Abstract
Fatigue is a common and often debilitating feature of multiple sclerosis (MS) that lacks reliably effective treatment options for most patients. Transcranial direct current stimulation (tDCS), a safe and well-tolerated type of noninvasive brain stimulation, is a low-cost and home-based approach with the potential to reduce fatigue in MS. We conducted a double-blind, sham-controlled, randomized clinical trial to compare active vs. low-dose (sham) tDCS paired with computer-based cognitive training, delivered as a home-based intervention, to reduce MS-related fatigue. Participants with MS-related fatigue, but without depression, were stratified by neurologic disability using the Extended Disability Status Scale (EDSS) and randomized to complete 30 daily sessions over six weeks of either active or sham tDCS paired with online cognitive training (BrainHQ). The primary outcome was the change in PROMIS Fatigue score from baseline to the end of the intervention. A total of 117 participants were randomized, with 92% completing all treatment sessions. Both groups showed significant reductions in fatigue, with no significant difference between them. This suggests that tDCS does not provide any additional benefit over cognitive training alone in reducing fatigue, but confirms the feasibility and tolerance of this home-based intervention.
Similar content being viewed by others
Introduction
Fatigue is a common and often debilitating feature of multiple sclerosis (MS) that lacks reliably effective treatment options for most patients1. Transcranial direct current stimulation (tDCS), a safe and well-tolerated type of noninvasive brain stimulation, is a low-cost and home-based approach with the potential to reduce fatigue in MS2.
Several controlled pilot studies have found that tDCS can be effective in reducing fatigue in MS3,4,5,6,7 and in other chronic conditions including fibromyalgia8, postpolio syndrome9, and Parkinson’s disease10,11. The mechanism by which tDCS reduces fatigue has not yet been established. Most studies examining tDCS for fatigue have used the dorsolateral prefrontal cortex (DLPFC) montage4,12,13,14, which is also effective in treating depression15,16, with the hypothesis that increasing activation in this region increases alertness and overall positive affect to mitigate fatigue17,18. However, some studies also showed fatigue reduction in MS with tDCS targeting the motor cortex or the somatosensory cortex3,19,20,21. Prior studies have varied in whether participants with depression were excluded, with some reporting fatigue improvements independent of mood and others noting concurrent improvements in both mood and fatigue3,4,14,22,23,24. These findings suggest that tDCS effects on fatigue may involve shared neural pathways or a mediating role of mood23. The variability in eligibility criteria and intervention parameters highlights the need for rigorously designed trials to clarify the relationship between mood and fatigue improvement.
In addition to the recognition of the necessity for repeated tDCS applications for any clinically meaningful effects, tDCS effects can be enhanced using combined application with an activity that engages the region where the stimulation is directed. This assumed mechanism of action of tDCS suggests that synergistic effects can be achieved through simultaneous task-related activation of the targeted brain area or network25).
Given the need for tDCS intervention to be delivered in repeated daily sessions over time and the burden that daily travel can place on patients26, we carefully developed and validated a protocol for home-based tDCS treatment via telehealth delivery27. This remotely supervised tDCS (RS-tDCS) protocol extends the standards of in-clinic intervention to the home setting using fail-safe equipment, live video supervision, and standardized stop criteria2,27. We combined tDCS with adaptive cognitive training (aCT; BrainHQ), which we had previously found to be effective for improving cognitive functioning in participants with MS when using a long period of training (60 h x 12 weeks) in a large home-based RCT28. In our first trial testing the intervention, we found an initial signal of cognitive benefit after only 10 × 20-minute sessions29. During this study, participants anecdotally reported significant reductions in their fatigue. Therefore, we specifically tested for fatigue reduction with the combined intervention in a pilot RCT4. The intervention delivered 20 sessions with the left anodal DLPFC montage paired with aCT. We found a significantly greater reduction in the Modified Fatigue Impact Scale (MFIS) score in the active vs. sham tDCS condition4. In that pilot study, the sham condition involved two 60-second periods in which the current ramped up and down, one at the beginning and one at the end of the session.
Our objective was to compare active vs. sham transcranial direct current stimulation (tDCS) combined with aCT delivered as a home-based intervention to reduce MS fatigue. Based on these pilot findings, we hypothesized that an intervention using active tDCS treatment would result in significantly greater fatigue reduction compared to sham tDCS treatment. We undertook a large RCT of home-based intervention extending to 30 days over 6 weeks of tDCS using a DLPFC montage paired with aCT in people with MS-related fatigue. In addition to increasing the number of daily sessions in this trial to achieve the most conservative blinding assurance30, we included a third (middle) period of current ramp-up/-down31. This design was modeled from rigorous sham protocol in tDCS depression trials31, to address the lack of robust blinding data at the time. While this approach reflected an abundance of caution, later studies, including our own, have shown that simpler sham protocols can achieve effective blinding without repeated ramp-up/-down periods2,32,33. It is also now recognized that repeated ramp-up/-down periods may have unintended active effects, which warrants further investigation30.
Results
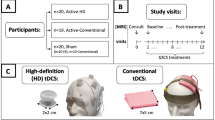
Participants were recruited from April 2019 to February 2021 (Fig. 1), and the follow-up period ended in June 2021. A total of n = 212 interested participants were prescreened; 120 participants consented, and 117 were randomized in an intent-to-treat design. Exclusion of three participants from randomization was due to personal reasons (n = 1), inability to come on-site for baseline assessment (n = 1), or inability to provide information to complete the screening (n = 1). A total of 117 participants initiated their assigned randomized intervention (n = 61 active, n = 56 sham), and 11 withdrew from the study (Fig. 1). A total of 105 randomized participants (90%) completed both the baseline and follow-up assessments of the primary outcome and were included in the analyses (n = 54 active, n = 51 sham).
Because the intervention was delivered remotely, the trial continued enrollment throughout the COVID-19 pandemic period until its completion. The protocol was modified to ship the study equipment to participants’ homes, and baseline (n = 54) and/or follow-up visits (n = 63) were administered remotely through video visits34,35.
The n = 117 individuals who were randomized completed an average of 26 ± 9 sessions (n = 4 participants withdrew before completing any sessions; n = 7 completed fewer than 10 sessions before withdrawing). Attrition was minimal with an overall high level of fidelity to treatment, with 92.0% of participants completing at least 25 of the 30 sessions to treatment end36,37,38.
Blinding effectiveness was tested by asking participants after the follow-up assessment to guess their group assignment (active or sham) at the end of the study visit. With the target that the proportion of correct guesses is significantly different from what would be expected by chance (50% in a two-arm trial)39, we found that 38% of the participants assigned to the active group and 52% of those in the sham group guessed that they received active tDCS (p’s > 0.5).
The interaction between the two treatment groups and the EDSS Low and High groups was not significant (p = 0.278, see Appendix Table 2). Therefore, we did not consider it in our following analyses.
Table 1 describes the demographic and clinical characteristics of the 117 randomized participants included in the intent-to-treat analyses.
Primary outcome—fatigue reduction
PROMIS Fatigue score decreased 19% in the sham group (baseline: 27.41 (95% confidence interval (CI): 25.93, 28.89); treatment end: 21.96 (95% CI: 20.06, 23.86)) and decreased 18% in the active group (baseline: 27.94 (95% CI: 26.52, 29.37); treatment end: 22.69 (95% CI: 20.88, 24.49)) (Table 2; Fig. 3).
The reduction was 5.45 (95% CI: 3.67, 7.23) in the sham group and 5.26 (95% CI: 3.71, 6.81) in the active group (Table 2, Fig. 3). Results were similar when participants were stratified by low vs. high EDSS score (Table 2; Fig. 2). See Fig. 3 for a closer look at the small differences in fatigue score changes between the sham and active groups, both by strata and in total.
The change in PROMIS Fatigue score from baseline to treatment end did not differ significantly between the active and sham tDCS intervention conditions for the full group (difference: 0.19; 95% CI: − 2.11, 2.49; p = 0.87) or when stratified by high (difference: 1.73; 95% CI: − 1.27, 4.72; p = 0.26) vs. low EDSS (difference: − 0.83; 95% CI: − 4.13, 2,47; p = 0.62) (Table 2).
Secondary outcomes
MFIS
The same pattern of findings resulted from analyses of change in the MFIS. The MFIS score decreased 17% in the sham group (baseline: 48.61 (95% confidence interval (CI): 44.27, 42.95); treatment end: 38.17 (95% CI: 33.70, 42.65)) and decreased 15% in the active group (baseline: 43.79 (95% CI: 39.74, 47.84); treatment end: 37.72 (95% CI: 33.43, 42.01)). As with the PROMIS Fatigue outcome, the change in MFIS from baseline to treatment end was not significantly different between the active and sham tDCS intervention conditions for the full group (difference: − 2.98; 95% CI: − 7.97, 2.01; p = 0.12).
Follow-up fatigue assessment
At the 3-month follow-up, we found that there remained a significant reduction in fatigue for both the active and sham conditions (p’s < 0.001), also without a separation. The mean change in PROMIS Fatigue scores was − 5.15 (SD = 6.57) for the sham tDCS group (n = 47) and − 4.91 (SD = 5.60) for the active tDCS group (n = 49). The independent samples t-test showed no significant difference between the groups (t(94) = − 0.185, p = 0.853).
To evaluate the potential bias in our results due to missing endpoint measurements in n = 12 participants, we compared the distributions of age, sex, race, ethnicity and the PROMIS Fatigue Baseline score of participants with missing endpoints to those with complete data using summary statistics and t-tests and Fisher’s exact tests (Appendix Table 1). No multiple comparison adjustments were used. There were no statistically significant differences between the two groups for any of these characteristics.
Safety and tolerability of the intervention
There was only one participant who required reduction in current intensity to 1.5 mA at the time of the tolerability test. This participant was assigned to the sham condition. No participants in the active condition required this adjustment. Throughout the intervention period, there were no serious adverse events, and the intervention was well tolerated. Across all participants in both conditions, n = 9 AEs were rated > 7 on the visual analog scale; in all cases, the discomfort subsided and the session resumed. One participant discontinued one session due to discomfort at the stimulation midway check-in point. Otherwise, all AEs were reported as mild in intensity and resolved after the end of the session.
Discussion
We completed one of the largest single-center trials focusing on fatigue in MS patients and evaluating the combined intervention of DLPFC tDCS paired with aCT (BrainHQ). The intervention far exceeded the average number of sessions of tDCS used in trial designs to date, enabled by our home-based delivery with the RS-tDCS protocol. We found that daily tDCS paired with aCT was a feasible home-based intervention, with high rates of treatment compliance and completion for people living with MS and fatigue. Reflecting the tremendous unmet treatment need and advantage of home-based access to treatment, we experienced relatively rapid recruitment (including during the COVID-19 pandemic restrictions). The active and sham tDCS arms were similar with respect to the primary outcome, showing a significant improvement from baseline to treatment end. The results suggest that fatigue was similarly reduced in each group. Therefore, in this non-depressed group of participants, we did not find a differential effect of DLPFC tDCS on fatigue.
Our findings do not indicate that active tDCS results in greater fatigue reductions than does sham tDCS. Not all studies of tDCS in MS have shown benefit in reducing fatigue. The most recent study used a dosing regimen of twice per week for 4 weeks (eight sessions) and found no difference in fatigue or fatigability after active vs. sham tDCS40. The small sample size and differences in dosing approaches among studies have made it difficult to draw broad conclusions about the potential benefit of tDCS in reducing fatigue in MS. tDCS must be delivered repeatedly over time as its effects on behavioral or clinical outcomes are cumulative37,41. However, appropriate dosing parameters remain largely undefined, and study protocols vary in dosing regimen and total duration of intervention.
It is possible that both the active and sham tDCS conditions were effective. Dosing parameters in tDCS remain an open question, and the parameters for sham tDCS used in trials are under-reported30 and variable. However, at the time of the trial design, there was an absence of verification for blinding efficacy and we followed the most recent RCTs in depression that used dual ramp-up/down periods in the sham condition31. Here, we employed an even more complex approach to the sham condition, with three periods of ramp-up/-down at each session. Without established parameters for sham tDCS, we chose this more complex approach following recent tDCS trials (e.g31). Since the trial was designed, however, both our group and many others have found that a ramp-up/-down period at the beginning and at the end of the stimulation period is sufficient for sham efficacy2. Therefore, the sham condition employed in this trial is both unusual and provides a higher level of stimulation than the conventional sham condition. Interestingly, the initial depression RCT that employed two ramps up/down with a constant current of 0.034 mA in the 20-minute stimulation for their sham condition also failed to discriminate in effect between active and sham conditions31.
We also hypothesize that the earlier reports of significant fatigue reduction with tDCS targeting the DLPFC5 (including those of our own pilot studies4) may have been secondary to improvement in mood. The most established effects of DLPFC tDCS are its effects on mood regulation, with interventions using prefrontal tDCS shown to be at least moderately effective as a treatment for depression15,16. A long time challenge to the study of fatigue is with its self-reported definition, which identifies neurological fatigue as well as aspects of daytime sleepiness and components of depression42. As an example, fatigue is a symptom in twelve categories of DSM-IV symptomatology43. Therefore, it is relatively less common for RCTs of fatigue to exclude on the basis of depression1, and therefore mood enhancing interventions may have secondary capture on measures of fatigue44.
Another consideration is that the aCT brain training, with its known engagement of frontal systems45, may have an independent and beneficial effect on fatigue. Therefore, our findings may suggest that aCT with BrainHQ benefits fatigue independent of the pairing with active or sham tDCS. For example, the antidepressant effects have been studied in similar aCT interventions, with some46,47,48 but not all reporting benefit49. However, to our knowledge, the fatigue response to aCT has not yet been specifically studied. Further, in an exercise study, aCT was found to result in improved physical endurance, which the authors posited was due to lower mental fatigue50. Therefore, the effects of aCT on fatigue specifically warrant further investigation.
Nonetheless, in the absence of any reliably effective and accessible treatment options, the finding of clinically significant fatigue reduction in the full group is notable. Without any intervention, fatigue as a symptom in MS tends to remain stable over similar time periods in the absence of intervention51. Further, this finding is consistent with the larger body of RCTs of interventions for fatigue in MS (including both pharmacological and behavioral approaches)1, where both the active and control intervention groups similarly improve.
In addition to our unconventional sham condition, limitations to our study also include the potential for effectiveness of other dosing parameters as well. The determination of optimal tDCS dosing for clinical efficacy is still in its early stages. With “underdosing” of the amount of sessions in clinical trials in tDCS to date, a strength is our 30 session intervention. However, it is possible that an even longer period of intervention would have resulted in discernable effect between active and sham. We believe that paired activity with the aCT during stimulation is a strength of the study, at least keeping “brain state” consistent across individuals and sessions. However, a 2 × 2 design would have allowed us to test the contribution of each intervention component both in combination and separately. While our dosing parameters were conventional in terms of the targeting (DLPFC montage), current intensity (2.0 mA), and session duration (20 min), alterations in these choices may have impacted the outcomes of the trial.
In conclusion, thirty sessions of home-based tDCS intervention paired with cognitive training were well-tolerated and feasible to deliver to people with MS. Both the active and sham tDCS groups experienced reductions in fatigue, with no significant difference between the two groups, suggesting that tDCS does not provide any additional benefit over cognitive training alone in reducing fatigue.
Methods
Trial design
This was a sham-controlled RCT that used a 6-week, double-blind, parallel-arm design following all CONSORT guidelines52 (Fig. 1). Participants were consented and enrolled by the study coordinators (P.B., M.S.). Randomization was determined and pre-programmed into REDCap by the unblinded statistician (X.L.). Randomization was stratified according to low vs. high Extended Disability Status Scale (EDSS) score (low: 0–3.0, high: 3.5–7.5) to control for representation of MS neurologic impairment between the intervention groups. Study tDCS devices were preprogrammed to deliver active or sham tDCS by an unblinded lab member who was otherwise not involved in the study to ensure double-blinded delivery of the intervention.
This study was approved by the New York University Langone Health Institutional Review Board, and all methods were performed in accordance with the ethical standards set forth by the Declaration of Helsinki. All participants provided written informed consent prior to any undergoing study procedures. The clinical trial is registered with clinicaltrials.gov (first posted date: 12/02/2019; registration number: NCT03838770).
Participants
Participants were 18–75 years old; diagnosed with clinically definite MS53, any subtype, and had at least moderate MS-related fatigue (Fatigue Severity Scale or FSS54 score ≥ 36). Given the potential influence of depression symptoms on fatigue and its self-reporting, participants were screened for potential clinically significant depression using the Beck Depression Inventory (BDI-Fast Screen55) score ≤ 10, validated for use in MS depression screening.
To ensure their ability to understand and participate in study procedures, potential participants were required to have no more than moderate cognitive impairment (Symbol Digit Modalities Test or SDMT56,57 age-normative z-score ≥ − 3.0) and estimated premorbid level of cognitive functioning in at least the average range (Wide Range Achievement Test or WRAT-458 reading recognition subtest standard score ≥ 85).
Participant eligibility was confirmed by an NYU Langone Health MS division neurologist (LK, LZ, JG, EC), who ensured that the participant met medical eligibility including no other primary neurological or psychiatric disorder than MS; was medically cleared to undergo tDCS (i.e., not pregnant or breastfeeding, no medical devices implanted in the head or neck, no skin disorders/sensitivity near the area of stimulation) and other study procedures; and was visually, physically, and cognitively competent to take part in study procedures. Participants had not had a relapse and had not begun any new pharmacological treatments for fatigue in the month prior to commencement of the study. Participants were compensated for their time with $50 after the baseline visit and $50 after the treatment end visit, for a total of $100.
Settings and locations
The study was conducted through the tDCS program in the Division of MS and Department of Neurology at NYU Grossman School of Medicine, located in the NYU Langone Health ambulatory care center in midtown New York, NY. Data were collected using REDCap. Screening, baseline, and end of intervention visits were completed either onsite or remotely using our HIPAA-compliant telehealth procedures. All intervention sessions were completed by the participants from home, following our RS-tDCS protocol.
Intervention
At the initial tDCS visit, participants were introduced to the study equipment and trained in its operation. In advance of the initial treatment session, participants underwent a 90-second tolerability test where the current was ramped up and down to the target stimulation intensity of 2 mA to ensure their comfort during the stimulation. If a participant reported a pain rating of 7 or higher (on a visual analog scale from 1 (minimal) to 10 (most severe)) or intolerable discomfort during the tolerability test, current strength was lowered from 2.0 mA to 1.5 mA. If intolerable discomfort persisted at the lower current intensity, the participant was discontinued from the study and did not move to their assigned study intervention.
Study equipment (Fig. 4)
Participants received a study kit including a laptop computer, tDCS device, and sponge electrodes. The study laptop (Lenovo or ASUS) was used to communicate with study staff through videoconferencing, collect data, and complete cognitive training exercises.
We used 1 × 1 tDCS mini-CT devices (Soterix Medical Inc., Woodbridge, NJ) for all tDCS treatment sessions. These devices have large buttons and built-in safety features for ease of at-home use2,27. They operate using a single-use unlock code to deliver a single dose of the preprogrammed stimulation (active or sham).
The mini-CT device holds 5-cm x 5-cm sponge electrodes on the head to optimize anodal stimulation of the left DLPFC. We used a bifrontal montage with the anode over F3 and the cathode over F459, following the 10–20 EEG system. Three different sizes of the headset according to the head circumference were provided to ensure optimal fit. The headset and sponges are designed to make assembly as easy and accurate as possible. For active tDCS, the device was programmed to ramp up to the target current intensity of 2 mA (for 30 s), provide constant current throughout the session (19 min), and then ramp down at the end (for 30 s). For sham tDCS, the device was programmed to provide three periods of 60-second ramp-up/-down during the 20-minute session: one at the beginning, one midway through the session, and one at the end.
Daily intervention sessions
Participants completed 30 remotely supervised tDCS treatment sessions from their home on consecutive business days (Monday through Friday) for 6 weeks. Stimulation was applied to the DLPFC for 20 minutes at an intensity of 2.0 mA. Participants simultaneously completed cognitive training exercises through Posit Science’s BrainHQ platform during the stimulation60. Five core BrainHQ exercises (‘Sound sweeps,’ ‘Memory grid,’ ‘Syllable stacks,’ ‘To do list training,’ and ‘In the know’) were rotated during 20-minute daily sessions over the 6-week treatment period. These exercises targeted key aspects of information processing, including processing speed, working memory, and executive function. BrainHQ’s closed-loop algorithm tracked progress and adjusted difficulty in real-time to balance challenge and engagement while ensuring consistent exposure to all exercises and maintaining session variety.
At each session, participants met with study staff via videoconference using the study laptops during each daily session visit so that staff could collect safety and tolerability data, ensure correct headset placement, provide the single-use unlock code to initiate the session, and supervise the tDCS session in real time.
Outcomes
After randomization, participants completed a baseline assessment that included self-reported fatigue inventories and a brief battery of cognitive tests (serving as secondary outcomes and reported elsewhere). Baseline measures were repeated at the end of intervention and again at a 3-month post-intervention follow-up.
Primary outcome
The primary outcome was the change in PROMIS Fatigue61 score from baseline to end of treatment after 30 sessions (6 weeks). This measure includes eight items that participants rated based on their experiences over the preceding week. Scores range from 8 to 40, with higher scores indicating more fatigue. This assessment was given at baseline, after 10 and 20 treatment sessions, at treatment end, and 2 weeks and 4 weeks after treatment end. Response to intervention was indicated by reduction in PROMIS Fatigue scores from baseline to end of the intervention, with a magnitude of reduction in the range of 4.5 points to be considered to be clinically meaningful change62,63,64.
Secondary outcomes
We included an additional self-reported fatigue measure, the MFIS, at the fatigue assessments. MFIS is a self-reported questionnaire of 21 items (with a total score ranging from 0 to 84) that assesses the effects of fatigue in terms of physical, cognitive, and psychosocial functioning.
To test for persisting effects of the intervention, the self-reported measures were also administered at the post-intervention follow-up and the 3-month follow-up.
Safety and tolerability of the intervention were measured via assessment of pain ratings and experiences of minor adverse events (AEs) after each tDCS session. Participants reported and rated any AEs they experienced using a visual analog scale from 1 (minimal) to 10 (most severe). Any pain rating above 7 led to discontinuation of the tDCS session, with the protocol defining criteria for study withdrawal in the case of 3-session discontinuations due to pain.
Sample size
The trial was designed to compare the pre- to post-intervention change after 30 sessions (6 weeks of treatment) in Patient Reported Outcomes Measurement Information System (PROMIS) Fatigue score as a primary outcome between groups receiving 30 sessions of either active or sham tDCS. With 60 participants randomized to each group (active or sham tDCS), the study would have 80% power to detect a difference between the groups. Sample size was estimated based on our pilot RCT (using a separate fatigue measure, the Modified Fatigue Impact Scale or MFIS)62, and powered for the most conservative estimate of meaningful change at a reduction of eight points on the PROMIS Fatigue scale62 (with a minimal clinically meaningful change of about 4.5 points63). Assuming a ± 0.5 standard deviation in the pre- vs. post change in PROMIS Fatigue score and a two-sided significance level of 0.05, a two-sample t-test (with the appropriate transformation of the measurements if required to meet the assumptions of the t-test) is appropriate to test the difference between groups. If a dropout rate of 5% is assumed, then approximately 54 participants for each of the active and sham control groups need to be randomized.
Analyses
An intention-to-treat analysis was performed on participants who completed both the baseline and end of the intervention assessments, totaling 105 participants (active tDCS: n = 54; sham tDCS: n = 51). Statistical analyses for primary and secondary outcomes were conducted on this data). The primary outcome of PROMIS Fatigue score change was compared between the treatment groups for stratified and non-stratified data using the two-sample two-sided t-test. The interaction of treatment groups and stratification groups was calculated and estimated using linear regression methods. No multiple testing adjustments were used. P values ≤ 0.05 were considered significant. This analysis was repeated for the secondary fatigue measure MFIS. R (version 4.0) and SAS (version 9.4) were used for analyses.
Data availability
The data supporting this study’s findings will be available from the corresponding author upon reasonable request. The data may not be shared publicly due to privacy or ethical restrictions. Requests for access will be reviewed by the Principal Investigator and will require a data use agreement.
References
Nourbakhsh, B. et al. Safety and efficacy of amantadine, modafinil, and methylphenidate for fatigue in multiple sclerosis: a randomised, placebo-controlled, crossover, double-blind trial. Lancet Neurol. 20, 38–48 (2021).
Pilloni, G. et al. Tolerability and feasibility of at-home remotely supervised transcranial direct current stimulation (RS-tDCS): single-center evidence from 6,779 sessions. Brain Stimul. 15, 707–716 (2022).
Ferrucci, R. et al. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation 34, 121–127 (2014).
Charvet, L. E. et al. Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult. Scler. J. 24, 1760–1769 (2018).
Ashrafi, A., Mohseni-Bandpei, M. A. & Seydi, M. The effect of tDCS on the fatigue in patients with multiple sclerosis: a systematic review of randomized controlled clinical trials. J. Clin. Neurosci. 78, 277–283 (2020).
Kan, R. L. D. et al. Effects of non-invasive brain stimulation in multiple sclerosis: systematic review and meta-analysis. Ther. Adv. Chronic Dis. 13, 20406223211069198 (2022).
Linnhoff, S., Fiene, M., Heinze, H. J. & Zaehle, T. Cognitive fatigue in multiple sclerosis: an objective approach to diagnosis and treatment by transcranial electrical stimulation. Brain Sci. 9, 1 (2019).
Loreti, E. H. et al. Effects of anodal transcranial direct current stimulation on the primary motor cortex in women with fibromyalgia: a randomized, triple-blind clinical trial. Neuromodulation 26, 767–777 (2023).
Acler, M. et al. Transcranial direct current stimulation (tDCS) for sleep disturbances and fatigue in patients with post-polio syndrome. Restor. Neurol. Neurosci. 31, 661–668 (2013).
Dobbs, B. et al. Generalizing remotely supervised transcranial direct current stimulation (tDCS): feasibility and benefit in Parkinson’s disease. J. NeuroEng. Rehabil. 15, 114 (2018).
Forogh, B. et al. Repeated sessions of transcranial direct current stimulation evaluation on fatigue and daytime sleepiness in Parkinson’s disease. Neurol. Sci. 38, 249–254 (2017).
Saiote, C. et al. Impact of transcranial direct current stimulation on fatigue in multiple sclerosis. Restor. Neurol. Neurosci. 32, 423–436 (2014).
Chalah, M. A. et al. Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J. Neurol. Sci. 372, 131–137 (2017).
Chalah, M. A., Lefaucheur, J. P. & Ayache, S. S. Long-term effects of tDCS on fatigue, mood and cognition in multiple sclerosis. Clin. Neurophysiol. 128, 2179–2180 (2017).
Razza, L. B. et al. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress. Anxiety 37, 594–608 (2020).
Fregni, F. et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int. J. Neuropsychopharmacol. 24, 256–313 (2021).
Silvers, J. A., Wager, T. D., Weber, J. & Ochsner, K. N. The neural bases of uninstructed negative emotion modulation. Soc. Cogn. Affect. Neurosci. 10, 10–18 (2015).
Friedman, N. P. & Robbins, T. W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 47, 72–89 (2022).
Tecchio, F. et al. Home treatment against fatigue in multiple sclerosis by a personalized, bilateral whole-body somatosensory cortex stimulation. Mult Scler. Relat. Disord. 63, 103813 (2022).
Workman, C. D., Kamholz, J. & Rudroff, T. Transcranial direct current stimulation (tDCS) for the treatment of a multiple sclerosis symptom cluster. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 13, 263–264 (2020).
Mortezanejad, M., Ehsani, F., Masoudian, N., Zoghi, M. & Jaberzadeh, S. Comparing the effects of multi-session anodal trans-cranial direct current stimulation of primary motor and dorsolateral prefrontal cortices on fatigue and quality of life in patients with multiple sclerosis: a double-blind, randomized, sham-controlled trial. Clin. Rehabil. 34, 1103–1111 (2020).
Ayache, S. S. et al. Prefrontal tDCS decreases pain in patients with multiple sclerosis. Front. Neurosci. 10, 147 (2016).
Hsu, W. Y., Cheng, C. H., Zanto, T. P., Gazzaley, A. & Bove, R. M. Effects of transcranial direct current stimulation on cognition, mood, pain, and fatigue in multiple sclerosis: a systematic review and meta-analysis. Front. Neurol. 12, 1 (2021).
Zakibakhsh, N. et al. Repeated prefrontal tDCS for improving mental health and cognitive deficits in multiple sclerosis: a randomized, double-blind, parallel-group study. J. Transl. Med. 22, 843 (2024).
Kronberg, G., Rahman, A., Sharma, M., Bikson, M. & Parra, L. C. Direct current stimulation boosts hebbian plasticity in vitro. Brain Stimul. 13, 287–301 (2020).
Shaw, M. T., Best, P., Frontario, A. & Charvet, L. E. Telerehabilitation benefits patients with multiple sclerosis in an urban setting. J. Telemed. Telecare 27, 39–45 (2021).
Charvet, L. E., Shaw, M. T., Bikson, M., Woods, A. J. & Knotkova, H. Supervised transcranial direct current stimulation (tDCS) at home: a guide for clinical research and practice. Brain Stimul. 13, 686–693 (2020).
Charvet, L. E. et al. Cognitive function in multiple sclerosis improves with telerehabilitation: results from a randomized controlled trial. PLoS ONE 12, e0177177 (2017).
Charvet, L. et al. Remotely supervised transcranial direct current stimulation increases the benefit of at-home cognitive training in multiple sclerosis. Neuromodul. J. Int. Neuromodul. Soc. 21, 383–389 (2018).
Fonteneau, C. et al. Sham tDCS: a hidden source of variability? Reflections for further blinded, controlled trials. Brain Stimul. 12, 668–673 (2019).
Loo, C. K. et al. International randomized-controlled trial of transcranial direct current stimulation in depression. Brain Stimul. 11, 125–133 (2018).
Arroyo-Fernández, R. et al. Effectiveness of transcranial direct current stimulation combined with exercising in people with fibromyalgia: a randomized sham-controlled clinical trial. Arch. Phys. Med. Rehabil. 103, 1524–1532 (2022).
Dinn, W. et al. Effectiveness of tDCS blinding protocol in a sham-controlled study. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 10, 401 (2017).
Settle, J. R., Robinson, S. A., Kane, R., Maloni, H. W. & Wallin, M. T. Remote cognitive assessments for patients with multiple sclerosis: a feasibility study. Mult. Scler. Houndmills Basingstoke Engl. 21, 1072–1079 (2015).
Eilam-Stock, T., Shaw, M. T., Sherman, K., Krupp, L. B. & Charvet, L. E. Remote administration of the symbol digit modalities test to individuals with multiple sclerosis is reliable: a short report. Mult. Scler. J. Exp. Transl. Clin. 7, 2055217321994853 (2021).
Zanão, T. A. et al. Impact of two or less missing treatment sessions on tDCS clinical efficacy: results from a factorial, randomized, controlled trial in major depression. Neuromodul. J. Int. Neuromodul. Soc. 17, 737–742 (2014).
Brunoni, A. R. et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 5, 175–195 (2012).
Martin, D. M., Liu, R., Alonzo, A., Green, M. & Loo, C. K. Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: effect of timing of stimulation. Exp. Brain Res. 232, 3345–3351 (2014).
Bang, H., Ni, L. & Davis, C. E. Assessment of blinding in clinical trials. Control Clin. Trials 25, 143–156 (2004).
Linnhoff, S., Haghikia, A. & Zaehle, T. Effects of repetitive twice-weekly transcranial direct current stimulations on fatigue and fatigability in people with multiple sclerosis. Sci. Rep. 13, 5878 (2023).
Shiozawa, P. et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 17, 1443–1452 (2014).
Braley, T. J. & Chervin, R. D. Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment. Sleep 33, 1061–1067 (2010).
Forbes, M. K. et al. Elemental psychopathology: distilling constituent symptoms and patterns of repetition in the diagnostic criteria of the DSM-5. Psychol. Med. 1, 1–9. https://doi.org/10.1017/S0033291723002544 (2023).
Phyo, A. Z. Z. et al. The efficacy of psychological interventions for managing fatigue in people with multiple sclerosis: a systematic review and meta-analysis. Front. Neurol. 9, 1 (2018).
Snowball, A. et al. Long-term enhancement of brain function and cognition using cognitive training and brain stimulation. Curr. Biol. 23, 987–992 (2013).
Motter, J. N. et al. Computerized cognitive training and functional recovery in major depressive disorder: a meta-analysis. J. Affect. Disord. 189, 184–191 (2016).
Koster, E. H. W., Hoorelbeke, K., Onraedt, T., Owens, M. & Derakshan, N. Cognitive control interventions for depression: a systematic review of findings from training studies. Clin. Psychol. Rev. 53, 79–92 (2017).
Legemaat, A. M. et al. Effectiveness of cognitive remediation in depression: a meta-analysis. Psychol. Med. 52, 1–16 (2021).
Routledge, K. M., Williams, L. M., Harris, A. W. F., Schofield, P. R. & Gatt, J. M. The impact of online brain training exercises on experiences of depression, anxiety and emotional wellbeing in a twin sample. J. Psychiatr. Res. 134, 138–149 (2021).
Dallaway, N., Lucas, S. J. E. & Ring, C. Concurrent brain endurance training improves endurance exercise performance. J. Sci. Med. Sport 24, 405–411 (2021).
Téllez, N. et al. Fatigue in multiple sclerosis persists over time. J. Neurol. 253, 1466–1470 (2006).
Cuschieri, S. The CONSORT statement. Saudi J. Anaesth. 13, S27–S30 (2019).
Thompson, A. J. et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 17, 162–173 (2018).
Krupp, L. B., LaRocca, N. G., Muir-Nash, J. & Steinberg, A. D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 46, 1121–1123 (1989).
Benedict, R. H. B., Fishman, I., McClellan, M. M., Bakshi, R. & Weinstock-Guttman, B. Validity of the Beck depression inventory-fast screen in multiple sclerosis. Mult. Scler. Houndmills Basingstoke Engl. 9, 393–396 (2003).
Strober, L. et al. Symbol digit modalities test: a valid clinical trial endpoint for measuring cognition in multiple sclerosis. Mult. Scler. J. 25, 1781–1790 (2019).
Smith, A. Symbol Digit Modalities Test (SDMT) Manual (Revised) Western Psychological Services (1982).
Wilkinson, G. S. & Robertson, G. J. WRAT 4: Wide Range Achievement Test (Psychological Assessment Resources Lutz, 2006).
Seibt, O., Brunoni, A. R., Huang, Y. & Bikson, M. The pursuit of DLPFC: non-neuronavigated methods to target the left dorsolateral pre-frontal cortex with symmetric bicephalic transcranial direct current stimulation (tDCS). Brain Stimul. 8, 590–602 (2015).
BrainHQ from Posit Science. BrainHQ from Posit Science. https://www.brainhq.com/
Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis
Nordin, Å., Taft, C., Lundgren-Nilsson, Å. & Dencker, A. Minimal important differences for fatigue patient reported outcome measures—a systematic review. BMC Med. Res. Methodol. 16, 62 (2016).
Yost, K. J., Eton, D. T., Garcia, S. F. & Cella, D. Minimally important differences were estimated for six patient-reported outcomes measurement information system-cancer scales in advanced-stage cancer patients. J. Clin. Epidemiol. 64, 507–516 (2011).
Amtmann, D., Bamer, A. M., Kim, J., Chung, H. & Salem, R. People with multiple sclerosis report significantly worse symptoms and health related quality of life than the US general population as measured by PROMIS and NeuroQoL outcome measures. Disabil. Health J. 11, 99–107 (2018).
Funding
This work was supported by the National Multiple Sclerosis Society (NMSS) [Grant Number 1803–30492].
Author information
Authors and Affiliations
Contributions
Conceptualization: LC. Methodology: LC, JDG, XL, MS, GP. Investigation: LC, PB, MS, GP, LZ, JDG, ML. Writing—Original Draft: LC, GP. Writing—Review and Editing: LC, JDG, XL, LZ, JG, AD, ML, MB, GP, LK. Funding Acquisition: LC. Supervision: LC.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Charvet, L., Goldberg, J.D., Li, X. et al. Home-based transcranial direct current stimulation paired with cognitive training to reduce fatigue in multiple sclerosis. Sci Rep 15, 4551 (2025). https://doi.org/10.1038/s41598-025-88255-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88255-2