Abstract
Conventional smear cytology (CSC) is a specific method used for breast tumor diagnosis in patients with nipple discharge. However, CSC tends to miss diagnose or even misdiagnose due to contaminating blood cells and other impurities. Thus, it is critical to develop more accurate and sensitive methods for clinical evaluation. Using nipple discharge collected directly from patients without aspiration, we performed liquid-based cytology (LBC) to analyze 111 collected samples for cytological evaluation for the first time. Following centrifugation to remove blood cells and protein contaminants, the sample of each nipple discharge was analyzed by light microscopy. In parallel, CSC was performed for comparison. Our results showed LBC has better diagnostic sensitivity than CSC (40.00% vs. 22.22%, \(\chi^{2}\) = 6.636, P = 0.01). The specificity was improved (LBC 100% vs. CSC 95.2%) and area under the curve was also enhanced (AUCLBC=0.700 vs. AUCCSC=0.587). Moreover, LBC images have a cleaner background, clearer field of view, uniform cell arrangement, and improved colour contrast, with an overall image quality significantly better than CSC. Therefore, LBC provides a new and better diagnostic method for early detection of breast tumors with nipple discharge.
Similar content being viewed by others
Introduction
Nipple discharge is a common complaint among patients presenting with breast problems. The causes of discharge are divided into functional and pathological. Functional discharge is usually a bilateral porous discharge, often caused by increased Prolactin associated with lactation, pituitary adenoma, or antipsychotics. Pathological discharge is spontaneous and intermittent, unrelated to pregnancy or lactation, and lasts from several months to several years. The most common cause of pathological discharge is intraductal papilloma, a typical precancerous lesion. The second is breast cancer, mostly intraductal carcinoma1. Sometimes, benign hyperplastic lesions can also lead to nipple discharge.
At present, specific clinical examinations of nipple discharge include ductoscopy, galactography, analysis of tumor markers in nipple discharge, cytological examination, etc. The nature and composition of nipple discharge vary greatly from patient to patient. Abnormal cell detection using cytological examination is a qualitative diagnosis, showing the nature of the lesion, assisting surgeons to identify the need for surgery, and design a surgical plan if necessary. However, conventional smear cytology (CSC) poses several issues. First, the cell detection rate is low (30.8–83%)2,3,4and, second, the image quality is poor because of overlapping cells and blood cell contamination5, either of which can lead to false-positive or false-negative results.
Liquid-based cytology (LBC) was invented by Hologic Gen-Probe, with Hutchinson first reporting its application to cervical cytology in 19916. LBC involves the use of automatic cell dyeing and high-adhesion slides to examine cells based on their physical properties. Some advantages of using this method include simple operation, clear background, contrastive color imaging, uniform distribution of cells, and feasibility for immunochemical staining and flow cytometry7. Besides automatic imaging, settling is a commonly used manual imaging method for LBC and involves the use of a special device that enriches the cells on the slide upon centrifugation, which improves the detection rate8. At present, LBC has been used in non-gynecological fields for the diagnosis of lung cancer, thyroid nodules, and other diseases9,10,11, as well as for the evaluation of nipple aspirate fluid12. No direct application in nipple discharge has been reported. Here, we describe the first application of LBC for direct nipple discharge analysis and compare diagnostic efficacy and image quality between LBC and CSC, illustrating that LBC can be used as an effective non-invasive diagnostic method for analyzing disease-associated nipple discharge.
Materials and methods
Patient selection
Inclusion criteria:
-
1)
Female patients with nipple discharge.
-
2)
Had not undergone any invasive examination (e.g., ductography, ductoscopy or biopsy) before cytology examination.
-
3)
Informed consent was obtained.
Exclusion criteria:
-
1)
Male patients.
-
2)
Patients were pregnant or lactating.
-
3)
Had taken oral antipsychotic medications over the past 6 months.
-
4)
Had undergone any invasive examination before cytology examination.
-
5)
Rejected informed consent.
A total of 101 patients with nipple discharge had received surgical treatment in the Department of Breast Surgery, Qilu Hospital of Shandong University. Among them, 10 had bilateral nipple discharge. A total of 111 nipple discharge samples were collected.
Approval for this study was obtained from the ethics committee of Qilu Hospital of Shandong University.
Sample collection
At the time of sample collection, the affected nipple was scrubbed with 75% alcohol and then dried with a sterile cotton swab. Nipple discharge is gently squeezed from around the breast. Finally, a sterile Eppendorf duct is used to collect nipple discharge. Half of the nipple discharge was taken for CSC, the other half for LBC (Fig. 1).
Liquid-based cytology examination
-
A)
Drain the discharge from the sterile EP tube into a centrifuge tube and add the cell preservation solution (Hanhe Medical Devices Co., LTD., Jinan, Shandong, China) to 1 ml.
-
B)
Add lymphocyte separation medium to 1:1 ratio.
-
C)
Put into a TD5Z-WS centrifuge (XIANGZHI Centrifuge Instrument Co., LTD., Changsha, Hunan, China), 2,000 r/min for 5 min.
-
D)
Aspirate the nucleated cell layer and the upper layer of the original fluid to another centrifuge tube.
-
E)
Put the centrifuge tube into TD4Z-WS auto-balancing centrifuge (XIANGZHI Centrifuge Instrument Co., LTD., Changsha, Hunan, China), 900 r/min for 4 min to obtain a glass slide.
-
F)
The glass slides were coded and placed on a staining rack.
-
G)
Add Wright-giemsa staining solution A + pitonate buffer (7:10) and stain for 25 min.
-
H)
Wash slides and dry.
-
I)
The slide was reviewed under a microscope.
Conventional smear cytologic examination
-
a.
A) Make smear manually with glass slides and place on the staining rack after coding.
-
b.
B) Add Wright-giemsa staining solution A + pitonate buffer (7:10) and stain for 25 min.
-
c.
C) Wash slides and dry.
-
d.
D) Review the slide under a microscope.
Report
Two professional cytopathologists evaluated slides, reported observed cell types (ductal epithelial cells, blood cells, etc.) and morphological characteristics, defined detected cancer cells or atypical hyperplasia cells as positive results, and if not, defined as negative results13.
Data analysis
-
1)
According to WHO classification of tumors, the lesions were divided into three groups: cancer, ADH (atypical ductal hyperplasia) plus/or papilloma and UDH (usual ductal hyperplasia) plus/or adenosis. Clinical data of all patients were collected to clarify the relationship between clinical features and lesion types. P values were calculated by paired χ2 test or Fisher exact test, and P < 0.05 was considered statistically significant.
-
2)
All reports were reviewed and tabulated to present the types of cells in the reports. A Venn diagram was made to show the detection of ductal epithelial cells in all cases, cancer and ADH plus/or papilloma.
-
3)
Cancer cells and atypical cells were defined as pathological cells. If pathological cells had been detected, the results were positive, otherwise, they were negative. In clinical treatment, both cancer and ADH (atypical ductal hyperplasia) plus/or papilloma had strong surgical indications, the two were combined and distinguished from UDH (usual ductal hyperplasia) plus/or adenosis. As a result, all cases be classified to “strong surgical indication group” and “weak surgical indication group”. The diagnostic validity of the two cytological examination methods was determined to include sensitivity, specificity, PPV, NPV and Kappa consistency test, drawn ROC curve, and performed ROC analysis.
SPSS 25.0 software was used for data processing and statistical analysis.
Results
Clinical and pathological data of patients
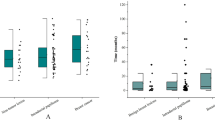
There were 111 nipple discharge samples from 101 patients included in this study (10 patients with bilateral nipple discharge). The mean age of the patients was 46.6 years (23–81 years, SD = 10.6 y). There were 25 samples from premenopausal patients and 86 samples from postmenopausal patients; 46 palpable breast lumps and 65 unpalpable. Nipple discharge with lumps was an indication of malignancy (P = 0.002). Of the 111 cases of discharge, there were 36 breast cancer, 51 papillomas, 3 ADH, and 21 UDH plus/or adenosis. Among the 36 breast cancers, the most common pathological type was DCIS (86.1%, 31/36). There were 40 cases of bloody discharge, 51 cases of serous discharge, 17 cases of clear water discharge, and 3 cases of milk discharge. The characteristics of discharge were not correlated with specific diseases (P = 0.019). There were no significant differences in age, number of ductal pores, menstrual status, family history, and breastfeeding history between those three groups (Table 1).
Cytological assessment of nipple discharge
Fewer miscellaneous cells in LBC
The cell types reported are shown in Fig. 2. The areas marked in blue blocks show cell detection in LBC, while red blocks marked cell detection in CSC. The LBC method could detect fewer blood cells and squamous epithelial cells, in another word, fewer miscellaneous cells.
Among 111 samples, ductal epithelial cells (including cancer cells, atypical cells, and normal ductal epithelial cells) were detected in 65 cases using LBC and in 34 cases using CSC. However, no ductal epithelial cells were detected by either method in 44 cases (Fig. 3. (a, b)). For breast cancer samples (n = 36), LBC identified ductal epithelial cells in 22 cases while CSC identified them in 14 cases (Fig. 3. (c, d)). For ADH plus/or papilloma (n = 54), LBC detected ductal epithelial cells in 35 cases compared to CSC which found them in only 17 cases (Fig. 3. (e, f)). As shown in the Venn diagram, there is concordance between these two methods. However, the ability of LBC to detect ductal epithelial cells was superior to that of CSC, particularly in breast cancer.
The diagnostic validity of LBC is better
In all the samples (n = 111), 36 cases of pathological cells were detected using LBC, and 21 cases were detected using CSC. Among samples from breast cancer (n = 36), 19 cases of pathological cells were detected with LBC, while only 11 were detected using CSC. Among samples from ADH plus/or papilloma (n = 54), 17 cases of pathological cells were detected using LBC compared to 9 using CSC. Among samples from UDH plus/or adenosis (n = 21), CSC detected pathological cells from one sample of bloody discharge, however, no case of pathological cells was detected using LBC.
When diagnostic validity was evaluated, we found LBC had higher sensitivity than CSC for breast tumors (52.78% vs. 30.56% for breast cancer; and 31.48% vs. 16.67% for intraductal papilloma). Breast cancer and ADH plus/or papilloma were grouped as strong surgical indication group and distinguished from strong surgical indication group (UDH plus/or adenosis). Based on this distinction, the diagnostic validity of these two methods were calculated. For LBC: sensitivity = 40%, specificity = 100%, positive predictive value = 100%, negative predictive value = 28%, and Kappa value = 0.201. For CSC: sensitivity = 22.22%, specificity = 95.22%, positive predictive value = 95.20%, negative predictive value = 22%, and Kappa value = 0 0.077 (Table 2). Chi-square test of the two groups of rates was performed: Sensitivity χ2 = 6.636, P = 0.01. That is, the sensitivity, specificity, positive predictive value, negative predictive value, and Kappa value of the LBC test were higher than those of CSC, and the difference in sensitivity was statistically significant. In receiver operating characteristic (ROC) curves of two methods (Fig. 4), AUCLBC = 0.700 (p = 0.004, 95% CI: 0.598 ~ 0.802). AUCCSC = 0.587 (p = 0.214, 95% CI: 0.463 ~ 0.711). The diagnostic validity of LBC is, therefore, better than CSC.
The image quality of LBC is better
The accuracy of diagnosis is related to the image quality, which can be greatly reduced by blood cells and cell debris in the sample. A bloody discharge of a breast cancer patient showed cells with large and deeply stained nuclei (Fig. 5. a-b). While the CSC image showed more blood cells in the background, damaged cell morphology, and many overlapped cells (a), the LBC image had a clean background, good morphological integrity, and no cell debris (b). In a bloody discharge of a patient with intraductal papilloma (Fig. 5 c-d), big ductal epithelial cells with increased nucleocytoplasmic ratio could be identified by both LBC and CSC. In the background of the CSC image, a large quantity of blood cells was arranged in clusters and the image was crowded, which sometimes obscured cancer cells. Moreover, the distribution of cancer cells was not concentrated, and the color contrast was not strong (c). However, the background of LBC is clean, cell distribution is concentrated, and the color is contrastive (d). In a sero-like discharge of intraductal papilloma (Fig. 5 e-f), the sample is rich in protein. In CSC, protein impurities adhere to the slide and appear purplish, which would shade cells (e). In LBC, the protein impurities are no longer attached to the slide after centrifugation (f). Overall, the cell preservation and image quality of LBC are superior to CSC.
Discussion
Breast cancer accounts for approximately 5–12% of pathologic nipple discharge in patients undergoing surgery14, most of which are DCIS1. Lesions with nipple discharge are mainly confined to lactiferous duct15. Invasive biopsy such as core needle puncture and fine needle aspiration puncture will destroy the lactiferous duct and lead to extravasation of the lesions, which is not beneficial to disease control. For patients with nipple discharge, cytological examination is presently the predominant method for clinical evaluation. Since the report of CSC in 196916, it has been widely used for pathological diagnosis. Although the sensitivity and specificity of cytology-based tests are comparable to that of imaging, cytology evaluation cannot replace imaging and is not recommended alone for clinical diagnosis3,]17,]18. LBC is an emerging and more sensitive cytological examination method than CSC, as reported here, with removal of impurities such as blood cells and mucus proteins during sample preparation to obtain a clearer image background. To date, LBC has been applied to nipple aspirate analysis using ductoscopy. The ductal epithelia could be damaged, resulting in ductal adhesion and disappearance of nipple discharge, making it difficult to position tumor locations. Moreover, ductoscopy often misses lesions in the terminal ductal lobular unit (TDLU). In this report, we successfully applied LBC for direct analysis of nipple discharge, and showed it represents a more sensitive diagnosis than CSC.
Two advantages of LBC over CSC include: (1) The image quality of LBC is superior to that of CSC; and (2) the diagnostic validity of LBC is better than that of CSC. In LBC, centrifugation helps remove impurities such as blood cells and mucus, which may increase the relative quantities of diagnostic cells to some extent. When nipple discharge is bloody, LBC produces a cleaner image than CSC. Moreover, during LBC examination, the cell preservation solution is added immediately after collecting nipple discharge that preserves better cell morphology, especially the intactness of the nucleus necessary for observing any atypic changes. After settling, the distribution of cells is more concentrated and uniform. For specimens with less cells, ductal epithelial cells can also be significantly enriched. In contrast, during CSC examination, nipple discharge is collected and placed directly onto the slide. When not treated in time, the specimen will dry out and lead to cell deformation and fragmentation. Cell overlapping and scattered distribution in CSC tend to fatigue the reader, resulting in bias. All the above factors lead to improved image quality of LBC.
In our analysis, the positive results of LBC did not completely cover match the CSC results. Two samples were positive for CSC but negative for LBC. The reasons may be: (1) The number of cells shed by discharge was limited, and there was sampling error in the examination process; (2) some background cells and proteins eliminated after centrifugation may provide clues to lesions; and (3) as LBC was not completely automated, some operations may be affected by artificial errors.
Some reasons for flash negative cytology may be: (1) The nipple discharge and breast lesion do not originate from the same mammary ductal system; (2) some of the lesion cells were not shed by discharge, which might be related to the differential expression of tumor adhesion factors; (3) Invasive ductal carcinoma is difficult to shed through the duct; and (4) ductal epithelial cells which entered nipple discharge might be out of shape, however the cognition of morphology features of pathological cells is still limited to tissue. Therefore, multiple samplings and multiple deliveries can be applied to reduce sampling errors. In addition, automatic staining and AI-aided automated cytology systems can also be used. AI can perform initial screening and then cytopathologists make the diagnosis of abnormal cells, with the potential to reduce artificial references and improve work efficiency. At the same time, cytopathologists need to receive special training to refresh their cognition and improve the sensitivity of diagnosis.
The sensitivity, specificity, PPV, NPV and AUC value of LBC were higher than those of CSC, as shown by compared diagnostic consistency of the cytology examination and pathology. Although Kappa values of the LBC were higher than those of CSC, neither method have poor diagnostic consistency with paraffin pathology due to both results < 0.4. Therefore, it is necessary to combine other specific tests in nipple discharge, such as the Thomsen-Freidenreich antigen test19and detection of tumor markers (CEA, CA15320, miRNA21, etc.).
In conclusion, LBC can be used as a new diagnostic method for cytological evaluation using direct nipple discharge without aspiration. However, due to sampling errors, LBC cannot completely replace CSC. If the sample volume was enough, patients should receive both examination methods to avoid misdiagnosis. If discharge volume was limited, LBC seems to be more effective to diagnose pathological nipple discharge. Moreover, LBC is also more conducive to the preservation of genetic material including protein, RNA and DNA22,]23, which may be used for subsequent molecular and immunocytochemical examination, while its application potential needs to be verified by follow-up experiments.
Data availability
The datasets generated during this study are available from the corresponding author on reasonable request.
References
Morrogh, M., Park, A., Elkin, E. B. & King, T. A. Lessons learned from 416 cases of nipple discharge of the breast. Am. J. Surg. 200 (1), 73–80. https://doi.org/10.1016/j.amjsurg.2009.06.021 (2010). Epub 2010/01/19.
Dolan, R. T., Butler, J. S., Kell, M. R., Gorey, T. F. & Stokes, M. A. Nipple discharge and the efficacy of duct cytology in evaluating breast cancer risk. Surgeon: J. Royal Colleges Surg. Edinb. Irel. 8 (5), 252–258 (2010). PubMed PMID: 20709281.
Jiwa, N. et al. Diagnostic Accuracy of Nipple Discharge Fluid Cytology: A Meta-Analysis and Systematic Review of the Literature. Annals of surgical oncology. ;29(3):1774-86. Epub 2021/11/29. (2022). https://doi.org/10.1245/s10434-021-11070-2. PubMed PMID: 34839426; PubMed Central PMCID: PMCPMC8627297 detection of breast cancer under the supervision of D. R. Leff and Z. Takats. The remaining authors have no conflicts of interest.
Grunwald, S. et al. Mammary ductoscopy for the evaluation of nipple discharge and comparison with standard diagnostic techniques. J. Minim. Invasive. Gynecol. 13 (5), 418–423. https://doi.org/10.1016/j.jmig.2006.05.004 (2006). Epub 2006/09/12.
Clark, S. E. et al. The investigation and management of unilateral nipple discharge. Ann. R. Coll. Surg. Engl. 102 (5), 369–374. https://doi.org/10.1308/rcsann.2020.0036 (2020). Epub 2020/04/03.
Hutchinson, M. L., Cassin, C. M. & Ball, H. G. 3rd. The efficacy of an automated preparation device for cervical cytology. Am. J. Clin. Pathol. 96(3), 300–305. https://doi.org/10.1093/ajcp/96.3.300 (1991) (Epub 1991/09/01).
Pathuthara, S. et al. Conventional versus liquid-based Cytology: Man versus Machine. J. Cytol. 40 (4), 169–176. https://doi.org/10.4103/joc.joc_54_23 (2023). Epub 2023/12/07.
Makde, M. M. & Sathawane, P. Liquid-based cytology: technical aspects. CytoJournal 19, 41 (2022). Epub 2022/08/06. doi: 10.25259/cmas_03_16_2021. PubMed PMID: 35928530; PubMed Central PMCID: PMCPMC9345114.
Kim, S. Y. et al. Combined use of conventional smear and liquid-based preparation versus conventional smear for thyroid fine-needle aspiration. Endocrine. ;53(1):157 – 65. Epub 2015/12/31. (2016). https://doi.org/10.1007/s12020-015-0835-z. PubMed PMID: 26714459.
Hashimoto, S. et al. Diagnostic efficacy of liquid-based cytology for solid pancreatic lesion samples obtained with endoscopic ultrasound-guided fine-needle aspiration: Propensity score-matched analysis. Dig. Endoscopy: Official J. Japan Gastroenterological Endoscopy Soc. 29 (5), 608–616. https://doi.org/10.1111/den.12827 (2017). Epub 2017/02/06.
Cao, C. et al. Diagnostic role of liquid-based cytology of bronchial lavage fluid in addition to bronchial brushing specimens in lung cancer. Tumori 107 (4), 325–328. https://doi.org/10.1177/0300891620960218 (2021). Epub 2020/09/26.
Masood, S. & Khalbuss, W. E. Nipple fluid cytology. Clinics in laboratory medicine. ;25(4):787 – 94, vii-viii. Epub 2005/11/26. (2005). https://doi.org/10.1016/j.cll.2005.08.010. PubMed PMID: 16308092.
Ahuja, S. & Malviya, A. Categorization of breast fine needle aspirates using the International Academy of Cytology Yokohama System along with Assessment of Risk of Malignancy and Diagnostic Accuracy in a Tertiary Care Centre. J. Cytol. 38 (3), 158–163. https://doi.org/10.4103/joc.Joc_31_21 (2021). Epub 2021/10/28.
Patel, B. K., Falcon, S. & Drukteinis, J. Management of nipple discharge and the associated imaging findings. Am. J. Med. 128 (4), 353–360. https://doi.org/10.1016/j.amjmed.2014.09 (2015). Epub 2014/12/03.
Vargas, H. I., Vargas, M. P., Eldrageely, K., Gonzalez, K. D. & Khalkhali, I. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am. Surg. 72 (2), 124–128 (2006). Epub 2006/03/16. PubMed PMID: 16536240.
Matsuda, M. & Koyama, H. [Cytologic diagnosis of the abnormal nipple discharge]. Gan no Rinsho Japan J. cancer Clin. 15 (12), 1039–1046 (1969). Epub 1969/12/01. PubMed PMID: 5392544.
Jiwa, N. et al. Nipple aspirate fluid and its use for the early detection of breast cancer. Annals of medicine and surgery 2022;77:103625. Epub 2022/06/01. (2012). https://doi.org/10.1016/j.amsu.2022.103625. PubMed PMID: 35638006; PubMed Central PMCID: PMCPMC9142541.
Carvalho, M. J. et al. What is the diagnostic value of nipple discharge cytology and galactography in detecting duct pathology? Eur. J. Gynaecol. Oncol. 30 (5), 543–546 (2009). Epub 2009/11/11. PubMed PMID: 19899412.
Deutscher, S. L. et al. Carbohydrate antigens in nipple aspirate fluid predict the presence of atypia and cancer in women requiring diagnostic breast biopsy. BMC cancer. 10, 519. https://doi.org/10.1186/1471-2407-10-519 (2010). Epub 2010/10/06.
Zhao, S. et al. Levels of CEA, CA153, CA199, CA724 and AFP in nipple discharge of breast cancer patients. Int. J. Clin. Exp. Med. 8 (11), 20837–20844 (2015). Epub 2016/02/18. PubMed PMID: 26885008; PubMed Central PMCID: PMCPMC4723853.
Zhang, K. et al. Identification of microRNAs in Nipple Discharge as potential diagnostic biomarkers for breast Cancer. Ann. Surg. Oncol. 22 (Suppl 3), S536–S544. https://doi.org/10.1245/s10434-015-4586-0 (2015). Epub 2015/05/16. PubMed PMID: 25976861.
Konofaos, P. et al. The role of ThinPrep cytology in the investigation of ki-67 index, p53 and HER-2 detection in fine-needle aspirates of breast tumors. J. BUON: Official J. Balkan Union Oncol. 18 (2), 352–358 (2013). Epub 2013/07/03. PubMed PMID: 23818345.
Sauer, T., Ebeltoft, K., Pedersen, M. K. & Kåresen, R. Liquid based material from fine needle aspirates from breast carcinomas offers the possibility of long-time storage without significant loss of immunoreactivity of estrogen and progesterone receptors. CytoJournal 7, 24. https://doi.org/10.4103/1742-6413.75665 (2010). Epub 2011/02/08.
Acknowledgements
We would like to thank Prof. Cheng-Ming Chiang (UT Southwestern Medical Center) and Prof. Yunyun Gong (University of Leeds ) for language editing and valuable comments.
Funding
This work was supported by grants from Shandong Provincial Natural Science Foundation, China (No. ZR2022MH248), the Postdoctoral Innovation Foundation of Shandong Province, China (No. 202103004).
Author information
Authors and Affiliations
Contributions
Jiang Zhu, Han Cong and Xiaotong Zhang (co-first authors): Data Curation and Analysis, Methodology, Investigation and Writing-Original Draft. Rong Ma, Pengyu Li (Correspondences) and Jiang Zhu: Conceptualization, Methodology, Funding Acquisition, Supervision and Writing-Review&Editing. Xiaoya Dong and Jianli Wang: Cytopathological evaluation and diagnosis. JUDE RANCHU MAIMO: Language editing.Song Zhao, Chaolu Hu, Yawen Wang and Kai Zhang: Patients admission and management, Conceptualization, Resources and Validation.All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was reviewed by the Ethics Committee of Qilu Hospital of Shandong University (No.2021-088). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, J., Cong, H., Zhang, X. et al. New method for cytological evaluation using direct nipple discharge without aspiration. Sci Rep 15, 4175 (2025). https://doi.org/10.1038/s41598-025-88456-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88456-9