Abstract
It is unclear whether diabetes mellitus (DM) affects the role of malnutrition in heart failure (HF). We evaluated the effect of the geriatric nutritional risk index (GNRI) on HF prognosis and DM’s role in this relationship. This single-centre retrospective cohort study included 540 HF patients with nutritional data grouped by DM status and GNRI score. The primary endpoint was all-cause mortality. Eighty-four patients (15.6%) were classified as malnourished (GNRI ≤ 98). Over a median follow-up of 4.0 years, 102 patients died. The DM/low GNRI (L-GNRI) group had the highest risk of all-cause death (HRadj: 3.253, 95% CI 1.643–6.474, P < 0.001) and cardiac death (HRadj: 3.411, 95% CI 1.606–7.243, P < 0.001) compared to the non-DM/high GNRI group. The adverse impact of L-GNRI was more pronounced in DM than in non-DM patients (Pinteraction < 0.05). In the total population and DM subgroup, GNRI was independently associated with an increased risk of all-cause and cardiac death after adjustment (all P < 0.05). In patients with DM, the GNRI classification significantly enhanced the predictive value of the model (all P < 0.05). A negative correlation between GNRI and HbA1c was observed only in patients with DM. Patients with HF with DM and malnutrition had the poorest prognosis. Poor glycemic control is related to increased malnutrition risk.
Similar content being viewed by others
Introduction
Heart failure (HF) is a leading cause of cardiovascular disease-related mortality and a major public health concern1. Malnutrition is common among patients with HF and is linked to poor prognosis, longer hospital stays, and reduced quality of life, especially in the advanced stages2,3. The prevalence of malnutrition in patients with HF ranges from 15 to 90%, depending on the setting and severity. HF can cause a loss of appetite, poor absorption, and decreased metabolism, leading to malnutrition4. Conversely, malnutrition can exacerbate HF progression, as inadequate energy intake causes chronic abnormalities and triggers a vicious cycle involving hormonal dysregulation and neurohormonal and inflammatory activation5. Tailored treatments for malnutrition in patients with HF may prevent deterioration and improve outcomes6. The geriatric nutritional risk index (GNRI) is a commonly used indicator of nutritional status. In patients with HF, a lower GNRI score is associated with an increased risk of death or major adverse cardiac events7.
Diabetes mellitus (DM) is a cardiovascular risk factor associated with an increased risk of malnutrition8. This is due to factors such as DM-related autonomic neuropathy leading to anorexia and gastroparesis, antidiabetic medications, and extreme dietary restrictions on blood sugar control9,10,11. Previous studies have demonstrated that lower glycated haemoglobin (HbA1c) levels in patients with DM are linked to poorer nutritional status, likely due to endothelial dysfunction and decreased nutrient intake from excessive dietary restrictions, leading to lower blood glucose levels12,13. Malnutrition and DM disrupt systemic metabolism, exacerbate inflammation, and promote the progression of cardiovascular diseases14,15. Although several studies have highlighted the predictive importance of malnutrition in patients with HF, there is a dearth of research on the differences related to DM.
This study aimed to assess the role of DM in the impact of the GNRI on the prognosis of patients with ischaemic heart failure with mildly reduced or reduced ejection fraction.
Results
Study population and baseline characteristics
The final study cohort consisted of 540 patients diagnosed with ischaemic HF, with a median age of 60 years (interquartile range: 52–66 years) and predominantly male representation (87.2%). Baseline characteristics for included and excluded patients are presented in Supplementary Table 1. Table 1 summarises the demographic, clinical, and laboratory data. Among all participants, the median GNRI was 104.0, with a malnutrition prevalence of 15.6%. The analysis revealed no statistically significant difference in malnutrition rates between HF patients with DM and those without DM (17.1% vs. 14.3%, P = 0.405). Compared with patients with H-GNRI, patients with L-GNRI were older, predominantly female, had a history of alcohol use and arrhythmia, and exhibited higher New York Heart Association (NYHA) classification and left ventricular ejection fraction (LVEF) (all P < 0.05). Expectedly, patients with L-GNRI had lower levels of total protein, albumin, prealbumin, cholinesterase, and high-density lipoprotein cholesterol but higher levels of brain natriuretic peptide (BNP) (all P < 0.05). In terms of inflammatory markers, the L-GNRI group exhibited elevated high-sensitivity C-reactive protein (hs-CRP) levels (3.37 [1.21–16.45] vs. 1.43 [0.55–3.45], P < 0.001). In contrast, the absolute leukocyte and lymphocyte counts in the L-GNRI group were significantly lower than those in the H-GNRI group (both P < 0.05). In addition, there was a balanced distribution of heart rate, systolic blood pressure, prevalence of hypertension, diabetes, hyperlipidemia, smoking history, family history, prior myocardial infarction, revascularisation procedures, and cardiovascular medication use between the two groups (all P > 0.05).
Impact of malnutrition or DM on adverse outcomes
The median follow-up duration was 4.0 years (IQR: 2.3–5.0 years). During this period, 102 patients experienced all-cause mortality, resulting in a mortality rate of 18.9%. Among these, 84 deaths were attributed to cardiac causes, accounting for 82.4% of the total cases. Kaplan–Meier analysis indicated no significant differences in all-cause and cardiac death rates between HF patients with DM and those without DM (hazard ratio (HR) = 1.403, 95% CI 0.951–2.069, P = 0.066; HR = 1.417, 95% CI 0.924–2.175, P = 0.109) (Supplementary Fig. 2a,b). Supplementary Fig. 2c and d illustrate the stratified Kaplan–Meier curves based on the GNRI, which revealed a significantly higher risk of all-cause and cardiac mortality in patients in the L-GNRI group (HR = 2.504, 95% CI 1.635–3.835, P < 0.001; HR = 2.828, 95% CI 1.789–4.472, P < 0.001). After multivariable adjustment, malnutrition remained independently associated with an increased risk of all-cause mortality and cardiac death (adjusted HR = 2.054, 95% CI 1.199–3.520, P = 0.009; adjusted HR = 2.465, 95% CI 1.381–4.400, P = 0.002) (Supplementary Table 2). When the GNRI was evaluated as a continuous variable, both univariable (HR = 0.947, 95% CI 0.922–0.973, P < 0.001; HR = 0.945, 95% CI 0.915–0.969, P < 0.001) and multivariable (adjusted HR = 0.967, 95% CI 0.932–1.003, P = 0.070; adjusted HR = 0.950, 95% CI 0.914–0.987, P = 0.009) analyses indicated that higher GNRI values corresponded to a reduced risk of all-cause and cardiac mortality (Table 2, Supplementary Table 3).
DM-related differences in the impact of malnutrition on adverse outcomes
To investigate the differential impact of the GNRI on adverse outcomes among patients with varying DM statuses, we categorised the participants into four groups based on nutritional status and DM presence. The Kaplan–Meier curves demonstrated that patients in the DM/L-GNRI group had a higher incidence of all-cause and cardiac death (log-rank P < 0.001) (Fig. 1a,b). Analyses before and after adjustment showed that the DM/L-GNRI group exhibited the highest risk of all-cause mortality compared to the non-DM/H-GNRI group (adjusted HR = 3.599, 95% CI 2.074–6.245, P < 0.001) (Table 3). In contrast, the DM/H-GNRI group (adjusted HR = 1.660, 95% CI 0.782–3.527, P = 0.187) and the non-DM/L-GNRI group (adjusted HR = 1.242, 95% CI 0.750–2.057, P = 0.400) showed no significant association with increased all-cause mortality (Table 3). Additionally, regarding cardiac mortality, the DM/L-GNRI group (adjusted HR = 3.411, 95% CI 1.606–7.243, P < 0.001) and the non-DM/L-GNRI group (adjusted HR = 2.262, 95% CI 1.026–4.987, P = 0.043) were significantly associated with an increased risk compared to the non-DM/H-GNRI group; however, the DM/H-GNRI group (adjusted HR = 1.233, 95% CI 0.701–2.170, P = 0.187) showed no significant correlation with increased cardiac death (Table 3). When the GNRI was analysed as a continuous variable, we found that an increase in GNRI values was associated with a reduced risk of all-cause mortality and cardiac death in patients with DM (adjusted HR = 0.954, 95% CI 0.913–0.996, P = 0.031; adjusted HR = 0.947, 95% CI 0.904–0.993, P = 0.024) (Supplementary Table 4). However, this relationship was not observed in the non-DM group, in which the HRs were 0.965 (95% CI 0.919–1.021, P = 0.215) for all-cause mortality and 0.943 (95% CI 0.887–1.003, P = 0.064) for cardiac death (Supplementary Table 4). Furthermore, the overall population and the DM subgroup demonstrated a linear relationship between the GNRI and the risk of all-cause mortality and cardiac death (Fig. 2). Conversely, the non-DM group showed no significant association between the GNRI and these adverse outcomes. The negative impact of the L-GNRI on all-cause mortality (interaction P = 0.002) and cardiac mortality (interaction P = 0.006) was significantly greater in patients with DM than in those without (Fig. 3a,b).
Association of continuous GNRI with all-cause and cardiac death. Restricted cubic spline curves for all population (a) DM (b) and non-DM (c) patients. Adjusted for sex, age, hyperlipidaemia; NYHA (III-IV), prior arrhythmia, prealbumin; cholinesterase, uric acid, BNP, beta-blocker, TPD, and LVEF. BNP brain natriuretic peptide, DM diabetes mellitus, GNRI geriatric nutritional risk index, HR hazard ratio, LVEF left ventricular ejection fraction, NYHA New York Heart Association, TPD total perfusion defects.
Association of GNRI and DM status with adverse outcomes. Forrest plots for all-cause death (a) and cardiac death (b). Abbreviations as in Fig. 2.
Incremental value of malnutrition in predicting adverse prognosis
In the overall population, malnutrition (GNRI ≤ 98) significantly predicted all-cause death (C-index: 0.583, 95% CI 0.535–0.630, P < 0.05) and cardiac death (C-index: 0.591, 95% CI 0.538–0.645, P < 0.05). Adding malnutrition to the full multivariable model significantly improved model fit (all-cause death: χ2 increased by 5.763. P = 0.016; cardiac death: χ2 increased by 8.232, P = 0.004) and prediction accuracy (all-cause death: C-index increase from 0.745 to 0.753, P < 0.001; cardiac death: C-index increase from 0.760 to 0.767, P < 0.001) (Table 4). The NRI was calculated using four risk categories (0–20%, 20–40%, 40–60%, and > 60%) and showed significant reclassification in the overall population when malnutrition was added to the full multivariable model (all-cause death: NRI = 20.2%, P < 0.001; cardiac death: NRI = 19.8%, P < 0.001). In patients with DM, malnutrition significantly predicts poor prognosis (all-cause death: C-index 0.610, 95% CI 0.546–0.6681, P < 0.05; cardiac death: C-index 0.605, 95% CI 0.533–0.676, P < 0.05). Adding malnutrition to the full multivariable model significantly improved the model fit (all-cause death: χ2 increased by 6.852. P = 0.008; cardiac death: χ2 increased by 5.032, P = 0.025), prediction accuracy (all-cause death: C-index increased from 0.781 to 0.797, P < 0.001; cardiac death: C-index increased from 0.791 to 0.802, P < 0.001), and reclassification (all-cause death: NRI = 32.9%, P < 0.001; cardiac death: NRI = 31.0%, P < 0.001) (Table 4). In non-DM patients, malnutrition significantly predicted poor prognosis (all-cause death: C-index 0.552, 95% CI 0.500–0.615, P < 0.05; cardiac death: C-index 0.576, 95% CI 0.507–0.651, P < 0.05). When GNRI ≤ 98 was added to the full multivariable model, its prediction accuracy became improved (all-cause death: C-index increase from 0.748 to 0.753, P < 0.001; cardiac death: C-index increase from 0.782 to 0.792, P < 0.001). However, for cardiac death, only an improvement in model fit was observed; no significant reclassification was observed (χ2 increased by 4.916, P = 0.027; NRI = 10.4%, P = 0.182). For all-cause deaths, adding GNRI ≤ 98 to the full multivariable model did not improve model fit and reclassification (χ2 increased by 1.484. P = 0.223; NRI = 6.2%, P = 0.545).
Relationship between inflammation, malnutrition, and DM
As shown in Supplementary Fig. 3, there were no significant differences in GNRI and hs-CRP levels between patients with and without DM. However, a significant negative correlation between GNRI and hs-CRP was observed in the overall population (r = − 0.245, P < 0.001), which was also evident in both the DM (r = − 0.228, P < 0.001) and non-DM groups (r = − 0.250, P < 0.001) (Supplementary Fig. 4). Comparisons between groups revealed that hs-CRP levels in the non-DM/L-GNRI group were significantly higher than those in the non-DM/H-GNRI (3.6 [1.6–13.4] vs. 1.3 [0.5–3.3], P < 0.001) and DM/H-GNRI (6 [1.6–13.4] vs. 1.6 [0.6–3.8], P = 0.004) groups (Supplementary Fig. 5). Similarly, hs-CRP levels in the DM/L-GNRI group were significantly higher compared to those in the non-DM/H-GNRI (3.1 [1.0–18.8] vs. 1.3 [0.5–3.3], P = 0.003) and DM/H-GNRI (3.1 [1.0–18.8] vs. 1.3 [0.5–3.3], P = 0.048) groups. Conversely, there was no significant difference in hs-CRP levels between the DM L-GNRI and non-DM L-GNRI groups (3.6 [1.6–13.4] vs. 3.1 [1.0–18.8], P = 1.000) (Supplementary Fig. 5).
Relationship between blood glucose status and GNRI in subgroups
This study further investigated the relationship between glucose levels and the GNRI. A total of 285 patients had available HbA1c data. The clinical characteristics of the patients with and without HbA1c are summarised in Supplementary Table 5. In the overall population, there was a marginal correlation between the GNRI and HbA1c levels (r = − 0.110, P = 0.063) (Supplementary Fig. 6a). In patients with DM, a weak negative correlation between GNRI and HbA1c was observed (r = − 0.195, P = 0.021); however, this correlation was not observed in non-DM patients (r = − 0.114, P = 0.171). Our analysis revealed no significant correlation between the GNRI and blood glucose levels, as observed in patients with DM (all P > 0.05) (Supplementary Fig. 6b). However, a weak correlation between the GNRI and blood glucose levels was observed in non-DM patients (r = 0.206, P = 0.015). Furthermore, among patients with DM, the L-GNRI group had higher HbA1c levels than the H-GNRI group (6.4 [5.8–7.6] vs. 6.8 [5.7–8.3], P = 0.019), whereas there was no significant difference in blood glucose levels (7.5 [6.2–11.3] vs. 7.1 [6.1–8.9], P = 0.240) (Supplementary Fig. 7).
Sensitivity analyses
To avoid potential biases arising from malnutrition and all-cause mortality due to ageing, a sensitivity analysis of young and middle-aged patients (aged ≤ 60 years) was performed. The Kaplan–Meier curves revealed that, compared with the DM/L-GNRI group, the DM/H-GNRI and non-DM/H-GNRI groups had significantly lower risks of all-cause mortality (42.1% vs. 5.6%, P < 0.05; 42.1% vs. 14.9%, P < 0.05). No significant difference in the risk of all-cause mortality was observed between the DM/L-GNRI and non-DM/L-GNRI groups (42.1% vs. 22.2%, P > 0.05). Furthermore, the multivariable Cox analysis demonstrated that malnutrition remained an independent predictor of all-cause mortality in patients with DM (adjusted HR = 0.944, 95% CI 0.892–1.000, P < 0.05). However, for non-DM patients, malnutrition was not an independent predictor of all-cause mortality (adjusted HR = 0.991, 95% CI 0.918–1.069, P = 0.813) (Supplementary Table 6).
Discussion
This study represents the first attempt to assess the differences related to DM in the impact of nutritional status on the prognosis of patients with HF. The main findings are as follows. First, the DM/L-GNRI group had the highest risk of adverse prognosis. After adjusting for potential confounding factors in patients with HF and DM, the GNRI remained independently associated with an increased risk of all-cause and cardiac mortality. Adding malnutrition significantly improved the model’s performance. Second, the research findings indicated a significant linear relationship between the GNRI and poor prognosis in patients with HF and DM. These associations were not observed in patients with HF without DM. Finally, subgroup analysis suggested that poor glycaemic control may be associated with an increased risk of malnutrition in patients with HF and DM. These findings highlight the prognostic significance of the interaction between nutritional status and DM status in patients with HF.
Symptoms of HF, such as breathing difficulties, significantly increase energy expenditure, often leading to anorexia and swallowing difficulties. Fluid retention, liver congestion, and inadequate intestinal perfusion can cause intestinal oedema, resulting in early satiety, nausea, and poor nutrient absorption22. Increased activity of the sympathetic nerve and the renin-angiotensin-aldosterone system leads to neurohormonal activation and increasing baseline energy consumption, inducing a catabolic state. The decrease in anabolic mediators (growth hormone, insulin-like growth factor-1, testosterone, and ghrelin) and increased oxidative stress caused by an upregulation in inflammatory mediators (tumour necrosis factor-α, interleukin-6, and hs-CRP) further exacerbate the anabolic-catabolic imbalance23. This significantly increases the risk of malnutrition and cachexia in patients with HF. Malnutrition is also a driving factor of HF progression. Numerous studies have identified malnutrition as an independent risk factor for poor outcomes in patients with HF2,3,7. Our findings align with those of previous studies, indicating that L-GNRI is independently associated with an increased risk of all-cause mortality and cardiac death in patients with HF. Malnutrition significantly worsens the prognosis of HF through mechanisms such as muscle loss, weakness, diminished immune function, heightened inflammatory responses, essential nutrient deficiencies, and mental health challenges24,25,26. The relationship between nutritional indices and outcomes may differ across ethnic groups, given genetic and dietary differences. A recent study showed that Black and White Americans, along with non-Hispanics, experience higher mortality rates from malnutrition than Native Americans, Asians, and Hispanics27. Dietary differences and food insecurity rates may explain this phenomenon. These findings highlight the importance of addressing malnutrition in clinical practice as it poses a significant risk to patient outcomes. Interventions to improve nutritional health can enhance survival rates and reduce the risk of cardiovascular events in patients with HF.
Patients with DM often exhibit a negative nitrogen balance owing to increased protein catabolism and excretion and decreased protein synthesis, increasing the likelihood of malnutrition. Malnutrition exacerbates insulin resistance, creating a vicious cycle of worsening metabolic and clinical outcomes. This study examined the interaction between malnutrition and DM and its impact on HF prognosis. Our findings indicate that patients with HF, DM, and L-GNRI are at the highest risk of all-cause and cardiac death. Similarly, Li et al. found a strong correlation between diabetes, malnutrition, and adverse clinical outcomes in patients with coronary artery disease14. A recent retrospective cohort study also highlighted that malnutrition is a significant independent risk factor for all-cause mortality in patients with acute myocardial infarction undergoing percutaneous coronary intervention, and patients with DM are at greater risk than those with normal glucose regulation or prediabetes28. Interestingly, these phenomena were not observed in patients with HF without DM, which aligns with Li et al.’s findings14. This suggests that DM may amplify the negative effects of malnutrition on prognosis. This may be because the interaction between DM and malnutrition exacerbates the inflammatory responses, leading to poor outcomes13,29. Consistent with this, we found that patients with HF, L-GNRI, and DM exhibited the highest hs-CRP levels, indicating severe inflammation. Elevated hs-CRP levels in these patients may be linked to chronic inflammatory responses and metabolic disturbances triggered by DM, severely affecting their nutritional status. In contrast, patients with HF without DM did not have the same mechanisms owing to the absence of persistent hyperglycaemia and associated metabolic burdens, resulting in milder inflammatory states. Therefore, attention should be paid to managing the nutritional and inflammatory statuses of patients with HF and DM.
In our subgroup analysis, we explored the relationship between glycaemic control and malnutrition. A significant negative correlation between the GNRI and HbA1c levels was observed only in patients with DM. Additionally, the results show that HbA1c levels in the L-GNRI group were significantly higher than in the H-GNRI group. Previous studies have also indicated that hyperglycaemia is associated with an increased prevalence of pre-frailty and frailty30. This relationship may be mediated by factors such as adipose tissue dysfunction, inflammatory responses, macrophage dysfunction, and increased oxidative stress31. However, the relationship between glycaemic abnormalities and frailty appears inconsistent, suggesting a U-shaped relationship between blood sugar levels and frailty32. Zaslavsky et al. confirmed a U-shaped association between glycaemic control and frailty33.
Patients with HF, DM, and L-GNRI had the highest risk of adverse outcomes. Even after adjusting for potential confounding factors, the GNRI remained independently associated with increased all-cause and cardiac death in these patients, a trend not observed in patients with HF without DM. This emphasises the importance of closely monitoring the nutritional status of patients with HF and DM. By evaluating the GNRI, healthcare providers can predict the prognosis and implement appropriate nutritional interventions. The study also found a significant linear relationship between the GNRI and adverse outcomes in patients with HF and DM. This provides new directions for exploring the underlying mechanisms of HF and DM and suggests potential clinical targets. Finally, subgroup analyses further indicated that poor glycaemic control may be associated with an increased risk of malnutrition in patients with HF and DM. These findings highlight the need for clinicians to focus on blood sugar management and nutritional status.
This study presents some limitations. First, it was conducted in a tertiary referral centre in China and included only ischemic HF patients who underwent SPECT examinations. This might limit the generalizability of the study results, necessitating further validation through large-scale multicenter studies. Second, although many risk factors were adjusted for, there may still be residual confounding factors that could influence the results. Third, we did not monitor changes in nutritional status over time. Dynamic changes in nutritional status may provide a more accurate predictive value for patients with HF and DM. Finally, prospective randomised controlled trials are needed to confirm the clinical benefits of nutritional support in patients with HF and diabetes mellitus.
In conclusion, patients with HF, DM, and malnutrition face the worst prognoses. DM amplifies the negative effects of the GNRI on adverse outcomes. Poor glycaemic control is associated with an increased risk of malnutrition.
Methods
Study population
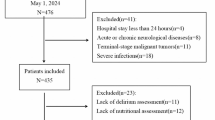
This single-centre, retrospective, longitudinal, observational study enrolled patients who were diagnosed with ischaemic HF and underwent clinically indicated 99mTc-sestamibi single-photon emission computed tomography (SPECT)-gated myocardial perfusion imaging (GMPI) and cardiac 18F-fluorodeoxyglucose positron emission tomography/CT scanning at Beijing Anzhen Hospital between 2016 and 2022. HF diagnosis was established through integrated clinical judgment following clinical guidelines16. HF was defined based on clinical symptoms, such as breathlessness, fatigue, left ventricular (LV) dysfunction, and abnormal LV geometry, resulting in reduced cardiac output or elevated intracardiac pressure at rest or during stress16. Standardised evaluations, including laboratory tests and clinical assessments, were conducted17. A flowchart of the patient inclusion process is shown in Supplementary Fig. 1. The exclusion criteria were left ventricular ejection fraction (LVEF) > 50%, insufficient clinical data (including body mass index [BMI], albumin, brain natriuretic peptide [BNP], and high-sensitivity C-reactive protein [hs-CRP]), and poor imaging quality. This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (Approval No. 2017024). Informed written or oral consent was obtained from all enrolled patients.
Data collection and definitions
Patient demographic and clinical data such as age, sex, BMI, heart rate, systolic blood pressure, diastolic blood pressure, medical history, clinical manifestations, laboratory values, and imaging examination results were retrieved from the electronic medical record system of Beijing Anzhen Hospital. Haematological analyses of all patients were conducted in the clinical laboratory of Beijing Anzhen Hospital. BMI was calculated as body weight in kilograms divided by the square of height in meters. Based on previous research18, the GNRI was calculated using the formula:
The ideal BMI was set to 22 kg/m2. If the actual BMI was greater than the ideal BMI, it was set to 17,18,19. Based on the GNRI values, the patients were divided into a low GNRI (L-GNRI) group (≤ 98) and a high GNRI (H-GNRI) group (> 98), as per previous studies3.
99mTc-sestamibi SPECT/CT GMPI and analysis
For 99mTc-sestamibi SPECT/CT GMPI at rest, a procedure similar to that described previously was used20. Data from the SPECT/CT GMPI were transmitted to a Siemens e.soft workstation (Siemens AG 2015). LVEF (%) and left ventricular mechanical synchrony (bandwidth [°]) were calculated using the QGS software version 3.1 (Cedars-Sinai Medical Center). Myocardial perfusion was analysed using the American Heart Association 17-segment and 5-point scoring system21. The total perfusion defect (TPD, %LV) was determined based on the number of hypoperfusion segments and the severity of the defect. Two experienced nuclear cardiologists, blinded to all clinical information, independently reviewed the images.
Endpoints and follow-up
The baseline time was defined as the day the patient underwent the SPECT examination. The patients were followed up until 1 January 2023. The primary outcome was all-cause death, and the secondary outcome was cardiac death. Events were determined by independent physicians who were blinded to the study. The outcomes were confirmed through a review of medical records, clinical visits, and interviews.
Statistical analysis
Data analysis was performed using SPSS software (version 22.0) and R (version 4.2.0). Categorical data are presented as counts and percentages, whereas continuous variables are shown as mean ± standard deviation or median with interquartile range. Normality was evaluated using the Kolmogorov–Smirnov test. Student’s t-test was used for normally distributed variables. Kruskal–Wallis and Mann–Whitney U tests were used for non-normally distributed variables. Chi-square or Fisher’s exact test was used to compare categorical data. Correlations were assessed using Pearson’s or Spearman’s coefficients. Kaplan–Meier curves were used to depict time-to-event data and log-rank tests were used for survival comparisons. Cox proportional hazard regression models were used to calculate hazard ratios and 95% confidence intervals (CIs) after adjusting for covariates. The nonlinear association between adverse endpoint events and the GNRI as a continuous variable was evaluated using a restricted cubic spline (RCS) model. The variables in the RCS model included sex, age, hyperlipidaemia, NYHA class (III–IV), prior arrhythmia, prealbumin, cholinesterase, uric acid, BNP, beta-blockers, TPD, and LVEF. The number of knots was based on the lowest value of the Akaike information criterion, and four knots were selected for the analysis. Model performance was evaluated using C-statistics. Fit was assessed using likelihood ratio and χ2 tests. The net reclassification index (NRI) method was used for formal risk reclassification analysis. All tests were two-sided, and significance was set at P < 0.05.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Heidenreich, P. A. et al. AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 145, e895–e1032. https://doi.org/10.1161/CIR.0000000000001063(2022) (2022).
Sze, S. et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: A comparison with body mass index. JACC Heart Fail. 6, 476–486. https://doi.org/10.1016/j.jchf.2018.02.018 (2018).
Pagnesi, M. et al. Impact of malnutrition in patients with severe heart failure. Eur. J. Heart Fail. 26, 1585–1593. https://doi.org/10.1002/ejhf.3285 (2024).
Driggin, E. et al. Nutrition assessment and dietary interventions in heart failure: JACC review topic of the week. J. Am. Coll. Cardiol. 79, 1623–1635. https://doi.org/10.1016/j.jacc.2022.02.025 (2022).
Adam, R. et al. Malnutrition and cachexia in heart failure. J. Parenter. Enter. Nutr. 40, 475–486. https://doi.org/10.1177/0148607114566854 (2015).
Bonilla-Palomas, J. L. et al. Nutritional intervention in malnourished hospitalized patients with heart failure. Arch. Med. Res. 47, 535–540. https://doi.org/10.1016/j.arcmed.2016.11.005 (2016).
Sun, T. et al. Prognostic impacts of geriatric nutritional risk index in patients with ischemic heart failure after percutaneous coronary intervention. Clin. Nutr. 42, 1260–1267. https://doi.org/10.1016/j.clnu.2023.05.023 (2023).
Ran, Q. et al. A nomogram model for predicting malnutrition among older hospitalized patients with type 2 diabetes: A cross-sectional study in China. BMC Geriatr. 23, 565. https://doi.org/10.1186/s12877-023-04284-4 (2023).
Umphonsathien, M. et al. Effects of intermittent very-low calorie diet on glycemic control and cardiovascular risk factors in obese patients with type 2 diabetes mellitus: A randomized controlled trial. J. Diabetes Investig. 13, 156–166. https://doi.org/10.1111/jdi.13619 (2022).
Madeira, T. et al. Nutritional intake and malnutrition in institutionalised and non-institutionalised older adults. Br. J. Nutr. 128, 921–931. https://doi.org/10.1017/S0007114521003925 (2022).
Marathe, C. S. et al. Gastrointestinal autonomic neuropathy in diabetes. Auton. Neurosci. 229, 102718. https://doi.org/10.1016/j.autneu.2020.102718 (2020).
Uyar, S. et al. Could insulin and hemoglobin A1c levels be predictors of hunger-related malnutrition/undernutrition without disease?. Clin. Lab. 64, 1635–1640. https://doi.org/10.7754/Clin.Lab.2018.180403 (2018).
Rajamanickam, A. et al. Impact of malnutrition on systemic immune and metabolic profiles in type 2 diabetes. BMC Endocr. Disord. 20, 168. https://doi.org/10.1186/s12902-020-00649-7 (2020).
Li, T. et al. Association of prognostic nutritional index level and diabetes status with the prognosis of coronary artery disease: A cohort study. Diabetol. Metab. Syndr. 15, 58. https://doi.org/10.1186/s13098-023-01019-8 (2023).
Wang, J. et al. A synergistic association between inflammation, malnutrition, and mortality in patients with diabetics. Front. Nutr. 9, 872512. https://doi.org/10.3389/fnut.2022.872512 (2022).
Ponikowski, P. et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 18, 891–975. https://doi.org/10.1002/ejhf.592 (2016).
Ponikowski, P. et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 37, 2129–2200. https://doi.org/10.1093/eurheartj/ehw128 (2016).
Cereda, E. & Pedrolli, C. The geriatric nutritional risk index. Curr. Opin. Clin. Nutr. Metab. Care 12, 1–7. https://doi.org/10.1097/MCO.0b013e3283186f59 (2009).
Bouillanne, O. et al. Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 82, 777–783. https://doi.org/10.1093/ajcn/82.4.777 (2005).
Yun, M. et al. Assessment of cerebral glucose metabolism in patients with heart failure by (18)F-FDG PET/CT imaging. J. Nucl. Cardiol. 29, 476–488. https://doi.org/10.1007/s12350-020-02258-2 (2022).
Zhang, X. et al. Long-term survival of patients with viable and nonviable aneurysms assessed by 99mTc-MIBI SPECT and 18F-FDG PET: A comparative study of medical and surgical treatment. J. Nucl. Med. 49, 1288–1298. https://doi.org/10.2967/jnumed.107.046730 (2008).
Sandek, A. et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 50, 1561–1569. https://doi.org/10.1016/j.jacc.2007.07.016 (2007).
Murphy, L. et al. Anabolism to catabolism: Serologic clues to nutritional status in heart failure. Curr. Heart Fail. Rep. 16, 189–200. https://doi.org/10.1007/s11897-019-00437-y (2019).
Wleklik, M. et al. The role of nutritional status in elderly patients with heart failure. J. Nutr. Health Aging 22, 581–587. https://doi.org/10.1007/s12603-017-0985-1 (2018).
Ruting, W. et al. The role of sarcopenia in heart failure with depression. Rev. Cardiovasc. Med. 23, 296. https://doi.org/10.31083/j.rcm2309296 (2022).
Ishikawa, Y. & Sattler, E. L. P. Nutrition as treatment modality in heart failure. Curr. Atheroscler. Rep. 23, 13. https://doi.org/10.1007/s11883-021-00908-5 (2021).
Kawano, B. et al. Racial and ethnic demographics in malnutrition related deaths. Clin. Nutr. ESPEN. 60, 135–138. https://doi.org/10.1016/j.clnesp.2024.01.018 (2024).
Ling, X. et al. Prognostic value of the prognostic nutritional index for patients with acute myocardial infarction undergoing percutaneous coronary intervention with variable glucose metabolism statuses: A retrospective cohort study. Diabetol. Metab. Syndr. 15, 207. https://doi.org/10.1186/s13098-023-01160-4 (2023).
Chun, Z. et al. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: Relationship with metabolic factors and complications. J. Mol. Med. 90, 175–186. https://doi.org/10.1007/s00109-011-0816-5 (2011).
Blaum, C. S. et al. Is hyperglycemia associated with frailty status in older women?. J. Am. Geriatr. Soc. 57, 840–847. https://doi.org/10.1111/j.1532-5415.2009.02196.x (2009).
Michael, B. S. et al. Physiological aging: Links among adipose tissue dysfunction, diabetes, and frailty. Physiology 32, 9–19. https://doi.org/10.1152/physiol.00012.2016 (2016).
Peter, H. et al. Frailty measurement, prevalence, incidence, and clinical implications in people with diabetes: A systematic review and study-level meta-analysis. Lancet Healthy Longev. 1, e106–e116. https://doi.org/10.1016/s2666-7568(20)30014-3 (2020).
Oleg, Z. et al. Glucose levels and risk of frailty. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 71, 1223–1226. https://doi.org/10.1093/gerona/glw024 (2016).
Funding
This work was supported by the National Natural Science Foundation of China (82171994, 81871377, and 82027804), Beijing Municipal Administration of Hospitals (ZYLX202110), and the Advanced Research Foundation of Capital Medical University (PYZ21121).
Author information
Authors and Affiliations
Contributions
All authors were involved in study conception and design. Zhiyong Shi prepared the materials and collected data. Zhiyong Shi were responsible for data analysis and interpretation. Zhiyong Shi was responsible for first drafted this manuscript. Xiaoli Zhang was in charge of supervision. Zhiyong Shi, Mingkai Yun, Haiyan Liu, Sijin Li and Xiaoli Zhang contributed to the discussion. Mingkai Yun, Haiyan Liu, Sijin Li and Xiaoli Zhang critically revised the manuscript. All authors commented on prior manuscript versions and agreed to the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Clinical Research Ethics Committee of the Beijing Anzhen Hospital, Capital Medical University (Approval No. 2017024). Written or oral informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, Z., Yun, M., Liu, H. et al. Impact of geriatric nutritional risk index and diabetes mellitus on prognosis in ischaemic heart failure with reduced ejection fraction. Sci Rep 15, 4226 (2025). https://doi.org/10.1038/s41598-025-88950-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88950-0