Abstract
The study reveals the level of polycyclic aromatic hydrocarbons (PAHs) in three local food crops planted within six selected mining sites in the State, compared with those that were planted 500 m away from the mining areas. Gas Chromatography-Flame Ionization detection (GC-FID) model was used to determine the PAHs content in the food crop samples. Mann-Whitney U Test Analysis, was used as the statistical tool to check for the significant difference between the PAH level in food crops within and 500 m away from the mining site, in order to determine the impact of the mining practice carried out in those sites on the cultivated food crops in those areas to PAHs contamination. The result of the analysis clearly showed a significant difference between the PAH level in the food crops grown within the mining area, and the ones grown 500 m away from the mining sites. BaP (Benzopyrene) equivalent concentration (BEC) and daily intake dosage (DDI). Also excess cancer risk (ILCR) were used to calculate the carcinogenic exposure limits and cancer risk evaluations from these food crops for the children and adults: with overall values of 2.01E-08, 6.01E-09, 1.54E-08, 8.08E-09, 7.90E-08, 1.26E-08, 1.94E-08, 6.26E-09, 8.63E-09, 5.74E-09, 1.23E-08, 3.90E-09 and 4.30E-09, 1.29E-09, 3.31E-09, 1.73E-09, 1.69E-08, 2.70E-09, 4.16E-09, 1.34E-09, 1.85E-09, 1.23E-09, 2.63E-09, 8.35E-10 respectively; for Enyigba, Ezza South, Ikwo, Akwatakwa, Ameri Ameka, Mpume-Akwaokuko. The increase of some of these PAHs such as Benzo (a) pyrene (BaP), 1,2-benzanthracene (BENA), benzo(k)fluoranthene (BkFLO), dibenzy (a, h)anthracene (DahANT) and benzo(b)fluoranthene (BbFLO), being carcinogenic and genotoxic, posed a great health risk to the indigenes/consumers of these food crops. Therefore, it becomes very important to enforce proper mining policies and practices in order to reduce the contamination of these PAHs on food crops. ILCR values gotten from across the six mining sites (sites A) were higher than those 500 m away sites B), even though these values when compared with the USEPA acceptable guideline value of 0.000001 (10−6), fell within the USEPA acceptable limit. However, the accumulation of these PAHs in the body tissues and organs, especially in young children, over the years, will expose consumers of these contaminated food crops in these mining areas, to PAH-related diseases, which can be very fatal.
Similar content being viewed by others
.
Introduction
Polycyclic aromatic hydrocarbons are organic solids that are mostly pale yellow, white or colourless1,2and cover more than two hundred compounds of organic substances which contain a minimum of1two or more fused aromatic rings3,4or benzene rings5. They can can occur naturally or by anthropogenic activities6,7. Polycyclic aromatic hydrocarbons are generally produced when organic materials undergo incomplete combustion8,9,10,11or are pyrolyzed8,9. The human source of PAH pollution can either be by pyrolysis or petrogenesis12,13.
The presence of the pollutants of PAHs in the aquatic, terrestrial, or atmospheric environment (where they are found in equal amounts) are abundant in those environments14; and food crops consumption are significant passageways through which humans are exposed to pollutants (such as PAHs) from the environment5. More than 70% of3non-smokers’ exposure to polycyclic aromatic hydrocarbons come from food consumption3,15, even though a small number of situations of a direct PAHs exposure as a result of combustion processes occur16. The three routes through which humans can be exposed to PAHs are through inhalation, skin contact and ingestion17; and since food and water consumption is important for the health of human beings18, the latter remains the major route through which these compounds enter the human system17. According to Şahin et al. (2021) eighty-eight to ninety-eight% of polycyclic aromatic hydrocarbons exposure is foodborne19; and researches on epidemic show that foodstuff that are exposed to polycyclic aromatic hydrocarbons is related with a number of human cancers20. So, PAHs have tendencies and potential to cause cancer and mutate DNA cells21,22,2,4,23.
Several activities of humans lead to environmental degradation24, and mining is one of these numerous activities. Mining, amongst other positive or negative effects it poses, can as well impact negatively on human health25. For instance, a study conducted by Hendyx et al. (2020) suggested that living close to surface coal mining sites was associated with high PAH exposures26. Another study on “distribution, source and ecological risk assessment of polycyclic aromatic hydrocarbons in groundwater in a coal mining area, China”, arrived at the conclusion that PAH concentrations were higher in the groundwater near the industrial park and the coal gangue storage area, than those in the ground water, located in the residential and rural areas27.
So mining being broadly considered to have adverse impacts on the environment28; releases toxic chemicals such as polycyclic aromatic hydrocarbons (PAHs) to the environment.
Mining operations, while important for economic growth, are environmentally harmful and trigger considerable land disturbance. The disturbances include mining dumps, tailings and slimes, compaction, heavy metal toxicity and acidity. Ebonyi State mining activities are carried out in the State, due to the presence of Lead, Zinc and Limestone deposits in the State. This State also has an arable land mass for agricultural produce. In the study therefore, three of the common food crops regularly grown in Ebonyi State are Okra, Cassava and Rice. Okra (Ok) (Abelmoschus esculentus(L.)) is a very significant vegetable crop, suggested to be cultivated in the tropics and subtropics29, which can either be cooked or eaten raw, and has a rich economic value30. According to Schafleitner et al. (2021) Nigeria is the second largest producers of Okra vegetable in the world31. Cassava (Ca) (Manihot esculenta Crantz) (a tuber crop with a high starch content used as one of the main sources of food especially in Asia and Africa32; hence, it is a profitable crop33). Rice (Ri) (Oryza sativa L.)is, among others, a most essential food produce for more than half of the populace worldwide34, a daily need of 3 billon persons35; and the number three of cereal grain, worldwide36).
This study then pivots on the determination of the level of PAHs and their associated health risk on the three food crops, from six selected mining areas in the State.
Materials and methodology
Study area
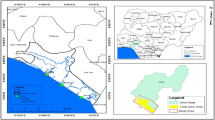
Out of all the mining sites in Ebonyi State, six mining sites (A sites) were randomly selected from six locations, namely: Enyigba, Ameri Ameka, Ezza South, Ikwo, Mpume Akwaokuku, and Akwatakwa; and 500 meters away (B sites), as seen in Fig. 1. For each A sites, in all the six locations, B sites were used as well. The coordinates for these sites are as follows: Enyigba (N: 06o 11.642’ E: 0080 08.380’ 263.4ft), Ameri Ameka (N: 06o 11.042’ E: 0080 06.110’ 149.3ft), Ezza South (N: 06o 09.929’ E: 0080 06.625’ 161.9ft), Ikwo (N: 06o 10.590’ E: 0080 07.438’196.8ft), Mpume Akwaokuku (N: 06o 30.268’E: 0080 16.352’ 226.6ft), and Akwatakwa (N: 06o 23.588’E: 0080 09.784’ 192.8ft).
Equipment and reagents
Gas Chromatography-Flame Ionization Detector (GC-FID), agate mortar, beaker, rotary vacuum evaporator, polypropylene cartridge column; Sodium Sulfate (Na2SO4), n-hexane, silica gel and methanol.
Food samples collection and preparation
The samples were collected based on the method described by37. The three different local food crops (Okra, Rice and Cassava), were each collected from the farmlands within the six mining sites (known as A sites) and then 500 m away from the mining sites (known as B sites or control sites). The samples were harvested from these farm sites with the consent of the farm owners. The total samples collected, among these three food crops samples were 36 in number (500 g each for the individual 36 samples). The edible parts of each of the three food samples were collected into air ventilated medium (to prevent spoilage), from each of the study sites to the lab for analysis, after the non-edible parts of the food crops, along with the bruised or the rotten parts were removed. The sample collection was done in accordance with acceptable guidelines for sample collection.
The PAHs in the food samples were analyzed according to38,39
Ten grams of the sample were weighed and properly homogenized. It was mixed with 60 g of anhydrous Na2SO4 in agate mortar to absorb moisture. The homogenate was placed into a 500 mL beaker. Extraction was carried out with 300 mL of n – hexane in 24 h. Crude extract obtained was evaporated using a rotary vacuum evaporator at 40oC, just to dryness.
One gram of silica gel that previously had been activated at 130 ºC for 10 h was carefully packed into 10 mL polypropylene cartridge column and 6 mL dichloromethane was used to condition the cartridge. The concentrated extract was then loaded onto the column and 50 mL pear shape flask was placed under the column to collect the eluate. A 10 mL dichloromethane was used to elute the column afterwards, and the total filtrate collected concentrated to just dryness using the rotary evaporator set at 38ºC. The residue was re-dissolved in 1 mL methanol and transferred into a 2 mL standard vial prior to quantification by Gas Chromatography.
The final extracts were analyzed by Gas Chromatograph-Buck M910 scientific gas chromatography equipped with combiPal autosampler and Flame Ionization Detector that allowed the detection of contaminants even at trace level concentrations (in the lower µg/g and µg/kg range) from the matrix to which other detectors do not respond. The GC conditions used for the analysis were, capillary column coated with VF-5 (30 m + 10 m EZ guard column x 0.25 mm internal diameter, 0.25 μm film thickness). The injector and detector temperature were set at 270 ºC and 300 ºC respectively. The oven temperature was programmed as follows: 70 ºC held for 2 min, ramp at 25 ºC/ min to 180 ºC, held for 1 min, and finally ramp at 5 ºC/ min to 300 ºC. Nitrogen was used as carrier gas at a flow rate of 1.0 mL/ min and detector make-up gas of 29 Ml min-1. The injection volume of the GC was 1.0 µL. The total run time for a sample was 31.4 min.
Quantification was performed from the GC profiles using the external standard method. The external standard was purchased from Supelco containing 16 PAH compounds (EPA 610 PAH mixture) including naphthalene [NAPH], acenapthylene [ACEN], acenapthene [ACENA], fluorene [FLU], phenanthrene [PHEN], anthracene [ANTH], fluoranthene [FLUO], pyrene [PYR], benzo(a)anthracene[BENA], chrysene [Chy], benzo(b)fluoranthene [BbFLO], benzo(k)fluoranthene [BkFLO], benzo(a)pyrene [BaP], dibenz(a, h)anthracene [DahANT], benzo(g, h,i)perylene [BghiPE], indeno(1,2,3-cd)pyrene [IP]. Quantification of PAH was performed by the linear regression method (r2 > 0.99) using five-point calibration curves established between the authentic standard concentrations and corresponding peak areas. Analysis of serial dilutions of PAH standard showed the limit of detection of the chromatographic method between 0.0007 and 0.016 ug/kg for the PAH compounds. The limit of quantification (LOQ) defined as the limit of detection divided by the sampling volume was in the range of 1.8 × 10−7 and 4.10 × 10−5 ug/kg. The recovery efficiency of the method was evaluated by the analysis of filters spiked with known concentration of standard PAH compounds. Most of the compounds provided high recoveries with mean values ranging between 70 and 80%. Field and laboratory blanks were routinely analyzed for quality control. Blanks levels of individual analytes were normally very low and in most cases not detectable.
The residue levels of PAHs were quantitatively determined by the external standard method using peak area. Measurement was carried out within the linear range of the detector. The peak areas whose retention times coincided with the standards were extrapolated on their corresponding calibration curves to obtain the concentrations.
Health risk assessment
Carcinogenic exposure and risk assessments
BEC (BaP equivalent concentration) in food, DDI (daily dietary intake exposure dosage), and ILCR (incremental lifetime cancer risk) were used to calculate the carcinogenic exposure limits (CELs) and cancer risk evaluations (CREs) from the food crops.
BEC - BaP equivalent concentration in food
TEF (toxic equivalent factors) for the polycyclic aromatic hydrocarbons (PAHs) were as follows: Fluorene (FLU) – 0.001, naphthalene (NAPH) – 0.001, phenanthrene (PHEN) – 0.001, acenaphthylene (ACEN) – 0.001, acenaphthene (ACENA) – 0.001, Fluoranthene (FLUO) – 0.001, benz(a)anthracene (BENA) – 0.1, benzo(a)pyrene (BaP) − 1, benzo (g, h,i)perylene (BghiPE) – 0.01, benzo(k)fluoranthene (BkFLO) – 0.1, dibenzy (a, h)anthracene (DahANT) – 1, pyrene (PYR) – 0.001 and benzo(b)fluoranthene (BbFLO) – 0.140.
Where: BEC is the PAH multiplied by BaP equivalent concentrations in food (ng g−1).
Ci is concentration of PAH congener in food (mg/kg).
TEFi is toxicity equivalent factor.
So, Eq. 1 represents the value of the BEC, which is the summation of the individual concentration of PAH level in food multiplied by the individual toxicity equivalent factor (TEF).
DDI - daily dietary intake exposure dosage assessment
Multiplying the BEC levels of the food samples by the ingestion rate, IR, (27 g/day) of the food crops is used to calculate the daily dietary intake (DDI) of PAHs; from Eq. 241.
Where: DDI = daily dietary intake (ng/day).
BEC = B[a]P equivalent concentration of PAHs in food (ng/g).
IR = ingestion rate of food per day (g/day) (27 g/day).
ILCR - incremental lifetime cancer risk
ILCR for PAHs related with day-to-day ingestion of BEC in then samples of cassava, okra and rice was calculated using the Eq. 3 formula41.
Where: ILCR - Incremental lifetime cancer risk.
CSF is the Benzo(a)pyrene oral cancer slope factor 7.3 mg/kg-day.
CF is the conversion factor (10−6 mg/ng).
ED is the exposure duration (63 years).
EF is the exposure frequency (156 days/year).
DDI is the daily dietary intake (ng/day).
BW of an average adult’s body weight (70 kg).
BW of an average adult’s body weight (15 kg)42.
AL is the average lifespan [total exposure duration = (70 years x 365 days = 25550 days).
Statistical analysis
The statistical tool for analyzing the result obtained was Microsoft Excel.Ink (2016) program. This Excel program was used to determine the composite Mean and Standard Deviation of the PAHs in the three food samples across the six mining sites. Then IBM SPSS Statistics 20 License Authorization Wizard was used to determine the ANOVA (Analysis of Variance) - at 0.05 level of significance; for the comparative analysis between the PAHs in the food crops harvested within the mining sites and those that were harvested 500 m away from the mining sites; by calculating their daily dietary intake (DDI) and their incremental lifetime cancer risk (ILCR).
Results and discussion
Tables 1 and 2 showed the values of the different PAHs across the three food samples within the mining sites and 500 m away, respectively. Tables 3 and 4 shows the Mann-Whitney U test and the mean rank values of the different PAHs in the food crops across the two sites.
Some of the PAHs have zero values (not detected): such as BENA, BghiPE and PYR PAHs within the mining sites; and NAPH, ACEN, ACENA, BENA, BghiPE, BkFLO, DahANT and PYR PAHs at 500 m away from the mining sites.
From the statistical U test analysis with Mann-Whitney U test from Table 3, the result indicated a significant difference of FLU PAH between the Okra grown at A and B sites; with values of the U Test and the significant difference, respectively: (U = 4.00,p = 0.026). Nevertheless, FLU in Cassava (U = 12.00,p = 0.394) and Rice (U = 8.00,p = 0.132) were non-significant. The values of FLU in Okra gotten from sites A were significantly higher with mean rank (MR = 8.83), when compared to that gotten from B sites (MR = 4.17), as shown in Table 4, confirming the reason why there was a significant difference between the sites A and B for FLU in Okra. The values of FLU in Cassava and Rice, gotten from A sites, were relatively the same when compared to that gotten from sites B.
In NAPH PAH, there were significant differences in Okra and Rice, with values: (U = 0, p = 0.002) and (U = 3.00,p = 0.015) respectively; while that of Cassava was non-significant; with values: U = 6.00,p = 0.65. The values of NAPH in Okra gotten from the A sites were significantly higher with mean rank (MR = 9.50), when compared to that gotten from B sites (MR = 3.50). Also the values of NAPH in Rice gotten from A sites were significantly higher with mean rank (M = 8.00), when compared to that gotten from B sites: (M = 4.00), confirming the reason why there were significant differences between sites A and B for NAPH in Okra and Rice. The values of NAPH in Cassava gotten from A sites were significantly higher with mean rank (M = 8.50), when compared to that gotten from B sites (M = 4.50); but for Cassava and Rice, the values of NAPH in them from A sites, were relatively the same when compared to those gotten from B sites.
The comparative values of PHEN PAH in Okra and Rice between the sites were also significant; but in Cassava, it was not. The values of the PHEN in Okra, Rice and Cassava were as follows: (U = 0,p = 0.02), (U = 4.50, p = 0.026) and (U = 9.00,p = 0.180) respectively. The values of PHEN in Okra and Rice gotten from A sites were significantly higher with mean rank (MR = 9.50) and (MR = 8.75) respectively, when compared to that gotten from B sites (MR = 3.50) and (MR = 4.25) respectively, confirming the reason why there were significant differences between sites A and B for PHEN in Okra and Rice. However, the values of PHEN in Cassava gotten from A sites were relatively the same when compared to that gotten from B sites.
ACEN PAH had significant difference values only in Rice (U = 3.00,p = 0.015); but in Cassava (U = 6.00,p = 0.065) and Okra (U = 9.00,p = 0.180), their values were non-significant. The values of ACEN in Rice gotten from A sites were significantly higher with mean rank (MR = 9.00), when compared to that gotten from B sites (MR = 4.00), confirming the reason why there were a significant difference between sites A and B for ACEN in Rice, but the values of ACEN in Okra and Cassava gotten from A sites were relatively the same when compared to that gotten from B sites.
ACENA PAH values in Okra and Cassava between A and B sites each had significant differences of (U = 3.00,p = 0.15) respectively; while the ACENA in Rice (U = 6.00,p = 0.065) between A and B sites had not significant difference. The values of ACENA in Okra and Cassava gotten from A sites were significantly higher with mean rank (MR = 9.00) and (MR = 9.00) respectively, when compared to that gotten from B sites (MR = 4.00) and (MR = 4.00) respectively, but the values of ACENA in Rice, gotten from A sites were relatively the same when compared to that gotten from B sites.
The statistical U test analysis, with Mann-Whitney U test from Table 3, showed that there was no significant difference (p > 0.05) in FLUO PAH in the Okra (U = 12.00,p = 0.394), Cassava (U = 8.00,p = 0.132) and Rice (U = 12.00,p = 0.394) samples between A and B sites, respectively. Therefore the values of FLUO in Okra, Cassava and Rice gotten from A sites were relatively the same when compared to that gotten from B sites.
For BENA, BghiPE and PYR PAHs, there were no significant differences as all the statistical values of these PAHs in the three food crops, for both A and B sites were all zero, making, U = 18.00 and p = 1.00. Therefore the values of BENA, BghiPE and PYR in Okra, Cassava and Rice gotten from A sites were relatively the same when compared to that gotten from B sites.
BaP PAH in Okra between A and B sites has a significant difference: U = 5.00,p = 0.041; while for Cassava (U = 14.00,p = 0.589) and Rice (U = 9.00,p = 0.180), there were no significant differences for each of them. Therefore the values of BPYR in Okra gotten from A sites were significantly lower with mean rank (MR = 4.33), when compared to that gotten from B sites (MR = 8.67); but for Cassava and Rice, the value of BPYR gotten from A sites were relatively the same when compared to that gotten from B sites.
BkFLO PAH in Okra, Cassava and Rice between A and B sites all had a significant difference of (U = 3.00,p = 0.015) for Okra and Cassava respectively; and (U = 0,p = 0.02) for Rice sample. Therefore the values of BkFLO in Okra, Cassava and Rice gotten from A sites were significantly higher with mean ranks (MR = 9.00), (MR = 9.00) and (MR = 9.50) respectively, when compared to that gotten from B sites (MR = 4.00), (MR = 4.00) and (MR = 3.50).
There was no significant difference for DahANT PAH in Okra (U = 6.00,p = 0.065), Cassava (U = 9.00, 0.180) and Rice (U = 9.00, 0.180) between the A and B sites. So the values of DahANT in Okra, Cassava and Rice gotten from A sites were relatively the same when compared to that gotten from B sites.
BbFLO PAH has a significant difference in Okra (U = 4.500,p = 0.026) between A and B sites; while there was no significant difference between A and B sites for Cassava (U = 11.500,p = 0.310) and Rice (U = 7.00,p = 0.093) samples. So, the values of BbFLO in Okra gotten from A sites were significantly lower with mean rank (MR = 8.75), when compared to that gotten from B sites (MR = 4.25). The values of BbFLO in Cassava and Rice, gotten from A sites were relatively the same when compared to that gotten from B sites.
Results from individual crops
Results from Tables 1 and 2 showed that FLU (in Okra), NAPH (in Okra and Rice), PHEN (in Okra and Rice), and ACEN (in Rice), were significantly high when compared to the ones that were 500 m away from the mining site (B sites). Therefore these food crops will potentially harm the indigents who consume them more, when compared to the level of harm they will cause to those that consume the food crop 500 m away from the mining sites, clearly showing that mining activities in A sites increased the level of PAHs in those food crops planted in those areas, to the detriment of the health of the indigenes/consumers.
However, PAHs of FLU (in Cassava and Rice), NAPH (in Cassava and Rice), PHEN (in Cassava), ACEN (in Okra and Cassava), and ACENA (in Rice), on the other hand, had no significant difference between A and B sites; and therefore will have relatively the same health impact on humans that consume the food crops planted in either/both sites. For these, crops mining activities were not the possible contributing factor to the presence of PAHs in them; which clearly shows that other factors like inherent crops characteristics3and/or environmental factors43, outside mining activities can be responsible as well must have contributed to PAHs presence in the food samples.
FLU for instance, being a low molecular weight PAH, may not directly have carcinogenic and genotoxic impact, but it is connected with potential indicators for cancer of the lung44. Therefore FLU, being found in a significant amount in the Okra crop poses a potential danger to the humans that consume the said food crop.
Likewise, the level of these high molecular PAHs were also significantly high in the following food crops in A sites. For instance, BaP (in Okra), BkFLO (in Okra, Cassava and Rice), and BbFLO (in Okra); were higher when compared with those in B sites. This clearly indicates that mining activities in those sites, contributed to the high level of these PAHs in A sites, thereby posing a great health danger to the consumers of those food crops.
However, PAHs like: FLUO (in Okra, Cassava and Rice), BaP (in Cassava and Rice), DahaANT (in Okra, Cassava and Rice), on the other hand, had no significant values difference between A and B sites; and therefore will have relatively the same health impact on humans that consume the food crops planted in either/both sites. PAHs of BENA, BghiPE and PYR all had zero values in the food crops across A and B sites. Therefore, they have no contributive ill-health impact on the consumers of those food crops.
Of the 13 PAHs classified as being cancer-causing and genotoxic, by the joint efforts of FAO/WHO: BaP, BENA, BkFLO, DahANT and BbFLO (discovered in a significant value in the current study) were reported as being cancer-causing and genotoxic, with BaP as being the PAH with the greatest considerable cancer-causing effect3. This finding is also confirmed by International Programme on Chemical Safety (IPCS, 1998)45. Therefore the presence of these PAHs in the food samples under considerations pose a great health challenge to the indigenes that consume these food crops.
Also among the three food crops that were analyzed across A and B sites, Okra samples (from Tables 1 and 2) accumulated more PAHs than the counterpart Cassava and Rice samples, in the following sequence: Okra > Rice > Cassava. This made it to have a higher overall significant difference across the PAHs. This is traceable to the inherent characteristics of Okra, as a vegetable, to store PAHs, as seen in studies like46. Therefore, Okra being a vegetable; and vegetables having the higher tendency to accumulate PAHs makes Okra in those mining sites to pose more risk of consumption than the other food crops under study.
Determination of PAHs in the food crops from composite mean values of the three food crops
Table 5; Figs. 2, 3, 4, 5, 6, 7 show the composite mean levels of the different PAHs within the mining sites (sites A) and 500 m away from the sites (sites B). From Enyigba, BkFLO PAH (being a carcinogenic PAH) showed the highest value in site A compared to other PAHs. PHEN PAH had the highest value in site A of Ezza South and Akwatakwa. For Ikwo mining site (site A), DahANT PAH (a carcinogenic PAH) showed the highest value. FLU PAH (a low molecular weight PAH) had had the highest value in site A of Ameri Ameka. For Mpume Akwaokuko, ACENA had the highest value in site A, followed by BkFLO PAH.
The overall result of the figures clearly show that the PAHs have more concentrations in sites A when compared with those in sites B (500 m away from the mining sites), with few exceptions where the concentration of the PAHs 500 m away from the mining sites (sites B) were higher; such as: FLUO in Mpume Akwaokuko, FLO in Enyigba, BaP in Ikwo, Enyigba, Ezza South and Ameri Ameka These exceptions can be attributed to other PAH-causative factors (PCF) such as inherent crops characteristics3and/or environmental factors43.
Table 5, and Figs. 8 and 9, indicate the overall summation mean concentration (in mg/kg) of PAHs in the food crops harvested from each of the six mining sites, and 500 m away from each of these sites. The result still showed that activities in the mining sites, as also seen in the following studies26,27:, increased the concentration of PAHs (both carcinogenic and non-carcinogenic) in the food crops form sites A, when compared to the concentration of PAHs in food crops from sites B with a significant difference value of p = 0.003, t(10) = 4.794.
Health risk assessment
Based on the result from Tables 6 and 7, the daily intake dosage (DDI) and the daily intake dosage of carcinogenic PAHs (CDDI) all showed that children and adult with body weights of 15 kg and 70 kg respectively are exposed to both carcinogenic and non-carcinogenic. However children were exposed more to the PAHs than the adults. A related study by Jia et al. (2018) on PAHs in vegetables also reveals that children had higher values of DDI (attuned by body weight), than in those of the teenage and adult stage47.
The same applies for the values of ILCR (incremental lifetime cancer risk for both the adult and the children. The ILCR values being higher in sites A than sites B were then compared with the USEPA acceptable guideline value of 0.000001 (10−6)48. The values for all the individual PAHs across the six mining sites (sites A and B), were found to be less than the USEPA acceptable value. That is, The ILCR estimate associated with consuming Okra, Cassava and Rice proposes 1 out of 10,000,000 in an adult population over a 70-year life expectancy is below the acceptable level of 0.000001 (10−6) and thus there was no significant health risk41. The same applies to the children population.
Nevertheless, accumulation of these PAHs over the years in the body tissues can still expose consumers of these contaminated food crops in these mining areas, to PAH-related diseases. A study was done by49on the occurrence of PAHs in the adipose tissue (AT) of Korean females: the result showed an accumulation of these PAHs in the AT of these females, with NAPH PAH concentration as being dominant. Another study by50showed that the PAHs level in the AT of French and Polish bariatric patients were higher when compared to the PAHs level in their body plasma, which suggests that these PAHs were accumulated in these adipose tissues. Another study by Onyedikachi et al. on PAHs in food crops in industrial areas in South Eastern Nigeria also opined that children as well as adult that live in these areas may be at grave danger owing to intake of PAHs in foodstuffs over a period of time as a result of bioaccumulation of these poisonous carcinogens51. Therefore, preventive measure must be employed to lower the impact of these PAHs in food crops, which will ultimately lower the human health risk exposures to these PAHs.
*Non-Carcinogenic PAHs, **Carcinogenic PAHs, \(\:\sum\:\text{P}\text{A}\text{H}\) = Total composite mean and SD concentration of all PAHs, \(\:\sum\:\text{C}\text{P}\text{A}\text{H}\) = Total composite mean and SD concentration of carcinogenic PAHs, Mean ± SD (site A) = composite mean concentration of PAHs in food crops within the mining sites, Mean ± SD (site B) = = composite mean concentration of PAHs in food crops from 500 m away from the mining sites.
Conclusion
The result of the study mostly showed that the level of PAHs in the food crops planted within the mining sites were higher than those planted 500 m away from the mining sites. This clearly points to the fact that mining activities has a great negative health influence on the consumption of food crops planted in those areas, due to the incremental contribution of PAHs they add to the local food crops planted with their areas, which has ultimately contributed to the health risks on the humans that consume those food crops. Also the Okra, a vegetable food crop: due to its inherent ability to accumulate organic contaminants, like PAHs, had a higher level of PAHs (for example BPYR: being a popular marker for PAHs) when compared to Cassava and Rice food samples, exposing those who consume the vegetable more at risk of PAHs pollutant than those who consume the Cassava and Rice crops. ILCR values gotten from across the six mining sites (4.30E-09 for Enyigba, 3.31E-09 for Ezza-South, 1.69E-08 for Ikwo, 4.16E-09 for Akwatakwa, 1.85E-09 for Ameri Ameka, 2.63E-09 for Mpume-Akwaokuko were higher than those 500 m away (1.29E-09 for Enyigba, 1.73E-09 for Ezza-South, 2.70E-09 for Ikwo, 1.34E-09 for Akwatakwa, 1.23E-09 for Ameri Ameka, 8.35E-10 for Mpume-Akwaokuko), were compared with the USEPA acceptable guideline value of 0.000001 (10 − 6). The values for all the individual PAHs across the six mining sites (sites A and B), were found to be less than the USEPA acceptable value. Nevertheless, accumulation of these PAHs over the years in the body tissues can still expose consumers of these contaminated food crops in these mining areas, to PAH-related diseases. Considering the research results, it is paramount for the government to enact strict laws that will be geared towards environmental protections and regulations as regards to food security and safety hence, reducing concentration of these toxic pollutants and their bioaccumulation in food crops. The study is limited to 500 m away from the mining site. Therefore, further distance(s) from the mining site should be considered to understand the correlation between distance variations and PAH concentrations and their significant effect on health risk assessment parameters. Also, future studies should be conducted on the seasonal variation of PAHs in the food crops. Special considerations on the impact of the PAHs contained in these food crops on pregnant women that reside within the mining areas should be conducted.
Data availability
Data is provided within the maniscript.
References
Abdel-Shafy, H. I. & Mansour, M. S. M. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt. J. Petroleum. 25 (1), 107–123. https://doi.org/10.1016/j.ejpe.2015.03.011 (2016).
Rengarajan, T. et al. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 5 (3), 182–189. https://doi.org/10.1016/S2221-1691(15)30003-4 (2015).
Sampaio, G. R. et al. Polycyclic Aromatic Hydrocarbons in Foods: Biological effects, legislation, occurrence, Analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 22 (6010), 1–30. https://doi.org/10.3390/ijms22116010 (2021).
Ewa, B. & Danuta, M. Š. Polycyclic aromatic hydrocarbons and PAH-related DNA adducts. J. Appl. Genet. 58, 321–330. https://doi.org/10.1007/s13353-016-0380-3 (2017).
Lawal, A. T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 3 (1), 1–89. https://doi.org/10.1080/23311843.2017.1339841 (2017).
Ingenbleek, L. et al. Polycyclic aromatic hydrocarbons in foods from the first regional total diet study in Sub-saharan Africa: contamination profile and occurrence data. Food Control. 103, 133–144. https://doi.org/10.1016/j.foodcont.2019.04.006 (2019).
Nežiková, B. et al. Bulk atmospheric deposition of persistent organic pollutants and polycyclic aromatic hydrocarbons in Central Europe. Environ. Sci. Pollut Res. 26, 23429–23441. https://doi.org/10.1007/s11356-019-05464-9 (2019).
Babaoglu, A. S., Karakay, M. & Öz, F. Formation of polycyclic aromatic hydrocarbons in beef and lamb kokorec: effects of different animal fats. Int. J. Food Prop. 20 (9), 1960–1970. https://doi.org/10.1080/10942912.2016.1225761 (2017).
Lee, B. Sources, Distribution and Toxicity of Polyaromatic Hydrocarbons (PAHs) in Particulate Matter. In (Ed.), Air Pollution. IntechOpen. (2010). https://doi.org/10.5772/10045
Maliszewska-Kordybach, B. Sources, Concentrations, Fate and Effects of Polycyclic Aromatic Hydrocarbons (PAHs) in the Environment. Part A: PAHs in Air. Polish Journal of Environmental Studies 8(3):131–136. Available via (1999). http://www.pjoes.com/Sources-Concentrations-Fate-and-Effects-of-Polycyclic Aromatic-Hydrocarbons-PAHs,87230,0,2.html of subordinate document. Accessed 14 July 2022.
Li, W. et al. Multi-year analyses reveal different trends, sources, and implications for Source-Related Human Health Risks of Atmospheric Polycyclic Aromatic Hydrocarbons in the Canadian Great Lakes Basin. Environ. Sci. Technol. 55 (4), 2254–2264. https://doi.org/10.1021/acs.est.0c07079?ref=pdf (2021).
Kafilzadeh, F. Distribution and sources of polycyclic aromatic hydrocarbons in water and sediments of the Soltan Abad River, Iran. Egypt. J. Aquat. Res. 41 (3), 227–231. https://doi.org/10.1016/j.ejar.2015.06.004 (2015).
Nasher, E., Heng, L. Y., Zakaria, Z. & Surif, S. Concentrations and sources of Polycyclic Aromatic hydrocarbons in the seawater around Langkawi Island, Malaysia. J. Chem. 2013, 1–10. https://doi.org/10.1155/2013/975781 (2013).
Patel, A. B., Shaikh, S., Jain, K. R., Desai, C. & Madamwar, D. Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches. Front. Microbiol. 11 (562813), 1–23. https://doi.org/10.3389/fmicb.2020.562813 (2020).
Zelinkova, Z. & Wenzl, T. The occurrence of 16 EPA PAHs in food – a review. Polycycl. Aromat. Compd. 35 (2–4), 248–284. https://doi.org/10.1080/10406638.2014.918550 (2015).
Rose, M. et al. Investigation into the formation of PAHs in foods prepared in the home to determine the effects of frying, grilling, barbecuing, toasting and roasting. Food Chem. Toxicol. 78, 1–9. https://doi.org/10.1016/j.fct.2014.12.018 (2015).
Hamidi, E. N., Hajeb, P., Selamat, J. & Razis, A. F. A. Polycyclic aromatic hydrocarbons (PAHs) and their Bioaccessibility in Meat: a Tool for assessing Human Cancer Risk. Asian Pac. J. Cancer Prev. 17, 16–22. https://doi.org/10.7314/APJCP.2016.17.1.15 (2016).
Ugbede, F. O. et al. Ingestion exposure of public to natural radionuclides and committed effective dose and cancer risk through tuber crops cultivated in Ebonyi State. Nigeria Environ. Monit. Assess. 195 (1385), 1–18. https://doi.org/10.1007/s10661-023-11992-2 (2023).
Şahin, T., Dalğa, S. & Ölmez, M. Polycyclic Aromatic Hydrocarbons (PAHs) and Their Importance in Animal Nutrition. In (Ed.), Animal Husbandry [Working Title]. IntechOpen. (2021). https://doi.org/10.5772/intechopen.101816
Wu, M. et al. Distribution and Health Risk Assessment on Dietary exposure of Polycyclic Aromatic hydrocarbons in vegetables in Nanjing, China. J. Chem. 2016 (1-8). https://doi.org/10.1155/2016/1581253 (2016).
Duan, X. et al. Dietary intake polycyclic aromatic hydrocarbons (PAHs) and Associated Cancer Risk in a cohort of Chinese urban adults: Inter- and intra-individual variability. Chemosphere 144, 2469–2475. https://doi.org/10.1016/j.chemosphere.2015.11.019 (2016).
Pashin, Y. V. & Bakhitova, L. M. Mutagenic and carcinogenic properties of polycyclic aromatic hydrocarbons. Environ. Health Perspect. 30, 185–189. https://doi.org/10.1289/ehp.7930185 (1979).
Lasota, J. & Błońska, E. Polycyclic aromatic hydrocarbons content in contaminated forest soils with different Humus types. Water Air Soil. Pollut. 229 (204), 1–8. https://doi.org/10.1007/s11270-018-3857-3 (2018).
Aiyesanmi, A. F., Ademefun, A. E., Ibigbami, O. A. & Adelodun, A. A. Polycyclic aromatic hydrocarbons and organochlorine pesticides in floodplain soils: a case study of Onuku River in Okitipupa. Nigeria Environ. Challenges. 5 (100351), 1–8. https://doi.org/10.1016/j.envc.2021.100351 (2021).
Haddaway, N. R. et al. Evidence of the impacts of metal mining and the effectiveness of mining mitigation measures on social–ecological systems in Arctic and boreal regions: a systematic map protocol. Environ. Evid. 8 (9), 1–11. https://doi.org/10.1186/s13750-019-0152-8 (2019).
Hendryx, M., Wang, S., Romanak, K. A., Salamova, A. & Venier, M. Personal exposure to polycyclic aromatic hydrocarbons in Appalachian mining communities. Environ. Pollut. 257, 1–19. https://doi.org/10.1016/j.envpol.2019.113501 (2020).
Jiang, C. et al. Distribution, source and ecological risk assessment of polycyclic aromatic hydrocarbons in groundwater in a coal mining area. China Ecol. Indic. 136, 1–10. https://doi.org/10.1016/j.ecolind.2022.108683 (2022).
Monjezi, M., Shahriar, K., Dehghani, H. & Namin, F. S. Environmental impact assessment of open pit mining in Iran. Environ. Geol. 58, 205–216. https://doi.org/10.1007/s00254-008-1509-4 (2009).
Temam, N., Mohammed, W. & Aklilu, S. Variability Assessment of Okra (Abelmoschus esculentus (L.) Moench) genotypes based on their qualitative traits. Int. J. Agron. 2021 (1-6). https://doi.org/10.1155/2021/6678561 (2021).
Dantas, T. L., Buriti, F. C. A. & Florentino, E. R. Okra (Abelmoschus esculentus L.) as a potential functional food source of Mucilage and Bioactive compounds with Technological Applications and Health benefits. Plants 10 (8), 1683:1–14. https://doi.org/10.3390/plants10081683 (2021).
Schafleitner, R. et al. The World Vegetable Center Okra (Abelmoschus esculentus) Core Collection as a source for flooding stress tolerance traits for breeding. Agriculture 11, 165:1–13. https://doi.org/10.3390/agriculture11020165 (2021).
Yabuta, S. et al. The Productivity of Cassava (Manihot esculenta Crantz) in Kagoshima, Japan, which belongs to the Temperate Zone. Agronomy 11, 1–18. https://doi.org/10.3390/agronomy11102021 (2021).
Daemo, B. B., Yohannes, D. B., Beyene, T. M. & Abtew, W. G. Biochemical Analysis of Cassava (Manihot esculenta Crantz) Accessions in Southwest of Ethiopia. J. Food Qual. 2022, 1–13. https://doi.org/10.1155/2022/9904103 (2022).
Xue, Y. & Xu, Z. An introduction to the China Rice Functional Genomics Program. Comp. Funct. Genom. 3, 161–163. https://doi.org/10.1002/cfg.147 (2002).
WoolstonC Rice. Nature 514 (S49). https://doi.org/10.1038/514S49a (2014).
Al Mamun, M. A. et al. Growth and trend analysis of area, production and yield of rice: a scenario of rice security in Bangladesh. PLoS ONE. 16 (12), 1–18. https://doi.org/10.1371/journal.pone.0261128 (2021).
Okeke, D. O., Ifemeje, J. C. & Eze, V. C. Determination of the heavy metals and physicochemical properties of borehole water within selected mining sitesin Ebonyi State, Nigeria. Int. J. Chem. Biol. Sci. 3 (2), 5–10. https://doi.org/10.25082/HE.2022.01.003 (2021).
AOAC. Official methods of analysis. Association of official analytical chemists, 16th ed. Washington DC, USA. (1995).
AOAC. Association of Analytical Chemistry. Methods for chemical Analysis. 2217–2280. (1990).
Kumar, V. et al. Determination of some carcinogenic PAHs with toxic equivalency factor along roadside soil within a fast developing northern city of India. J. Earth Syst. Sci. 123 (3), 479–489. https://doi.org/10.1007/s12040-014-0410-7 (2014).
Acquah-Baidoo, D., Affrifah, N. S., Afoakwa, E. O. & Saalia, F. K. Exposure assessment of polycyclic aromatic hydrocarbons from the consumption of processed cowhide (Wele), a west African delicacy. Sci. Afr. 20, 1–8. https://doi.org/10.1016/j.sciaf.2023.e01694 (2023).
Haque, R., Ali, M., Ahmed, W. & Rahman, M. Assessment of metal(loid)s pollution in water and sediment from an urban river in Bangladesh: an ecological and health risk appraisals. Case Stud. Chem. Environ. Eng. 6 (100272), 1–11. https://doi.org/10.1016/j.cscee.2022.100272 (2022).
Bansal, V. & Kim, K-H. Review of PAH contamination in food products and their health hazards. Environ. Int. 84, 26–38. https://doi.org/10.1016/j.envint.2015.06.016 (2015).
Alhamdow, A. et al. Fluorene exposure among PAH-exposed workers is associated with epigenetic markers related to lung cancer. Occup. Environ. Med. 77, 488–495. https://doi.org/10.1136/oemed-2020-106413 (2020).
Purcaro, G., Moret, S. & Conte, L. S. Polycyclic aromatic hydrocarbons. Encyclopedia Food Health. 406–418. https://doi.org/10.1016/B978-0-12-384947-2.00550-X (2016).
Somtrakoon, K., Chouychai, W. & Lee, H. Removal of Anthracene and Fluoranthene by Waxy Corn, Long Bean and Okra in lead-contaminated soil. Bull. Environ. Contam. Toxicol. 95, 407–413. https://doi.org/10.1007/s00128-015-1587-4 (2015).
Jia, J., Bi, C., Zhang(Jim, J., Jin, X. & Chen, Z. Characterization of polycyclic aromatic hydrocarbons (PAHs) in vegetables near industrial areas of Shanghai, China: sources, exposure, and cancer risk. Environ. Pollut. 241, 750–758. https://doi.org/10.1016/j.envpol.2018.06.002 (2018).
Tongo, I., Ogbeide, O. & Ezemonye, L. Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicol. Rep. 4, 55–61. https://doi.org/10.1016/j.toxrep.2016.12.006 (2017).
Moon, H-B., Lee, D-K., Lee, Y. S. & Kannan, K. Occurrence and accumulation patterns of polycyclic aromatic hydrocarbons and synthetic musk compounds in adipose tissues of Korean females. Chemosphere 86 (5), 485–490. https://doi.org/10.1016/j.chemosphere.2011.10.008 (2012).
Mlyczynska, E. et al. Concentration of polyclic aromatic hydrocarbon (PAHs) in human serum and adipose tissues and stimulatory effect of Naphthalene in adipogenesis in 3T3-L1 cells. Int. J. Mol. Sci. 24 (1455), 1–19. https://doi.org/10.3390/ijms24021455 (2023).
Onyedikachi, U. B., Belonwu, C. D. & Wegwu, M. O. The determination of polycyclic aromatic hydrocarbons in some foods from industrialized areas in South Eastern Nigeria: human health risk impact. Ovidius University Annals of Chemistry 30 (2019) 37–43. (2019). https://doi.org/10.2478/auoc-2019-0007
Acknowledgements
The authors acknowledge Springboard Lab, where the analyses of the samples were carried out.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by O.N.N. and D.O.O. O.N.N.O, I.K.I and D.O.O reviewed the study. The first draft of the manuscript was written by O.N.N and D.O.O. and all authors reviewed the previous versions of the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Okoye, O., Ijoma, I.K. & Okechukwu Okeke, D. Health risk assessment of polycyclic aromatic hydrocarbons in common food crops within and outside selected mining areas of Ebonyi State, Nigeria. Sci Rep 15, 24982 (2025). https://doi.org/10.1038/s41598-025-87623-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87623-2