Abstract
MXenes, as a novel two-dimensional lamellar material, has attracted much attention. However, MXenes lamellar are prone to collapse and stacking under hydrogen bonding and interlayer van der Waals forces, which affects their electrochemical and capacitive deionization performance. A three-dimensional Ni-1,3,5-benzenetricarboxylate/Ti3C2Tx (Ni-BTC/Ti3C2Tx) composite electrode material was developed to enhance the electrochemical and capacitive deionization performance. The uniformly decorated Ni-BTC can prevent MXenes from aggregation and provide a large specific surface area and rich pore structure. As a substrate supporting Ni-BTC, MXenes can effectively disperse the growth of Ni-BTC and enhance the ion transport rate. In addition, the unique three-dimensional structure of Ni-BTC/Ti3C2Tx provides horizontal charge transfer paths like two-dimensional nanosheets and has unique vertical charge transfer paths between nanosheets. Therefore, the Ni-BTC/Ti3C2Tx exhibits an exceptional chromium(VI) removal rate of 94.1%. The electrosorption capacity of the Ni-BTC/Ti3C2Tx for chromium(VI) is 124.5 mg g−1, much higher than that of the pure Ti3C2Tx (55.5 mg g−1). The superior CDI efficiency accomplished through the Ni-BTC/Ti3C2Tx electrode is due to the unique three-dimensional network structure and synergistic effect of the pseudocapacitance generated by the unique assembly of Ni-BTC and Ti3C2Tx. Ni-BTC/Ti3C2Tx is a promising CDI electrode material that can be used for capacitive deionization.
Similar content being viewed by others
Introduction
Heavy metal wastewater has been discharged with the rapid economic increase, especially in the mining, metallurgy, casting, and chemical industries. These heavy metal wastewater entering the water environment will cause water resource pollution and ecological damage1,2. In particular, chromium(VI) has substantial toxicity and can invade the human body through the skin, respiratory tract, and digestive tract3,4. Long-term or short-term exposure to chromium(VI) or inhalation of chromium(VI) is carcinogenic. Therefore, heavy metal industrial wastewater discharge has become a bottleneck problem restricting sustainable economic and social development. Finding reasonable and efficient methods for treating heavy metal wastewater protects the ecological environment and alleviates water crises5,6,7.
Traditional methods for treating heavy metals in wastewater include extraction, ion exchange, chemical precipitation, and membrane separation8,9,10,11. However, these treatment methods face difficulties in operation, regeneration, and the risk of secondary pollution12,13,14. With the strengthening of people’s environmental awareness, there is an urgent need to develop a more efficient, lower energy consumption, lower cost, and environmentally friendly method for treating wastewater containing heavy metal ions. Capacitive deionization technology (CDI) has the advantages of low energy consumption, in situ regeneration, and no secondary pollution to the environment15,16,17. It has great potential and broad application prospects in seawater desalination and sewage treatment.
Electrode materials are essential to capacitive deionization technology18,19,20. Developing and researching high-performance electrode materials are the core links to improving capacitive deionization technology’s separation efficiency and practicality and reducing process energy consumption. Currently, electrode materials for capacitive deionization technology are mainly carbon materials21,22,23,24. Although carbon materials have made significant progress as CDI electrode materials, they are limited by the specific surface area available for ion adsorption and the influence of coexisting ion repulsion effects, resulting in low deionization levels25,26,27. Therefore, developing CDI electrode materials with better performance still has significant theoretical and practical significance.
Faraday electrode material, through redox reaction, achieves the deintercalation of ions while avoiding the co-ionic effect to improve capacitive deionization performance28,29,30. MXenes have attracted much attention as a new two-dimensional lamellar Faraday electrode material, which has been gradually used in catalysis, secondary batteries, capacitor deionization, and other fields due to its high specific capacitance, good conductivity, good mechanical properties, and unique layered structure31,32,33,34. However, MXenes nanosheets are prone to collapse and stacking under the action of hydrogen bonds and interlayer van der Waals forces. They exhibit severe swelling behavior in aqueous solution, which affects their electrochemical properties as electrode materials35,36. Therefore, improving MXenes materials’ stability and maintaining good structure and properties have become urgent needs.
Composite materials provide a new idea for solving the above problems. Composites integrate the functions of different monomer components and derive new functions, which can significantly improve the performance of materials. MOFs are widely used in gas storage37,38, sustained drug release39, chemical sensing40, and catalysis41,42,43 due to their abundant internal pores, high specific surface area, easy components control, and adjustable pore size. However, the electrical conductivity of MOF materials could be better, so the application of MOF materials in the electrochemical direction is limited.
Among various MOFs, Ni-1,3,5-benzenetricarboxylate (Ni-BTC) exhibits a relatively large specific surface area, stable porous structure, long-term stability, and good electrolyte accessibility44,45. Therefore, we composite Ni-BTC with Ti3C2Tx MXene materials to compensate for their shortcomings and build new three-dimensional structure Ni-BTC/Ti3C2Tx composite materials. Introducing Ni-BTC can reduce the aggregation and enlarge interlayer spaces of Ti3C2Tx MXene nanosheets, which is beneficial to the transport of electrolyte ions. At the same time, the electrical conductivity of the MOF material is improved, and additional pseudocapacitance contribution is provided. In addition, the unique three-dimensional structure of Ni-BTC/Ti3C2Tx provides horizontal charge transfer paths and unique vertical charge transfer paths between nanosheets. As expected, the Ni-BTC/Ti3C2Tx electrode showed a high removal efficiency of 94.1% with a removal capacity of 124.5 mg g−1 for chromium(VI). The results of this study show that the Ni-BTC/Ti3C2Tx electrode is an ideal CDI electrode material that can be used for heavy metal removal.
Experimental
Preparation of samples
Synthesis of Ti3C2Tx
Ti3C2Tx was prepared in a typical method. 1.5 g Ti3AlC2 powder was added to the etchant solution, which consisted of 1.5 g LiF and 30 mL 6 mol L−1 HCl. Then, the obtained mixture was in a 100 mL reactor and maintained at 90 °C for 5 days. We were shaking the reactor three times a day. The product was repeatedly centrifuged and rinsed with deionized water and ethanol. After centrifuge washing, the product was added to 25 mL, 60 g L−1 LiCl aqueous solution for 2 h and then centrifuged repeatedly with deionized water.
Preparation of Ni-BTC/Ti3C2Tx
2.25 mmol Ni (NO3) 2 6H2O and a certain amount of Ti3C2Tx were added to 20 mL of ethanol. 1.25 mmol H3BTC was dissolved in 10 mL of ethanol. Mixed these two solutions and transferred them to a reactor for 24 h at 150ºC. The product was thoroughly rinsed with ethanol and freeze-dried.
The material characterization is transferred to Supporting Information.
Electroabsorption experiments
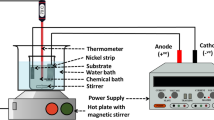
The hybrid capacitance deionization device was composed of Ni-BTC/Ti3C2Tx composites and activated carbon (Fig. S1), and the capacitance deionization properties of Ni-BTC/Ti3C2Tx composites were studied. The electrodes comprised 80 wt% of as-prepared Ni-BTC/Ti3C2Tx composites or activated carbon, 10 wt% of acetylene black, and 10 wt% of PTFE. The obtained slurries were coated on nickel foam. The Ni-BTC/Ti3C2Tx electrode was set to 2 × 2 cm2. The loading of electrode materials (as-prepared Ni-BTC/Ti3C2Tx composites or activated carbon, acetylene black, and PTFE) was controlled to ~ 20 mg. The simulated wastewater’s chromium(VI) concentration is 30 mg L−1. The removal efficiency (R) and electroabsorption capacity (qe) are determined according to the formula (1) and (2):
where C0 and Ce are the initial and final concentration (mg L−1) of chromium (VI) ions in simulated wastewater; V (L) is the volume of simulated wastewater, and m is the mass (g) of Ni-BTC/Ti3C2Tx composites.
Results and discussions
Materials characterization
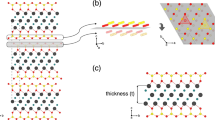
The preparation process of Ni-BTC/Ti3C2Tx by in-situ growth method is shown in Fig. 1. Accordion-like Ti3C2Tx was obtained by etching Ti3AlC2 with LiF-HCl etchant in a sealed reactor. Then, using Ti3C2Tx as substrate, Ni2+ ions were anchored to the surface and interlayer of Ti3C2Tx via electrostatic interactions. Finally, H3BTC was added to the solution, and Ni-BTC was uniformly grown in situ on the surface and interlayer of Ti3C2Tx. The resulting Ni-BTC can prevent Ti3C2Tx from self-stacking. Meanwhile, the unique three-dimensional structure of Ni-BTC/Ti3C2Tx can provide horizontal charge transfer paths alongside nanosheets and unique vertical charge transfer paths between nanosheets.
XRD spectra verified the stripping process of Ti3AlC2. As shown in Fig. 2a, the dominant diffraction peak (002) (2θ = 9.45°) of Ti3AlC2 shifts to a lower angle (~ 7.19°) in the Ti3C2Tx plot, while the characteristic (104) peak (2θ = 38.7°) in Ti3AlC2 completely disappears in Ti3C2Tx, indicating the successful etching of the Al layer46,47. Ni-BTC/Ti3C2Tx was also characterized. The main diffraction peaks corresponding to simulated Ni-BTC and the characteristic (002) peaks of Ti3C2Tx can be observed in the Ni-BTC/Ti3C2Tx, suggesting the successful combination of Ti3C2Tx and Ni-BTC48. Ti3C2Tx and Ni-BTC can bind to each other due to the electrostatic interactions and chelation between the Ti3C2Tx and the metal ions in Ni-BTC. Their typical diffraction peak (002) shifts slightly at a small angle compared to that of Ti3C2Tx, from 7.19° to 5.82° (as shown in Fig. 2a). This result indicates that the layer spacing becomes larger under the influence of Ni-BTC.
FTIR spectra of Ti3C2Tx and Ni-BTC/Ti3C2Tx are given in Fig. 2b. In the FTIR spectrum of Ni-BTC/Ti3C2Tx, the characteristic peaks located at 1619 and 1571 cm−1, attributed to asymmetric vibrations of –COO−, and at 1438 and 1375 cm−1, due to symmetric vibrations of –COO−. The broadband at 3412 cm−1 could be assigned to the hydrogen-bonded hydroxyl in Ti3C2Tx. This result indicates that Ni-BTC has been combined with Ti3C2Tx.
The SSA and pore structure of Ti3C2Tx and Ni-BTC/Ti3C2Tx were studied (Fig. 3a, b). The isotherm of Ni-BTC/Ti3C2Tx shows an H3 hysteresis loop and typical type IV equilibrium isotherm, indicating the presence of mesoporous and macroporous pores. The isotherm of Ti3C2Tx shows a type II equilibrium isotherm. Type II isotherms reflect typical physical adsorption processes on nonporous adsorbents. Figure 3b shows the corresponding pore size distribution curves of Ti3C2Tx and Ni-BTC/Ti3C2Tx obtained by the BJH method. From Fig. 3b, there are mesopores and macropores in Ni-BTC/Ti3C2Tx and a small number of micropores in Ti3C2Tx. The pore volume of Ni-BTC/Ti3C2Tx (0.264 m3 g−1) is much higher than that of Ti3C2Tx (0.022 m3 g−1). The BET surface area of Ni-BTC/Ti3C2Tx (75.13 m2 g−1) is higher than that of Ti3C2Tx MXene (9.061 m2 g−1). These results confirm that Ni-BTC plays a key role in avoiding Ti3C2Tx MXene layer stacking and increasing the pore volume and SSA, thus providing higher collision frequencies and more active sites for chromium(VI) removal. Electroabsorption performance is highly dependent on the SSA and pore structure of the electrode material. The large SSA and multi-level pore structure can shorten the diffusion distance of ions and form a good ion transfer pathway, thus accelerating ion transport. This characteristic is better for enhancing capacitor deionization performance.
From Fig. 4a, b, the Ti3C2Tx has an accordion-like structure and a smooth surface with a layer thickness of about 30 nm. When Ni2+ was added into the suspension of Ti3C2Tx, Ni2+ was uniformly anchored to the surface of Ti3C2Tx due to electrostatic interactions, and Ni-BTC was uniformly grown on the surface of Ti3C2Tx after H3BTC was added. From Fig. 4c, d, some small particles uniformly adhere to the surface of Ti3C2Tx material. According to the XRD analysis results, these small particles are the Ni-BTC, indicating the successful assembly of Ni-BTC and Ti3C2Tx. Meanwhile, the Ni-BTC/Ti3C2Tx presents a unique three-dimensional network structure, which can provide rich charge transport paths. Furthermore, TEM images of Ni-BTC/Ti3C2Tx (Fig. S2) show many small particles with an average size of 25 nm on the Ti3C2Tx. This unique structure can provide more attachment sites for removing chromium(VI) ions. Meanwhile, Ni-BTC particles are uniformly distributed in the continuous Ti3C2Tx MXene network, with an open structure and excellent electrical conductivity. This property facilitates the transport of electrons and ions. The mapping images of Ni-BTC/Ti3C2Tx are presented in Fig. 4e. The results showed that Ni-BTC/Ti3C2Tx comprises C, O, F, Ti, and Ni, indicating that Ni-BTC was successfully polymerized on the Ti3C2Tx.
XPS analysis was further studied to investigate the chemical composition and state of Ti3C2Tx and Ni-BTC/Ti3C2Tx. The full survey of XPS (Fig. S3) confirmed the coexistence of C, Ti, O, F, and Ni elements in Ni-BTC/Ti3C2Tx, in agreement with the aforementioned elemental mapping and XRD. C, Ti, O, and Ni valence states were further analyzed using the high-resolution spectrum. The high-resolution C 1s spectrum of Ni-BTC/Ti3C2Tx (Fig. 5a) exhibits fitted peaks at 288.5 (O=C–O), 286.3 (C-O), 284.8 eV (C–C/C=C), and 283.2 eV (C–Ti)49,50. The high-resolution Ti 2p spectrum of Ni-BTC/Ti3C2Tx (Fig. 5b) can be deconvoluted into four peaks at 464.1, 462.9, 458.6, and 457.4, corresponding to Ti3+ 2p1/2, Ti2+ 2p1/2, Ti3+ 2p3/2 and Ti2+ 2p3/2, respectively51. The high-resolution O 1 s spectrum of Ni-BTC/Ti3C2Tx (Fig. 5c) displays four peaks at 529.7, 531.2, 531.8, and 533.1 eV, which are ascribed to Ti–O, Ni–O, C=O, and C–O48,52. The high-resolution Ni 2p spectrum of Ni-BTC/Ti3C2Tx (Fig. 5d) shows two peaks, 856.2 and 873.8 eV, and two satellite (sat.) peaks, 861.2 and 879.6 eV, which are associated with Ni 2p3/2 and Ni 2p1/2 of Ni2+, respectively48.
Electrochemical performance
Cyclic voltammetry tests were performed to evaluate the ion storage capacity of Ti3C2Tx and Ni-BTC/Ti3C2Tx. Figure 6a compares the electrochemical performance of Ti3C2Tx and Ni-BTC/Ti3C2Tx at a scan rate of 50 mV s−1. The prominent redox peaks on the cyclic voltammetry curve indicate that Ti3C2Tx and Ni-BTC/Ti3C2Tx composite materials are typical pseudo-capacitive electrodes. The charge storage ability of the Ti3C2Tx and Ni-BTC/Ti3C2Tx electrodes can be obtained based on the integral area. From Fig. 6a, the total area of the Ni-BTC/Ti3C2Tx curve is more significant than that of Ti3C2Tx, indicating that the Ni-BTC/Ti3C2Tx composite has higher specific capacitance than Ti3C2Tx. By comparison, the Ni-BTC/Ti3C2Tx electrode material has higher reactivity, more extensive polarization current, and better energy storage performance than the pure Ti3C2Tx electrode material.
An electrochemical impedance test is an essential means to evaluate the electrochemical properties of electrode materials. Figure 6b is a Nyquis diagram of Ti3C2Tx and Ni-BTC/Ti3C2Tx, from which it can be seen that the Nyquis curve of the materials has a typical mixing control process. The high-frequency region is the kinetic control of the electrode reaction (charge mass transfer) process, and the low-frequency region is the diffusion control process53,54. After compositing with Ni-BTC, the internal resistance decreases, mainly because the introduction of Ni-BTC avoids stacking Ti3C2Tx MXene layers and unique three-dimensional charge transfer paths, thereby improving the electrical conductivity of Ni-BTC/Ti3C2Tx. With the introduction of Ni-BTC, the slope of the low-frequency straight line increases, and the interface diffusion of Ni-BTC/Ti3C2Tx is also improved. According to the above electrochemical performance test results, it can be inferred that Ni-BTC/Ti3C2Tx is a highly promising CDI electrode material with extensive prospects in the field of CDI55.
Figure 6c shows the CV curves of Ni-BTC/Ti3C2Tx at scanning rates ranging from 1 to 100 mV s−1. With the increase in scanning speed, the integrated area of the CV curve gradually increases. Even at scan rates up to 100 mV s−1, the consistent shape of the Ni-BTC/Ti3C2Tx CV curve indicates that the material has excellent rate performance. When the scan rate is 10 mV s−1, it is calculated that the specific capacitance of Ni-BTC/Ti3C2Tx is 139.4 F g−1. The CDI efficiency of the electrode material is affected by the capacitance of the electrode. Therefore, comparing the specific capacitance of Ni-BTC/Ti3C2Tx with that of MOF and MXenes-based materials previously reported, the three-dimensional Ni-BTC/Ti3C2Tx composite prepared in this study is a good CDI electrode material.
To further investigate the electrochemical mechanism of the three-dimensional Ni-BTC/Ti3C2Tx composite electrode, the charge-storage process was analyzed by the power law equation:
where ip and v are the peaks current and the scan rate, the values of a and b are constants, and the size of the b value can indicate the type of charge storage mechanism. When the b-value is 0.5, the diffusion-controlled process dominates the storage mechanism. When the b-value is 1, the capacitive control process dominates the storage mechanism56,57. Figure 6d is log(i)−log(ν) curve of Ni-BTC/Ti3C2Tx at 1–100 mV s−1. According to Fig. 6d, the b value of the Ni-BTC/Ti3C2Tx is 0.72, demonstrating that the three-dimensional Ni-BTC/Ti3C2Tx composite electrode is ascribed to both capacitance and diffusion control processes.
Capacitive deionization performance
To investigate the capacitive deionization performance of Ni-BTC/Ti3C2Tx, the capacitive deionization experiments of chromium(VI) on Ti3C2Tx and Ni-BTC/Ti3C2Tx electrodes were conducted. In the capacitive deionization experiment, the chromium(VI) concentration is 30 mg L−1, and the voltage is 4.0 V. Figure 7a shows the comparison of electrosorption performance between Ti3C2Tx and Ni-BTC/Ti3C2Tx. The removal efficiency of Ni-BTC/Ti3C2Tx exhibits a significant improvement compared to Ti3C2Tx. The improved removal efficiency of Ni-BTC/Ti3C2Tx is because Ni-BTC/Ti3C2Tx three-dimensional composite electrode materials can avoid the stacking problem of Ti3C2Tx MXene and provide rich charge transfer paths, which is beneficial for the transfer and diffusion of electrolyte ions and electrons. Moreover, introducing Ti3C2Tx MXene can improve Ni-BTC’s conductivity and provide additional pseudo-capacitance contribution. For comparison, the adsorption test of Ni-BTC/Ti3C2Tx was studied. Without any applied voltage, the adsorption capacity of chromium(VI) by Ni-BTC/Ti3C2Tx (18.39 mg g−1) is much lower than that with an applied voltage.
The kinetic behavior of Ti3C2Tx and Ni-BTC/Ti3C2Tx in chromium(VI) aqueous solution was studied further to analyze the process and mechanism of capacitive deionization. From Fig. 7a, chromium(VI) is rapidly removed in the first 30 min and gradually reaches equilibrium. This is due to surface adsorption in the initial stage and a high concentration of chromium(VI), rapidly diffusing to the surface of Ti3C2Tx and Ni-BTC/Ti3C2Tx for adsorption. In the wake of the progress of the adsorption process, a part of the adsorption site on the adsorbent is occupied, leading to a decrease in the adsorption rates. Moreover, the concentration of chromium(VI) in the solution decreases as the adsorption process progresses, leading to a decrease in chromium(VI) adsorption rate by Ti3C2Tx and Ni-BTC/Ti3C2Tx.
The electroabsorption behavior of Ti3C2Tx and Ni-BTC/Ti3C2Tx has been explored using pseudo-first-order, pseudo-second-order, and elovich kinetic models. The kinetic fitted curves of Ti3C2Tx and Ni-BTC/Ti3C2Tx are shown in Fig. 7b–d. The relevant kinetic parameters are calculated by fitting the intercepts and slopes of the linear equations listed in Table S1. The R2 values of the pseudo-second-order model of Ti3C2Tx and Ni-BTC/Ti3C2Tx are 0.9996, greater than the R2 values of the other two models. In addition, the actual saturated adsorption capacity qe(exp) is closer to that of Ti3C2Tx and Ni-BTC/Ti3C2Tx calculated by pseudo-second-order model. It can be seen that the adsorption kinetics of chromium(VI) by Ti3C2Tx and Ni-BTC/Ti3C2Tx conform to the pseudo-second-order model, which proves that the chemisorption processes of chromium (VI) by Ti3C2Tx and Ni-BTC/Ti3C2Tx occupies a significant part in electrosorption process.
In addition, to evaluate the rate-determining step, the Weber-Morris formula was used to simulate further the adsorption behavior of chromium on Ti3C2Tx and Ni-BTC/Ti3C2Tx (Fig. 7e and Table S1). The intra-particle diffusion model diagram of Ti3C2Tx and Ni-BTC/Ti3C2Tx is divided into three phases. The rapid movement of chromium(VI) from the liquid phase to the outer surface of Ti3C2Tx and Ni-BTC/Ti3C2Tx is the first stage, which is the mass transfer process stage. The second phase is the capture process, where the chromium(VI) is adsorbed onto the inner surface of Ti3C2Tx and Ni-BTC/Ti3C2Tx. The third stage is the diffusion of chromium(VI) into macropores and mesopores. The adsorption rate is the lowest in this stage. The adsorption rate constant Kintra of Ni-BTC/Ti3C2Tx is much larger than that of Ti3C2Tx, demonstrating the rapid chromium(VI) adsorption by Ni-BTC/Ti3C2Tx.
Cyclic stability experiments were conducted to further study the performance of Ni-BTC/Ti3C2Tx electrodes. Figure 7f illustrates that the electroabsorption capacity did not significantly reduce after five cycles, indicating that Ni-BTC/Ti3C2Tx has excellent reusability. Therefore, Ni-BTC/Ti3C2Tx should be a promising electrode material for electroabsorption.
Ni-BTC nanoparticles were grown on Ti3C2Tx MXene using in-situ growth and hydrothermal methods to prepare three-dimensional Ni-BTC/Ti3C2Tx composite electrode materials, thereby avoiding the stacking problem of Ti3C2Tx MXene and providing rich charge transfer paths, improving the conductivity of MOF materials, and providing additional pseudocapacitive contributions. In the chromium(VI) removal process, three-dimensional Ni-BTC/Ti3C2Tx composite electrode material can provide more ion accumulation and electroabsorption sites, which can be more effective in removing chromium(VI). The XPS analysis of Ni-BTC/Ti3C2Tx after the CDI processes was performed (Fig. S4). An additional Cr 2p peak at 576–589 eV appears after the CDI process. The bonding and chemical valence states of Cr were further analyzed using the high-resolution spectrum. The high-resolution Cr 2p spectrum of Ni-BTC/Ti3C2Tx-Cr (Fig. S4b) can be deconvoluted into four peaks at 576.1, 585.2, 579.2, and 588.1, corresponding to Cr3+ 2p3/2, Cr3+ 2p1/2, Cr6+ 2p3/2 and Cr6+ 2p1/2, respectively, which suggested that chromium(VI) removal process was accompanied by its reduction to chromium(III). The mechanism of removing chromium(VI) from three-dimensional Ni-BTC/Ti3C2Tx composite electrode material includes the following aspects: first, chromium(VI) is adsorbed onto the surface and diffused to the porous structure of Ni-BTC/Ti3C2Tx through physical and chemical adsorption. Second, the electrosorption is because of the electrostatic interaction between Ni-BTC/Ti3C2Tx and chromium(VI)58,59,60. Third, part of the chromium(VI) is reduced to chromium(III). Several removal methods synergistically enhance efficiency, achieving high chromium(VI) removal during electroabsorption.
Table 1 compares the specific capacitance and electroabsorption capacity of Ni-BTC/Ti3C2Tx with previous studies61,62,63,64,65,66,67. The removal efficiency was achieved 100% by using bael fruit shell derived AC at a voltage of 15 V. It is found that the Ni-BTC/Ti3C2Tx as electrode presented high specific capacitance and electroabsorption capacity of chromium(VI). The high specific capacitance and electroabsorption capacity of chromium(VI) may be ascribed to the combined advantages of the large SSA, the multi-level pore structure, and the high pseudo-capacitance of functionalized Ni-BTC/Ti3C2Tx.
Conclusions
In this work, we successfully constructed a Ni-BTC/Ti3C2Tx composite electrode with a three-dimensional charge transfer path and used it as a chromium(VI)-adsorbed electrode in CDI batteries. Based on the adsorption of metal ions by the abundant groups of Ti3C2Tx MXene, the Ni-BTC nanoparticles were grown on Ti3C2Tx by in-situ growth method, and the three-dimensional Ni-BTC/Ti3C2Tx composite electrode materials were prepared. Incorporating Ni-BTC reduces the strong interfacial interaction of Ti3C2Tx and prevents the aggregation of Ti3C2Tx. As a substrate supporting Ni-BTC, Ti3C2Tx can effectively disperse the growth of Ni-BTC and improve the ion transport rate. Ni-BTC/Ti3C2Tx exhibits superior capacitive deionization performance due to the three-dimensional charge transfer pathway and synergistic effect generated by the unique assembly of Ni-BTC and Ti3C2Tx. As expected, the Ni-BTC/Ti3C2Tx electrode showed a high removal efficiency of 94.1% and a high electroabsorption capacity of 124.5 mg g−1. Thus, the three-dimensional Ni-BTC/Ti3C2Tx composite electrode obtained in this work could be suitable for recovering heavy metals from an aqueous solution in CDI.
Data availability
The data that support the findings of this study are available from the corresponding author on request.
References
Mao, M. L., Yan, T. T., Shen, J. J., Zhang, J. P. & Zhang, D. S. Capacitive removal of heavy metal ions from wastewater via an electro-adsorption and electro-reaction coupling process. Environ. Sci. Technol. 55, 3333–3340 (2021).
Wang, H. et al. Bimetallic FeNi nanoparticles immobilized by biomass-derived hierarchically porous carbon for efficient removal of Cr(VI) from aqueous solution. J. Hazard. Mater. 423, 127098 (2022).
Qiu, Y. et al. Removal mechanisms of Cr(VI) and Cr(III) by biochar supported nanosized zero-valent iron: Synergy of adsorption, reduction and transformation. Environ. Pollut. 265, 115018 (2020).
Liang, C., Fu, F. & Tang, B. Mn-incorporated ferrihydrite for Cr(VI) immobilization: Adsorption behavior and the fate of Cr(VI) during aging. J. Hazard. Mater. 417, 126073 (2021).
Saravanan, A., Senthil Kumar, P., Ramesh, B. & Srinivasan, S. Removal of toxic heavy metals using genetically engineered microbes: Molecular tools, risk assessment and management strategies. Chemosphere 298, 134341 (2022).
Wang, S. Y. et al. Highly efficient adsorption and immobilization of U(VI) from aqueous solution by alkalized MXene-supported nanoscale zero-valent iron. J. Hazard. Mater. 408, 124949 (2021).
Rahman, M. A. et al. Removal of arsenate from contaminated waters by novel zirconium and zirconium-iron modified biochar. J. Hazard. Mater. 409, 124488 (2021).
Wu, H., Kim, S. Y., Miwa, M. & Matsuyama, S. Synergistic adsorption behavior of a silica-based adsorbent toward palladium, molybdenum, and zirconium from simulated high-level liquid waste. J. Hazard. Mater. 411, 125136 (2021).
Wang, T. C. et al. Novel Cu(II)–EDTA decomplexation by discharge plasma oxidation and coupled Cu removal by alkaline precipitation: Underneath mechanisms. Environ. Sci. Technol. 52, 7884–7891 (2018).
Khurshid, H., Mustafa, M. R. U. & Isa, M. H. Adsorption of chromium, copper, lead and mercury ions from aqueous solution using bio and nano adsorbents: A review of recent trends in the application of AC, BC, nZVI and MXene. Environ. Res. 212, 113138 (2022).
Galán, B., Castañeda, D. & Ortiz, I. Removal and recovery of Cr(VI) from polluted ground waters: A comparative study of ion-exchange technologies. Water Res. 39, 4317–4324 (2005).
Awual, M. R., Hasan, M. M. & Znad, H. Organic–inorganic based nano-conjugate adsorbent for selective palladium(II) detection, separation and recovery. Chem. Eng. J. 259, 611–619 (2015).
Li, J. et al. Functionalization of biomass carbonaceous aerogels and their application as electrode materials for electro-enhanced recovery of metal ions. Environ. Sci Nano 4, 1114–1123 (2017).
Zhang, Y., Zhou, J., Wang, D., Cao, R. & Li, J. Performance of MXene incorporated MOF-derived carbon electrode on deionization of uranium(VI). Chem. Eng. J. 430, 132702 (2022).
Tang, W. W. et al. Electro-assisted adsorption of Zn(II) on activated carbon cloth in batch-flow mode: Experimental and theoretical investigations. Environ. Sci. Technol. 53, 2670–2678 (2019).
Oladunni, J. et al. A comprehensive review on recently developed carbon based nanocomposites for capacitive deionization: From theory to practice. Sep. Purif. Technol. 207, 291–320 (2018).
Gamaethiralalage, J. G. et al. Recent advances in ion selectivity with capacitive deionization. Energy Environ. Sci. 14, 1095–1120 (2021).
Gao, T. et al. Mesoporous carbon derived from ZIF-8 for high efficient electrosorption. Desalination 451, 133–138 (2019).
Zhang, J. et al. Enhanced capacitive deionization of saline water using N-doped rod-like porous carbon derived from dual-ligand metal–organic frameworks. Environ. Sci. Nano 7, 926–937 (2020).
Kim, J., Kim, J., Kim, J. H. & Park, H. S. Hierarchically open-porous nitrogen-incorporated carbon polyhedrons derived from metal-organic frameworks for improved CDI performance. Chem. Eng. J. 382, 122996 (2020).
Lu, J. M. et al. Functionalization of mesoporous carbons derived from pomelo peel as capacitive electrodes for preferential removal/recovery of copper and lead from contaminated water. Chem. Eng. J. 433, 134508 (2022).
Dai, M. et al. Electrosorption of As(III) in aqueous solutions with activated carbon as the electrode. Appl. Surf. Sci. 434, 816–821 (2018).
Liu, X. J. & Wang, J. L. Electro-assisted adsorption of Cs(I) and Co(II) from aqueous solution by capacitive deionization with activated carbon cloth/graphene oxide composite electrode. Sci. Total Environ. 749, 141524 (2020).
Liu, P. Y., Yan, T. T., Zhang, J. P., Shi, L. Y. & Zhang, D. S. Separation and recovery of heavy metal ions and salt ions from wastewater by 3D graphene-based asymmetric electrodes via capacitive deionization. J. Mater. Chem. A 5, 14748–14757 (2017).
Wang, Y. et al. Layered metal oxide nanosheets with enhanced interlayer space for electrochemical deionization. Adv. Mater. 35, 2210871 (2023).
Ma, J., Cheng, Y., Wang, L., Dai, X. & Yu, F. Free-standing Ti3C2Tx MXene film as binder-free electrode in capacitive deionization with an ultrahigh desalination capacity. Chem. Eng. J. 384, 123329 (2020).
Bao, W. et al. Porous Cryo-dried MXene for efficient capacitive deionization. Joule 2, 778–787 (2018).
Pasta, M., Wessells, C. D., Cui, Y. & Mantia, F. L. A desalination battey. Nano Lett. 12, 839–843 (2012).
Lee, J., Kim, S., Kim, C. & Yoon, J. Hybird capacitive deionization to enhance the desalination performance of capacitive techniques. Energ. Environ. Sci. 7, 3683–3689 (2014).
Wang, S. Y. et al. Copper removal and recovery from electroplating effluent with wide pH ranges through hybrid capacitive deionization using CuSe electrode. J. Hazard. Mater. 457, 131785 (2023).
Wang, D. et al. Direct synthesis and chemical vapor deposition of 2D carbide and nitride MXenes. Science 379, 1242–1247 (2023).
Tyndall, D. et al. Understanding the effect of MXene in a TMO/MXene hybrid catalyst for the oxygen evolution reaction. Nature 7, 1–11 (2023).
Zhang, K. J. et al. Highly efficient capacitive deionization of copper(II) ions from wastewater in symmetric Ti3C2Tx MXene-based electrode: Performance, optimization and deionization mechanism. J. Environ. Chem. Eng. 12, 112019 (2024).
Mu, W. J. et al. Removal of radioactive palladium based on novel 2D titanium carbides. Chem. Eng. J. 358, 283–290 (2019).
Liu, N. N. et al. Ti3C2-MXene partially derived hierarchical 1D/2D TiO2/Ti3C2 heterostructure electrode for high-performance capacitive deionization. Adv. Sci. 10, 2204041 (2023).
Zang, X. B. et al. Enhancing capacitance performance of Ti3C2Tx MXene as electrode materials of supercapacitor: From controlled preparation to composite structure construction. Nanomicro Lett. 12, 1–24 (2020).
Li, S. J. et al. Complete dehydrogenation of N2H4BH3 over noble-metal-free Ni0.5Fe0.5-CeOx/MIL-101 with high activity and 100% H2 selectivity. Adv. Energy Mater. 8, 1800625 (2018).
Torad, N. L. et al. MOF-derived hybrid nanoarchitectured carbons for gas discrimination of volatile aromatic hydrocarbons. Carbon 168, 55–64 (2020).
Li, Z. L. et al. Surface nanopore engineering of 2D MXenes for targeted and synergistic multitherapies of hepatocellular carcinoma. Adv. Mater. 30, 1706981 (2018).
Rakhi, R. B., Nayak, P., Xia, C. & Alshareef, H. N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 6, 36422 (2016).
Yan, J. M. et al. AuPd–MnOx/MOF–Graphene: An efficient catalyst for hydrogen production from formic acid at room temperature. Adv. Energy Mater. 5, 1500107 (2015).
Zhou, H. et al. Engineering the Cu/Mo2CTx (MXene) interface to drive CO2 hydrogenation to methanol. Nat. Catal. 4, 860–871 (2021).
Li, X. R., Wang, C. L., Xue, H. G., Pang, H. & Xu, Q. Electrocatalysts optimized with nitrogen coordination for high-performance oxygen evolution reaction. Coordin. Chem. Rev. 422, 213468 (2020).
Gan, Q., He, H., Zhao, K., He, Z. & Liu, S. Morphology-dependent electrochemical performance of Ni-1,3,5-benzenetricarboxylate metal-organic frameworks as an anode material for Li-ion batteries. J. Colloid Interface Sci. 530, 127–136 (2018).
Maniam, P. & Stock, N. Investigation of porous Ni-based metal-organic frameworks containing paddle-wheel type inorganic building units via high-throughput methods. Inorg. Chem. 50, 5085–5097 (2011).
Wang, W. T. et al. When MOFs meet MXenes: Superior ORR performance in both alkaline and acidic solutions. J. Mater. Chem. A 9, 3952–3960 (2021).
Shang, M., Chen, X., Li, B. & Niu, J. A fast charge/discharge and wide-temperature battery with a germanium oxide layer on a Ti3C2 MXene matrix as anode. ACS Nano 14, 3678–3686 (2020).
Zhu, Z. H. et al. In situ self-assembled macroporous interconnected nanosheet arrays of Ni-1,3,5-benzenetricarboxylate metal organic framework on Ti mesh as high-performance oxygen evolution electrodes. J. Colloid Interf. Sci. 639, 274–283 (2023).
Ran, F. et al. Ultrathin 2D metal-organic framework nanosheets in situ interpenetrated by functional CNTs for hybrid energy storage device. Nanomicro Lett. 12, 46 (2020).
Meng, J. et al. Identification of phase control of carbon-confined Nb2O5 nanoparticles toward high-performance lithium storage. Adv. Energy Mater. 9, 1802695 (2019).
Halim, J. et al. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 362, 406–417 (2016).
Zhou, J. et al. Discovery of quantitative electronic structure-OER activity relationship in metal-organic framework electrocatalysts using an integrated theoretical-experimental approach. Adv. Funct. Mater. 31, 2102066 (2021).
Zhang, H. et al. Ni-Al layered double hydroxide with regulated interlayer spacing as electrode for aqueous asymmetric supercapacitor. Chem. Eng. J. 368, 905–913 (2019).
Tarimo, D. J., Oyedotun, K. O., Mirghni, A. A., Sylla, N. F. & Manyala, N. High energy and excellent stability asymmetric supercapacitor derived from sulphur-reduced graphene oxide/manganese dioxide composite and activated carbon from peanut shell. Electrochim. Acta 353, 136498 (2020).
Liu, Y. et al. Partial oxidation of FeS nanoparticles enhances Cr(VI) sequestration. Environ. Sci. Technol. 56, 13954–13963 (2022).
Cao, L. et al. Bimetallic sulfide Sb2S3@FeS2 hollow nanorods as high-performance anode materials for sodium-ion batteries. ACS Nano 14, 3610–3620 (2020).
Zhao, C. T. et al. Enhanced sodium storage capability enabled by super wide-interlayer-spacing MoS2 integrated on carbon fibers. Nano Energy 41, 66–74 (2017).
Liu, S. et al. Precisely modulating the branching functional groups of MIL-53(Al) for highly efficient sequestration of uranium. J. Environ. Chem. Eng. 11, 109393 (2023).
Wang, T., Sun, Y., Bai, L., Han, C. & Sun, X. Ultrafast removal of Cr(VI) by chitosan coated biochar-supported nano zero-valent iron aerogel from aqueous solution: Application performance and reaction mechanism. Sep. Purif. Technol. 306, 122631 (2023).
Ding, J. et al. Adsorption and reduction of Cr(VI) together with Cr(III) sequestration by polyaniline confined in pores of polystyrene beads. Environ. Sci. Technol. 52, 12602–12611 (2018).
Gaikwad, M. S. & Balomajumder, C. Simultaneous electrosorptive removal of chromium(VI) and fluoride ions by capacitive deionization (CDI): Multicomponent isotherm modeling and kinetic study. Sep. Purif. Technol. 186, 272–281 (2017).
Gaikwad, M. S., Balomajumder, C. & Tiwari, A. K. Acid treated RHWBAC electrode performance for Cr(VI) removal by capacitive deionization and CFD analysis study. Chemosphere 254, 126781 (2020).
Mohanraj, P., AllwinEbinesar, J. S. S., Amala, J. & Bhuvaneshwari, S. Biocomposite based electrode for effective removal of Cr (VI) heavy metal via capacitive deionization. Chem. Eng. Commun. 207, 775–789 (2020).
Feng, J., Xiong, S., Ren, L. & Wang, Y. Atomic layer deposition of TiO2 on carbonnanotubes membrane for capacitive deionization removal of chromium from water. Chin. J. Chem. Eng. 45, 15–21 (2022).
Bharath, G. et al. Enhanced electrochemical performances of peanut shell derived activated carbon and its Fe3O4 nanocomposites for capacitive deionization of Cr(VI) ions. Sci. Total Environ. 691, 713–726 (2019).
Nguyen, T. K. A., Huynh, T. V. & Doong, R. Enhanced capacitive deionization of Cr(VI) using functionalized metal carbide 2D framework and badam tree leaf-derived carbon as the asymmetric electrode materials. Chem. Eng. J. 475, 146439 (2023).
Hou, S. et al. Synergistic conversion and removal of total Cr from aqueous solution by photocatalysis and capacitive deionization. Chem. Eng. J. 337, 398–404 (2018).
Acknowledgements
This work was supported by Hebei Natural Science Foundation (No.E2022411007), Collaboration & Innovation Platform Project of National Independent Innovation Demonstration Zone (Fuzhou, Xiamen & Quanzhou) (No.3502ZCQXT2023004), Science and Technology Planning Project from Hebei Provincial Department of Education (ZD2022135), National Natural Science Foundation of China (52302369).
Author information
Authors and Affiliations
Contributions
X.Z. Conceptualization, Investigation, Methodology, Resources, Writing-original draft; Z.W. Formal analysis, Investigation, Writing-review & editing; X.G. Investigation, Resources. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, X., Wang, Z. & Guo, X. Confinement-induced Ni-based MOF formed on Ti3C2Tx MXene support for enhanced capacitive deionization of chromium(VI). Sci Rep 15, 3727 (2025). https://doi.org/10.1038/s41598-025-87642-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87642-z