastillo, C. L. et al. Antimicrobial effect of titanium dioxide nanoparticles, in Antimicrobial Resistance-A One Health Perspective (2020).
Venkatasubbu, G. D. et al. Toxicity mechanism of titanium dioxide and zinc oxide nanoparticles against food pathogens. Colloids Surf., B. 148, 600–606 (2016).
Cao, H. et al. Electron storage mediated dark antibacterial action of bound silver nanoparticles: Smaller is not always better. Acta Biomater. 9 (2), 5100–5110 (2013).
Weng, S. et al. Synthesis, characterization, antibacterial activity in dark and in vitro cytocompatibility of Ag-incorporated TiO 2 microspheres with high specific surface area. J. Mater. Science: Mater. Med. 29, 1–10 (2018).
Hu, D. & Wang, L. Physical and antibacterial properties of polyvinyl alcohol films reinforced with quaternized cellulose. J. Appl. Polym. Sci. 133(25) (2016).
Acknowledgements
The authors gratefully thank the National Research Centre for the financial support of the in-house project no. 13010307.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Ahmed Ghanem, Abdelrahman Badawy, Ahmed Youssef, Naema S. Yehia, Farag A. Issa, Manal A. Nofal: Conception, Experimental design, Experimental carrying out, Characterization, Data analysis and interpretation, Reagents and materials contribution, Analysis tools, and writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghanem, A.F., Badawy, A.A., Youssef, A.A. et al. Polyvinyl alcohol film comprising biochar modified titanium dioxide nanocomposites as decoloring and disinfectant agents. Sci Rep 15, 11423 (2025). https://doi.org/10.1038/s41598-025-87432-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87432-7