Abstract
Lower urinary tract symptoms (LUTS) significantly affect patient quality of life. Treatment options for bladder outlet obstruction (BOO) due to benign prostatic hyperplasia (BPH) (a common cause of LUTS) are insufficient to relieve discomfort. As the incidence of BPH is increasing, new pharmacological targets for LUTS treatment are required. Corticotropin-releasing hormone (CRH) is a neuropeptide that controls normal micturition in rodents. Herein, we investigated the role of spinal CRH in regulating micturition in sham and BOO rats, and evaluated CRH as a therapeutic target for bladder dysfunction in BOO model Sprague–Dawley rats. Histological analysis, cystometry with intrathecal administration of CRH agonists/antagonists, western blotting, and real-time PCR assessed the role of CRH and its receptors (CRHR1 and CRHR2) in micturition in sham and BOO rats. CRH administration shortened the voiding interval, while pretreatment with antagonists against CRHR2 (but not CRHR1) suppressed CRH-induced frequent voiding. Western blotting confirmed CRHR1 expression in the dorsal root ganglia (DRG) and bladder, but not the spinal cord, of rats. Real-time PCR showed higher CRHR2 mRNA expression in the spinal cord and DRG than in the bladder in both groups. Overall, spinal CRH facilitates the micturition reflex via CRHR2, and is a promising therapeutic target for LUTS.
Similar content being viewed by others
Introduction
The function of the lower urinary tract (LUT) is regulated by a complex interaction between the central nervous system and peripheral organs, including the bladder. Lower urinary tract symptoms (LUTS) is a condition characterized by inaccuracies in various storage and voiding behaviors. LUTS is associated with a decrease in the quality of life (QoL) of both patients and carers, due to the social and economic burden1,2. Bladder outlet obstruction (BOO), most commonly induced by benign prostatic hyperplasia (BPH), causes both storage and voiding dysfunction, and commonly requires pharmacological or surgical treatment3,4. However, these treatments are often insufficient, and with the increasing global prevalence of BPH, there is currently a demand for new pharmaceutical targets for the treatment of LUTS5.
Corticotropin-releasing hormone (CRH), a 41-amino acid neuropeptide, and its two G protein-coupled receptors (type1 [CRHR1] and type2 [CRHR2]) have been recognized as the central components of stress responses via the hypothalamus-pituitary-adrenocortical (HPA) axis and the sympatho-adrenomedullary (SA) system6. CRH also acts as a neuromodulator, controlling the micturition reflex at the pontine and spinal level7. The intracerebroventricular application of CRH facilitates the micturition reflex via CRHR1 and the glutamatergic receptor8. Meanwhile, the intrathecal administration of CRH at the lumbar level in rats results in mixed responses, including reduced or increased micturition volume9,10. Therefore, the role of spinal CRH in the regulation of micturition is controversial, and no previous report has yet investigated whether spinal CRH contributes to pathophysiological conditions, including BOO.
Given this content, the present study aimed to clarify the role of spinal CRH in the regulation of the micturition reflex in both sham and BOO rats, and to evaluate the potential of CRH as a new therapeutic target for bladder dysfunction caused by BOO. To achieve this, we first investigated the pharmacological effects of intrathecal CRH agonists and antagonists on micturition in sham and BOO rats. Second, we investigated the expression of CRHR1 and CRHR2 in the spinal cord, dorsal root ganglia (DRG), and bladders of sham and BOO rats.
Materials and methods
Ethical approval and animals
All experiments were conducted in 8-week-old male Sprague–Dawley rats (Japan SLC, Hamamatsu, Japan), housed in a temperature-controlled environment with a 12 h day/night cycle and free access to food and water. All animal experiments were performed in accordance with the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan) and were approved by the Institutional Committee of Laboratory Animal Experimentation (Animal Research Center of Yokohama City University Graduate School of Medicine, approval numbers: FA21-051 and FA24-004).
BOO model development
The details of the surgery for the BOO model have been described previously11. In brief, rats were anesthetized with isoflurane, and placed in the supine position. The abdominal cavity was then opened via a midline incision to expose the urethrovesical junctions. The proximal urethra was loosely tied with a 19 G needle using 3-0 silk and 5-0 proleneⓇ thread, and the needle was removed to produce partial BOO. The same procedure was performed in the sham rats without tying the thread.
Histological analysis of bladder collagen deposition and bladder weight
To characterize BOO rats, we examined the histological changes in the bladder at 14, 28, and 42 days following BOO surgery using our previous method. In brief, sham and BOO rats were deeply anaesthetized with isoflurane, while the isolated bladder was fixed in 4% paraformaldehyde-phosphate buffered saline, embedded in paraffin and cut into 5 μm sections. Masson’s trichrome staining was performed to analyze fibrosis in the detrusor muscle layer and the whole bladder wall12. Collagen deposition was determined in 3 randomly selected sections. Images were analyzed using Adobe software and ImageJ software (http://imagej.nih.gov/ij/).
Cystometry and intrathecal implantation
The methods for cystometry and intrathecal catheter implantation methods have been described previously11,13,14. In brief, rats were deeply anesthetized with isoflurane, and a PE-50 catheter (Clay Adams, Parsippany, NJ, USA) was inserted into the bladder via the bladder dome and secured using a purse-string suture with a 5-0 nylon thread. Four days following the implantation of the bladder catheter, the end of the PE-50 catheter was exposed outside the bladder under isoflurane anesthesia. The rats were then placed in the prone position to expose the spine, a PE-10 intrathecal catheter (Clay Adams) was inserted between the lumbar vertebrae, and the tip was advanced to the level of the sixth lumbar spinal cord. Correct catheter positioning at the L6-S2 was confirmed during necropsy. The rats were then placed in a restraint cage (KN-326; Natsume Seisakusho Co., Ltd., Tokyo, Japan), and allowed to recover from anesthesia for 120 min prior to initiating measurements. The bladder catheter was connected to a DX-100 pressure transducer (Nihon Kohden, Tokyo, Japan) and KDS100 microinjection syringe pump (Muromachi Kikai Co., Ltd., Tokyo, Japan) via a three-way stopcock. First, saline was instilled at a rate of 10 mL/h until micturition occurred, after which the residual volume was measured by collecting the natural dripping postvoid residue through the bladder catheter for 5 min. Once the CMG trace was stable, the initial three micturition cycles were used as the baseline. CRH (10 μg /10 μl), Antalarmin (CRHR1 antagonist) 20 μg /10 μl, and Antisauvagine-30 (CRHR2 antagonist) 20 μg /10 μl were subsequently administered intrathecally, flushing was performed with 5 μl of saline, and CMG measurements were continued. Values were measured 30 min to 1 h after drug administration. The group pretreated with CRH antagonists received CRH 30 min following treatment CRHR1,2 antagonists. The saline-treated group was managed similarly.
The following CMG parameters were analyzed: baseline pressure (BP; minimum bladder pressure), micturition pressure (MP; maximum bladder pressure during micturition), inter-voids internal (IVI), voided volume (VV), post void residual volume (PVR), bladder capacity (BC; bladder infusion volume), voiding efficiency (VE), and mean amplitude and number of non-voiding contractions (NVCs). NVCs were defined as bladder contractions without micturition with an amplitude of at least 1 mmHg.
Western blotting
Fourteen days after BOO surgery, two BOO rats and two sham rats were sacrificed. The bladder and 6th lumbar spinal cord and L6 level DRG were harvested and snap-frozen. Based on previous reports, we used SD rat liver as the positive control15 and SD rat spleen as the negative control. The excised tissue was dissolved in radioimmunoprecipitation assay (RIPA) buffer (R0278; Sigma-Aldrich) containing a protease inhibitor cocktail (P8340; Sigma-Aldrich) and a phosphatase inhibitor cocktail (P2850, P5726; Sigma-Aldrich). The lysate was subsequently centrifuged at 15,000 × g for 30 min at 4 °C, and the supernatant was collected as the target sample. After determination of the protein concentration using the Bio-Rad protein assay (Bio-Rad, Hercules, CA), 20 μg of total cell lysate was electrophoresed with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to the Trans-Blot Turbo Transfer Pack Mini (Bio-Rad, Hercules, CA) and electrophoretically transferred to Immobilon-P membranes (Millipore, Billerica, MA). The transferred membranes were subsequently blocked with blocking reagent N102 (Nichiyu Corporation, Tokyo, Japan), and incubated with specific antibodies in Tris buffered-saline containing Tween 20 (TBST; 150 mM NaCl, 20 mM Tris, 0.05% Tween 20) at 4 °C overnight. The primary antibodies used were rabbit anti-CRHR1 (1:500, Novus Biologicals, USA) and rabbit anti-CRHR2 (1:500, Novus Biologicals, Colorado, USA ; Abcam, Cambridge, UK). The β-actin antibody was obtained from Sigma-Aldrich (St. Louis MO). The membranes were washed with TBST three times for 10 min each, after which they were treated with horseradish peroxidase (HRP)-conjugated secondary antibodies at RT for 1 h, and then washed with TBST three times for 10 min each. Specific signals were detected using the LAS 4000 imaging system.
Real time PCR
Fourteen days after BOO surgery, six BOO and six sham-operated rats were sacrificed. The spinal cord (L6), DRG, and bladder body were dissected, and the tissues were snap frozen in liquid nitrogen for storage. Total RNA was subsequently extracted from tissues using ISOGEN (NIPPON GENE, Tokyo, Japan) and reverse transcribed to cDNA using the ReverTra Ace™ qPCR RT Master Mix (TOYOBO, Tokyo, Japan) according to the manufacturer’s instructions. For the relative quantification of mRNA expression, real-time PCR was performed using TaqMan™ Fast Advanced Master Mix (Thermo Fisher Scientific, USA) and gene-specific primers on a StepOnePlus Real-Time PCR System. The primer sequences used are shown in the Appendix. The primer sequences for CRHR1 and CRHR2 are commercially available (Thermo Fisher Scientific, USA). Relative expression levels of each receptor were calculated from threshold count values using standard curve methods, with ACTB serving as an internal control for normalization.
Drugs
CRH (Sigma-Aldrich) was obtained in powdered form, and diluted in sterile saline (1 μg /1 μl). Antalarmin (Sigma-Aldrich) was dissolved in DMSO (> 10 mg/ml) and administered at a concentration of 20 μg /10 μl. Antisauvagine-30 was obtained from Med Chem Express (New jersey, USA), dissolved in saline, and administered at a concentration of 20 μg /10 μl. These concentrations and dosages were selected based on previously reported data9,10.
Statistical analysis
All data are shown as the mean ± SEM. Differences between sham and BOO rats were analyzed using an unpaired Student’s t-test and Mann–Whitney U test. Dunnʼs multiple comparisons test was applied to compare parameters obtained in BOO rats at POD14 with POD28 and POD42, and to analyze the results of RT-PCR. Results were analyzed using the Wilcoxon matched-pairs signed rank test to compare CMG parameters before and after drug administration in sham and BOO rats. Statistical analysis was performed using Prism, version 7, with p < 0.05 considered to indicate statistically significant differences.
Results
Histological examination and bladder weight
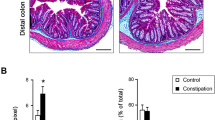
The bladder weight and bladder-to-weight ratio were significantly higher in BOO rats than in sham rats (Fig. 1A). Additionally, the bladder weight of BOO rats was significantly increased on POD 28 and 42 compared to POD 14. Masson’s trichrome staining revealed no significant difference in the percentages of detrusor muscles and collagen between BOO and sham rats (Fig. 1B); however, the percentage of muscular tissue in the entire bladder layer was significantly higher in BOO rats than in sham rats on POD 14 and POD28 (Figs.1C, D).
Histological Characteristics of the bladder in sham and BOO rats showing the ratio of bladder weight to body weight. (A) The percentage of fibrous tissue in the bladder smooth muscle, (B) and the percentage of detrusor muscle in the bladder (C). Masson trichrome staining of the bladders of sham and BOO rats at postoperative days 14, 28, and 42 (D). *p < 0.05, **p < 0.01 : significant difference from the sham rats (unpaired Student’s t-test and Mann–Whitney U test). ##p < 0.01, significant difference between postoperatively days 14, 28, and 42 in BOO rats (Dunn’s multiple comparison test).
Cystometry and intrathecal application of CRH agonists and antagonists in sham and BOO rats
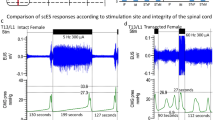
When comparing the baseline values of sham and BOO rats on POD14, BOO rats had significantly higher BC, PVR, and number and amplitude of NVCs compared to sham rats. In contrast, VE was significantly higher in sham rats than in BOO rats (Table 1). Intrathecal administration of CRH shortened the IVI and significantly reduced the VV and BC in both sham and BOO rats compared to baseline (Figs. 2A, B and 3A, B). Further, no significant changes in the MP, BP, PVR, VE, and the number and amplitude of NVC were observed (Tables 2 and 3). The intrathecal administration of Antalarmin (a CRHR1 antagonist) and Antisauvagine-30 (a CRHR2 antagonist) did not change any of the CMG parameters (Tables 2 and 3).
Effect of intrathecal treatment with CRH and CRH antagonists in sham rats at day 14 post-operatively. (A) Bladder pressure trace following intrathecal administration of CRH alone (upper trace) or with pre-administration of Antalarmin (middle trace) or Antisauvagine-30 (lower trace) followed by CRH treatment. (B) Comparison of the voiding volumes following intrathecal administration of CRH alone, Antalarmin alone, and Antisauvagine-30 alone, and pre-administration of vehicle, Antalarmin, and Antisauvagine-30 followed by CRH administration. *p < 0.05, : significant difference compared to the baseline (Wilcoxon matched-pairs signed rank test).
Effect of intrathecal CRH and CRH antagonists in BOO rats at day 14 post-operatively. (A) The bladder pressure trace following intrathecal administration of CRH alone (upper trace) and pre-administration of Antalarmin (middle trace) and Antisauvagine-30 (lower trace) followed by CRH application. (B) Comparison of voiding volume following the intrathecal administration of CRH alone, Antalarmin alone, and Antisauvagine-30 alone, or following the pre-administration of vehicle, Antalarmin, and Antisauvagine-30 followed by CRH administration. *p < 0.05 : significant difference compared to the base (Wilcoxon matched-pairs signed rank test).
Cystometry and intrathecal pretreatment of antagonists followed by CRH agonists in sham and BOO rats
Intrathecal pretreatment with Antisauvagine-30 prevented the shortening of IVI and the reduction in VV induced by intrathecal CRH administration in both sham and BOO rats (Figs. 2A, B and 3A, B). Pretreatment with antalarmin significantly reduced the shortening of IVI and VV in both sham and BOO rats compared with the pretreatment baseline, showing similar results to those observed with CRH administration.
Western blotting and real time PCR of the sacral spinal cord, DRG, and bladder in sham and BOO rats
Western blot analysis confirmed the presence of the CRHR1 protein in the DRG and bladders of sham and BOO rats. However, no CRHR1 protein was observed in the spinal cords of sham and BOO rats (Fig. 4A and Supplementary Fig. 1). Unfortunately, a specific and reliable band of the CRHR2 protein could not be obtained using two different antibodies in this study.
Analysis of CRH receptor expression. (A) Western blot results showing the expression of CRHR1 in the bladder and DRG, but not in the L6 spinal cord in both sham and BOO rats. SD Rat liver was used as the positive control, and SD rat spleen was used as the negative control. Uncropped data was displayed in Figure S1. (B) RT-PCR showing the expression of CRHR1 and CRHR2 in the L6 spinal cord and DRG in both sham and BOO rats, with little expression in the bladder. *p < 0.05, **p < 0.01 : significant difference compared to the bladder (Dunnʼs multiple comparisons test).
Real-time PCR confirmed the mRNA expression of CRHR2 in L6 and DRG, but not in the bladder of both sham and BOO rats, with significantly higher expression in the L6 than in the bladder (Fig. 4B). In contrast, CRHR1 was observed in the L6 and DRG, similar to CRHR2, but not in the bladder. The expression of CRHR1 in the L6 was significantly higher than that in the bladder. The expression of CRHR1 and 2 showed no difference in the bladder, L6, or DRG between sham and BOO rats.
Discussion
In the present study, BOO rats at POD 14 were used to evaluate the effects of CRH on BOO micturition, following the observation of compensatory hypertrophy of the muscle tissue in histological evaluations at that time point. At POD 14, BOO rats showed almost 50% lower VE, higher PVR, and greater NVC compared to sham rats, as previously reported using a similar BOO rat model16,17. We thought that this BOO model represented the phenotype of patients with voiding difficulty, high PVR, and urgency caused by BOO, which are commonly observed in clinical settings18.
This is the first study to investigate the pharmacological effects of CRH targeting the central CRH in BOO rats. The intrathecal administration of CRH reduced the shortened IVI and VV without changing the MP, PVR, or VE, and this response was counteracted by intrathecal administration of antisauvagine-30, a CRHR2 antagonist. These reactions occurred in both sham and BOO rats, which is consistent with a previous report in normal rats9. These findings further indicate that the intrathecal administration of CRH promotes micturition, with CRHR2 being the primary site of action, and that similar responses occur in BOO and healthy models. Interestingly, our study showed no influence of the application of CRHR2 antagonists alone on the rat micturition cycle. However, intrathecal pretreatment with CRHR2 antagonists attenuated the facilitated effects of CRH, indicating that CRH modulated the micturition efferent pathway, not the afferent pathway, via action of CRHR2. These findings suggest that CRH agonists may promote micturition in patients with BOO-induced urinary retention, potentially enabling them to become catheter-free.
Previous reports have shown a different or even opposite influence of spinal CRH on the micturition reflex in rodents, in which the intrathecal administration of CRH and overexpression of CRH in the Barrington nucleus increased bladder capacity, whereas the intrathecal administration of CRHR1 receptor antagonists decreased BC in male SD rats10,19. Another study showed that intrathecal CRHR2 antagonists, but not CRHR1 antagonists, attenuated bladder hypersensitivity in rats with bladder inflammation20,21 and electrical foot shock stress22, indicating that spinal CRHR2 may modulate sensitivity to bladder afferent pathways. Factors that could account for these differences from other reports include the concentration and total amount of CRH administered intrathecally, as well as differences in restraint during cystometry. For example, in the report by Kiddoo et al., the dosage of CRH was 6 μg/14 μl; cystometry was performed in the unanesthetized, unrestrained state; and intrathecal administration of CRH increased both the BC and VV, while administration of antalarmin increased urinary frequency and decreased BC10. Also, Kiddoo et al. studied normal rat bladders with a 24-h recovery period after bladder and intrathecal catheter implantation, whereas our group used sham rats with a 4-day recovery period after bladder catheter and immediate insertion of the intrathecal catheter. Intriguingly, the role of CRH showed heterogeneity not only at the spinal level but also at the brainstem level. Stimulation of CRH-positive neurons in the Barrington nucleus of mice led to bladder contractions when the mice were under urethane anesthesia7,23. In contrast, stimulation of CRH-positive neurons in the Barrington nucleus in awake, anesthetic-free mice increased bladder capacity and appeared to inhibit voiding24,25. These discrepancies may be explained by differences in animal strain or experimental design.
In terms of drug dosage, Klausner et al. previously compared the effects of intrathecal administration of CRH at doses of 1.5, 3.0, 6.0, and 9.0 μg, reporting that at 6.0 μg, a maximal reduction in IVI and VV was observed compared to baseline9. On the basis of this finding, we utilized CRH dosage of 6.0 μg, throughout this study. Also, we kept the drug-volume of intrathecal application at less than 20 µL, as intrathecal administration of more than 20 µL has been reported to affect the brain26. Overall, these reports have shown that spinal CRH plays an important role in the physiology of micturition.
Regarding the expression of CRHR1 and CRHR2, CRHR1 has been reported to be expressed in the central nervous system regions such as the hypothalamus, hippocampus, and amygdala27, as well as in the liver15. CRHR2 gene expression has been confirmed in the lumbosacral spinal cord, Th13-S1 DRG, hypothalamus, and raphe nucleus6,28,29. Consistent with these reports, RNA expression of CRHR2 was detected in both the L6 spinal cord and DRG of sham and BOO rats, without any significant expression discrepancies. These results indicate that CRHR2 expression in not regulated in BOO. In contrast, western blot analysis did not detect CRHR1 protein expression in L6 cells; however, RT-PCR confirmed its mRNA expression in L6 cells. This result is thought to be due to feedback effects such as the lack of CRHR1 protein expression.
This study did not identify a strong expression of either CRHR1 or CRHR2 in the bladders of sham and BOO rats. However, in previous reports, CRHR2 mRNA and protein were expressed in rat bladders, whereas CRHR1 was not30,31. Reports confirming the expression of CRHR1 and CRHR2 in the rat bladder are limited. In other studies, both CRHR1 and CRHR2 have been reported to be expressed in the urinary tracts of humans and cats. In the human bladder, CRHR1 is primarily expressed in the submucosal layer, whereas CRHR2 is primarily expressed in urothelial cells30. In cats, CRHR1 and CRHR2 expression has been confirmed at the protein level in the urothelium29. Therefore, CRH in the urinary tract may be of interest as a potential pharmacological target.
This study has several limitations. Firstly, we did not investigate the effects of systemic administration of CRH and CRH antagonists on rat micturition. Considering the potential for the clinical application of this knowledge, the systemic effects of these drugs should be evaluated. Second, we did not evaluate the protein expression of CRHR2 in the bladder, L6, and DRG. Although we tested two antibodies, reproducibility was not achieved. Third, cystometry was performed under unanesthetized restraint conditions. To replicate the natural environment better, experiments under unrestrained conditions should also be considered.
In summary, the results of the present study show that the intrathecal administration of CRH resulted in frequent urination, an effect which could be counteracted by treatment with a CRHR2 antagonist, whereas pretreatment with a CRHR1 antagonist had no effect. Protein expression of CRHR1 was observed in the bladder and DRG, but not in the L6. Although CRHR2 protein expression could not be confirmed, gene expression was detected in the L6 and DRG, with minimal expression in the bladder. These results indicate no differences between the sham and BOO rats. Overall, these results indicate that the spinal CRH contributes to both the physiology and pathophysiology of the bladder through CRHR2.
Data availability
The datasets generated and analyzed in the current study are not publicly available, but are available from the corresponding author upon reasonable request.
References
Irwin, D. E. et al. Prevalence, severity, and symptom bother of lower urinary tract symptoms among men in the EPIC study: Impact of overactive bladder. Eur. Urol. 56, 14–20 (2009).
Irwin, D. E. et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur.Urol. 50, 1306–14 (2006).
Lerner, L. B. et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE PART I-Initial work-up and medical management. J. Urol. 206, 806–817 (2021).
Lerner, L. B. et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE PART II-surgical evaluation and treatment. J. Urol. 206, 818–826 (2021).
Collaborators GBDBPH. The global, regional, and national burden of benign prostatic hyperplasia in 204 countries and territories from 2000 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2022; 3: e754-e76
Deussing, J. M. & Chen, A. The corticotropin-releasing factor family: Physiology of the stress response. Physiol. Rev. 98, 2225–2286 (2018).
Ito, H. et al. Probabilistic, spinally-gated control of bladder pressure and autonomous micturition by Barrington’s nucleus CRH neurons. Elife. 9, e56605 (2020).
Hata, Y. et al. Stimulation of brain corticotropin-releasing factor receptor type1 facilitates the rat micturition via brain glutamatergic receptors. Biochem. Biophys. Res. Commun. 607, 54–59 (2022).
Klausner, A. P. et al. The role of corticotropin releasing factor and its antagonist, astressin, on micturition in the rat. Autonom. Neurosci. Basic Clin. 123, 26–35 (2005).
Kiddoo, D. A. et al. Impact of state of arousal and stress neuropeptides on urodynamic function in freely moving rats. Am. J. Physiol. Regul. Integr. Comp. Physiol.. 290, R1697–R1706 (2006).
Sugiyama, R. et al. Synergic suppressive effect of silodosin and imidafenacin on non-voiding bladder contractions in male rats with subacute bladder outlet obstruction. LUTS Lower Urinary Tract Sympt. 9, 94–101 (2015).
Ito, H. et al. Long-term caloric restriction in rats may prevent age related impairment of in vitro bladder function. J. Urol. 193, 2123–2130 (2015).
Füllhase, C. et al. Spinal cord FAAH in normal micturition control and bladder overactivity in awake rats. J. Urol. 189, 2364–2370 (2013).
Ito, H. et al. Preventive effects of long-term caloric restriction on aging related in vivo bladder dysfunction and molecular biological changes in the bladder and dorsal root ganglia in rats. J. Urol. 196, 1575–1583 (2016).
Zhao, Y., Wang, M. Y., Hao, K., Chen, X. Q. & Du, J. Z. CRHR1 mediates p53 transcription induced by high altitude hypoxia through ERK 1/2 signaling in rat hepatic cells. Peptides 44, 8–14 (2013).
Yoshida, S. et al. Therapeutic effect of TAC-302, a cyclohexenoic fatty alcohol derivative, on bladder denervation-related storage and voiding dysfunctions in rats. Neurourol. Urodyn. 37, 2106–2113 (2018).
Liu, C. et al. Sulforaphane ameliorates bladder dysfunction through activation of the Nrf2-ARE pathway in a rat model of partial bladder outlet obstruction. Oxid. Med. Cell Longev. 2016, 7598294 (2016).
Gravas, S., Cornu, J.-N., Gacci, M. et al. EAU Guidelines on Management of Non-Neurogenic Male Lower Urinary Tract Symptoms (LUTS) including Benign Prostatic Obstruction (BPO); Available at: http://www.uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/, (2021).
McFadden, K., Griffin, T. A., Levy, V., Wolfe, J. H. & Valentino, R. J. Overexpression of corticotropin-releasing factor in Barrington’s nucleus neurons by adeno-associated viral transduction: Effects on bladder function and behavior. Eur. J. Neurosci. 36, 3356–3364 (2012).
Ness, T. J., DeWitte, C. & Randich, A. Neonatal cystitis leads to alterations in spinal corticotropin releasing factor receptor-type 2 content and function in adult rats following bladder re-inflammation. Brain Res. 1788, 147927 (2022).
Ness, T. J., DeWitte, C. & Randich, A. The double insult of neonatal cystitis plus adult somatic inflammation results in corticotropin releasing factor type II receptor-dependent bladder hypersensitivity in female rats. J. Pain. 23, 2167–2178 (2022).
Robbins, M. T. & Ness, T. J. Footshock-induced urinary bladder hypersensitivity: Role of spinal corticotropin-releasing factor receptors. J. Pain. 9, 991–998 (2008).
Hou, X. H. et al. Central control circuit for context-dependent micturition. Cell. 167(73–86), e12 (2016).
Verstegen, A. M. J., Vanderhorst, V., Gray, P. A., Zeidel, M. L. & Geerling, J. C. Barrington’s nucleus: Neuroanatomic landscape of the mouse “pontine micturition center”. J. Comp. Neurol. 525, 2287–2309 (2017).
Garcia DuBar, S. et al. Somatostatin neurons in the mouse pontine nucleus activate GABA(A) receptor mediated synaptic currents in locus coeruleus neurons. Front. Synaptic Neurosci. 13, 754786 (2021).
Pavcovich, L. A. & Valentino, R. J. Central regulation of micturition in the rat the corticotropin-releasing hormone from Barrington’s nucleus. Neurosci. Lett. 196, 185–188 (1995).
Locci, A., Yan, Y., Rodriguez, G. & Dong, H. Sex differences in CRF1, CRF, and CRFBP expression in C57BL/6J mouse brain across the lifespan and in response to acute stress. J. Neurochem. 158, 943–959 (2021).
Korosi, A. et al. Corticotropin-releasing factor, urocortin 1, and their receptors in the mouse spinal cord. J. Comp. Neurol. 502, 973–989 (2007).
Hanna-Mitchell, A. T., Wolf-Johnston, A., Roppolo, J. R., Buffington, T. C. & Birder, L. A. Corticotropin-releasing factor family peptide signaling in feline bladder urothelial cells. J. Endocrinol. 222, 113–121 (2014).
Jhang, J. F. et al. Dysregulation of bladder corticotropin-releasing hormone receptor in the pathogenesis of human interstitial cystitis/bladder pain syndrome. Sci. Rep. 9, 19169 (2019).
LaBerge, J., Malley, S. E., Girard, B., Corrow, K. & Vizzard, M. A. Postnatal expression of corticotropin releasing factor (CRF) in rat urinary bladder. Autonom. Neurosci. Basic Clin. 141, 83–93 (2008).
Acknowledgements
None.
Funding
No funding was provided for this research.
Author information
Authors and Affiliations
Contributions
R.S., R.J., S.K., T.T., G.N., D.U., Y.I., M.K., K.M., H.H., K.K., M.T., K.F., K.M., N.A., and H.I. were involved in Protocol/project development. R.S. conducted Data collection or management. Data analysis was performed by R.S., R.J., T.N., T.F., H.T., M.H., S.K., G.N., and H.I. The main manuscript text and figures was wrote by R.S. and H.I. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were performed in accordance with the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan) and were approved by the Institutional Committee of Laboratory Animal Experimentation (Animal Research Center of Yokohama City University Graduate School of Medicine, approval numbers: FA21-051 and FA24-004).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shinoki, R., Jikuya, R., Nirei, T. et al. Spinal CRH facilitates the micturition reflex via the CRH2 receptor in rats with normal bladder and bladder outlet obstruction. Sci Rep 15, 3604 (2025). https://doi.org/10.1038/s41598-025-87990-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-87990-w