Abstract
Capillary blood collection presents advantages such as reduced invasiveness over venous serum for syphilis diagnosing. This study aimed to compare diagnostic accuracy between capillary and venous blood samples for syphilis diagnosis. Individuals aged ≥ 18 years were included in a cross-sectional study. Syphilis screening was done using Rapid tests (RT) followed by collection of serum capillary and venous samples for VDRL and TPHA test. Sensitivity, specificity, and Kappa coefficient were calculated. Of 191 participants, 115 RT + and 76 RT-. Diagnostic properties did not significantly differ between capillary and venous samples. Capillary VDRL showed 99% sensitivity and 100% specificity, mirroring TPHA results. Furthermore, there was significant agreement between sample types for both serological tests (p < 0.001). Capillary sampling offers comparable diagnostic accuracy to venous collection, regardless of serum quality. Capillary sampling holds promise, particularly in developing countries and large-scale testing initiatives.
Similar content being viewed by others
Introduction
Syphilis is a multisystem bacterial infection caused by Treponema pallidum subsp. pallidum (T. pallidum), which primarily spreads through sexual transmission or vertical transmission during pregnancy1,2. It remains a significant contributor to perinatal morbidity and mortality, as well as serious complications such as neurosyphilis, which is particularly prevalent in developing countries3.
Despite being a preventable and curable infection, the global burden of syphilis persists4. In 2020, an estimated 7 million adults aged 15 to 49 acquired syphilis worldwide5. In Brazil, between 2011 and 2022, there were 1,115,529 reported cases of acquired syphilis with 60.7% occurring in men and 39.3% in women6.
The majority of cases are asymptomatic, contributing to the perpetuation of the transmission chain in the population. Hence, there is a critical need for diagnostic tools that not only enhance access but also ensure accuracy and efficiency in diagnosis2,3,5.
Serological tests, including Treponemal and Non-treponemal tests are the primary diagnostic methods for syphilis. These tests typically utilize venous serum obtained through venous whole blood collection, which is the gold standard sample in diagnostic assays1. Capillary blood collection for blood collection has emerged as a promising alternative. This approach is less invasive and eliminates the need for complex laboratory infrastructure7,8. However, validation of this collection method and its suitability for syphilis laboratory tests has not yet been completely established.
Objective
The aim of this study was to compare diagnostic properties of capillary versus venous blood samples for serological syphilis testing.
Materials and methods
Study design and participants
We utilized data from the Health Information and Monitoring of Sexually Transmitted Infections (SIM study), a study conducted between May 20, 2022 and October 28, 2022, which aimed to evaluate strategies to enhance treatment compliance and follow-up among participants9. To access syphilis positive participants, a single-center, cross-sectional study was done using a mobile heath unit that was placed in highly populated areas across Porto Alegre city during the recruitment period. Participants aged 18 years or older were invited to answer an interview and provided both capillary and venous blood samples after giving written informed consent.
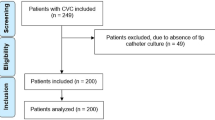
A structured questionnaire was administered to collect information on demographic and behavioral characteristics, as well as sexually transmitted infections (STI). Data were accessed for research purposes between May 20, 2022, and October 28, 2023. Point-of-care tests such as lateral-flow immunochromatographic rapid testing (RT), were conducted for STI screening. Positive results were subsequently confirmed through laboratory tests for syphilis. This study was performed according to the Standards for Reporting Diagnostic Accuracy Studies (STARD) guidelines (http://www.equator-network.org/reporting-guidelines/stard) as outlined in Fig. 1.
Screening tests
Rapid tests (RT), specifically lateral flow immunochromatographic assays, were performed for Syphilis (Bioclin Syphilis Bio, Brazil, MG), HIV (ABON™ HIV 1/2/O Tri-line Human Immunodeficiency Virus Rapid Test Device, China, Hangzhou), HCV (ABON™ HCV Hepatitis C Virus Rapid Tests Device, China, Hangzhou) and HBV (Bioclin BIOLISA HBsAg, Brazil, MG). The results were interpreted according to the manufacturer’s instructions. Whole blood samples obtained through finger prick collection were used for testing. These tests are validated for capillary blood samples and approved by the Brazilian Health Regulatory Agency (Anvisa).
Participants were subsequently categorized into two groups based on the syphilis screening results: RT- (negative test) and RT+ (positive test) groups. Additionally, all participants in the RT- group reported no clinical history of syphilis in the questionnaire, corroborating the test results. Furthermore, all participants who tested positive for Syphilis and had positive HIV, HCV, or HBV rapid tests were excluded from the study. This exclusion criterion was implemented to mitigate potential cross-reactions and impacts on nontreponemal test results. As this was a methodological validation study, we followed the manufacturer’s protocols for nontreponemal tests10 and the literature findings11.
Specimen collection and processing of samples
Trained nurses collected blood samples from a finger prick (capillary - test index) and from venipuncture (venous - reference standard) for each participant. The protocols for sampling procedures, including material selection, finger prick technique, and processing for syphilis laboratory tests was described in the Supplementary material.
-
(1)
Capillary blood collection was conducted by nurses using a SARSTEDT® safety lancet (Nümbrecht, NRW) at the designated site for the ring or middle finger puncture. The capillary blood collection was carefully performed in the central region of the participant’s distal phalanx to ensure adequate blood volume. Subsequently, blood was directly dripped into BD Microtainer® Amber SST™ tubes (Franklin Lakes, New Jersey), each capable of holding 600µL of whole blood.
To maintain serum quality and adhere to sampling standards, some procedures were followed:
-
(a)
Discarding the first drop of blood with gauze helps to remove any contaminants or debris from the puncture site.
-
(b)
Avoiding rubbing the finger on the microcapillary collection tube: to prevent introducing additional contaminants or disrupt the integrity of the blood sample, affecting the accuracy of test results.
-
(c)
Do not apply excessive pressure to the finger during collection to prevent hemolysis, which can affect the quality of the serum and compromise test results.
-
(2)
The collection of venous blood samples was conducted via peripheral venipuncture using a BD Vacutainer® SST™ II Advance Tubes (Franklin Lakes, New Jersey), following standard protocols. We collected 8,5mL of whole blood that were appropriately labeled and stored at a controlled temperature until laboratory analysis12,13.
The samples were transported from the collection site to the Clinical Epidemiology Laboratory (Epiclin), the central laboratory in our study, maintaining a temperature range of 2ºC to 8ºC. Microcapillary collection tubes were then centrifuged at 10,000 rpm for 90 s, yielding an average of 300µL of serum. Venous samples underwent centrifugation at 2,000 rpm for 10 min, resulting in 4.5 mL of serum, following the manufacturer’s instructions. Subsequently, serum aliquots of 110µL were utilized in syphilis tests, categorized by type of collection: either capillary serum or venous serum.
Serum was visually assessed to determine whether the appearance of hemolysis and/or lipemia could potentially influence the testing results. Therefore, all sera were classified as visually adequate (without hemolysis/lipemia) or non-adequate (presence of hemolysis/lipemia).
Laboratory tests
Capillary and venous serum were compared using standard syphilis serological assays in the laboratory. Non-treponemal testing was conducted using the modified Venereal Disease Research Laboratory (VDRL) test, also known as the unheated serum reagin (USR) test (V.D.R.L test - Wiener lab, AR). The treponemal test was conducted using Treponema Pallidum Haemagglutination Assay (TPHA) test (RANDOX SYP-TPHA test - Randox laboratories LTD, UK). Both the VDRL and TPHA tests were carried out according to the manufacturer’s instructions14. TPHA was chosen because of its widespread use and availability in the country as well as its accuracy measures, which are similar to those of other treponemal test14,15.
As for the VDRL test, the results were expressed as reactivity, categorized as either non-reactive (NR) or reactive, and quantitative titers were reposted as NR; 1:1; 1:2; 1:4; 1:8; 1:16; and ≥ 1:32. For the TPHA test results were expressed as non-reactive, reactive, inconclusive, defined according to the manufacturer’s instructions by cell standard. Samples were coded and randomized for execution of techniques and interpretation of results. The tests were conducted by experienced laboratory technicians who were masked to the sample result screening (RT- or RT+) and type of collection (whether capillary or venous).
Sample size calculation
The sample size required for reliability of results was 150 subjects: 75 RT + and 75 RT-. The study employed a confidence level of 95% and aimed for a confidence interval with an amplitude of 10%, utilizing the Wald method. Anticipated sensitivity was 98.4%, and expected specificity stood at 94.9%16. The PSS Health online tool was used for this calculation.
Statistical analysis
The association of sociodemographic variables with RT positivity was investigated using the Chi-square test. Sensitivity and specificity for each type of sample were calculated according to VDRL and TPHA, considering the venous sample as a gold standard when compared to the capillary sample. The impact of serum quality in the result of tests was also evaluated. McNemar’s paired statistical test and the Kappa coefficient of agreement were used to evaluate agreement. Inconclusive results for TPHA were excluded from analyzes. All analyses employed a 5% significance level and were developed in R statistical software (version 4.1.3; R Core Team 2022, http://www.r-project.org/).
Ethics declarations
This study was approved by the Institutional Review Board of Hospital Moinhos de Vento under protocol number 3.745.031/2019 - CAAE: 26185219.0.0000.5330. All procedures adhered to the national guidelines for ethics committees and the ethical standards outlined in the Declaration of Helsinki17.
Results
From 191 participants included in the analysis (Figs. 1), 115 (60%) were positive for syphilis based on Rapid Test (RT+), and 76 (40%) tested negative (RT-). The majority of the sample were females, aged over 49 years old, declared themselves as white, e and have a secondary school (Table 1). Participants with RT + for syphilis significantly declare themselves as black (27.2%) (p = 0.011) and with a higher proportion of elementary school (38.3%) (p < 0.001) by adjusted residual analyses similar to official notification data18.
There were no differences in concordance analysis according to type of sampling (capillary vs. venous) for VDRL (p = 0.5) and TPHA (p > 0.9) (Table 2). Kappa coefficients demonstrated high and significant concordance of results between capillary and venous collection methods for VDRL (k = 0.975, p < 0.001) and TPHA (k = 0.921, p < 0.001). In all cases where there was a discrepancy in the VDRL quantitative titers, this difference did not exceed one dilution, either for more or less, as shown in Fig. 2 (lower panel).
In the comparison of diagnostic accuracy for syphilis across the types of sample collection, both the VDRL and TPHA tests demonstrated high sensitivity (VDRL 99%, TPHA 99%) and specificity (VDRL 100%, TPHA 100%) with high PPV and NPV (Table 3). Regarding the visual aspect of the serum, 113 capillary serum samples (59%) and 187 venous serum samples (98%) were judged as adequate. Hemolysis was the predominant in capillary serum samples (40%), with only one sample showing lipemia. There were no differences in diagnostic properties in the sensitivity analysis by sample adequacy (Table 3).
Discussion
This study represents the first attempt to compare the diagnostic properties of capillary serum samples with venous samples for syphilis diagnosis. We found high agreement, sensitivity and specificity of capillary samples for both treponemal and non-treponemal serological tests. Moreover, the diagnostic accuracy remained consistently high when we evaluated the samples based on serum quality and collection method.
Previous research has predominantly focused on capillary samples only, without gold standard comparison19,20,21. Furthermore, our findings propose that a sample obtained through capillary blood collection offers a viable alternative for both screening and definitive diagnosis of syphilis, contingent upon the chosen protocol (traditional, reverse or ECDC)3.
The capillary collection technique offers advantages over venipuncture, particularly for large-scale studies, being technically less complex to obtain the samples, and with previous studies indicating the minimization of pain22. Thoroughly described and validated, the technique can be replicated by healthcare professionals and students, especially in remote locations and situations requiring the use of microvolumes of blood for diagnosis. It was suggested by Freeble and colleagues as an efficient and economical method that facilitates the syphilis diagnosis, especially in individuals where venipuncture is known to be difficult8. This technique even allows for self-collection and postal sending, with analysis conducted via microparticle chemiluminescent immunoassay. However, the impact of the capillary collection technique on sample quality and diagnostic results has not been fully explored since its creation7,8,23. Hemolysis, a factor inherent to the capillary collection methodology, can interfere with the results in some analyses as fasting glycemia24. However, we demonstrate that it does not affect syphilis diagnostic accuracy, as evidenced by high sensitivity, specificity and agreement between the type collection (index vs. standard).
Treponemal tests (such as TPHA) detect antibodies against T. pallidum proteins used for screening and diagnostic confirmation, while non-treponemal tests (such as VDRL) detect antibodies against lipid antigens released from damaged host cells and are widely used in monitoring of the disease. Therefore, a direct comparison of performance between the two tests (VDRL vs. TPHA) was not evaluated in the study. However, when observing the performance of each of them individually, in relation to the type of collection, we identified similar diagnostic accuracy. These findings are significant as these tests are also part of algorithms for the serological diagnosis of syphilis, which are applied differently in the Americas and Europe3.
It is important to note that the sensitivity and specificity of reference standard serological tests vary depending on the clinical stage of the disease and the type of methodology employed3,25. False positives may occur in non-treponemal tests such as VDRL due to cross-reactions, whereas treponemal tests such as TPHA may remain reactive even in treated cases, a condition known as “serofast”26. These intrinsic methodological limitations can impact the accuracy of the tests.
Although this study did not aim to evaluate other STIs, the lack of information may limit the generalizability of the findings regarding co-infections. Therefore, further studies investigating the impact of co-infections on diagnostic tests for syphilis using capillary blood collection are warranted. Additionally, due to logistic constraints, we only have access to a commercial test for either treponemal or non-treponemal test.
Conclusions
Despite syphilis being a centuries-old disease, with curative and inexpensive treatment, its diagnosis and clinical management remain complex. In addition to the immune evasion of T. Pallidum, difficulties in testing and early treatment are associated with the increased prevalence and incidence of the disease in several countries. We show that the capillary blood collection method is promising, especially in developing countries and/or in large-scale testing approaches that require a rapid and less invasive collection procedure. In addition to the high diagnostic accuracy, the minimization of pain and reproducibility of the technique demonstrate its applicability in the diagnosis of syphilis. Prospective studies evaluating treatment, clinical stage and other commercial serological tests for the diagnosis of syphilis are strongly recommended, as the diagnostic properties associated with the manufacturer or composition of each commercial test may vary.
Data availability
The raw data for dataset are not publicly available to preserve individuals’ privacy under the Brazil General Personal Data Protection Law. Data are available from the corresponding author (Eliana Márcia Wendland, elianawend@gmail.com) for researchers who meet the criteria for access to confidential data.
References
Peeling, R. et al. Syphilis Nat. Reviews Disease Primers 3, (2017).
Satyaputra, F., Hendry, S., Braddick, M., Sivabalan, P. & Norton, R. The laboratory diagnosis of Syphilis. J. Clin. Microbiol. 59, e0010021 (2021).
Luo, Y., Xie, Y. & Xiao, Y. Laboratory diagnostic tools for syphilis: current status and future prospects. (2021). https://doi.org/10.3389/fcimb.2020.574806. PMID: 33628742; PMCID: PMC7897658.
Syphilis -- Global. (2024). https://www.who.int/health-topics/syphilis
Orbe-Orihuela, Y. C., Sánchez-Alemán, M. Á., Hernández-Pliego, A., Medina-García, C. V. & Vergara-Ortega, D. N. Syphilis as re-emerging disease, antibiotic resistance, and vulnerable population: global systematic review and meta-analysis. Pathogens 11, 1546 (2022).
DATHI | Indicadores Sífilis. (2024). http://indicadoressifilis.aids.gov.br/
Freeble, C. R. J. & Orsburn, B. Capillary tube technique for serologic screening of syphilis. Public. Health Rep. 1896, 68 (1953).
Page, M. et al. Are all blood-based postal sampling kits the same? A comparative service evaluation of the performance of dried blood spot and mini tube sample collection systems for postal HIV and syphilis testing. Sex. Transm Infect. 97, 209–214 (2021).
Wendland, E. et al. Health information and monitoring of sexually transmitted infections (SIM study): a single-center, parallel, three-arm randomized controlled trial protocol for enhancing adherence to syphilis treatment and follow-up. Trials 23, 445 (2022).
Wiener lab. (2024). https://wiener-lab.com.ar/
Matthias, J. et al. Frequency and characteristics of Biological false-positive test results for Syphilis reported in Florida and New York City, USA, 2013 to 2017. J. Clin. Microbiol. 57, e00898–e00819 (2019).
Scales, K. A practical guide to venepuncture and blood sampling. Nurs. Stand. 22, 29–36 (2008).
SÍFILIS - Estratégias para. Diagnóstico no Brasil (2010). https://bvsms.saude.gov.br/bvs/publicacoes/sifilis_estrategia_diagnostico_brasil.pdf
Park, I. U., Tran, A., Pereira, L. & Fakile, Y. Sensitivity and specificity of Treponemal-specific tests for the diagnosis of Syphilis. Clin. Infect. Dis. 71, S13–S20 (2020).
Woznicová, V. & Vališová, Z. Performance of CAPTIA SelectSyph-G enzyme-linked immunosorbent assay in Syphilis testing of a high-risk population: analysis of discordant results. J. Clin. Microbiol. 45, 1794–1797 (2007).
Negash, M., Wondmagegn, T. & Geremew, D. Comparison of RPR and ELISA with TPHA for the diagnosis of syphilis: Implication for updating syphilis point-of-care tests in Ethiopia. J. Immunol. Res. 1–7 (2018). (2018).
World medical association declaration of Helsinki. ethical principles for medical research involving human subjects. JAMA 310, 2191 (2013).
Soares, E. O. Saúde da População Negra em Porto Alegre (2013). https://lproweb.procempa.com.br/pmpa/prefpoa/cgvs/usu_doc/boletim_53_especial_racaocor.pdf
Bednova, V., Babiĭ, A., Toporovskiĭ, L. & Chistiakova, T. Immunofermentnyĭ analiz s kapilliarnoĭ krov’iu pri serodiagnostike sifilisa [Immunoenzyme analysis with capillary blood in the serodiagnosis of syphilis]. Vestn Dermatol. Venerol 21–24 (1990).
Benzaken, A. et al. Field performance of a rapid point-of-care diagnostic test for antenatal syphilis screening in the Amazon region, Brazil. Int. J. STD AIDS. 22, 15–18 (2011).
García Luna, J. et al. Diagnostic performance of two rapid tests for syphilis screening in people living with HIV in Cali, Colombia. PLoS One. 18, e0282492 (2023).
Woods, K. et al. Patient preferences for capillary vs. venous INR determination in an anticoagulation clinic: a randomized controlled trial. Thromb. Res. 114, 161–165 (2004).
Davies, J. A. V. A rapid micro-hinton and capillary hinton test for syphilis. J. Pediatr. 10, 802–808 (1937).
Heenan, H., Lunt, H., Chan, H. & Frampton, C. Capillary samples and hemolysis: further considerations. J. Diabetes Sci. Technol. 11, 847–848 (2017).
Morshed, M. G. & Singh, A. E. Recent trends in the serologic diagnosis of Syphilis. Clin. Vaccine Immunol. 22, 137–147 (2015).
Cao, W., Thorpe, P. G., O’Callaghan, K. & Kersh, E. N. Advantages and limitations of current diagnostic laboratory approaches in syphilis and congenital syphilis. Expert Rev. Anti Infect. Ther. 21, 1339–1354 (2023).
Funding
This work was supported by the Brazilian Ministry of Health (Institutional Development Support Program of the Brazilian National Health System – PROADI-SUS) and the Associação Hospitalar Moinhos de Vento (AHMV).
Author information
Authors and Affiliations
Contributions
AVS and ACMR: contributed equally to this work.AVS (Roles): Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing.ACMR (Roles): Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing.GTS (Roles): Conceptualization, Investigation, Methodology, Validation, Writing – review & editing.IAV (Roles): Conceptualization, Methodology, Validation, Writing – review & editing.CNO (Roles): Conceptualization, Investigation, Data curation, Validation, Writing – review & editing.SB (Roles): Conceptualization, Methodology, Writing – review & editing.LGP (Roles): Conceptualization, Methodology, Formal analysis, Software, Writing – review & editing.VSR (Roles): Conceptualization, Methodology, Formal analysis, Software, Writing – review & editing.ESB (Roles): Methodology, Investigation, Writing – review & editing.TMD (Roles): Methodology, Investigation, Writing – review & editing.GFMP (Roles): Validation, Funding acquisition, Supervision, Writing – review & editing.FMAS (Roles): Validation, Funding acquisition, Investigation, Supervision, Writing – review & editing.EMW (Roles): Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
dos Santos, A.V., da Rocha, A.C.M., dos Santos, G.T. et al. Accuracy of capillary blood sampling for diagnosing syphilis infection. Sci Rep 15, 5243 (2025). https://doi.org/10.1038/s41598-025-88329-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88329-1