Abstract
Hepatitis B virus (HBV) whole genome sequencing (WGS) is currently limited as the DNA viral loads (VL) of many clinical samples are below the threshold required to generate full genomes using current sequencing methods. We developed two pan-genotypic viral enrichment methods, using probe-based capture and tiled amplicon PCR (HEP-TILE) for HBV WGS. We demonstrate using mock samples that both enrichment methods are pan-genotypic (genotypes A-J). Using clinical samples, we demonstrate that HEP-TILE amplification successfully amplifies full genomes at the lowest HBV VL tested (30 IU/ml), and the PCR products can be sequenced using both Nanopore and Illumina platforms. Probe-based capture with Illumina sequencing required VL > 300,000 IU/ml to generate full length HBV genomes. The capture-Illumina and HEP-TILE-Nanopore pipelines had consensus sequencing accuracy of 100% in mock samples with known DNA sequences. Together, these protocols will facilitate the generation of HBV sequence data, enabling a more accurate and representative picture of HBV molecular epidemiology, cast light on persistence and pathogenesis, and enhance understanding of the outcomes of infection and its treatment.
Similar content being viewed by others
Introduction
Worldwide an estimated 254 million people are living with chronic Hepatitis B virus (HBV) infection (CHB)1. Despite the availability of prophylactic vaccines and suppressive antiviral therapies, the annual toll of approximately 1.1 million HBV-related deaths in 20221 highlights the persistent global challenge faced. HBV whole genome sequencing (WGS) provides genetic insights into the pathogen at population and individual levels2. At a population level, WGS improves our understanding and monitoring of HBV drug resistance, vaccine efficacy, diagnostic escape and tracking of outbreaks. In individuals, WGS has the potential to contribute to personalised healthcare, e.g. informing modification of antiviral regimens to take into account antiviral resistance associated mutations (RAMs), and risk-stratified hepatocellular carcinoma (HCC) surveillance3.
There are several challenges associated with HBV WGS. Target enrichment using probe based capture, HBV specific polymerase chain reaction (PCR) or rolling circle amplification (RCA)4,5 is required when performing HBV WGS due to frequently low DNA viral loads (VL)6, the tiny genome (3.2 kB), and abundant host nucleic acid background. Sequencing low VL samples, although challenging, is important at both an individual and population level, in order to understand mechanisms of transmission, persistence, immunological control and clearance, breakthrough viraemia on treatment, and for characterising occult HBV infection. HBV has ten distinct genotypes (A–J), with > 8% nucleotide divergence, which are geographically distributed and associated with infection outcomes7. This diversity leads to challenges in designing pan-genotypic capture probes and PCR primers. The structure of the HBV genome also presents challenges for WGS. In the blood, HBV DNA is present predominantly in a relaxed-circular (rc-DNA) form, a circular, partially double-stranded (ds)DNA configuration (Fig. 1A)8; neither positive nor negative DNA strand is continuous.
Design of HBV targeted enrichment methods. (A) HBV genome structure: relaxed circular DNA (rc-DNA). The complete negative strand is discontinuous, with a ‘nick’ and covalently bound polymerase. The positive strand is incomplete with a variable length and RNA tail. (B) Probe design schematic for HBV probe based enrichment. An initial set of probes (red) are designed based on non-recombinant whole genomes downloaded from HBVdb20. Additional probes (orange) are added in regions of high diversity, using an extended reference sequence to account for the circular genome, and to account for insertions/deletions, then the final probe panel confirmed. Blue dots indicate 5’ biotinylation. (C) Multiplex tiling PCR for the circular HBV genome. Generation of tiled schemes from linear reference genomes leaves a gap in coverage for a circular genome. An additional amplicon spanning the start/end of the linear reference ensures a theoretical maximum 100% genome coverage can be obtained. Primer positions and amplicon length for the final HEP-TILE scheme (hbv/600/v2.1.0). Numbering of nt positions based on X02763 reference genome. Created in BioRender. Lumley, S. (2024) BioRender.com/r40b439.
We set out to optimise and validate workflows for HBV WGS. Due to limitations in sensitivity of our RCA protocol4 (related to difficulty in generating a fully circular template), we initially used probe-based enrichment, a strategy which has previously been validated across diverse pathogens9,10,11. The method is based on the design of biotinylated single-stranded DNA probes, designed to hybridise with target viral sequences within a sequencing library, and selectively capture HBV DNA, thereby enriching the final library for HBV DNA and increasing on-target sequencing yield (Fig. 1B).
Second, due to limitations in sensitivity of probe-based enrichment, we developed a tiled amplicon approach for HBV whole genome sequencing (HEP-TILE) (Fig. 1C) using HBV-specific primers designed using PrimalScheme3, successor to PrimalScheme12,13. PrimalScheme3 includes key updates to allow the generation of WGS from pathogens with circular genomes, with primers able to deal with greater pathogen diversity. The HBV sequencing wet and dry lab workflows build on the widely used ARTIC-Network nCoV-2019 sequencing protocol14 and fieldbioinformatics15, both of which have been used extensively worldwide during the SARS-CoV-2 pandemic.
Scale up of WGS requires development of open access, easily deployable sequencing methods ideally building on existing infrastructure and expertise. By developing the protocols described here, we aim to facilitate the generation of HBV sequence data globally, to obtain a more accurate and representative picture of HBV genetics and molecular epidemiology.
Methods
Samples
Mock samples
We prepared mock HBV samples using plasmids containing ~ 1.3 copies of the HBV genome representing genotypes A-J in a pUC57 backbone, (genotypes A-F and J from Bannister et al.16, G and I designed in-house and produced by GeneArt) (Supplementary Table 1). We spiked plasmids into non-infected human serum (Merck, H5667) at a concentration of 105 plasmid copies/ml.
Clinical samples
We used 63 plasma samples collected between 2011 and 2023 from adults with CHB in three settings: (1) outpatient clinics in Oxford (UK)6,17, (2) outpatient clinics in Tygerberg Hospital, Cape Town (Stellenbosch University) and public sector hospital services in Bloemfontein (University of the Free State), South Africa6, and (3) participants of the Uganda Liver Disease Study (Kalungu District, south west Uganda)18,19. We selected samples to represent a range of VL and genotypes; methods were developed iteratively and based on available samples (Fig. 2, Supplementary Table 2).
Overview of sequencing workflows. Numbers of samples sequenced at each stage indicated. Due to sample availability, project stage, and local infrastructure, we used different subsets of samples to validate each method. In the clinical sample set from South Africa, if residual extract was available after capture, two further aliquots were taken forward for HEP-TILE enrichment and sequencing on Nanopore and Illumina. Created in BioRender. Lumley, S. (2024) BioRender.com/k41p399.
Written informed consent was provided by participants at enrollment. Approval for this work was provided as follows, all methods were performed in accordance with the relevant protocols:
-
UK patients: Oxford Research Ethics Committee A (ref. 09/H0604/20).
-
South Africa/Stellenbosch patients: Stellenbosch University (ref. N17/01/013) and OxTREC (ref 1–18).
-
South Africa/Bloemfontein patients: University of the Free State (ref. UFS-HSD2018/0193-0001) and OxTREC (ref 1–18).
-
Uganda patients: Uganda Virus Research Institute Research and Ethics Committee (ref. GC/127/19/04/711), Uganda National Council for Science and Technology, and Oxford Tropical Research Ethics Committee (ref 50 − 18).
We collected blood samples in EDTA tubes. To separate plasma, we centrifuged whole blood at 1800 rpm for 10 min. We harvested the supernatant and stored it at − 80 °C.
Quantification of HBV DNA VL was performed on the clinically validated Abbott M2000 platform by Oxford University Hospitals (OUH) diagnostic microbiology laboratory for UK samples, and with the Cobas Ampliprep/Taqman HBV test for Ugandan and South African samples (performed by the Clinical Diagnostic Laboratories for MRC/UVRI/LSHTM Research Institute Uganda and the NHLS Tygerberg Virology Laboratory respectively). VL ranged from 1.5 to > 8.3 log10 IU/ml (i.e. above the assay limit of quantification) (Supplementary Table 2).
Host depletion and nucleic acid extraction
For mock and clinical samples initially being enriched by capture, we performed host depletion with micrococcal nuclease (NEB catalogue number M0247S, 250 µl plasma with 1.25 µl MNase and 23.75 µl MNase buffer incubated for 20 min at 37 °C) followed by extraction using the Kingfisher Apex Magmax viral/pathogen nucleic acid isolation kit (two aliquots of MNase-treated plasma eluted into 50 µl of kit elution buffer, combined followed by a SPRIselect bead clean up to reduce volume to 40 µl).
For samples from UK and Uganda for HEP-TILE Nanopore sequencing, we extracted DNA using the QIAgen MinElute Virus Spin kit from either 200–400 µl input plasma. Double volume (400 µl) extraction was used if VL was < 3 log10 IU/ml (with double volume protease, ethanol and buffer AL). We used carrier RNA as per manufacturer’s instructions, and eluted into 45 µl H2O. The sample volume and extraction method used for each sample is detailed in Supplementary Table 2.
Public data for probe/primer design
We based our probe and primer design on downloads of all available HBV non-recombinant whole genomes from the Hepatitis B Virus Database (HBVdb)20 on 31st January 2019 for probe design and 15th February 2024 for primer design (Supplementary Table 3). In addition for HEP-TILE, genomes for genotypes I and J were sourced from McNaughton et al.7.
HBV probe based enrichment
Probe design
We used RaxML21 with a general time reversible model with Gamma model of rate heterogeneity (“-m GTRGAMMA” option in RAxML) to infer a maximum likelihood phylogeny of HBV full genomes. Next, we used RAxML to infer the ancestral sequences and as input we used the midpoint rooted tree and the sequence of our isolates with the GTRGAMMA option. We used the ancestral sequence at the root of the tree to design the first set of probes assuming that this sequence on average has the least amount of divergence relative to all other isolates.
As the HBV genome is circular, we added 120 bases from the beginning of the ancestral root sequence to the end of the sequence to ensure that capture probes cover the break point which is used to present the genome linearly. We then divided the ancestral root sequence into 120 nucleotide (nt) segments with 60 bases overlap which resulted in an initial set of 55 probes (Fig. 1B). Genotype G has an insertion of 36 bases relative to other genotypes in the core gene. As the ancestral root sequence contained this insertion, we also designed a probe of 120 nt which lacked this insertion. Furthermore, genotype D has a deletion of 33 bases in the pre-S1 region relative to all other genotypes. To ensure a probe covers this region for genotype D, we designed a probe of 120 nt that lacked this region.
Our previous work in HCV probe-based sequence capture demonstrated that probes of 120 bases long can tolerate up to 20% divergence relative to their target sequence before the efficiency of capture drops11. To ensure that the designed probes are within a maximum of 20% divergence of each viral sequence, we divided the dataset based on genotype and created a consensus sequence for each genotype. We then aligned the probes to each viral sequence and measured the proportion of mismatches between each probe and the isolate. For each viral sequence, if a continuous region of ≥ 60 bases diverged from the probe sequences by > 20%, a new probe was designed for the region using the genotype consensus sequence. As a quality control step, we removed any potential sequences that contained an “N” as we assumed that the sequence may be of low quality. Additionally we counted the number of ambiguous nucleotides and any viral sequence containing ≥ 5 ambiguous nucleotides was also removed. The final probe set contained 74 probes.
The probe sequences and the set of HBV genome sequences that were used for their design are presented in supplementary Tables 4 and can also be downloaded from the following webpage: https://figshare.com/articles/dataset/HBV_probe_sequences/22127015.
Library preparation, hybrid capture and sequencing protocol
We prepared libraries for Illumina sequencing using the NEBNext Ultra II FS DNA library preparation kit (protocol for use with inputs < 100 ng). We used an input of 26 µL extracted DNA for the fragmentation/end preparation reaction and fragmented for 3 min, with a negative (mastermix only) control. We performed indexing PCR using NEBNext multiplex Oligos Unique Dual Index and 13 amplification cycles. We pooled the amplified libraries corresponding to each aliquot in equivolume proportions to generate a final multiplex library. We purified the pool using SPRIselect beads, eluted into a final volume of 20 µL and subsequently quantified using High Sensitivity dsDNA Qubit assay (Invitrogen) and Agilent 2100 Bioanalyzer high sensitivity DNA protocol.
We concentrated a 4.4 µg aliquot of the final multiplexed library using the manufacturer’s AMPure XP Bead DNA concentration protocol, then enriched for HBV using the custom-designed probe panel (IDT Technologies) and xGen Hybridization and Wash Kit (IDT Technologies) following manufacturer’s ‘tube protocol’. We amplified the final enriched library (12 cycles on-bead PCR), repurified (eluting into 12 µL EB), quantified using High Sensitivity dsDNA Qubit assay (Invitrogen) and Agilent 2100 Bioanalyzer high sensitivity DNA protocol, then sequenced on the Miseq v3 with 2 × 300nt paired-end reads or on a partial lane of Novaseq X 2 × 150nt paired-end reads.
Data analysis—capture Illumina pipeline
We trimmed de-multiplexed sequence read-pairs of low-quality bases using QUASR (version 7.01)22, trimmed adapter sequences with CutAdapt (version 4.8)23 and Skewer (version 0.2.2)24 and subsequently discarded if either read had < 50 bp sequence remaining. We mapped the cleaned read pairs to human reference genome hg19 using Bowtie2 (version 2.2.4)25 and excluded from further analyses (-minins 0, -maxins 1000). All nonhuman read pairs were mapped using BWA (version 0.7.10)26,27 mem tool with default parameters to a set of 44 HBV references covering all known HBV genotypes and subgenotypes to choose an appropriate reference7. We chose the HBV reference with the greatest number of HBV reads mapping to it as the genetically closest reference to the sequenced isolate. Next, we re-mapped all non-human read pairs to the closest HBV reference. We then used Picard markduplicates tool (version 1.111)28 to remove duplicate read pairs (where read pairs starting in the same place and ending in the same place on the genome are assumed to be PCR duplicates). A base was called at the consensus level if allele depth was above x5, with HBV genome coverage calculated by determining the percentage of bases called at the consensus level.
HBV tiled amplicon scheme (HEP-TILE)
PrimalScheme 3
We developed a pan-genotypic HBV scheme using an early version of PrimalScheme329, the successor to PrimalScheme13, a web-based primer design tool for developing multiplex primer schemes. A number of changes were made in PrimalScheme3 to enable us to generate an overlapping (tiled) amplicon scheme which covered the circular HBV genome, utilising a number of discrete primers at each position to handle intraspecies diversity.
Usually an amplicon scheme results in a short sequence at each end of the reference genome which is not covered due to primer placement constraints and downstream primer-trimming. With the conventionally designated ‘start site’ for the circular HBV genome being within the overlapping surface and reverse transcriptase genes (Fig. 1), this results in the loss of epidemiologically and clinically relevant information. PrimalScheme3 introduces an option to add an amplicon spanning the start/end of circular genomes, resulting in 100% genome coverage.
Intra-species diversity has posed an issue for amplicon sequencing, as variation within the primer binding sites reduces primer binding efficacy. The original PrimalScheme identified conserved primer binding sites by heavily penalising those with variation within the input genomes. This approach is effective for closely related genomes, such as outbreak strains, but for diverse inputs a different approach is needed. PrimalScheme3 handles diversity with the use of ‘primer clouds’ i.e. a discrete set of 3’-anchored primers which cover all mutations above a user-specified frequency, without the use of ambiguous bases. This approach reduces the negative effects of adding additional primers to a multiplexed PCR, such as primer-primer interactions, and mispriming.
Multiplex primer pool design for HBV (HEP-TILE)
We aligned HBVdb20 HBV genomes from each genotype (A-H) separately using MAFFT30, and phylogenetically downsampled each genotype to 0.95 relative tree length using TreeMMer31. We combined each downsampled genotype’s genomes with genotype I and J sequences from7, aligned with MAFFT30, and input into PrimalScheme3 command line tool, with the options; --ampliconsize 600, --minbasefreq 0.02, --backtrack and –minoverlap 20.
Initially, we developed a 500 bp, eight amplicon scheme (Supplementary Table 5)29,32, however when trialled, one amplicon spanning 1700–2000 nt consistently dropped out in samples with VL < 5 log10 IU/ml (Supplementary Fig. 1A and 1B). We hypothesise that is explained by the structure of HBV DNA: in low VL samples only rc-DNA is present, which has discontinuities in both the positive and negative DNA strands in that region leading to amplicon dropout. HBV pgRNA and double stranded linear DNA are also not continuous in that region. In contrast, in higher VL samples, covalently closed circular DNA (cccDNA) is also present in the plasma33,34. cccDNA has continuous positive and negative strands which act as a template for this amplicon, even if present at a very low percentage of the overall viral population in the blood, this could still provide a significant number of copy templates/ml, for example if VL 1010 IUml, if cccDNA was present at 0.1% of the viral population this would give 107 copies/ml cccDNA template. We identified a suitable location for primers spanning this region, taking into account the structure of rc-DNA, and re-designed a 600 bp primer scheme (hbv/600/v2.1.0) (Supplementary Table 6, supplementary Fig. 1C–F), resulting in a six amplicon scheme with amplicons ~ 600–715 bp long, generating a theoretical 100% HBV genome coverage (Fig. 1C). This final scheme uses 131 primers to cover the HBV diversity present in our reference dataset.
HEP-TILE PCR and sequencing protocol
Amplification using primer scheme hbv/600/v2.1.0 and library preparation were performed by adapting the nCoV-2019 LoCost v3 sequencing protocol14, with the removal of the reverse transcriptase step (full protocol on protocols.io35). Extracted DNA from samples with VL > 6 log10 IU/ml were diluted 10 fold prior to PCR. Negative (mastermix only) controls were added at the PCR and library prep stages. We used the native barcoding kit SQK-NBD114.96 and R10.4.1 MinION flowcells (Oxford Nanopore Technologies, ONT), multiplexing up to 96 samples per run and sequencing for 72 h. For Illumina sequencing of tiled amplicons, we fragmented the amplicons and performed library preparation using the NEBNext Ultra II FS DNA kit, multiplexing up to 96 samples per run and sequenced on a partial lane of Novaseq X 150PE.
Data analysis—HEP-TILE Nanopore pipeline
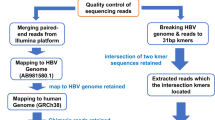
We basecalled reads and demultiplexed data using dorado (0.7). We generated a novel pipeline hbv-fieldbioinformatics29,36 to produce consensus genomes. Reads are mapped against representative genomes from all genotypes7, with the genome with the most mapped reads being selected as the primary reference. To handle the circular genome, an intermediate circular primary reference genome is created, where the sequence of the circular amplicon is appended to the 3′ end of the reference. Reads are then remapped to the circular reference with minimap2 (2.26), primers are trimmed, followed by variant calling by LongShot and Medaka (0.4.5, 1.11.3). Variants and read depth for the appended region are then mapped back to their corresponding position on the original reference. A base was called at the consensus level if allele depth was over ×20, with HBV genome coverage calculated by determining the percentage of called consensus bases.
Data analysis—HEP-TILE Illumina pipeline
Similar to the capture-based Illumina pipeline, we initially used QUASR (version 7.01)22 to filter out poor de-multiplexed sequencing reads. We then trimmed primer sequences by using CutAdapt (version 4.8)23 and Skewer (version 0.2.2). Human-like reads were excluded with Bowtie2 (version 2.2.4)25. We extended all 44 HBV genome references by adding their first 300 bases to the end and mapped the remaining non-human reads to these new HBV reference sequences to identify the genetically closest HBV isolate. In the next two rounds, we mapped the deduplicated reads to (1) the closest HBV isolate and (2) a fine-tuned consensus sequence based on mapping in (1). In the final stage, we cut reads that cross the conventional genome end and combined mappings in the conventional and appended regions. A base was called at the consensus level if allele depth was over ×5, with HBV genome coverage calculated by determining the percentage of called consensus bases.
Determining clinically relevant mutations
HBV genotypes, including recombinants, were determined using the online NCBI genotyping tool37. Resistance associated mutations and vaccine escape mutations were called using the online Geno2pheno HBV tool38.
Software
Graphs were produced using the ggplot2 package in R version 4.2.239, figures were produced with Biorender with a licence to publish40.
Results
Targeted enrichment with probe based capture
Sequencing mock HBV samples enriched with probe based capture using lllumina
We prepared and sequenced 10 mock samples (plasmids with genotypes A-J, 5 log10 copies/ml) in duplicate, with/without probe-based capture on the Illumina Novaseq X, libraries were diluted to 10pM and pooled prior to sequencing. Without capture, a median of 391 (IQR 153–1858) HBV reads per million reads sequenced was generated across 20 mock samples. With capture a median of 563,956 (IQR 397523–896635) HBV reads per million reads sequenced was generated (Supplementary Table 1). Enrichment with probe-based capture led to the generation of full length HBV consensus sequences for all genotypes (Fig. 3A, Supplementary Table 1).
Sequencing clinical HBV samples enriched with probe based capture using Illumina
We enriched a subset of 23 clinical samples (HBV VL 4.21 to > 7.20 log10 IU/ml, as optimisation work established ~ 4 log10 IU/ml as a conservative estimate for the threshold at which full genomes could be obtained at ×5 depth, (Fig. 2) using probe based capture and sequenced the samples on Illumina Novaseq X. A median of 22,377 (IQR 3267 − 135,184) HBV reads per million reads sequenced was generated. The relationship between HBV VL and percentage reads on target is shown in (Fig. 4A, D). Full length (> 98%) genomes at x1 coverage were generated in 18/23 samples, with partial genomes in 5/23. Full length genomes above a minimum x5 read depth (minimum required by bioinformatic pipeline to call base at the consensus level) were generated in 7/23 samples, all of which had VL > 5.5 log10 IU/ml (~ 300,000 IU/ml). Below VL 5.5 log10 only partial genomes at x5 depth were generated (Supplementary Table 2). The negative controls had 0% consensus coverage of the HBV genome.
HBV WGS using capture and amplicon based target enrichment. Top row: Relationship between viral load and percentage of reads on-target (i.e. HBV) for (A) probe-based capture sequenced on Illumina, (B) HEP-TILE sequenced on Nanopore and (C) HEP-TILE sequenced on Illumina. Bottom row: Relationship between viral load and percentage of genome with high confidence consensus for (D) probe-based capture sequenced on Illumina (x5 minimum depth), (E) HEP-TILE sequenced on Nanopore (x20 minimum depth) and (F) HEP-TILE sequenced on Illumina (x5 minimum depth). Points are coloured by the percentage genome coverage. Notes. Panels E and F show an outlier with viral load 5.41 log10 IU/ml, with disproportionately 0% genome coverage. Repeat VL was 3 log10 IU/ml. There is one sample, HEP-1529, in the lower left corner of Fig. 4B with 0.55% reads (260 reads) mapped to the HBV genome, which led to 45% coverage as shown in supplementary Table 2. This is mathematically possible as the minimum theoretical number of reads to obtain a whole genome at x20 depth in a 6 amplicon scheme is only 120 reads.
Targeted enrichment with HEP-TILE tiled amplicon PCR
Sequencing mock HBV samples enriched with HEP-TILE using Nanopore and Illumina
We prepared and sequenced the same 10 mock samples in duplicate, enriching using the HEP-TILE tiled amplicon scheme and sequenced on Nanopore MinION and Illumina Novaseq X. With HEP-TILE Nanopore a median of 997,016 (IQR 994,669–999,427) HBV reads per million reads sequenced was generated. With HEP-TILE Illumina a median of 977,441 (IQR 963,459–982,631) HBV reads per million reads sequenced was generated (Supplementary Table 1). Enrichment with HEP-TILE led to the generation of full-length HBV sequences across genotypes on both platforms (Fig. 3B, C).
Sequencing clinical HBV samples enriched with HEP-TILE using Nanopore
We enriched a subset of 50 clinical samples (HBV VL 1.50 to > 8.23 log10 IU/ml, Fig. 2) using the HEP-TILE protocol and sequenced on the Nanopore MinION. A median of 769,194 (IQR 415,794–999,529) HBV reads per million reads sequenced was generated. The relationship between HBV VL and percentage reads on target is presented (Fig. 4B, E).
Full genome coverage (x1 depth) was generated in 40/50 samples. Full-length HBV consensus genomes at x20 depth (the minimum required to accurately call a mutation), were generated in 40/50 samples, down to the lowest VL tested (1.5 log10 IU/ml). Three isolates were tested with VL 1.5–2 log10 IU/ml. Partial genomes were obtained for the remaining 10 samples. These low coverage samples tended to have lower VL (Fig. 4), although this did not reach statistical significance (p = 0.16, Mann Witney U test, VL not normally distributed). We did not observe any relationship between HBV genotype and inability to produce whole genomes (Supplementary Fig. 2). The negative controls had 0% consensus coverage of the HBV genome.
Sequencing clinical HBV samples enriched with HEP-TILE using Illumina
We selected a subset of 10 samples (based on sample availability) for HEP-TILE amplification, followed by fragmentation and sequencing on Illumina (Novaseq X 150PE partial lane) (Fig. 2). A median of 956,800 (IQR 759,122–977,744) HBV reads per million reads sequenced was generated. The relationship between HBV VL and percentage reads on target is shown in (Fig. 4C, F). Full genome coverage (x1 depth) was generated in 7/10 samples. Full-length consensus HBV genomes (minimum x5 read depth) were generated in 7/10 samples, partial genomes were obtained for 2/10 samples and the pipeline failed to generate a consensus from 1 sample due to low read coverage and anomalous mapping mismatches. The negative controls had 0% consensus coverage of the HBV genome.
Methods comparison
Accuracy of consensus genomes
We prepared and sequenced all 10 mock samples using all three workflows, with each sample processed in duplicate (capture Illumina, HEP-TILE Illumina, HEP-TILE Nanopore). Comparison of consensus sequences generated from mock samples to the known plasmid sequence, demonstrated 100% accuracy of consensus genomes produced with capture-Illumina and HEP-TILE Nanopore and 99.98% with HEP-TILE Illumina (Table 1). Four samples sequenced with HEP-TILE Illumina had mismatches or bases designated ‘N’, however the median number of mismatches and N’s was 0. There were no indels for any of the methods.
Comparison of capture vs. HEP-TILE ability to generate whole genomes from high viral load clinical samples
We sequenced a subset of 10 clinical samples (> 5 log10 IU/ml) using all three workflows so that we could compare the ability of capture vs. HEP-TILE to generate HBV WGS at high VL. At x1 depth, capture-Illumina and HEP-TILE Nanopore generated full genomes in 8/10 samples, with HEP-TILE Illumina in 7/10 samples. At depths required to confidently call consensus (x5 for Illumina, x20 for Nanopore), full genomes were generated in 4/10 samples for capture-Illumina, 8/10 for HEP-TILE Nanopore and 7/10 for HEP-TILE Illumina (Supplementary Table 2).
Determining genotypes from HBV WGS data
We identified clinical samples of genotype A-E, in addition to genotype B/C and D/E recombinants (see “Methods”) (Supplementary Table 2). We were able to call polymorphisms at sites of previously reported resistance associated mutations (RAMs) and vaccine escape mutations (VEMs)38 (details in Supplementary text).
Discussion
Summary of outputs
We have developed two pan-genotypic viral enrichment methods for HBV WGS: HEP-TILE and probe based capture. Both techniques effectively enrich HBV genomes from all known genotypes (A-J), as evidenced by application to mock samples, and increase the percentage of sequencing reads mapping to the HBV genome, therefore reducing the amount of sequence data needed to obtain full genome coverage. We used HBV WGS to call HBV genotypes, including recombinants, which may be missed with partial-genome sequencing, although as for any short read sequencing, recombinants can only be confidently called if breakpoints occur within an amplicon or read. HEP-TILE capitalises on the rapid global expansion of technical expertise and infrastructure for amplicon based WGS that occurred during the SARS-CoV-2 pandemic. Having a toolbox of diverse sequencing methods allows the most appropriate method to be selected according to local infrastructure, cost and throughput requirements (Table 2).
Comparison between methods
HEP-TILE offers the greatest flexibility to sequence across a range of HBV VL, able to sequence full genomes down to 1.50 log10 IU/ml (~ 30 IU/ml, lowest tested here), using standard extraction from 200 to 400 µl plasma. The primers are designed to be pan-genotypic (A-J), unlike other current schemes designed for genotypes A-E41,42. The ~ 600–715 bp amplicons used in HEP-TILE improve sensitivity compared to single amplicon schemes43, enhancing sequencing success from low VL or degraded samples. While HEP-TILE amplicons can be sequenced on either Nanopore or Illumina, the use of the ONT native barcoding kit described here (ligation based) rather than ONT rapid barcoding kit or NEB-Ultra-II-FS kit (both requiring fragmentation) enables the sequencing of whole amplicons. Although shorter amplicon schemes (300–600 bp) can facilitate transfer to Illumina platforms without amplicon fragmentation, HBV diversity and genome structure prohibit the generation of a shorter pan-genotypic scheme (Supplementary Fig. 1). Generally, shorter amplicons improve sensitivity and platform transferability, however longer 1–2 amplicon schemes or schemes generating concatenated full genomes are useful for haplotype reconstruction4,41. The use of two primer pools, pooled equivolume, (instead of individual reactions for each amplicon) simplifies the PCR step and eliminates the need for re-balancing of individual amplicons post-PCR required by other methods44. An alternative enrichment method using Cas9-guided ribonucleoproteins has been developed for sequencing from primary human hepatocytes with high copy numbers of viral DNA, utility for sequencing low viral load blood samples is yet to be seen45.
We report percent genome coverage at x1 depth in addition to the percentage of the genome with consensus calls to allow comparison with other published methods presenting coverage at x1 depth. We use a higher threshold of x20 depth to call bases at a consensus level for Nanopore, and x5 for Illumina to improve certainty of the consensus base call (higher threshold for Nanopore used due to the higher error rate of Nanopore sequencing technology).
Table 2 compares the costs of each workflow, estimated per sample based on an initial batch of 1000 samples. Cost per sample decreases with multiplexing (e.g. 96 samples for Nanopore, more possible for Illumina). Due to the initial cost of purchasing capture probes (~£6300 for this panel), sequencing smaller sample sets with capture becomes prohibitively expensive. Costs for Illumina sequencing vary significantly depending on whether the option of purchasing a partial lane on a high throughput platform is available.
Real world application of methods
Although full genome HBV sequencing coverage should be achievable for the majority of samples using HEP-TILE, if samples at extreme ends of the VL spectrum are pooled on the same run, uneven read distribution is expected between samples, with loss of coverage for the lowest VL samples. Therefore batching samples by VL and normalising the mass of PCR product being taken forwards into library preparation is recommended. Viral enrichment by PCR is highly sensitive to amplicon contamination from previous experiments therefore caution should be taken to keep work areas, equipment and reagents free of contamination, and multiple negative controls should be included. To avoid in-silico ‘contamination’ double barcoding is required when demultiplexing to eliminate chimeric reads which may otherwise lead to mis-assignment of reads to incorrect barcodes46. These wet lab and bioinformatic principles are also applicable to sequencing other targets.
Although our capture approach can be used to improve the efficiency of viral sequencing at VL > ~ 5.50 log10 IU/ml, its efficacy for producing full-length consensus genomes diminishes at lower VLs, as observed elsewhere47. Additional/alternative host depletion and high volume (e.g. 5 ml48) extraction is likely to be required to reliably obtain full consensus genomes using this method in samples with VL < 5.5 log10 IU/ml.
Caveats and limitations
The results presented here are from an iterative set of experiments and protocol development, rather than a pre-planned head-to-head comparison. This limits the direct comparability of workflows due to insufficient sample volumes of the original South Africa sample set. This meant we were unable to sequence all samples using all three enrichment approaches. Furthermore, the extraction method changed over time. Nevertheless, these data showcase the capabilities of each workflow.
Correlation between VL and percentage genome coverage was more variable than anticipated. Factors such as storage duration, shipping conditions, and repeated freeze-thaw cycles may all influence sample quality, although DNA is generally robust. Poor sample quality or inaccurate VL reporting (different sample/timepoint tested or lab/reporting error) may explain instances where samples that were initially reported to have high VL yielded lower coverage than expected (e.g. sample 1116 for which VL was reported as 5.41 log10 IU/ml but on repeat on our sample was 3 log10 IU/ml).
The probes and primers were designed to be pan-genotypic, however only take into account the diversity of sequences on HBVdb, which contains relatively few genotype F-H sequences, and under-represents certain global populations49. A strength of our study is the use of mock samples, which allowed us to assess performance across all genotypes, including rarer genotypes F-J that were unlikely to be present in our cohorts, however might still be present in other cohorts globally. Analysis of rarer genotypes is often missing in reports of sequencing schemes, however will become increasingly important as more HBV WGS is performed from diverse global regions, and known HBV sequence diversity expands. While we report these methods as pan-genotypic using mock samples generated from plasmids containing HBV genotypes A-J, it is important to note that we only tested one variant per genotype. Some genotypes have multiple subtypes; future studies could test enrichment methods with a wider range of subgenotypes.
Sequencing low VL samples has inherent stochasticity, depending on the presence of viral DNA in a sample or extract, and assay chemistry. Future work will test HEP-TILE performance at viral loads < 1.5 log10 IU/ml, using high volume extraction to increase the chance of sampling viral DNA (e.g. extraction from 5 ml serum/plasma48), increasing the number of PCR cycles from 35 to 40 and pooling products from PCRs performed in duplicate or triplicate. Analysis based on consensus sequences does not identify minority variants, Future work could compare the ability of capture or HEP-TILE to accurately quantify low abundance minority variants, requiring a panel of control material at varying VLs with known variant percentages, sequenced with multiple technical replicates. Finally, enrichment methods could be trialled on different sample types, for example liver biopsies where high host background is a challenge.
Conclusion
Collectively, these protocols will facilitate the wider generation of HBV sequence data. This data will lead to a more accurate and representative global picture of HBV molecular epidemiology, cast light on persistence and pathogenesis, and enhance understanding of treatment responses and clinical outcomes. These aspirations align with high profile international goals for elimination of HBV as a public threat.
Data availability
Data for this study is available within the manuscript, supplementary information files and online links. Sequences for samples where full length genomes were generated were deposited to the European Nucleotide Archive (ENA) under the study PRJEB79403 for Nanopore data and PRJEB79773 for Illumina.
References
Global hepatitis report 2024: action for access in low- and middle-income countries. World Health Organization (2024, accesed 7 May 2024). Available: https://www.who.int/publications/i/item/9789240091672
Houldcroft, C. J., Beale, M. A. & Breuer, J. Clinical and biological insights from viral genome sequencing. Nat. Rev. Microbiol. 15, 183–192 (2017).
Lok, A. S. Personalized treatment of hepatitis B. Clin. Mol. Hepatol. 21, 1–6 (2015).
McNaughton, A. L. et al. Illumina and Nanopore methods for whole genome sequencing of hepatitis B virus (HBV). Sci. Rep. 9, 7081 (2019).
Lumley, S. F. et al. Pan-genotypic probe-based enrichment to improve efficiency of Hepatitis B virus sequencing. bioRxiv 2023, 2023.02.20.529276. https://doi.org/10.1101/2023.02.20.529276 (2023).
Downs, L. O. et al. Bimodal distribution and set point HBV DNA viral loads in chronic infection: retrospective analysis of cohorts from the UK and South Africa. Wellcome Open. Res. 5, 113 (2020).
McNaughton, A. L., Revill, P. A., Littlejohn, M., Matthews, P. C. & Ansari, M. A. Analysis of genomic-length HBV sequences to determine genotype and subgenotype reference sequences. J. Gen. Virol. 101, 271–283 (2020).
McNaughton, A. L. et al. Insights from deep sequencing of the HBV genome-unique, tiny, and misunderstood. Gastroenterology 156, 384–399 (2019).
Briese, T. et al. Virome capture sequencing enables sensitive viral diagnosis and Comprehensive Virome Analysis. MBio 6, e01491–e01415 (2015).
Depledge, D. P. et al. Specific capture and whole-genome sequencing of viruses from clinical samples. PLoS ONE 2011, e27805. https://doi.org/10.1371/journal.pone.0027805 (2011).
Bonsall, D. et al. ve-SEQ: Robust, unbiased enrichment for streamlined detection and whole-genome sequencing of HCV and other highly diverse pathogens. F1000Res 4, 1062 (2015).
PrimalScheme: primer panels for multiplex PCR (2024, accessed 7 May 2024). https://primalscheme.com/.
Quick, J. et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat. Protoc. 12, 1261–1276 (2017).
Quick, J. nCoV-2019 sequencing protocol v3 (LoCost) v3. https://doi.org/10.17504/protocols.io.bp2l6n26rgqe/v3 (2020).
fieldbioinformatics: The ARTIC field bioinformatics pipeline. Github. https://github.com/artic-network/fieldbioinformatics (2024).
Bannister, E. et al. Analysis of the in vitro replication phenotype of African hepatitis B virus (HBV) genotypes and subgenotypes present in Australia identifies marked differences in DNA and protein expression. Virology 540, 97–103 (2020).
Downs, L. O. et al. Electronic Health Informatics Data To Describe Clearance Dynamics of Hepatitis B Surface Antigen (HBsAg) and e Antigen (HBeAg) in Chronic Hepatitis B Virus infection. MBio 10, 859. https://doi.org/10.1128/mBio.00699-19 (2019).
O’Hara, G. et al. Liver function tests and fibrosis scores in a rural population in Africa: a cross-sectional study to estimate the burden of disease and associated risk factors. BMJ Open. 10, e032890 (2020).
Asiki, G. et al. The general population cohort in rural south-western Uganda: a platform for communicable and non-communicable disease studies. Int. J. Epidemiol. 42, 129–141 (2013).
Hayer, J. et al. HBVdb: a knowledge database for Hepatitis B Virus. Nucleic Acids Res. 41, D566–D570 (2013).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Gaidatzis, D., Lerch, A., Hahne, F. & Stadler, M. B. QuasR: quantification and annotation of short reads in R. Bioinformatics 31, 1130–1132 (2015).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Jiang, H., Lei, R., Ding, S-W. & Zhu, S. Skewer: a fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinform. 15, 182 (2014).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. bwa: Burrow-Wheeler Aligner for short-read alignment (see minimap2 for long-read alignment). Github. https://github.com/lh3/bwa (2024).
Picard (2024, acessed 25 Jun 2024). http://broadinstitute.github.io/picard.
nickloman, Rowe, W. et al. ChrisgKent/hbv-fieldbioinfomatics: 1.4. Zenodo. https://doi.org/10.5281/ZENODO.13341402 (2024).
Katoh, K. & Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 26, 1899–1900 (2010).
Menardo, F. et al. Treemmer: a tool to reduce large phylogenetic datasets with minimal loss of diversity. BMC Bioinform. 19, 164 (2018).
primerschemes/hbv/500/v1.1.0 at main · quick-lab/primerschemes. Github. https://github.com/quick-lab/primerschemes/tree/main/primerschemes/hbv/500/v1.1.0 (2024).
Chen, Y., Sze, J. & He, M-L. HBV cccDNA in patients’ sera as an indicator for HBV reactivation and an early signal of liver damage. World J. Gastroenterol. 10, 82–85 (2004).
Takkenberg, R. B. et al. Validation of a sensitive and specific real-time PCR for detection and quantitation of hepatitis B virus covalently closed circular DNA in plasma of chronic hepatitis B patients. J. Med. Virol. 81, 988–995 (2009).
Lumley, S., Kent, C., Quick, J. & Matthews, P. HEP-TILE: HBV whole genome sequencing (nanopore protocol) v1. https://doi.org/10.17504/protocols.io.5jyl82bedl2w/v1 (2024).
ChrisKBio hbv-fieldbioinfomatics: The ARTIC field bioinformatics pipeline for HBV analysis. Github. https://github.com/ChrisgKent/hbv-fieldbioinfomatics (2024).
Genotyping [2024, accessed 17 Jun 2024). https://www.ncbi.nlm.nih.gov/projects/genotyping/formpage.cgi.
Geno2pheno hbv (2024, acessed 17 Jun 2024). https://hbv.geno2pheno.org/index.php.
Ripley, B. D. The R project in statistical computing. MSOR Connect. 1, 23–25 (2001).
Scientific image and illustration software (2024, accessed 26 Jun 2024). http://www.biorender.com.
Stenbäck, J. B. et al. Accurate and cost-efficient whole genome sequencing of hepatitis B virus using Nanopore. medRxiv 2024, 2024.08.12.24311345. https://doi.org/10.1101/2024.08.12.24311345 (2024).
Tshiabuila, D. et al. An Oxford Nanopore Technology-based Hepatitis B Virus sequencing protocol suitable for genomic Surveillance within Clinical Diagnostic settings. medRxiv. https://doi.org/10.1101/2024.01.19.24301519 (2024).
Günther, S. et al. A novel method for efficient amplification of whole hepatitis B virus genomes permits rapid functional analysis and reveals deletion mutants in immunosuppressed patients. J. Virol. 69, 5437–5444 (1995).
Ringlander, J., Andersson, M. E., Prakash, K., Larsson, S. B. & Lindh, M. Deep sequencing of hepatitis B virus using Ion Torrent fusion primer method. J. Virol. Methods 299, 114315 (2022).
Goldsmith, C. et al. Cas9-targeted nanopore sequencing reveals epigenetic heterogeneity after de novo assembly of native full-length hepatitis B virus genomes. Microb. Genomics. https://doi.org/10.1099/mgen.0.000507 (2021).
Xu, Y. et al. Detection of viral pathogens with multiplex nanopore MinION sequencing: be careful with cross-talk. Front. Microbiol. 9, 2225 (2018).
Berg, M. G. et al. Advanced molecular surveillance approaches for characterization of blood borne hepatitis viruses. PLoS One 15, e0236046 (2020).
Fu, M. X. et al. Ultrasensitive PCR system for HBV DNA detection: risk stratification for occult hepatitis B virus infection in English blood donors. J. Med. Virol. 95, e29144 (2023).
Delphin, M. et al. Under-representation of the WHO African region in clinical trials of interventions against hepatitis B virus infection. Lancet Gastroenterol. Hepatol. 9, 383–392 (2024).
Funding
Open Access funding provided by The Francis Crick Institute
This research was funded in whole, or in part, by the Wellcome Trust [220549/Z/20/Z, 206298/B/17/Z, 220171/Z/20/Z]. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this Submission. SFL is funded by a Wellcome Doctoral Training Fellowship (grant number 220549/Z/20/Z). JQ/NL/CK are funded by the Wellcome Trust through the ARTIC Network Collaborative Award (Grant number 206298/B/17/Z). MAA is supported by a Sir Henry Dale Fellowship jointly funded by the Royal Society and Wellcome Trust (220171/Z/20/Z). PCM has funding from the UCLH NIHR Biomedical Research Centre, and core funding from the Francis Crick Institute (ref CC2223).
Author information
Authors and Affiliations
Contributions
Conceptualization: SFL, JQ, MAA, PCM. Methodology: SFL, CK, DJ, HC, AT. Software: CK, HC, SW, JQ, MAA. Formal Analysis: CK, HC, SFL. Investigation: SFL, CK, DJ, HC, GA, EW, MD, AT, SW, YW, G M-C, MK, KO, JC, CT. Resources: BK, TGM, CdL, JM, MVS, DG, RN, EB, PCM. Data curation: CK, HC, JQ, MAA. Writing (original draft): SL, CK. Writing (review and editing): all authors. Visualisation: SL, CK. Supervision: JQ, MAA, PCM. Funding acquisition: SFL, JQ, MAA, PCM.
Corresponding authors
Ethics declarations
Competing interests
Chris Kent has received an honorarium from Illumina for a talk on PrimalScheme3. No other authors declare competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lumley, S.F., Kent, C., Jennings, D. et al. Whole genome sequencing of hepatitis B virus using tiled amplicon (HEPTILE) and probe based enrichment on Illumina and Nanopore platforms. Sci Rep 15, 5795 (2025). https://doi.org/10.1038/s41598-025-87721-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87721-1