Abstract
This study details the hydrothermal synthesis and characterization of SnO2/nanocellulose (NC) nanocomposites, highlighting their potential for advanced applications. X-ray diffraction (XRD) analysis confirmed the amorphous nature of the composites, with a broad peak at 25.6° and a rightward shift indicating strong SnO2-NC interactions. A sharp peak at 32.26° in the SnO2-20 sample signified a rutile structure with single-plane crystallinity. UV-Vis absorption spectra showed a significant blue shift, with bandgap values reaching 5.86 eV for SnO2-5, compared to 3.6 eV for pure SnO2, demonstrating enhanced optical properties. Photoluminescence (PL) spectra revealed prominent emissions at 384 nm and 486 nm, attributed to oxygen vacancies and defect states, which enhance luminescence and support optoelectronic applications. FTIR spectroscopy identified a peak at 3430 cm−1, confirming abundant hydroxyl groups on cellulose surfaces and high sample purity. Scanning electron microscopy (SEM) images showed uniformly distributed SnO2 nanoparticles on an amorphous NC matrix, with rod-like features contributing to irregular morphologies that impact defect density and optical behavior. These structural and optical improvements enhance charge transport and reduce carrier recombination, establishing SnO2-NC nanocomposites as promising materials for optoelectronic devices, gas sensors, and photocatalysis. The integration of eco-friendly nanocellulose offers a sustainable pathway for developing next-generation technologies with superior performance.
Similar content being viewed by others
Introduction
Tin dioxide (SnO2), a wide-bandgap semiconductor with a bandgap energy of approximately 3.6 eV1, has garnered significant attention from researchers due to its outstanding electrical, optical, and gas-sensing properties2,3. Its unique features, such as high carrier mobility4, excellent transparency in the visible and near-infrared regions5, and remarkable chemical stability6, make it a highly versatile material for applications in transparent conducting electrodes (TCEs)7,8, photocatalysis9,10, gas sensors11,12, and lithium-ion batteries (LIBs)13,14. Nevertheless, the performance of SnO2 is limited by challenges such as high surface recombination rates of photogenerated charge carriers15 and low electrical conductivity in its bulk form16, which necessitate ongoing research and optimization for specific applications.
For instance, Karthik et al.17 highlighted the advantages of microwave-assisted green synthesis in producing SnO2 nanoparticles with enhanced optical properties and efficient photocatalytic degradation capabilities, emphasizing the method’s sustainability and practicality. Similarly, Pakiyaraj et al.18demonstrated that annealing Al-Zn co-doped SnO2 thin films significantly improves their structural, optical, and electrical properties, which are crucial for advanced optoelectronic devices. Additionally, Gurushankar et al.19investigated the optoelectronic performance of SnO2 thin films under varying temperatures, establishing a clear relationship between processing conditions and properties such as light management and conductivity. These studies collectively highlight the adaptability and potential of SnO2 in advancing optoelectronic technologies.
Nanocellulose (NC), a bio-derived material obtained from cellulose through techniques such as acid hydrolysis, enzymatic treatment, and mechanical shearing20,21,22,23, has emerged as an ideal partner for metal oxides in nanocomposites due to its numerous advantageous properties. With a high aspect ratio and large surface area, NC facilitates enhanced interaction with target molecules24, while its excellent mechanical strength contributes to the development of robust and durable nanocomposites25,26. Its inherent biodegradability11 further aligns with the growing demand for sustainable materials27, making it highly attractive for diverse technological applications.
Nanocellulose imparts lightweight characteristics, sufficient mechanical strength, and commendable flexibility to composites, attributes that have garnered significant attention for optoelectronic applications. Metal oxide/cellulose nanocomposites, such as ZnO/cellulose, TiO2/cellulose, and Fe2O3/cellulose, have been extensively explored for their unique functionalities. When incorporated with cellulose, SnO2 maintains its stability under UV radiation and harsh conditions, providing superior performance compared to other oxides like ZnO and TiO228,29. These properties make SnO2/cellulose nanocomposites suitable for transparent electrode (TCE) and optoelectronic applications, offering an edge over ZnO- and CuO-based counterparts, which often experience charge trapping due to poor particle dispersion30,31.
The integration of SnO2 and cellulose leverages cellulose’s biocompatibility, sustainability, and flexibility, while enhancing the optical and electronic properties of SnO2. This synergy is particularly advantageous for developing advanced nanocomposites tailored for non-invasive sensors, UV photodetectors19, and flexible optoelectronic devices like wearable sensors and foldable displays32,33. The uniform dispersion of SnO2 nanoparticles within the cellulose matrix minimizes recombination losses, reduces brittleness, and enhances optical, electrical, and photocatalytic properties8,34,35,36,37. Furthermore, NC’s high aspect ratio and inherent conductivity enable the creation of conductive pathways within the SnO2 matrix, reducing grain boundaries and shortening charge transport paths.
NC also acts as a scaffold, preventing recombination of photogenerated electron-hole pairs in SnO2, which improves photocatalytic performance in pollutant degradation and photocatalytic water splitting38,39,40,41,42,43. The combination of SnO2’s wide bandgap, high electron mobility, and optical transparency with NC’s mechanical flexibility and biodegradability positions these composites as promising candidates for optoelectronic applications like light-emitting diodes, photodetectors, and gas sensors44. The bio-derived cellulose matrix enhances gas adsorption and diffusion within the SnO2-NC composite, increasing sensitivity and selectivity for gas-sensing applications26,45,46,47,48,49,50,51. Mahadeva and Kim52 explores hybrid nanocomposites of cellulose and tin oxide, focusing on their synthesis, structural characterization, and mechanical properties. The work highlights the material’s potential in electrical applications, demonstrating notable tensile strength and electrical characteristics essential for advanced material science.
Yempally et al.53 emphasized the potential of cellulose acetate in sensor applications when combined with nanomaterials like graphene, which significantly enhance its mechanical and electrical properties. Similarly, Ansari et al.54 reviewed nanocellulose-based composites in electronics, highlighting their utility in flexible and sustainable sensor technologies). Meanwhile, Karthik et al.55 demonstrated the superior optical and photocatalytic properties of SnO2 synthesized via microwave-assisted green synthesis and Pakiyaraj et al.18reported enhanced structural and optical properties in Al-Zn co-doped SnO2 thin films. Recently, Leo et al.56 reported fabrication of tin oxide-cellulose nanocomposites for flexible and thin supercapacitor applications. It emphasizes the hybrid material’s unique properties, such as mechanical flexibility and enhanced electrochemical performance, making it promising for energy storage devices.
Hydrothermal synthesis stands out as a versatile, environmentally benign, and scalable method for fabricating SnO2/cellulose nanocomposites57. This approach ensures uniform nanoparticle dispersion and precise control over the structural and compositional properties of thin films, optimizing their optoelectronic behavior10,58,59. The rational design and meticulous characterization of SnO2/cellulose nanocomposites are crucial for unlocking their potential in applications like TCEs, flexible optoelectronics, and energy storage devices60,61. The enhanced surface area and porosity of NC further promote gas adsorption and light absorption, boosting the performance of SnO2-based devices in gas sensing and energy harvesting applications26,45,46,47,48,49,50,51,52.
The integration of cellulose into SnO2 nanocomposites not only enhances their eco-friendliness and biodegradability but also provides a sustainable and cost-effective alternative to conventional materials, ensuring long-term reliability for advanced technological applications62,63,64. The SnO2/Cellulose nanocomposite exhibits a significant blue shift in the UV-Vis absorption spectra larger than what is typically observed for ZnO or TiO2/Cellulose composites, suggesting better suitability for UV-based optoelectronic applications. The presence of oxygen vacancies in SnO2 is particularly beneficial for optoelectronics, These defect states improve charge separation and enhance light emission, outperforming other metal oxide/Cellulose composites, which often suffer from recombination losses. SnO2’s superior stability under UV radiation and harsh conditions, coupled with the mechanical flexibility imparted by cellulose, makes this composite more durable and versatile for applications such as transparent electrodes and wearable sensors. The uniform dispersion of SnO2 nanoparticles within the cellulose matrix reduces grain boundaries, facilitating improved charge transport compared to other metal oxides like CuO or Fe2O3 when paired with cellulose. By combining these features, the SnO2/Cellulose nanocomposite emerges as a superior material for optoelectronic applications, offering a unique balance of optical, structural, and mechanical properties.
This study focuses on synthesizing SnO2/cellulose nanocomposites and comprehensively characterizing their structural, optical, and morphological properties, aiming to contribute to their emerging role in sustainable optoelectronic technologies.
Experimental
Materials
AR grade Cellulose (CDH, 99%), Sulphuric Acid (Ranbaxy, 98%), and LR grade Tin chloride dehydrate (SnCl2·2H2O, 98%), Ammonium nitrate (NH4NO3, 99%), Ethanol (C2H5OH, 95%), and Sodium Hydroxide (NaOH, 98%) were purchased from Fisher Scientific and processed without further purification. The distilled (DI) water used throughout the experiment was of high purity.
Hydrothermal synthesis of SnO2/cellulose nanocomposite
The synthesis of SnO2/Cellulose nanocomposite is being done by a well-known hydrothermal method, and the whole process was completed in two steps.
Nano cellulose preparation
10 g of cellulose were weighed and dispersed in 100 mL of distilled water (DI water). An excessive NaOH solution was prepared by dissolving 1.6 g of NaOH in 100 mL of DI water. The cellulose dispersion was mixed with the NaOH solution using vigorous stirring for 1 h. The resulting slurry was washed with DI water until reaching pH 7 and then filtered. The filtered slurry was dried for 6 h at 80 °C in a hot air oven. The treated cellulose was dispersed in 50 mL of DI water and added 1 mL of sulfuric acid. Stirring continued for 2 h, followed by neutralization with a known amount of DI water to halt hydrolysis, resulting in the formation of the slurry Nano cellulose (NCl).
Synthesis of SnO2/cellulose nanocomposite
A milky white solution of SnO2 was prepared by dissolving 0.1 M SnCl2·2H2O in a mixture of 20 mL DI water and 20 mL ethanol. 0.1 M NH4NO3 was added to the solution, and stirring continued for 30 min at 40 °C. Different volumes (5 mL, 10 mL, and 20 mL) of this solution were mixed with 10 mL of the NCl solution. Stirring was continued for an additional 30 min at a constant temperature of 40 °C. The resulting solutions were transferred to stainless steel-covered, Teflon-lined autoclaves and glass pieces (1x. 1 cm2) to prepare films on glass substrates. The autoclaves were placed in a hot air oven for 6 h at 180 °C. After cooling to room temperature, nanocomposite-coated films were retrieved using pointed tweezers. The obtained white solution was centrifuged, washed with DI water and absolute ethanol, and dried at 60 °C for 2 hours. The resulting samples were labelled as SnO2-5, SnO2-10, and SnO2-20. Pure SnO2 and nano cellulose (NCl-HT) were prepared separately for comparison purposes.
Schematic representation of Synthesis of SnO2/Cellulose Nanocomposite shown in Fig. 1.
Characterization
The obtained samples underwent comprehensive structural, optical, compositional, and morphological analyses using various instrumental techniques. XRD analysis was conducted using a PANalytical Empyrean instrument equipped with Cu Kα radiation (λ = 0.15406 nm). The scan range was set from 5° to 80° to examine the crystalline structure of the samples. The morphology of the nanocomposite was observed using a scanning electron microscope (SEM). The specific instrument utilized was a Jeol or Hitachi model, providing high-resolution imaging capabilities. FTIR spectra were acquired using a designated FTIR spectrometer, Spectrum 2 (PerkinElmer), equipped with a deuterated triglycine sulfate (DTGS) detector. The spectra were recorded in the wavenumber range of 4000–400 cm−1 with a resolution of 4 cm−1. This analysis facilitated the identification of functional groups present in the samples, aiding in compositional characterization.UV-visible spectra were recorded using a Perkin Elmer Lambda 25 spectrophotometer within the wavelength range of 200 nm to 800 nm. This analysis enabled the investigation of the optical properties of the samples. PL spectra were obtained using a Perkin Elmer LS55 spectrofluorometer. This technique allowed for the assessment of the samples’ compositional and optical characteristics based on emitted light under specific excitation conditions, following standard protocols for analysis.
Results and discussion
Structural studies
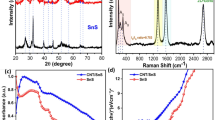
The structural investigation of the as-prepared nanocomposite samples was conducted using X-ray diffraction (XRD), and the diffractograms of NCL-HT and cellulose/SnO2 nanocomposite films are depicted in Fig. 2. The XRD diffractogram of NCL-HT revealed an amorphous character, characterized by broad and less pronounced peaks centered at 22.27°. This observation is indicative of the amorphous nature of the cellulose component in the nanocomposite11,65,66. This information contributes to the understanding of the structural properties of nanocomposite materials, laying the groundwork for further analysis and interpretation of their properties and potential applications.
A broad peak, centred at 26.6° and exhibiting a rightward shift, was observed in the XRD pattern of the cellulose/SnO2 nanocomposites. This phenomenon confirms the interaction between SnO2 and the cellulose moiety, indicating the successful formation of cellulose/SnO2 nanocomposites. A sharp peak observed at 33.9° in the XRD pattern of the SnO2-20 sample signifies its single-plane crystalline nature. However, this peak was absent in the XRD patterns of the other samples, indicating their amorphous nature. The observed peak in the XRD pattern of the SnO2-20 sample corresponds to rutile (JCPDS file No. 41-1445). The standard lattice parameters for rutile are a = b = 4.738 Å and c = 3.187 Å.
The average particle size of SnO2 was calculated as 65 nm using the Debey-Scherrer formula67,68,
where D is the nanoparticles crystalline size, K represents the Scherrer constant (0.98), λ denotes the wavelength (1.54), β denotes the full width at half maximum (FWHM).
These XRD findings provide valuable insights into the structural properties of the cellulose/SnO2 nanocomposites and underscore the crystalline nature of the SnO2-20 sample, contributing to a deeper understanding of their potential applications. The lattice constant for the crystal planes of (101) has been calculated by Eq. 2 and found as a = b = 4.747 Å and c = 3.206 Å. These are consistent with the standard values.
The slight right shift in the 2θ values and the change in lattice parameters are observed for the nanocomposites that indicate the compressive strain in the samples which may be due to displacement of the unit cells about their normal position and might be due to dislocations, surface restructuring, lattice vacancies and interstitials etc. There is no other peak in the XRD pattern of the samples was detected, which implies the purity of the prepared samples.
Fourier transform infrared spectroscopy (FTIR)
The composition of the prepared samples was investigated using Fourier Transform Infrared Spectroscopy (FTIR). Palates with KBr were prepared, with KBr serving as the reference material for all samples, as illustrated in Fig. 3. The FTIR spectra revealed distinctive peaks characteristic of various functional groups present in the samples. Notably, the peak centred at 3430 cm− 1 confirmed the presence of abundant hydroxyl groups, which are typically found on the surface of natural cellulose fibres. This finding underscores the suitability of cellulose as a substrate for metal oxide deposition. Additionally, the presence of key functional groups was identified through specific peaks: The peak at 2909 cm− 1 indicated C-H stretching. Peaks associated with C = O stretching, CH2 symmetric bending, C-N stretching, C-H bending, and C-O stretching were observed in the range of 1651 –1576 cm− 1, 1449 cm− 1, 1353 cm− 1, 1242 cm− 1, and 1033 cm− 1, respectively. These peaks collectively confirm the rich variety of stretching and bending modes of different bonds present in cellulose. Moreover, peaks near 1135 cm− 1 and 620 cm− 1 were of high intensity and broad, indicating the presence of SnO269. This observation suggests the high degree of purity of the prepared samples, further validating their suitability for subsequent analyses and applications.
Morphological study
The morphological characteristics of cellulose/SnO2 nanocomposites and pretreated mercerized cellulose (NCL-HT) films were analyzed using scanning electron microscopy (SEM) and EDX spectra, as shown in Fig. 4. The SEM image of NCL-HT (Fig. 4a) revealed an amorphous structure with randomly arranged rod-like features, highlighted by a yellow circle. This distinctive surface morphology, characterized by an irregular and porous texture, demonstrates the suitability of NCL-HT as a substrate for metal oxide nanoparticle deposition.
Figure 4(b) and (c) illustrate the SnO2-5 and SnO2-10 samples, which exhibit undefined shapes and unsmooth, irregular surfaces. These features contribute to an amorphous structure that increases defect density, introducing states capable of influencing light emission and absorption. In contrast, the SEM image of SnO2-20 (Fig. 4d) shows the formation of nanospheres (marked with small yellow circles), indicating improved surface uniformity. The red circle in Fig. 4(d) highlights ultra-fine SnO2 nanoparticles deposited on the cellulose matrix. The amorphous carbon matrix of NCL-HT, rich in hydroxyl groups, provides an ideal substrate for the formation of cellulose/SnO2 nanocomposites. This morphology influences structural characteristics and optical properties, as confirmed by complementary XRD analyses. The unsmooth surfaces of SnO2-5 and SnO2-10 introduce a high density of defect states, such as oxygen vacancies, which serve as charge carrier recombination centres. These defects can modulate photoluminescence (PL) intensity, affecting optical behaviour. In comparison, the smoother surface of SnO2-20, marked by the presence of nanospheres, enhances light scattering and absorption, improving interaction with light and optoelectronic performance. The interplay between surface morphology and optical properties underscores the potential applications of these nanocomposites in optoelectronic devices.
The EDX elemental mapping was also done of the prepared samples of the nanocomposites and the NCL-HT for further determination of the existence of C, O and Sn elements. Table 1 represents the atomic and weight% of these elements in the samples.
UV-vis absorption studies
The UV-vis absorption spectra were recorded in the wavelength range of 200 nm to 800 nm for the samples of pure SnO2 and the cellulose/SnO2 nanocomposites, as depicted in Fig. 5(a). A notable observation is the comparison between the spectra of pure SnO2 and the cellulose/SnO2 nanocomposites. The cellulose/SnO2 nanocomposites exhibited a significant blue shift compared to pure SnO2, as evident from Fig. 5(a). Furthermore, a distinct hump near the 225 nm wavelength was observed in the spectra of the three nanocomposites. In contrast, no absorption was detected in the spectrum of NCl-HT. This observation suggests that the nanocomposites and NCl-HT are nearly transparent in the visible region.
A Tauc plot, representing (αhυ)2 versus (hυ)67,68, was constructed to determine the direct band gap of the as-prepared nanocomposite samples, as shown in Fig. 5(b). The calculated direct band gaps were found to be 5.86 eV, 5.58 eV, and 5.55 eV for SnO2-5, SnO2-10, and SnO2-20 samples, respectively. These values highlight the large optical band gaps exhibited by the nanocomposites. For comparison, the direct band gap of pure SnO2 is reported to be approximately 3.6 eV for bulk material70. The significantly larger band gaps observed in the SnO2 nanocomposites can be attributed to factors such as reduced particle size, the quantum confinement effect, and morphological changes due to the composite formation.
The absorption coefficient indicates that only a small fraction of refracted light is absorbed by the nanocomposites, emphasizing their potential in optoelectronic applications. Moreover, the large optical band gaps render them suitable for use in optical sensors, optical wavelength limiters, and photocatalytic systems.
The observed higher optical band gaps underscore the critical role of morphology in determining the optical properties of the SnO2 nanocomposites. The shift from the bulk SnO2 band gap to the larger band gaps of the composites demonstrates the impact of nanoscale structuring and the composite’s unique characteristics on their electronic structure and light interaction.
Photoluminescence (PL) studies
The photoluminescence (PL) spectra of the prepared nanocomposite films were recorded at room temperature, as shown in Fig. 6. PL analysis is a crucial technique for probing the optoelectronic properties of materials, providing insights into defects and crystalline quality. While SnO2 nanoparticles have been less extensively studied via PL, this technique remains valuable for understanding the excitation fine structure and defect states of materials69. The PL spectra of the cellulose/SnO2 nanocomposites were recorded under excitation at 320 nm, which corresponds to an energy absorbed by the valence electrons, promoting them to the conduction band before transitioning to metastable states. The emission spectra are characterized by broad peaks, which are often associated with intrinsic defects such as oxygen vacancies that are commonly observed in metal oxides. Specifically, the spectra exhibit a broad, high-intensity emission at approximately 384 nm, with a smaller peak at 486 nm. These peaks are attributed to the presence of oxygen vacancies, which serve as recombination centers for charge carriers trapped at metastable energy levels. These defects are largely surface-related, which is typical for nanocomposite materials, and contribute significantly to the observed luminescence. Moreover, compositional studies further corroborate the presence of these vacancies. Overall, the PL analysis offers important insights into the optical characteristics and defect structures of the nanocomposite films, which are pivotal for their potential applications in optoelectronics.
Comparative details highlighting the advantages of research compared to other similar studies
Several work reported with cellulose or carbon composites71,72,73. SnO2 Nanoflower–Nanocrystalline Cellulose Composites as Anode Materials highlights the use of nanocrystalline cellulose (CNC) as a template for SnO2 nanoflowers (NFs) to address volume expansion and aggregation issues in lithium-ion battery anodes. SnO2@Rice Husk Cellulose Composite via a hydrothermal method The study focused on mitigating structural collapse during charge/discharge cycles. SnO2@Carbon Nanocomposites also reported with a carbon matrix, incorporating cellulose-derived carbon for enhanced conductivity. These references represent key advancements in the integration of SnO2 and cellulose or its derivatives for applications in energy storage and gas sensing. However we have reported Cost-effective, eco-friendly, and compatible route with renewable cellulose materials Offers insights into defect-related optical properties and a flexible substrate for SnO2 deposition. Offers insights into defect-related optical properties and a flexible substrate for SnO2 deposition. Provides detailed optical characterization, including defect-driven luminescence, for optoelectronic applications. Comparative table emphasizing the advantages of the present study shown below (Table 2).
Conclusion
In this study, we successfully synthesized cellulose/SnO2 nanocomposites using a hydrothermal method and conducted comprehensive structural, optical, compositional, and morphological analyses. XRD results confirmed the amorphous nature of the composites, with peak shifts indicating strong interactions between SnO2 and the cellulose substrate. UV-Vis absorption spectra demonstrated a significant blue shift compared to pure SnO2, highlighting the nanocomposites’ potential for optoelectronic applications. Furthermore, PL analysis revealed the presence of defects, such as oxygen vacancies, suggesting the formation of metastable energy levels within the band gap, which are critical for luminescence processes. These findings underscore the potential of cellulose/SnO2 nanocomposites in diverse applications, including photocatalysis, gas sensing, and advanced optoelectronic devices. Future research could explore optimizing synthesis conditions to tailor defect states and enhance specific properties further. Additionally, investigating the photocatalytic and sensing performance under varied environmental conditions could expand the scope of practical applications. Advanced computational studies could also provide deeper insights into the interaction mechanisms between cellulose and SnO2, guiding the design of next-generation nanocomposites with enhanced functionality. This work lays the foundation for sustainable, high-performance materials that align with the growing demand for eco-friendly technologies.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Batzill, M. & Diebold, U. The surface science of tin dioxide. Prog. Surf. Sci. 89(1–3), 162–254. https://doi.org/10.1016/j.progsurf.2005.09.002 (2014).
Jang, E. & Ehrman, S. H. Transparent conducting films of SnO2: Sn prepared by atmospheric pressure chemical vapor deposition using tetrakis(dimethylamino) tin. Appl. Phys. Lett. 83(4), 738–740 (2003).
Goswami, Y. C. et al. Enhancing photocatalytic performance of SnO2/ZnS nanocomposites synthesized via dual-step precipitation and ultrasonicated hydrothermal route. Curr. Appl. Phys. 68, 275–283. https://doi.org/10.1016/j.cap.2024.10.011 (2024).
Goswami, Y. C., Kumar, V. & Rajaram, P. Sonochemical growth of SnO2 hollow nanostructures on cellulose acetate flexible substrates. Mater. Lett. 128, 425–428. https://doi.org/10.1016/j.matlet.2014.04.174 (2014).
Valenzuela, M., Ponce, A. M., Estrada, W. & López-Ríos, T. E. Synthesis and characterization of highly transparent SnO2 thin films. Thin Solid Films 475(1–2), 147–152 (2005).
Umar, A., Yusoff, M. M., Ahmad, Z. A. & Zaidi, M. A. Chemical stability of SnO2 nanoparticles: Effect of preparation methods. Int. J. Nanomed. 8, 779–787 (2013).
Mohapatra, R., Kaundal, J. B. & Goswami, Y. C. Synthesis of reduced graphene oxide/zinc sulfide nano composites with sonochemical route. Chalcogenide Lett. 18(5), 255–262. https://doi.org/10.15251/CL.2021.185.255 (2021).
Ohyama, M., Koike, H. & Yamazoe, M. Novel finding of H2S sensing with SnO2 thin films. Sens. Act. B Chem. 8(1), 3–8 (1992).
Nagaich, S., Goswami, Y.C. Shor’s Algorithm for Quantum Numbers Using MATLAB Simulator. in 2015 Fifth International Conference on Advanced Computing & Communication Technologies, Haryana, India 165–168. https://doi.org/10.1109/acct.2015.16 (2015).
Goswami, Y. C. et al. Synthesis of SnO2 nanostructures by ultrasonic-assisted sol–gel method. J. SolGel Sci. Technol. 69, 617–624. https://doi.org/10.1007/s10971-013-3241-0 (2014).
Sharma, R., Kumar, V. & Goswami, Y. C. Excellent flexible tin oxide-metal sulfide nanocomposites grown by spin coating chemical route. Chalcogenide Lett. 18(8), 473–479. https://doi.org/10.15251/CL.2021.188.473 (2021).
Li, N., Wang, C., Zheng, C., Fu, H. & Wu, Y. SnO2 hollow microspheres for high-performance lithium-ion battery anodes. ACS Nano 7(8), 7325–7334 (2013).
Zhu, J., Zhu, X., Guo, S. & Li, J. Tin oxide anodes for lithium ion batteries. Nano Res. 7(3), 161–176 (2014).
Nguyen, N. T. et al. Photocatalytic H2 evolution over Pt-loaded SnO2 nanoparticles prepared by a microwave-assisted method. Int. J. Hydrogen Energy 37(14), 10407–10413 (2012).
Lakshminarayanan, V. & Bhattacharya, I. Advances in Optical Science and Engineering 533–539 (Springer, 2015).
Pal, D., Singh, G., Goswami, Y. C. & Kumar, V. Synthesis of randomly oriented self-assembled CuS nanorods by co-precipitation route. J. Mater. Sci. Mater. Electron. 30, 15700–15704. https://doi.org/10.1007/s10854-019-01953-2 (2019).
Karthik, K., Revathi, V. & Tatarchuk, T. Microwave-assisted green synthesis of SnO2 nanoparticles and their optical and photocatalytic properties. Mol. Cryst. Liq. Cryst. 671(1), 17–23. https://doi.org/10.1080/15421406.2018.1542080 (2018).
Pakiyaraj, K., Kirthika, V. & Karthik, K. Effect of annealing on the structural, morphological, optical and electrical properties of Al-Zn co-doped SnO2 thin films. Mater. Res. Innov. 24(4), 193–201. https://doi.org/10.1080/14328917.2019.1628498 (2019).
Gurushankar, K., Grishina, M., Gohulkumar, M. & Kannan, K. Computational and experimental studies on SnO2 thin films at various temperatures. Comput. Opt. 47(1), 53–61. https://doi.org/10.18287/2412-6179-CO-1151 (2023).
Singh, R., Goswami, Y.C., Goswami, R. Semi-empirical and experimental investigation on coordination behavior of S-methyl β-N-(4-methoxyphenylmethyl) methylene dithiocarbazate Schiff base towards Co (II), Ni (II), and Cu (II) metal ions. J. Chem. 21 (2011).
Verma, H. P. S., Kumar, A. & Sharma, S. K. Extraction of nanocellulose from wheat straw: A review. Carbohyd. Polym. 119, 108–120 (2015).
Habibi, Y., Lucia, R. & Rojas, O. J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Soc. Rev. 43(2), 4419–4442. https://doi.org/10.1021/cr900339w (2014).
Li, X. et al. Hierarchically porous SnO2@cellulose nanofiber composites for high performance lithium ion batteries. Adv. Funct. Mater. 26(19), 3541–3548 (2016).
Dufresne, A. Nanocellulose: A new material for reinforcement, additives, and biocomposites. Macromolecules 40(7), 2284–2299. https://doi.org/10.1039/9781849735315-00001 (2007).
Fernandes, M. N., Mano, A. P. & Rocha, I. M. Nanocellulose as a versatile bio-based building block for emerging breathable barriers. J. Mater. Sci. 52(1), 303–339 (2017).
Jawaid, M. G., Rahman, A. R., Zainuddin, M. P., Bakri, L. C. T. & Khalil, A. Cellulose-based polymer composites: Fabrication techniques and their impact on properties. Carbohyd. Polym. 87(4), 833–842. https://doi.org/10.1016/j.carbpol.2011.04.043 (2012).
Nasir, M. S. & Rahman, M. A. A review of nanocellulose in biocomposite technology. Nano Rev. 12(1), 1–22. https://doi.org/10.22452/mnij.vol3no1.1 (2017).
Amutha, T. et al. Optical and electrical properties of pure and doped tin oxide nanoparticles. Partic. Sci. Technol. 41(2), 268–276. https://doi.org/10.1080/02726351.2022.2080618 (2022).
Choudhury, S. et al. UV stability of SnO2-based composites. ACS Appl. Mater. Interf. (2019).
Motsoeneng, R. G., Kortidis, I., Ray, S. S. & Motaung, D. E. Designing SnO2 nanostructure-based sensors with tailored selectivity toward propanol and ethanol vapors. ACS Omega 4(9), 13696–13709. https://doi.org/10.1021/acsomega.9b01079 (2019).
Gupta, K., Singh, M., Charge transport in metal oxide-cellulose nanocomposites. Mater. Sci. Semicond. Process. (2021).
Li, Y., Wu, Z., Wang, Q. & Zhang, X. Enhanced photoconductivity of SnO2-cellulose composites. Appl. Phys. Lett. 112(3), 112–115 (2018).
Goswami, Y., Begzaad, S. & Kaundal, J. Highly luminescent Cu doped SnO2 nanocomposites and their photocatalytic application as excellent methylene dye removal. J. Adv. Sci. Res. 13(4), 94–103. https://doi.org/10.55218/JASR.202213417 (2022).
Takahashi, T., Sato, S., Mori, T. & Tanihara, H. Gas-sensing characteristics of SnO2 films prepared by a spray pyrolysis method. Sens. Act. B Chem. 134(2), 1034–1039 (2008).
Liu, X., Hu, J., Zheng, S., Wu, Z. & Shen, C. High-performance transparent conducting films of SnO2 nanocrystals embedded in amorphous SiO2 network. Adv. Funct. Mater. 21(12), 2432–2439 (2011).
Kumar, N., Purohit, L. P. & Goswami, Y. C. Spin coating of ZnS nanostructures on filter paper and their characterization. Phys. E Low-Dimens. Syst. Nanostruct. 83, 333–338. https://doi.org/10.1016/j.physe.2016.04.025 (2016).
Šuligoj, A., Pavlović, J., Arčon, I., Rajić, N. & Novak Tušar, N. SnO2-containing clinoptilolite as a composite photocatalyst for dyes removal from wastewater under solar light. Catalysts 10(2), 253. https://doi.org/10.3390/catal10020253 (2020).
Batzill, M. & Diebold, U. The surface and materials science of tin oxide. Progress in Surface Science 79, 47–154 (2005).
Mota-Morales, J. D. & Morales-Narváez, E. Transforming nature into the next generation of bio-based flexible devices: New avenues using deep eutectic systems. Matter 4(7), 21412162. https://doi.org/10.1016/j.matt.2021.05.009 (2021).
Goswami, Y. C., Goswami, R. & Chirova, T. K. Highly effective antifungal and antibacterial properties of ZnO, ZnS, FeS2 and SnO2 nanoparticles against various fungal and bacterial isolates. Orient. J. Chem. https://doi.org/10.13005/ojc/400222 (2024).
Kumar, V., Rajaram, P. & Goswami, Y. C. Sol gel synthesis of SnO2/CdSe nanocomposites and their optical structural and morphological characterizations. Optik 127(5), 2490–2494. https://doi.org/10.1016/j.ijleo.2015.11.167 (2016).
Li, Y. et al. Hydrothermal synthesis of flower-like SnO2 nanostructures for gas sensors. J. Am. Chem. Soc. 131(40), 14990–14991 (2009).
Luo, D. et al. Tuning shell numbers of transition metal oxide hollow microspheres toward durable and superior lithium storage. ACS Nano 11(11), 11521–11530. https://doi.org/10.1021/acsnano.7b06296 (2017).
Ahmad, M. B., Kumar, S., Dixit, A. & Ahuja, S. SnO2/cellulose nanofibril composite nanofibers for high performance lithium ion battery anodes. ACS Appl. Mater. Interf. 5(14), 6404–6411 (2013).
Jianping, Li. et al. H2S sensing properties of the SnO2-based thin films. Sens. Act. B Chem. 65(1–3), 111–113. https://doi.org/10.1016/S0925-4005(99)00406-2 (2000).
Liu, B., Zheng, H., Zhang, H. & Wang, X. Tin dioxide (SnO2) nanoparticles for photocatalysis applications. Nanoscale 5(14), 6871–6886 (2013).
Nguyen, N. T. et al. Photocatalytic H2 evolution over Pt-loaded SnO2 nanoparticles prepared by a microwave-assisted method. Int. J. Hydrog. Energy 37(14), 10407–10413 (2012).
Singh, R., Kumar, D., Goswami, Y. C. & Sharma, R. Synthesis, spectral studies and quantum-chemical investigations on S-benzyl β-N-(4-biscyno diethylamino phenylmethylene) dithiocarbazate. Arab. J. Chem. 12(7), 1537–1544. https://doi.org/10.1016/j.arabjc.2014.10.022 (2019).
A. Kumar et al., Tunable morphology in hydrothermal synthesis of SnO2 nanocomposites. J. Phys. Chem. C, 2022.
T. Nakamura et al., Flexible optoelectronics using cellulose-based composites. Adv. Mater. 2020.
Kumar, N., Purohit, L. P. & Goswami, Y. C. Synthesis of Cu doped ZnS nanostructures on flexible substrate using low-cost chemical method. AIP Conf. Proc. https://doi.org/10.1063/1.4929188 (2015).
Mahadeva, J. & Kim, J. Hybrid nanocomposite based on cellulose and tin oxide: Growth, structure, tensile and electrical characteristics. Sci. Technol. Adv. Mater. 12, 055006 (2011).
Yempally, S., Hegazy, S. M., Aly, A., Kannan, K. & Sadasivuni, K. K. Non-invasive diabetic sensor based on cellulose acetate/graphene nanocomposite. Macromol. Symp. 392, 2000024. https://doi.org/10.1002/masy.202000024 (2020).
Ansari, J. R. et al. Nanocellulose-based materials/composites for sensors. In Micro and Nano Technologies Nanocellulose Based Composites for Electronics (eds Thomas, S. & Pottathara, Y. B.) 185–214 (Elsevier, 2021). https://doi.org/10.1016/B978-0-12-822350-5.00008-4.
Karthik, K., Revathi, V. & Tatarchuk, T. Microwave-assisted green synthesis of SnO2 nanoparticles and their optical and photocatalytic properties. Mol. Crystals Liq. Crystals 671(1), 17–23. https://doi.org/10.1080/15421406.2018.1542080 (2018).
Leo, C. P. et al. Fabrication of hybrid tin oxide-cellulose nanocomposite as the flexible and thin supercapacitor. Authorea https://doi.org/10.22541/au.167243591.14437019/v1 (2023).
Goswami, Y. C., Singh, R., Sharma, R. & Kumar, D. Optical characterization of cadmium sulphide films grown by newly modified hot substrate chemical bath colloidal route. Contemp. Eng. Sci. 5(1), 1–8 (2012).
Hossain, M. H., Chowdhury, M. A., Hossain, N., Islam, M. A. & Mobarak, M. H. Advances of lithium-ion batteries anode materials—A review. Chem. Eng. J. Adv. 16, 100569. https://doi.org/10.1016/j.ceja.2023.100569 (2023).
Sharma, R., Singh, R., Goswami, Y. C., Kumar, V. & Kumar, D. Highly luminescent CdS nanoparticles synthesized using microwave irradiation of Dithiocarbazate ligand as a single molecular precursor source. J. Aust. Ceram. Soc. 57, 697–703. https://doi.org/10.1007/s41779-021-00563-8 (2021).
Liu, H. et al. Electrostatic self-assembly of 0D–2D SnO2 quantum Dots/Ti3C2Tx MXene hybrids as anode for lithium-ion batteries. Nano-Micro Lett. 11, 65. https://doi.org/10.1007/s40820-019-0296-7 (2019).
Goriparti, S., Miele, E., De Angelis, F. & Di Fabrizio, E. Remo Proietti Zaccaria, Claudio Capiglia, Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sour. 257, 421–443. https://doi.org/10.1016/j.jpowsour.2013.11.103 (2014).
Liu, W. et al. Cellulose nanopaper: Fabrication, functionalization, and applications. Nano-Micro Lett. 14, 104. https://doi.org/10.1007/s40820-022-00849-x (2022).
Roy, R., Eldhose, M., George, C. & Joseph, A. Nanocellulose-Based (Bio)composites for Optoelectronic Applications. In Handbook of Biopolymers (eds Thomas, S. et al.) (Springer, 2023).
Wen, H. et al. Advancements in transparent conductive oxides for photoelectrochemical applications. Nanomaterials 14, 591. https://doi.org/10.3390/nano14070591 (2024).
Šuligoj, A., Pavlović, J., Arčon, I., Rajić, N. & Novak Tušar, N. SnO2-containing clinoptilolite as a composite photocatalyst for dyes removal from wastewater under solar light. Catalysts 10(2), 25. https://doi.org/10.3390/catal1002025 (2020).
Singh, R., Goswami, Y. C. & Goswami, R. Semi-empirical & experimental investigation on coordination behavior of S-methyl β-N-(4-methoxyphenylmethyl) methylene dithiocarbazate Schiff base towards Co (II), Ni (II) and Cu (II) metal ions. J. Chem. 68, 275–283 (2011).
Karthik, K., Pushpa, S., Madhukara Naik, M. & Vinuth, M. Influence of Sn and Mn on structural, optical and magnetic properties of spray pyrolysed CdS thin films. Mater. Res. Innov. 24(2), 82–86. https://doi.org/10.1080/14328917.2019.1597436 (2019).
Kiran, T., Ahmed, H. M. P., Begum, N. S., Kannan, K. & Radhika, D. Structural, morphological and optical studies of sol-gel engineered Sm3+ activated ZnO thin films for photocatalytic applications. Phys. Chem. Solid State 21(3), 433–439. https://doi.org/10.15330/pcss.21.3.433-439 (2020).
Triantafyllou, E. et al. Microwave-assisted extraction of cellulose from aloe vera plant residue and preparation of CNC/PVA hydrogels. Molecules https://doi.org/10.20944/preprints202410.2095.v1 (2024).
Karmaoui, M. et al. A study on functional materials for energy and environmental applications. ACS Omega 3(10), 13227–13238. https://doi.org/10.1021/acsomega.8b02122 (2018).
Tran, Q. N., Kim, I. T., Park, S., Choi, H. W. & Park, S. J. SnO2 nanoflower-nanocrystalline cellulose composites as anode materials for lithium-ion batteries. Materials 13, 3165. https://doi.org/10.3390/ma13143165 (2020).
Hu, Z. et al. SnO2@rice husk cellulose composite as an anode for superior lithium ion batteries. New J. Chem. 43, 8755. https://doi.org/10.1039/C9NJ01435G (2019).
Tang, H. et al. Facile synthesis of hierarchical CNF/SnO2/Ni nanostructures via self-assembly process as anode materials for lithium ion batteries. R Soc Open Sci. 5(6), 171522. https://doi.org/10.1098/rsos.171522 (2018).
Acknowledgements
The authors thank the ASEAN India Science and Technology Division (AISTDF), Department of Science and Technology, India, for funding this research (IMRC/AISTDF/CRD/2018/000029). The authors are also thankful to ITM University Gwalior India, DRI Myanmar and LIPI Indonesia for providing necessary infrastructure facilities.
Author information
Authors and Affiliations
Contributions
R. Bisauriya and Aye Aye Hlaing carried out lab work, R. Goswami and T. T. Moe analysed the results and prepared the figures. YCG wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Goswami, Y.C., Bisauriya, R., Goswami, R. et al. Hydrothermal synthesis of SnO2/cellulose nanocomposites: optical, Structural, and morphological characterization. Sci Rep 15, 9752 (2025). https://doi.org/10.1038/s41598-025-87948-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87948-y