Abstract
As a naturally occurring reducing and oxidizing agent, hydrogen peroxide (H2O2) has a role in several biotic and abiotic processes. Hence, the onsite, precise, and rapid determination of H2O2 is crucial. Herein, we propose a method for colorimetric detection of H2O2 on the basis of hindered formation of gold/silver core/shell nanoparticles. We used ascorbic acid (AA) as the electron donor to reduce silver ions (Ag+) to be shelled around gold nanoparticles and iron(III) edta as an accelerator reactant. Upon reduction of Ag+, owing to the formation of core/shell nanoparticles, the color of the system changes from pink to yellow/orange in the spherical nanoparticles and from pink to purple/blue/green/yellow/orange in the nanorods. The nanorods distinguished color in a rainbow manner for higher concentrations of H2O2, and spherical nanoparticles were critical in the sensitive detection of lower concentrations of H2O2. H2O2 scavenges AA electrons and therefore inhibits core/shell formation and, consequently, restrains the system’s spectral shift and color change. This characteristic was exploited to measure different concentrations of H2O2. Under well-optimized conditions, various concentrations of H2O2 ranging from 1.0 to 50 µΜ have shown an acceptable linear relationship with different colors and, with a limit of detection (LOD) of 230 nM. Furthermore, various real samples were examined to confirm the practicality of our developed probe.
Similar content being viewed by others
Introduction
The existence of reactive oxygen species is the inevitable consequence of aerobic respiration in living cells. Even though these highly oxidative molecules are considered mainly cytotoxins, their presence is highly influential on the ability of a cell to live properly. One of these simultaneously crucial and cruel substances is hydrogen peroxide (H2O2). H2O2 is a colorless, unscented liquid with mild acidity. First synthesized by Louis Jacques Thénard in 1818, the history of the chemical and biological functions of this ancient molecule dates back to the Archean eon (2.5 to 3.9 billion years ago)1, and its versatile nature has increasingly captured attention for its pivotal roles in biological and nonbiological systems. By the year 2022, the production of H2O2 had rocketed to approximately 2190 thousand tons per year2, and H2O2 is among the 100 most important chemicals in the world given its wide range of consumption; H2O2 is produced at several concentrations on the basis of its commercial usage, for example, diluted concentrations of H2O2 (3–5%) are used in medicine and dentistry, whereas concentrated H2O2 (90–98%) is used in military and aerospace purposes. Several methods have been developed to produce H2O2 on large scales, and the method that dominates its production industrially is the anthraquinone autoxidation process3.
H2O2 is a double-edged sword, as we can consider its numerous beneficial roles in all kingdoms of life, such as being a signaling molecule and running crucial pathways in cells4, and having antimicrobial effects while having hormonal regulatory effects in plant cells5. Due to its relatively unreactive characteristics, H2O2 is a perfect molecule for fulfilling the requirements of a signaling molecule. It is produced enzymatically, is further degraded in cells and can oxidize thiol groups of proteins, which is another perfect characteristic of a signaling molecule6, and is a stress response molecule and metabolic regulator in bacterial cells7. However, the accumulation of H2O2 can lead to oxidative stress, which is a harmful phenomenon that is upstream of many harmful cellular consequences, such as DNA and protein damage, lipid peroxidation, and metabolic disruption. H2O2 can be disastrous in many aspects (e.g., cancer, inflammation, and aging), as accumulated H2O2 can cause damage to both eukaryotic cells and their prokaryotic counterparts. This normal aerobic metabolite can occur at a concentration of approximately 10 nm inside cells8.
There are a variety of methods for the detection of H2O2, including spectrophotometry (e.g., ultraviolet‒visible spectroscopy (UV‒Vis), surface-enhanced Raman spectroscopy (SERS)9, nuclear magnetic resonance (NMR)10), fluorescence11, luminescence12, and electrochemical, and enzyme-based13 techniques. All of these methods are expensive and require technical expertise, which is time-consuming. One of the most frequent methods for the quantitative detection of H2O2 is based on the oxidizing property of H2O2. In this approach, a peroxidase enzyme catalyzes the oxidation of a colorless, chromogenic substrate (e.g., 3,3′,5,5′-tetramethylbenzidine (TMB)) by H2O2 to turn it into a fluorescent, luminescent, or colored molecule. The concentration of H2O2 can be detected by measuring the light absorbance via spectroscopy14. The most utilized peroxidase enzyme in this system is horseradish peroxidase (HRP)15. However, there are several drawbacks to the use of enzymatic methods such as HRP. The activity of the enzyme is strongly affected by the concentration of H2O2 and pH; at pH values lower than 7.0, a rapid decrease in the initial velocity of the enzyme is encountered, indicating its limit of application in certain pH ranges16. In the same study, it was shown that at concentrations of H2O2 greater than 20 mM, a zero-order reaction rate and an extreme decrease in the initial velocity of molecular oxygen production gradually occurred, which raises problems in the detection of higher concentrations of H2O216. Other problems associated with enzymatic methods include their high cost in terms of preparation and assay, and their ability to be inhibited by several compounds. Moreover, enzymes are highly sensitive to environmental factors (e.g., pH, temperature, and electrolytes)17.
Recently, researchers have focused on the design of H2O2 sensors based on plasmonic nanoparticles such as gold (AuNPs), silver (AgNPs), and copper (CuNPs) nanoparticles. These nanoparticles have special and unique colorimetric features due to a phenomenon called localized surface plasmon resonance (LSPR). In LSPR, the incidence of a light beam with a specific wavelength to the surface of a noble metal nanoparticle can cause a collective oscillation of conduction electrons near the nanoparticle surface. As a result, the reflected light has a different wavelength than the irradiated light. In plasmonic nanoparticles, the wavelength of the reflected light is in the visible range, which makes it favorable for utilization in colorimetric sensing systems18,19,20,21,22, especially rapid and onsite sensors. The color features of plasmonic nanoparticles are strongly dependent on the type, shape, and size of the particles23,24,25.
A colorimetric method has been reported for the detection of H2O2 at concentrations between 0.6 and 200 µM. This method is based on the formation of Au@Ag nanocubes26. In another study, Au/Ag core/shells were utilized as an electrochemical electrode to sense H2O227. In another study, a hybrid material was synthesized using AgNPs and cellulose nanowhiskers as probes for H2O2 sensing28. A research has also established a portable colorimetric sensor based on CuNPs for the detection of H2O2 and uric acid29. Additionally, a visual colorimetric H2O2 sensor was fabricated on the basis of the Fenton reaction with AuNPs30. An H2O2 sensor must be accurate and highly sensitive simultaneously because of its applications in the health and food industries. Most of the abovementioned colorimetric sensors do not meet these qualifications. For example, the limit of detection (LOD) of a sensor established by Yeh et al.26 is 1.11 µM, with r2 = 0.904 at concentrations lower than 200 µM, and 0.60 µM, with r2 = 0.941 at concentrations lower than 40 µM. This level of accuracy and LOD is not sufficient, especially in regard to clinical applications.
Some reagents used to detect H2O2 can be considered toxic materials. For example, in 2019, a method was developed on the basis of the preparation of hydrogel particles, and the compound used to interact with H2O2 was methylene blue (MB), which can be reduced and change color from blue to pink, resulting in a colorimetric sense of H2O2 detection31. However, compounds such as methylene blue are considered cytotoxins and can interrupt vital cellular processes. It interferes with cell membranes and is considered a mutagenic compound because it can be inserted between DNA bases, interrupting replication and the DNA repair process32.
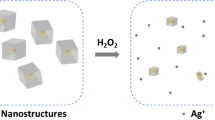
In the present study, we developed a sensitive, fast, and precise colorimetric sensing system for the detection of hydrogen peroxide on the basis of the inhibition of the growth of gold/silver core/shell nanoparticles utilizing iron(III) edta as an accelerator reactant (Fig. 1). Moreover, the inhibition resulted in a redshift and a decrease in the spectrum peak, which was measured via a UV‒Vis spectrophotometer. Furthermore, characterization of the core/shell and nanoparticles was performed via dynamic light scattering (DLS) and transmission electron microscopy (TEM). Additionally, the developed sensor does not have the disadvantages of the previous methods mentioned above.
Materials and methods
Materials
Ascorbic acid (AA or AAH-), silver nitrate (AgNO3), cetyltrimethylammonium bromide (CTAB), sodium borohydride (NaBH4), 2-(n-morpholino) ethane sulfonic acid (MES), ethylenediaminetetraacetic acid iron(III) sodium salt (iron(III) edta), 5-bromo-2-hydroxybenzoic acid (5-BrSA), hydrogen peroxide (H2O2) (to standardize H2O2, it was quantitatively oxidized by titration with potassium permanganate), and hydrogen tetrachloroaurate (HAuCl4) were purchased from Sigma‒Aldrich. For all of the experiments, we used deionized water (18.2 MΩ) for solution preparation.
Instrumentation
A Lambda (PerkinElmer, USA) spectrophotometer was used for absorbance spectral recording at room temperature. A PHILIPS MC 10 TH microscope was used to obtain transmission electron microscopy images at an accelerating voltage of 100 kV. Dynamic light scattering analysis, employing a Zetasizer Viscotec 802 (DLS), was utilized to determine the size of the nanoparticles. The probe images were captured with a Xiaomi Poco X3 GT smartphone.
Synthesis of spherical AuNPs
The three-step seed-mediated method was used to prepare spherical AuNPs33. (A) Primary seed mixture was made by adding 0.125 mL of HAuCl4 (0.01 M) to 5.0 mL of CTAB (0.10 M) solution. After that, 0.30 mL of fresh NaBH4 (0.01 M) was combined with the prepared solution while being vigorously stirred. The hue of the solution subsequently changed from yellow to brownish-yellow. Stirring was stopped immediately after 2 h. (B) A secondary seed mixture was made by adding 0.167 mL of HAuCl4 (0.01 M) to 15 mL of CTAB (0.10 M) solution. After that, 0.10 mL of 0.1 M solution was combined with the prepared solution. Two minutes later, 5.0 mL of the primary seed mixture was added, and NPs with dimensions of 10 nm were synthesized. (C) To synthesize NPs with a diameter of 25 nm, 0.5 mL of HAuCl4 (0.01 M) was mixed with 45 mL of CTAB (0.10 M) solution. Next, 0.25 mL of AA (0.1 M) was combined with the solution. In the final step, 5.0 mL of the secondary seed mixture was added after 2 min. The synthesized spherical NPs had a red–purple color and an absorption peak at 524 nm.
Synthesis of AuNRs
Rod-shaped gold nanoparticles (AuNRs) were synthesized via a slightly modified version of a previously reported seed-mediated protocol34. The primary seed solution was prepared by adding 0.125 mL of HAuCl4 (0.01 M) and then 0.30 mL of fresh NaBH4 (0.01 M) to 4.7 mL of CTAB solution (0.10 M). The hue of the solution changed from yellow to brownish yellow within one minute. Before use, this solution was stirred for 2 h at laboratory temperature. To prepare the AuNRs, 10 mg of 5-BrSA was mixed with 5.0 mL of 0.1 M CTAB solution. After the dissolution of 5-BrSA, 96 μL of 0.01 M AgNO3 was added. The mixture was mildly stirred at laboratory temperature for 15 min, after which 5.0 mL of 0.001 M HAuCl4 solution was added to the mixture. After 120 min of prereduction, 26 μL of 0.1 M ascorbic acid solution was added while vigorously stirred. Finally, 16 μL of seed mixture was injected into the growth solution. The stirring was stopped immediately after 30 s, after which the mixture was left undisturbed at laboratory temperature for a minimum of 4 h. The AuNRs were finally synthesized, and the color of the solution changed to brown moderately.
Sensing procedure for H2O2
Various concentrations of H2O2 were added to a solution containing MES buffer (final concentration of 5.0 mM, pH 6.5), ascorbic acid (final concentration of 200 µM), and edta iron(III) sodium salt solution (final concentration of 20 µM). The above solution was inoculated into a solution containing 100 µL of AuNSs or AuNRs and AgNO3 (final concentration of 300 µM) such that the total volume was 1.0 mL. The final solution was shaken thoroughly, and after 10 min of incubation, the absorbance was recorded in the range of 350–900 nm.
Selectivity study
To show how selective the developed sensor is, some common ions that are usually present in real samples were added to the standard H2O2 solution and measured through the procedure mentioned in section “Sensing procedure for H2O2”. These ions include NaNO2, NaNO3, KCl, MgCl2, NaCl, CaCl2, NaCO3, Na2SO4, FeCl3, and CoCl2 at a final concentration of 1.0 mM.
Real sample analysis
To investigate the operativity of the sensor for real, complex samples, the quantity of H2O2 was measured in deMan Rogosa Sharpe (MRS) medium (0.1X, 0.025% V/V), tap water (5% V/V), tomato leaf extract (0.01X and 0.04X), and tomato fruit extract (0.01X and 0.04X). The tap water samples were used without preparation. Tomato fruit and leaf samples were prepared according to the QuEChERS method35. All the samples were spiked with 15 µM H2O2 solution and evaluated by the sensor via the procedure described in section “Sensing procedure for H2O2”.
Results and discussion
Principle of H2O2 sensing
The AuNSs were utilized to develop the sensor, which possesses a characteristic LSPR band centered at 524 nm (Fig. 2A). TEM images revealed that the diameter of the AuNSs was 20 nm (Fig. 2F). The LSPR peak could be blue shifted whenever the Ag° shells around the AuNSs forming Au/Ag core/shell nanostructures. The formation of Au–Ag core/shell nanostructures and their inhibition in the presence of H2O2 were utilized to develop a colorimetric sensor for H2O2 detection. To do this, two solutions, A and B, were prepared. Solution A consisted of AuNSs, AgNO3, and deionized water. Solution B consisted of AA, iron(III) edta, deionized water, and MES buffer. When solutions A and B were mixed, a blueshift in the UV‒Vis spectrum (Fig. 2B, blue line) and a gradual color change in the solution were observed, possibly because of the reduction of Ag+ to Ago, which could be shelled around the AuNSs (Eq. 1).
(A, C, F) Absorbance spectrum, intensity size distribution, and TEM image of as-prepared AuNSs. Absorbance spectra, intensity size distributions, and TEM images illustrating Au–Ag core/shell formation in the absence (B: red line) and presence (D, G, B: blue line) of iron(III) edta, and the inhibition of core/shell formation in the presence of H2O2 (E, H, B: green line). The concentrations of silver ions (Ag+), MES buffer (B), ascorbic acid (AA), iron(III) edta, and H2O2 were 210 µM, 5.0 mM (pH 7.0), 150 µM, 25 µM, and 125 µM, respectively.
To prove the effect of iron(III) edta (Fig. 1), the spectra of the same solution without iron(III) edta were recorded (Fig. 2, line red), which has a much lower blueshift than the blue line. In the absence and presence of iron(III) edta, the LSPR peak is centered at 520 and 418 nm, respectively. Notably, higher concentrations of ascorbic acid can reduce more Ag+ ions and the same blue shift will be observed. However, since the basis of the present work is the oxidation of hydrogen peroxide by ascorbate, a lower concentration of AA is preferred to increase the sensitivity of hydrogen peroxide detection. By adding hydrogen peroxide to solution B, hydrogen peroxide as an electron-scavenging agent, can scavenge ascorbate electrons via reactions 5 to 8 accelerated by iron(III) edta; consequently, it restrains the growth of Au/Ag core/shell nanostructures. The mentioned effect was dubbed the “inhibition reaction”.
Both core/shell formation and inhibition reactions were accelerated by iron(III) edta due to its potential to maximize the electron donation of ascorbic acid (Fig. 2B). The initiation of the core/shell formation reaction starts with the direct reduction of iron(III) edta by ascorbic acid, which produces ascorbate radicals that react with another ferric chelate to complete the initiation process (Eqs. 2 and 3). This production of ascorbic acid radicals along with iron(II) edta, can readily reduce Ag+ to produce Ag° (+ 1.57 v) that shells around the AuNSs (Eq. 4), which results in a blueshift of the spectrum (Fig. 2B) and a change in color from pink to yellow.
The initiation reaction of the inhibition reaction process completely resembles the core/shell formation reaction, in which ascorbic acid radicals and ferrous chelates are produced, which can further reduce hydrogen peroxide. This reaction of H2O2 with ferrous chelate is called the Fenton reaction36, and at the end of this series of reactions, water is produced, and ascorbic acid gets completely oxidized (Eqs. 5–8). Therefore, the formation of Au/Ag core/shell nanostructures is inhibited in the presence of hydrogen peroxide (Fig. 2B, green line). There are arguments regarding Eqs. 5 and 6 that instead of iron(III) edta and hydroxide, oxidoiron(II) is the product in Eq. (5) at pH values above 536, and with respect to Eq. (6), AAH. Is completely deprotonated37.
TEM images were used to verify the formation of Au/Ag core/shell nanostructures and the inhibition process in the presence of H2O2. As shown in Fig. 2F–H, the size of the 20 nm AuNSs (Fig. 2F) increased to 30 nm as the Ag° shells surrounded them (Fig. 2G), and owing to the inhibition reaction in the presence of H2O2, the size of the core/shell did not grow more than 25 nm (Fig. 2H). The differences in size were confirmed by DLS, as illustrated in Fig. 2C–E, in which the average size of the AuNSs in the presence of H2O2 (Fig. 2E) was less than the core/shell itself (Fig. 2D), and both were greater than the average size of the AuNSs themselves (Fig. 2C).
Optimization of the sensing conditions
Some influential parameters, such as pH, ascorbic acid concentration, silver nitrate concentration, iron(III) edta concentration, and analysis time, were optimized to boost the stability, sensitivity, accuracy, and precision of the developed sensing system. The difference between the spectrum area of the sensor in the absence (So) and presence (S) of H2O2 was regarded as the output signal of the measurement of H2O2.
Optimizing the concentration of ascorbic acid
As ascorbic acid is exposed to H2O2, it becomes oxidized to dehydroascorbic acid according to Eqs. (9–11)38 that reduces H2O2 instead of Ag+ ions during the redox reaction (Eq. 1). In other words, H2O2 prevents ascorbic acid from turning silver ions into silver atoms. Therefore, the presence of H2O2 inhibits the formation of core/shell nanostructures; consequently, color changes and shifts in the absorbance spectrum are not observed.
The quantity of ascorbic acid in the sensing system has a major effect on the sensitivity of the developed sensor. Figure 3A illustrates how the reaction rate of core/shell formation changes in the presence of different concentrations of ascorbic acid. As the concentration of ascorbic acid increased, the rate of the reaction grew gradually. Finally, concentration 200 µM was chosen since the responses at concentration 250 µM had low repeatability which originated from highly fast core/shell formation reaction.
(A) The bar plots demonstrating the effect of ascorbic acid on the formation of Au/Ag core/shell nanostructures (AuNSs = 100 μL, MES buffer = 5.0 mM, pH 6.5, iron(III) edta = 20 µM, Ag = 300 µM). (B) The bar plots demonstrating the effect of silver ions on the formation of Au/Ag core/shell nanostructures (AuNSs = 100 μL, MES buffer = 5.0 mM, pH 6.5, iron(III) edta = 20 µM, AA = 200 µM).
Optimizing the concentration of AgNO3
In this study, we used AgNO3 aqueous solution as the source of Ag+ to subsequently be reduced by an electron donor; in this case, the interplay of ascorbic acid and iron(III) edta would donate the electron to Ag+ (Eq. 4). It is suggested that the electrochemical differentiation between iron(II) edta and Ag+ is the cause for the electron donation from ferrous to Ag+ which is reduced to Ag° that can then make a shell around gold nanostructures, and the intensity of core/shell formation is indicated by a change in color. To optimize the concentration of silver ions in the solution, we prepared six concentrations of AgNO3 (75, 150, 225, 300, and 375 µM), which gave us the range of no response to the maximum response, which was 300 µM (Fig. 3B), for that we selected the optimum concentration of AgNO3.
The effect of pH
Experiments have shown that the pH of the sensing system affects the stability and sensitivity of the developed sensor. The concentration of H+ ions strongly influences the yield of the Au/Ag core/shell formation reaction. This reaction spontaneously occurs at basic pH in the presence of AuNPs, Ag+ ions, and ascorbic acid according to Eq. (1). MES buffer was utilized to stabilize the pH of the system. As shown in Fig. 4A, the difference in the spectrum area rose with the rise in pH which means that the reaction yield was much greater under basic conditions. However, although the response was more at a pH of 7.0 than 6.5, as the best buffering power of MES buffer is between 5.5 and 6.7, and core/shell formation reaction is faster at higher pHs (i.e., 7.0), it ends in the low repeatability at pH of 7.0 that led us to choose pH 6.5 as the optimal pH.
The effect of iron(III) edta
Ethylenediaminetetraacetic acid (EDTA) has a high affinity for iron(III) and together they form a complex that can solubilize ferric ions in water that are otherwise insoluble39. Iron(III) edta shows a yellow color when it is in aqueous form and has a strong ultraviolet absorbance band with a peak at 260 nm at acidic pH values. Ferric chelate can directly be involved in the oxidation of ascorbic acid, which can give rise to ascorbate free radicals. The rate-determining step in this reaction is the one-electron oxidation of ascorbic acid, which can be completed by the involvement of the second ferric chelate that can complete the reduction of ascorbic acid that produces dehydroascorbic acid, which can further be oxidized to produce oxalate and threonate. In the developed sensor, owing to the greater oxidation potential of Ag+ than of Fe2+, the electron from Fe2+ can be donated to Ag+, which gives rise to Ag°, which can be shelled over Au.
It has also been suggested that by the interplay of ascorbic acid and iron chelate, a mixture of ascorbate radical anions is produced at neutral pH37, which can reduce Ag+ to Ag°, which can be shelled around AuNPs. We can also consider the direct reduction of Ag+ by intact ascorbic acid, which is not involved in the reaction with ferric chelate. The rate of the oxidation of ascorbic acid by H2O2 can be drastically accelerated by iron(III) edta. The ferric chelate is reduced by ascorbic acid, which gives rise to ascorbate free radicals and is propagated by a chain reaction initiated by the reaction of ferrous chelate with H2O2 to produce hydroxyl radicals that react with ascorbic acid to produce ascorbate radicals40. It has also been suggested that this reaction at pH values above 5 produces oxidoiron(2 +) (FeO2+-EDTA) instead, which contradicts Eq. (5)36. The ascorbate free radical then reduces ferric chelate to be completely oxidized, and the chain reaction is subjected to termination by the reaction of ascorbate free radicals and hydroxyl radicals to produce dehydroascorbic acid and water. This chained reaction has been suggested to be 42 times faster than the direct reduction of hydrogen peroxide by ascorbic acid40,41.
As mentioned, iron(III) edta enhances the ability of ascorbic acid to donate electrons to Ag+ ions. This occurs in the absence of hydrogen peroxide, which reduces Ag+ to Ag°. The Ag° then forms a shell around the nanoparticles, intensifying the color of the nanoparticle solution. However, in the presence of hydrogen peroxide, the electrons donated by ascorbic acid are scavenged to reduce hydrogen peroxide, leaving Ag+ in the cation form and unable to form core/shell nanostructures. To optimize the concentration of iron(III) edta, we tested different concentrations ranging from 0.0 to 35 µM. Without iron(III) edta, the response of core/shell formation after 10 min was similar to or slightly less than when adding hydrogen peroxide. This suggests that in the mixture, the reaction does not proceed as expected. By adding iron(III) edta, the core/shell formation response increases until it reaches an optimal level of approximately 20 µM (Fig. 4B). At this concentration, the maximum difference between the spectrum area of the blank and the sample is observed, making it the chosen optimized level of iron(III) edta.
Optimizing the analysis time
As mentioned in section “Principle of H2O2 sensing”, after adding solution B (Buffer + AA + iron(III) edta) to solution A (AuNSs + AgNO3), core/shell formation starts immediately and is indicated by color changes from pink to yellow. To achieve the maximum response, the absorbance of the probe was investigated every 2 min. After 10 min, the response reached 90% of the complete response; hence, we chose 10 min as the best analysis time (Fig. 5A).
Selectivity study
Several likely interfering ions that might exist under many real conditions (i.e., NaNO2, KCl, MgCl2, NaCl, CaCl2, Na2SO4, FeCl3, and CoCl2) were used to investigate the selectivity of the developed sensor (Fig. 5B). The results indicated that these ions produce a negligible change in the response, which verifies the selectivity of the sensor.
Calibration curve
To investigate the sensitivity of the developed system under optimum conditions, several concentrations of hydrogen peroxide were measured by the sensor. The results indicated that a rise in the hydrogen peroxide concentration would cause a gradual redshift in the LSPR peak. This means that the presence of H2O2 inhibited the formation of the Au/Ag core/shell nanostructures. The calibration curve illustrates a linear relationship between the signal response (So-S) and the hydrogen peroxide concentration in the range of 1.0–50.0 µM (with an equation of y = 2.64x + 34.45 and a correlation coefficient of 0.998) (Fig. 6).
The limit of detection (LOD) was calculated to be 230 nM, which is lower than the expected concentration of hydrogen peroxide in rainwater, which was calculated to be 6.9 µM42, and the concentration of hydrogen peroxide excreted out of the probiotic bacteria Lactobacillus johnsonii NCC 533 which is 1.0 mM43. This shows the potential of the developed sensor to detect H2O2 in both biological and non-biological systems. To validate this hypothesis, 15 µM H2O2 was spiked into the complex deMan Rogosa Sharpe (MRS) medium, and the sensor was practical for hydrogen peroxide detection with 90% recovery. Compared with electrochemical sensors, the developed sensor shows better linear characteristics, along with a lower LOD44, or when Au nanocubes are used but there is a lack of iron(III) edta in the reaction26.
Real sample analysis
To prove the applicability of the designed sensor in real conditions, the amount of H2O2 was measured in several real samples including MRS medium, tap water, tomato fruit extract, and tomato leaf extract. Table 1 summarizes the results of real sample analysis. The values of recovery and relative standard deviation (RSD) were in the range of 88.0 to 94.7 and 1.8 to 2.9, respectively.
The comparison of different colorimetric sensors based on plasmonic nanoparticles for the measurement of H2O2 is provided in Table 2. This comparison demonstrates that our method is applicable to more real samples, has short analysis time, and exhibits a competent linear range compared to most existing colorimetric nanosensors. Furthermore, in this work not only there is no need to functionalize nanoparticles, but also no enzyme is utilized.
AuNR-based sensor
Another sensing system that is similar to the previously mentioned system has been developed using AuNRs. AuNRs possess the characteristic of longitudinal and transversal LSPR bands at 683 and 513 nm, respectively. The longitudinal peak could be blueshifted whenever Ag° shells were present around the AuNRs52,53,54,55,56. The response of the sensor to different concentrations of H2O2 is indicated in Fig. 7. Utilizing AuNRs in the design of sensors is extraordinarily advantageous over the use of AuNSs because of their unique color properties57,58,59. Au/Ag core/shell nanorods are able to reflect light with several hues, including red, pink, violet, blue, green, and yellow, with different tints, which provides excellent naked-eye detection ability (Fig. 7).
Conclusion
In summary, in this study, the inhibition of gold/silver core/shell formation was exploited via both nanospheres (AuNSs) and nanorods (AuNRs) to detect H2O2. Although AuNRs give better color distinction in a rainbow manner in higher concentrations, AuNSs were critical in determining lower concentrations of H2O2. Both core/shell formation and inhibition reactions were shown to be accelerated by iron(III) edta, and these characteristics are suggested to be due to a chained reaction, starting with the reduction of ferric chelate to ferrous chelate that in the end maximizes the electron donation capacity of ascorbic acid. In the case of nanospheres, the developed sensor has been shown to have an LOD of 230 nM, offering greater accuracy and improved linear characteristics with increasing concentrations compared to similar sensors. To validate the applicability of the sensor, H2O2 in various real samples including MRS medium, tap water, and tomato extract were tested; The results were promising in the case of H2O2 presence detection. The on-site and rapid detection of H2O2 is always needed in medicine and industry. The developed sensor is fast, accurate, on-site, and cheap to detect H2O2 in biological and non-biological systems.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Koppenol, W. H. & Sies, H. Was hydrogen peroxide present before the arrival of oxygenic photosynthesis? The important role of iron(II) in the Archean ocean. Redox Biol. 69, 103012. https://doi.org/10.1016/j.redox.2023.103012 (2024).
https://www.chemanalyst.com/industry-report/hydrogen-peroxide-market-191.
Ranganathan, S. & Sieber, V. Recent advances in the direct synthesis of hydrogen peroxide using chemical catalysis—A review. Catalysts 8, 379. https://doi.org/10.3390/catal8090379 (2018).
Neill, S. J., Desikan, R., Clarke, A., Hurst, R. D. & Hancock, J. T. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 53, 1237–1247. https://doi.org/10.1093/jexbot/53.372.1237 (2002).
Parveen, N. et al. Auxin crosstalk with reactive oxygen and nitrogen species in plant development and abiotic stress. Plant Cell Physiol. 63, 1814–1825. https://doi.org/10.1093/pcp/pcac138 (2023).
Koppenol, W. H. & Sies, H. Ancient molecule’s 200th anniversary. Nature 559, 181. https://doi.org/10.1038/d41586-018-05674-0 (2018).
Mongkolsuk, S. & Helmann, J. D. Regulation of inducible peroxide stress responses. Mol. Microbiol. 45, 9–15. https://doi.org/10.1046/j.1365-2958.2002.03015.x (2002).
Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 289, 8735–8741. https://doi.org/10.1074/jbc.R113.544635 (2014).
Li, Y. et al. A simple enzyme-free SERS sensor for the rapid and sensitive detection of hydrogen peroxide in food. Analyst 145, 607–612. https://doi.org/10.1039/C9AN01964B (2020).
Kakeshpour, T., Metaferia, B., Zare, R. N. & Bax, A. Quantitative detection of hydrogen peroxide in rain, air, exhaled breath, and biological fluids by NMR spectroscopy. Proc. Natl. Acad. Sci. 119, e2121542119. https://doi.org/10.1073/pnas.212154211 (2022).
Abo, M. et al. Development of a highly sensitive fluorescence probe for hydrogen peroxide. J. Am. Chem. Soc. 133, 10629–10637. https://doi.org/10.1021/ja203521e (2011).
Liu, J. et al. Turn-on luminescent probe for hydrogen peroxide sensing and imaging in living cells based on an iridium (III) complex–silver nanoparticle platform. Sci. Rep. 7, 8980. https://doi.org/10.1038/s41598-017-09478-6 (2017).
Liu, Y. et al. Horseradish peroxidase supported on porous graphene as a novel sensing platform for detection of hydrogen peroxide in living cells sensitively. Biosens. Bioelectron. 87, 101–107. https://doi.org/10.1016/j.bios.2016.08.015 (2017).
Zhang, W., Ma, D. & Du, J. Prussian blue nanoparticles as peroxidase mimetics for sensitive colorimetric detection of hydrogen peroxide and glucose. Talanta 120, 362–367. https://doi.org/10.1016/j.talanta.2013.12.028 (2014).
Song, Y., Wang, L., Ren, C., Zhu, G. & Li, Z. A novel hydrogen peroxide sensor based on horseradish peroxidase immobilized in DNA films on a gold electrode. Sens. Actuators B Chem. 114, 1001–1006. https://doi.org/10.1016/j.snb.2005.07.061 (2006).
Hernández-Ruiz, J., Arnao, M. B., Hiner, A. N., García-Cánovas, F. & Acosta, M. Catalase-like activity of horseradish peroxidase: Relationship to enzyme inactivation by H2O2. Biochem. J. 354, 107–114. https://doi.org/10.1042/0264-6021:3540107 (2001).
Motsenbocker, M. A. Sensitivity limitations encountered in enhanced horseradish peroxidase catalysed chemiluminescence. J. Biolumin. Chemilumin. 2, 9–16. https://doi.org/10.1002/bio.1170020104 (1988).
Mohammadi, A., Ghasemi, F. & Hormozi-Nezhad, M. R. Development of a paper-based plasmonic test strip for visual detection of methiocarb insecticide. IEEE Sens. J. 17, 6044–6049. https://doi.org/10.1109/JSEN.2017.2731418 (2017).
Taefi, Z., Ghasemi, F. & Hormozi-Nezhad, M. R. Selective colorimetric detection of pentaerythritol tetranitrate (PETN) using arginine-mediated aggregation of gold nanoparticles. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 228, 117803. https://doi.org/10.1016/j.saa.2019.117803 (2020).
Bigdeli, A. et al. Optical nanoprobes for chiral discrimination. Analyst 145, 6416–6434. https://doi.org/10.1039/D0AN01211D (2020).
Ghasemi, F. et al. Paper-based optical nanosensors–A review. Anal. Chim. Acta 1238, 340640. https://doi.org/10.1016/j.aca.2022.340640 (2023).
Sepahvand, M., Ghasemi, F. & Hosseini, H. M. S. Plasmonic nanoparticles for colorimetric detection of nitrite and nitrate. Food Chem. Toxicol. 149, 112025. https://doi.org/10.1016/j.fct.2021.112025 (2021).
Orouji, A., Ghasemi, F. & Hormozi-Nezhad, M. R. Machine learning-assisted colorimetric assay based on Au@Ag nanorods for chromium speciation. Anal. Chem. 95, 10110–10118. https://doi.org/10.1021/acs.analchem.3c01904 (2023).
Abdali, M., Ghasemi, F., Hosseini, H. M. S. & Mahdavi, V. Different sized gold nanoparticles for array-based sensing of pesticides and its application for strawberry pollution monitoring. Talanta 267, 125121. https://doi.org/10.1016/j.talanta.2023.125121 (2024).
González, A., Noguez, C., Beránek, J. & Barnard, A. Size, shape, stability, and color of plasmonic silver nanoparticles. J. Phys. Chem. C 118, 9128–9136. https://doi.org/10.1021/jp5018168 (2014).
Yeh, I.-H., Tadepalli, S. & Liu, K.-K. Au@Ag nanostructures for the sensitive detection of hydrogen peroxide. Sci. Rep. 12, 19661. https://doi.org/10.1038/s41598-022-24344-w (2022).
Yang, X., Wang, Y., Liu, Y. & Jiang, X. A sensitive hydrogen peroxide and glucose biosensor based on gold/silver core–shell nanorods. Electrochim. Acta 108, 39–44. https://doi.org/10.1016/j.electacta.2013.06.017 (2013).
Teodoro, K. B., Migliorini, F. L., Christinelli, W. A. & Correa, D. S. Detection of hydrogen peroxide (H2O2) using a colorimetric sensor based on cellulose nanowhiskers and silver nanoparticles. Carbohydr. Polym. 212, 235–241. https://doi.org/10.1016/j.carbpol.2019.02.053 (2019).
Ma, C. et al. Enzyme-free and wide-range portable colorimetric sensing system for uric acid and hydrogen peroxide based on copper nanoparticles. Talanta 255, 124196. https://doi.org/10.1016/j.talanta.2022.124196 (2023).
Sang, Y. et al. A visual detection of hydrogen peroxide on the basis of Fenton reaction with gold nanoparticles. Anal. Chim. Acta 659, 224–228. https://doi.org/10.1016/j.aca.2009.11.031 (2010).
Lee, K. M. & Kim, H. One-step preparation of hydrogel particles that show rapid detection of hydrogen peroxide: The dual role of new methylene blue. Dyes Pigments 170, 107546. https://doi.org/10.1016/j.dyepig.2019.107546 (2019).
Ginimuge, P. R. & Jyothi, S. D. Methylene blue: Revisited. J. Anaesthesiol. Clin. Pharmacol. 26, 517–520 (2010).
Jana, N. R., Gearheart, L. & Murphy, C. J. Seeding growth for size control of 5–40 nm diameter gold nanoparticles. Langmuir 17, 6782–6786. https://doi.org/10.1021/la0104323 (2001).
Scarabelli, L., Grzelczak, M. & Liz-Marzán, L. M. Tuning gold nanorod synthesis through prereduction with salicylic acid. Chem. Mater. 25, 4232–4238. https://doi.org/10.1021/cm402177b (2013).
Mahdavi, V. et al. Pesticide residues in green-house cucumber, cantaloupe, and melon samples from Iran: A risk assessment by Monte Carlo Simulation. Environ. Res. 206, 112563. https://doi.org/10.1016/j.envres.2021.112563 (2022).
Koppenol, W. H. Ferryl for real. The Fenton reaction near neutral pH. Dalton Trans. 51, 17496–17502. https://doi.org/10.1039/d2dt03168j (2022).
Bielski, B. H. J. Chemistry of ascorbic acid radicals. Am. Chem. Soc. https://doi.org/10.1021/ba-1982-0200.ch004 (1982).
Chausson, M. et al. Block copolymers of the type poly (caprolactone)-b-poly (ethylene oxide) for the preparation and stabilization of nanoemulsions. Int. J. Pharm. 362, 153–162. https://doi.org/10.1016/j.ijpharm.2008.06.007 (2008).
Xue, H., Sigg, L. & Kari, F. G. Speciation of EDTA in natural waters: Exchange kinetics of Fe-EDTA in river water. Environ. Sci. Technol. 29, 59–68. https://doi.org/10.1021/es00001a007 (1995).
Grinstead, R. R. The oxidation of ascorbic acid by hydrogen peroxide. Catalysis by ethylenediaminetetraacetato-iron(III). J. Am. Chem. Soc. 82, 3464–3471. https://doi.org/10.1021/ja01498a057 (1960).
Deutsch, J. C. Ascorbic acid oxidation by hydrogen peroxide. Anal. Biochem. 255, 1–7. https://doi.org/10.1006/abio.1997.2293 (1998).
Deng, Y. & Zuo, Y. Factors affecting the levels of hydrogen peroxide in rainwater. Atmos. Environ. 33, 1469–1478. https://doi.org/10.1016/S1352-2310(98)00239-8 (1999).
Hertzberger, R. et al. H2O2 production in species of the Lactobacillus acidophilus group: A central role for a novel NADH-dependent flavin reductase. Appl. Environ. Microbiol. 80, 2229–2239. https://doi.org/10.1128/AEM.04272-13 (2014).
Yang, X., Bai, J., Wang, Y., Jiang, X. & He, X. Hydrogen peroxide and glucose biosensor based on silver nanowires synthesized by polyol process. Analyst 137, 4362–4367. https://doi.org/10.1039/c2an35407a (2012).
Rivero, P. J. et al. A self-referenced optical colorimetric sensor based on silver and gold nanoparticles for quantitative determination of hydrogen peroxide. Sens. Actuators B Chem. 251, 624–631. https://doi.org/10.1016/j.snb.2017.05.110 (2017).
Nitinaivinij, K., Parnklang, T., Thammacharoen, C., Ekgasit, S. & Wongravee, K. Colorimetric determination of hydrogen peroxide by morphological decomposition of silver nanoprisms coupled with chromaticity analysis. Anal. Methods 6, 9816–9824. https://doi.org/10.1039/C4AY02339K (2014).
Aygun, A., Sahin, G., Tiri, R. N. E., Tekeli, Y. & Sen, F. Colorimetric sensor based on biogenic nanomaterials for high sensitive detection of hydrogen peroxide and multi-metals. Chemosphere 339, 139702. https://doi.org/10.1016/j.chemosphere.2023.139702 (2023).
Liu, Y. et al. A label-free plasmonic nanosensor driven by horseradish peroxidase-assisted tetramethylbenzidine redox catalysis for colorimetric sensing H2O2 and cholesterol. Sens. Actuators B Chem. 389, 133893. https://doi.org/10.1016/j.snb.2023.133893 (2023).
Lu, M. et al. Gold nanoparticle etching induced by an enzymatic-like reaction for the colorimetric detection of hydrogen peroxide and glucose. Anal. Methods 11, 4829–4834. https://doi.org/10.1039/C9AY01599J (2019).
Lin, W.-Z. et al. A colorimetric sensor for the detection of hydrogen peroxide using DNA-modified gold nanoparticles. J. Taiwan Inst. Chem. Eng. 89, 49–55. https://doi.org/10.1016/j.jtice.2018.05.005 (2018).
Vinayagam, R. et al. Structural characterization of marine macroalgae derived silver nanoparticles and their colorimetric sensing of hydrogen peroxide. Mater. Chem. Phys. 313, 128787. https://doi.org/10.1016/j.matchemphys.2023.128787 (2024).
Sepahvand, M. & Ghasemi, F. Colorimetric silver ion detection based on silver metallization of gold nanorods. ChemistrySelect 9, e202400080. https://doi.org/10.1002/slct.202400080 (2024).
Orouji, A., Abbasi-Moayed, S., Ghasemi, F. & Hormozi-Nezhad, M. R. A wide-range pH indicator based on colorimetric patterns of gold@ silver nanorods. Sens. Actuators B Chem. 358, 131479. https://doi.org/10.1016/j.snb.2022.131479 (2022).
Naseri, A. & Ghasemi, F. Analyte-restrained silver coating of gold nanostructures: An efficient strategy to advance multicolorimetric probes. Nanotechnology 33, 075501. https://doi.org/10.1088/1361-6528/ac3704 (2021).
Miryousefi, N., Varmazyad, M. & Ghasemi, F. Synthesis of Au@ Ag core-shell nanorods with tunable optical properties. Nanotechnology https://doi.org/10.1088/1361-6528/ad572b (2024).
Ghorbanian, E., Ghasemi, F., Tavabe, K. R. & Sabet, H. R. A. Formation of plasmonic core/shell nanorods through ammonia-mediated dissolution of silver (I) oxide for ammonia monitoring. Nanoscale Adv. 6, 3229–3238. https://doi.org/10.1039/D4NA00216D (2024).
Sepahvand, M., Ghasemi, F. & Hosseini, H. M. S. Thiol-mediated etching of gold nanorods as a neoteric strategy for room-temperature and multicolor detection of nitrite and nitrate. Anal. Methods 13, 4370–4378. https://doi.org/10.1039/D1AY01117K (2021).
Cao, J., Sun, T. & Grattan, K. T. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B Chem. 195, 332–351. https://doi.org/10.1016/j.snb.2014.01.056 (2014).
Rao, H., Xue, X., Wang, H. & Xue, Z. Gold nanorod etching-based multicolorimetric sensors: Strategies and applications. J. Mater. Chem. C 7, 4610–4621. https://doi.org/10.1039/C9TC00757A (2019).
Acknowledgements
The authors would like to gratefully acknowledge Prof. Willem H. Koppenol for his precious comments. Additionally, the financial support of the Agricultural Biotechnology Research Institute of Iran is acknowledged.
Author information
Authors and Affiliations
Contributions
F.G.H. conceived the original idea and with the contributions of M.H. and A.H.Q.S. developed the study. M.H. and A.H.Q.S. performed the experiments and wrote the manuscript. F.G.H. provided advice, material, and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hemmati, M., Selakjan, A.H.Q. & Ghasemi, F. Iron(III) edta-accelerated growth of gold/silver core/shell nanoparticles for wide-range colorimetric detection of hydrogen peroxide. Sci Rep 15, 4050 (2025). https://doi.org/10.1038/s41598-025-88342-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88342-4