Abstract
Blood heparin-binding protein (HBP) for diagnosing bacteremia in patients with sepsis has not been fully investigated. This study aims to explore the diagnostic value of blood HBP in predicting bacteremia in patients with sepsis compared with procalcitonin (PCT), Interleukin 6 (IL-6), C-reactive protein (CRP), white blood count (WBC), and neutrophil. In this observational study, we enrolled consecutive 146 patients, who were divided into two groups as the bacteremia group (n = 57) and the control group (n = 89). HBP, PCT, IL-6, CRP, WBC, and neutrophil were measured. The Chi-squared test and Student’s t-test were used to compare the baseline characteristics. The area under the receiver operating characteristic curve (AUC) was calculated to describe the diagnostic accuracy of the biomarkers for predicting bacteremia in patients with sepsis. Spearman’s correlation was used to analyze associations between the biomarkers. The concentration of HBP (204.13 ± 87.30 ng/mL ) in the bacteremia group was significantly higher than that in the control group (81.43 ± 61.53). HBP achieved the largest AUC for predicting bacteremia, with a value of 0.88 (95% confidence interval [CI], 0.82–0.94 ). This value was higher than those of the other biomarkers: 0.78 (95% CI 0.69–0.86) for PCT, 0.59 (95% CI 0.48–0.70) for IL-6, 0.56 (95% CI 0.45–0.67) for WBC, 0.73 (95% CI 0.64–0.83) for CRP, and 0.64 (95% CI 0.53–0.74) for neutrophil. The best cut-off value of blood HBP for identifying bacteremia was 95.69 ng/mL, with a sensitivity of 88.64%, a specificity of 68.06%, a positive predictive value of 64.1%, and a negative predictive value of 89.71%. A significant association was found between HBP and CRP (Spearman’s rho = 0.528, p < 0.01). However, the correlations among PCT, IL-6, WBC, and neutrophil (Spearman’s rho < 0.5, p < 0.01) were relatively weak. Blood HBP may be a useful auxiliary diagnostic marker that is preferable over PCT, IL-6, CRP, WBC, and neutrophil in identifying bacteremia in patients with sepsis.
Similar content being viewed by others
Introduction
Sepsis is an ongoing and substantial challenge in global healthcare, and it significantly contributes to morbidity and mortality rates associated with hospitalization1. Sepsis and septic shock are significant global healthcare challenges, which affect millions of individuals annually2,3. Sepsis can develop in any individual who is infected, and its incidence among hospitalized patients is estimated to be as high as 1–2%4. Sepsis is characterized as a critical condition where organ dysfunction arises due to an imbalanced host response to infection, which poses a life-threatening situation5. The pathophysiological process of sepsis involves an intricate disruption of the delicate equilibrium between pro- and anti-inflammatory responses6. The activation of immune cells by invading microorganisms or damaged tissue elements can trigger the release of proinflammatory cytokines, reactive oxygen species, and enzymes7. The widespread use of antibiotics in recent years has resulted in a rise in antibiotic resistance, which significantly impacts the effectiveness of treatment outcomes5,8. Long-term sepsis leads to immunosuppression, which is characterized by increased release of anti-inflammatory cytokines, immunocyte death, decreased HLA-DR expression, increased expression of negative costimulatory molecules, and expansion of immunomodulatory cells5. Research indicates that sepsis leads to substantial reductions in CD4 and CD8 T cells, which significantly impair immune responses and diminish the ability of the host to combat microbial threats9. Early detection and timely intervention in the critical hours following the onset of sepsis greatly enhance prognosis and overall patient outcomes10,11. Therefore, discovering new biomarkers that can enable the early, precise, and effective identification of pathogens is crucial. Newer markers such as heparin-binding protein (HBP) are being increasingly explored for sepsis diagnosis to address the aforementioned challenges7,12.
HBP, which is also known as the cationic antimicrobial protein of 37 kDa (CAP37) or azurocidin, is a protein found in neutrophil granules; it was first discovered and identified in 1984 by Shafer et al.13. HBP is swiftly released from activated neutrophils during the initial stages of inflammation, as a response to infections14. This characteristic positions HBP as a promising biomarker with increasing relevance in the diagnosis of infectious diseases15,16. The protein has demonstrated its superior performance to procalcitonin (PCT), Interleukin 6 (IL-6), and C-reactive protein (CRP) in early detection of infections17,18,19. Similarly, HBP has exhibited a more potent diagnostic capacity for sepsis20,21,22. To date, blood culture, which is the current important indicator for sepsis diagnosis and management, has inherent limitations. It requires several days to produce conclusive results and can yield false negatives due to pre-emptive antibiotic administration. However, existing evidence on the accuracy of HBP to predict positive blood bacterial cultures in septic patients is still lacking. This research aims to assess the value of blood HBP in identifying bacteremia in patients with sepsis.
Methods
Study design and patient population
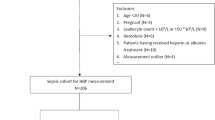
This retrospective observational study was conducted at the Chinese PLA General Hospital between December 2021 and August 2023. Eligible subjects who were older than 18 years of age and met the SEPSIS-3 criteria were enrolled in the study23. Exclusion criteria were subjects who had undergone operation and experienced trauma within the past 24 h, which may be affect the expression of HBP, those receiving immunosuppressive therapy (e.g., organ transplants, rheumatological, and autoimmune diseases), pregnant individuals, and those younger than 18 years. Patients who had already received empiric antibiotic therapy prior to sampling were not excluded from the study. The project protocol was approved by the ethics committee of the Chinese PLA General Hospital and was conducted in accordance with the relevant guidelines/recommendations. Patient informed consent was waived given the retrospective nature of this study. Demographic data of the patients, including age, sex, laboratory test findings, duration of intensive care unit (ICU) stay, clinical outcomes, and causes of ICU admission, were obtained from their medical records. Based on the clinical manifestations and blood culture results, the patients were categorized into two groups: the bacteremia group and the control group.
Blood sample collection and analysis
Blood samples were collected from patients when patients were diagnosed with sepsis during 24 h. If sepsis is diagnosed upon admission to the ICU, a blood sample will be collected at that time. Our department has been using standardized blood culture collection methods to prevent contamination during the blood culture process. In order to prevent blood culture contamination from affecting the accuracy of results, infections of unknown origin have been excluded from this study. Blood samples were collected from the patients for HBP, PCT, IL-6, CRP, WBC, neutrophil, and culture analyses. The blood samples were collected in 5 ml sodium citrate anticoagulation tubes and centrifuged at 3,000 rpm for 10 min immediately. The HBP level was measured using a commercially available immunofluorescence dry quantitation method assay kit (Joinstar Biomedical Technology Co., Ltd., Hangzhou, China). The assay kit had a detection range for HBP of 5.9–300 ng/mL. The detection methods for PCT, IL-6, and CRP were electrochemiluminescence, chemiluminescence, and nephelometry, respectively.
Statistical analysis
Baseline characteristics were compared using the Chi-squared test, while group comparisons and subgroup analyses were conducted using the Student’s t-test. The diagnostic accuracy of blood biomarkers was assessed by calculating the area under the receiver operating characteristic curve (AUC). Considering the positive correlations observed in previous studies among HBP, PCT, WBC, IL-6, CRP, and neutrophil, Spearman’s correlation analysis was conducted to examine the associations among these biomarkers. The analysis involved controlling the effect of one biomarker while assessing the correlation between the two others. Statistical significance was defined as a p-value less than 0.05. All statistical analyses were performed using GraphPad Prism version 9.0 (GraphPad Software (https://www.graphpad.com), USA) and SPSS version 29.0 (IBM Corporation, USA).
Results
Baseline characteristics
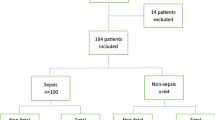
Blood samples were collected from 171 patients who were diagnosed with sepsis and underwent blood culture. However, 25 patients were ultimately excluded due to surgeries or trauma within the past 24 h, which could potentially affect the expression of HBP24,25. A total of 146 samples were analyzed, including 57 samples from the bacteremia group and 89 samples from the control group. Table 1 presents a comprehensive summary of the baseline characteristics for 146 patients. No statistically significant differences were observed in the demographic characteristics and medical conditions between the two groups, and no statistically significant differences were found in the proportion of causes of ICU admission between the two groups. The liver function, kidney function, brain natriuretic peptide, and ICU days showed no significant differences between the two groups.
Bacteria of positive blood cultures
As summarized in Tables 2 and 57 patients were tested positive for bacterial growth in their blood cultures, which resulted in 64 instances of blood culture-positive bacteria. Gram-positive and Gram-negative mixed infections were found in 7 of the patients who tested positive in blood culture. Other patients showed a positive culture only from a single Gram-positive or Gram-negative bacterium. Among these bacteria, the most prevalent Gram-positive bacterium was Staphylococcus, which accounted for 43.8%. The main sources of coagulase - negative staphylococci include pneumonia(10, 35.72%), gastrointestinal tract(5, 17.86%), urinary tract infection (5, 17.86%), central venous catheters (2, 7.14%), and other foci of infections (6, 21.43%). The most prevalent Gram-negative bacterium was Klebsiella pneumoniae, which accounted for 17.2%. Other bacteria recorded included Acinetobacter baumannii, Escherichia coli, Enterococcus, Pseudomonas aeruginosa, Clostridium septicum, Enterobacter aerogenes, Eliza meningosepticum, Clostridium perfringens, Klebsiella oxytoca, Serratia marcescens, and Proteus mirabilis.
Value of blood HBP for predicting bacteremia in patients with sepsis
We found that blood parameters (HBP, PCT, IL-6, CRP, and WBC) universally increased in the bacteremia group compared with the control group (Fig. 1). The concentration of HBP (204.13 ± 87.30 ng/mL) in the bacteremia group was significantly higher than that in the control group (81.43 ± 61.53 ng/mL). The receiver operating characteristic (ROC) curve for the discrimination of bacteremia and blood culture negative showed that blood HBP achieved the largest AUC of 0.88 (95% confidence interval [CI], 0.82–0.94), which was higher than those of the other biomarkers: 0.78 (95% CI 0.69–0.86) for PCT, 0.59 (95% CI 0.48–0.70) for IL-6, 0.73 (95% CI 0.64–0.83) for CRP, 0.56 for WBC (95% CI 0.45–0.67), and 0.64 (95% CI 0.53–0.74) for neutrophil (Fig. 2). The best cut-off value of blood HBP for identifying bacteremia was 95.69 ng/mL, with a sensitivity of 88.64%, a specificity of 68.06%, a positive predictive value of 64.1%, and a negative predictive value of 89.71% (Table 3). The sensitivity and specificity of PCT for distinguishing bacteremia and blood culture negative were found to be 95.45 and 51.39%, respectively. Those of IL-6, CRP, WBC, and neutrophil were found to be 34.09 and 84.72, 68.2 and 70.8, 33.33 and 83.34%, and 52.3% and 76.4%, respectively (Table 3).
Correlation between blood biomarkers
A strong association existed between HBP and CRP (Spearman’s rho = 0.528, p < 0.001). However, the correlations among PCT, IL-6, WBC, and neutrophil (Spearman’s rho < 0.5, p < 0.001/ p < 0.05) were relatively weak (Table 4; Fig. 3).
Diagnostic accuracy of blood HBP in patients who received empiric antibiotics in ICU
A total of 102 patients were documented to have received empiric antimicrobial therapy prior to sampling in ICU. No statistical significance was found in the concentrations of blood HBP, PCT, IL-6, CRP, WBC, and neutrophil between the patients who received empiric antibacterial therapy (n = 102) and those who did not in ICU (n = 44) (Figure S1).
Diagnostic accuracy of blood HBP in identifying Gram-positive and Gram-negative bacteria
There was no significant difference in the levels of HBP, PCT, IL-6, CRP, WBC and neutrophil between the Gram-positive group and Gram-negative group (Figure S2). We then further evaluated the diagnostic efficiency of these parameters using ROC analysis. The receiver operating ROC curve analysis was conducted to evaluate the ability of various parameters (HBP, PCT, IL-6, CRP, WBC, and neutrophil) to discriminate between Gram-positive and Gram-negative bacterial infections. However, the areas under the curves of HBP, PCT, IL-6, CRP, WBC and neutrophil were 0.535, 0.583, 0.422, 0.538, 0.424 and 0.436 respectively. The results indicated that none of these parameters showed discriminatory power in distinguishing between Gram-positive and Gram-negative bacterial infections (Figure S3).
Discussion
We found that HBP achieved the largest AUC as a diagnostic marker compared with PCT, IL-6, CRP, WBC, and neutrophil for diagnosing bacteremia in patients with sepsis. The concentrations of HBP, PCT, IL-6, CRP, WBC, and neutrophil were significantly higher in the bacteremia patients than in the blood culture negative patients. In addition, the subgroup analysis failed to ascertain the ability of blood HBP in identifying Gram-positive and Gram-negative bacterial infection and the influence on HBP levels of empiric antibiotics.
Our data confirmed previous research suggesting that the concentration of HBP was significantly increased in bacterial sepsis patients compared with those with non-bacterial infection26,27. However, plasma HBP levels appeared to be the most reliable parameter for distinguishing between severe sepsis and mild infection7. At a cut-off level of 15 ng/ml, the sensitivity for HBP in diagnosing severe sepsis was 87%, and the specificity was 95%28. Another previous study showed the best cut-off value of concentration of HBP for identifying sepsis and non-sepsis patients was 30 ng/mL29. By contrast, a cut-off value of HBP ≥ 95.69 ng/mL gave a sensitivity of 88.64% and a specificity of 68.06% in diagnosing bacteremia in patients with sepsis in the present study. In differentiating sepsis patients with positive blood culture results, HBP displayed enhanced diagnostic ability compared with PCT, IL-6, CRP, WBC, and neutrophil counts. This insight is helpful for rapid treatment decision making by clinicians.
HBP is a granular protein that is generated during the maturation process in the bone marrow and later released by neutrophils at the site of inflammation, and bacteria can induce the synthesis and secretion of HBP by neutrophils7. In healthy individuals, blood levels of HBP are exceedingly low. Upon the onset of an infection, certain pathogens can infiltrate the bloodstream and stimulate neutrophils to secrete HBP, which results in an elevation of HBP levels in the blood. The production and release of HBP have been documented in various bacterial infections30. Previous studies have shown that blood HBP could have a significant clinical potential for early identification of patients with sepsis28. Clinicians should exercise caution when dealing with sepsis patients who present increased blood HBP levels. In such cases, the presence of bacterial infections should be considered until a negative blood culture is obtained. Despite the numerous advancements in technology, obtaining a blood culture result still typically takes at least 24 h to 48 h. As a result, the subsequent delays in diagnosing and treating infections can have detrimental effects on patient care within the ICU. Therefore, HBP could serve as a useful additional tool for stratifying patients with possible bacteremia in sepsis, but it cannot substitute for blood culture results.
The correlation between HBP and PCT, IL-6, WBC, and neutrophil was weak in our study, although a previous study showed that plasma HBP was positively correlated with serum PCT, WBC, and neutrophil in patients31. However, in keeping with another previous study32, our study demonstrated significant correlation between HBP and CRP. This finding aligns with the conclusion drawn in a previous study, which indicated that plasma HBP levels serve as significant biomarkers for assessing inflammatory response in patients with sepsis33.
Unexpectedly, our results indicated that patients with sepsis who received empirical antimicrobial therapy did not exhibit a significant difference in HBP levels compared with those who did not. Our results are consistent with the findings of a previous study34. Blood HBP could serve as a valuable marker in resolving diagnostic dilemmas encountered in these patients. In our study, we also evaluated the diagnostic accuracy of blood HBP in identifying Gram-positive and Gram-negative bacteria. However, no statistical significance was found in blood HBP levels for identifying Gram-positive and Gram-negative bacteria.
The novelty of our study is that we demonstrated the efficacy of blood HBP in predicting bacteremia in patients with sepsis. Furthermore, our study included patients who received antibiotic therapy prior to blood culture, which aligns more closely with real-world clinical scenarios. By contrast, previous studies predominantly excluded these patients.
Our study has some limitations. First, we only recruited patients with bacterial infection and did not include those with infection of other microbes, such as fungi and viruses. Second, the numbers of cases in positive blood culture were small. Third, the majority of our patients had complications that could potentially impact the levels of biomarkers. For instance, patients with cardiovascular diseases tend to exhibit higher expression of HBP. These factors should be considered when interpreting the biomarker results for accurate assessment and treatment decisions in prospective studies. Finally, Due to the diverse sources of patients in our department, such as those after surgical operations, from the emergency department, and transferred from other hospitals, etc., many patients are very likely to have been treated with antibiotics before they are admitted to the ICU. Nevertheless, we are unable to obtain the data of each patient prior to their admission to the ICU. Therefore, the actual proportion of empirical antibiotic application may far exceed the data we presented. This is also one of the possible reasons for the insignificant difference in the expression levels of HBP between the empirical antibiotic application and the no empirical antibiotic application in our study. In the future, strict prospective studies may be able to improve this result.
Conclusions
Blood HBP may serve as a useful auxiliary diagnostic marker, which is preferable over PCT, IL-6, CRP, WBC, and neutrophil in identifying bacteremia in patients with sepsis. However, blood culture still plays a very important role in the diagnosis of sepsis. Strict prospective studies may be needed in the future to evaluate the diagnostic role of HBP for bacteremia in the ICU setting.
Data availability
All data generated or analyzed during this study are included in this published article. WSY should be contacted for data requests related to this study.
Abbreviations
- HBP:
-
Heparin-binding protein
- PCT:
-
Procalcitonin
- IL-6:
-
Interleukin 6
- CRP:
-
C-reactive protein
- WBC:
-
White blood counts
- BNP:
-
Brain natriuretic peptide (NT-pro)
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- ICU:
-
Intensive care medicine
References
Oczkowski, S. et al. Surviving sepsis campaign guidelines 2021: highlights for the practicing clinician. Pol. Arch. Intern. Med. 132, 7–8 (2022).
Fleischmann-Struzek, C. et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 46 (8), 1552–1562 (2020).
Fleischmann, C. et al. Assessment of global Incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am. J. Respir Crit. Care Med. 193 (3), 259–272 (2016).
Huang, M., Cai, S. & Su, J. The pathogenesis of Sepsis and potential therapeutic targets. Int. J. Mol. Sci., 20(21). (2019).
Liu, D. et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med. Res. 9 (1), 56 (2022).
Das, U. N. Infection, inflammation, and immunity in Sepsis. Biomolecules, 13(9). (2023).
Linder, A., Soehnlein, O. & Akesson, P. Roles of heparin-binding protein in bacterial infections. J. Innate Immun. 2 (5), 431–438 (2010).
Huang, Y. et al. Bacterial growth-induced Tobramycin smart release self-healing hydrogel for pseudomonas aeruginosa-Infected burn wound healing. ACS Nano. 16 (8), 13022–13036 (2022).
McBride, M. A. et al. Immune checkpoints: Novel therapeutic targets to Attenuate Sepsis-Induced Immunosuppression. Front. Immunol. 11, 624272 (2020).
Rello, J. et al. Sepsis: a review of advances in management. Adv. Ther. 34 (11), 2393–2411 (2017).
Han, X. et al. Heparin-binding protein-enhanced quick SOFA score improves mortality prediction in sepsis patients. Front. Med. (Lausanne). 9, 926798 (2022).
Fisher, J. & Linder, A. Heparin-binding protein: a key player in the pathophysiology of organ dysfunction in sepsis. J. Intern. Med. 281 (6), 562–574 (2017).
Shafer, W. M., Martin, L. E. & Spitznagel, J. K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect. Immun. 45 (1), 29–35 (1984).
Tapper, H. et al. Secretion of heparin-binding protein from human neutrophils is determined by its localization in azurophilic granules and secretory vesicles. Blood 99 (5), 1785–1793 (2002).
Kong, Y. et al. Accuracy of heparin-binding protein for the diagnosis of nosocomial meningitis and ventriculitis. Crit. Care. 26 (1), 56 (2022).
Kyriazopoulou, E. et al. Heparin-binding protein levels predict unfavorable outcome in covid-19 pneumonia: a post hoc analysis of the save trial. Shock 61 (3), 395–399 (2024).
Meng, Y. et al. Blood heparin-binding protein and neutrophil-to-lymphocyte ratio as indicators of the severity and prognosis of community-acquired pneumonia. Respir Med. 208, 107144 (2023).
Ma, J. et al. A diagnostic test: combined detection of heparin-binding protein, procalcitonin, and C-reactive protein to improve the diagnostic accuracy of bacterial respiratory tract infections. J. Thorac. Dis. 14 (3), 721–728 (2022).
Zuo, L. et al. Heparin-binding protein as a biomarker for the diagnosis of sepsis in the intensive care unit: a retrospective cross-sectional study in China. BMJ Open. 14 (6), e078687 (2024).
Katsaros, K. et al. Heparin binding protein for the early diagnosis and prognosis of Sepsis in the Emergency Department: the prompt Multicenter Study. Shock 57 (4), 518–525 (2022).
Wu, Y. L. et al. Accuracy of heparin-binding protein in diagnosing Sepsis: a systematic review and Meta-analysis. Crit. Care Med. 49 (1), e80–e90 (2021).
Taha, A. M. et al. Diagnostic and prognostic value of heparin-binding protein in sepsis: a systematic review and meta-analysis. Med. (Baltim). 103 (25), e38525 (2024).
Shankar-Hari, M. et al. Developing a new definition and assessing New Clinical Criteria for septic shock: for the Third International Consensus definitions for Sepsis and septic shock (Sepsis-3). Jama 315 (8), 775–787 (2016).
Johansson, J. et al. Heparin-binding protein (HBP): an early marker of respiratory failure after trauma? Acta Anaesthesiol. Scand. 57 (5), 580–586 (2013).
Sterner, N. et al. The Dynamics of Heparin-Binding Protein in cardiothoracic Surgery-A pilot study. J. Cardiothorac. Vasc Anesth. 35 (9), 2640–2650 (2021).
Kjölvmark, C. et al. Distinguishing asymptomatic bacteriuria from urinary tract infection in the elderly - the use of urine levels of heparin-binding protein and interleukin-6. Diagn. Microbiol. Infect. Dis. 85 (2), 243–248 (2016).
Zhou, Y. et al. Usefulness of the heparin-binding protein level to diagnose sepsis and septic shock according to Sepsis-3 compared with procalcitonin and C reactive protein: a prospective cohort study in China. BMJ Open. 9 (4), e026527 (2019).
Linder, A. et al. Heparin-binding protein: an early marker of circulatory failure in sepsis. Clin. Infect. Dis. 49 (7), 1044–1050 (2009).
Bergquist, M. et al. TNFR1, TNFR2, neutrophil gelatinase-associated lipocalin and heparin binding protein in identifying sepsis and predicting outcome in an intensive care cohort. Sci. Rep. 10 (1), 15350 (2020).
Chen, S. et al. Suilysin stimulates the release of heparin binding protein from neutrophils and increases vascular permeability in mice. Front. Microbiol. 7, 1338 (2016).
Xiao, X. et al. Diagnostic value of plasma heparin-binding protein and the heparin-binding protein-to-albumin ratio in patients with community-acquired Pneumonia: a retrospective study. BMC Infect. Dis. 23 (1), 777 (2023).
Liu, P. et al. Heparin-binding protein as a biomarker of severe sepsis in the pediatric intensive care unit: a multicenter, prospective study. Clin. Chim. Acta. 539, 26–33 (2023).
Xue, H. & Yu, F. Changes in Heparin-binding protein, Procalcitonin, and C-Reactive protein within the first 72 hours predict 28-Day mortality in patients admitted to the Intensive Care Unit with septic shock. Med. Sci. Monit. 29, e938538 (2023).
Linder, A. et al. Heparin-binding protein: a diagnostic marker of acute bacterial meningitis. Crit. Care Med. 39 (4), 812–817 (2011).
Author information
Authors and Affiliations
Contributions
ZM conceived and designed this study. MMY and Jie Gao collected data. JWZ designed and performed the statistical analyses. WSY wrote the first draft of the manuscript. FHZ, HJK and HL reviewed and modified the final manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The project protocol was approved by the ethics committee of the Chinese PLA General Hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mao, Z., Yang, W., Gao, J. et al. Accuracy of blood heparin-binding protein (HBP) for diagnosis bacteremia in patients with sepsis. Sci Rep 15, 5702 (2025). https://doi.org/10.1038/s41598-025-89241-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89241-4
Keywords
This article is cited by
-
Recent advances in biomarkers for detection and diagnosis of sepsis and organ dysfunction: a comprehensive review
European Journal of Medical Research (2025)