Abstract
Epoxidized vegetable oils present a viable substitute for polymers derived from petroleum. This research focuses on the impact of a process parameter on the epoxidation of palm stearin when zeolite ZSM-5 is used as a catalyst. This study synthesized peracetic acid as the oxidizing agent by combining hydrogen peroxide and acetic acid, adjusting molar ratios relative to palm stearin. The optimal relative conversion oxirane (RCO) percentage reached 43.06% at 70 °C, 200 rpm stirring speed, and 0.8 g of catalyst. The acetic acid to palm stearin molar ratio was 1:1, and the hydrogen peroxide to palm stearin ratio was 0.5:1. Both palm stearin and its epoxide derivative have been studied using Fourier-transform infrared spectroscopy, showing the appearance of an oxirane ring at a wavenumber of 1240 cm⁻¹. Kinetic modelling demonstrates that the simulation and experiment show a reasonable discrepancy, considering several assumptions that have been made. After 100 iterations, the reaction rate constant obtained as follows: \(\:{k}_{11}\) =0.01 mol⋅L−1⋅min−1, \(\:{k}_{12}\) = 1.85 mol⋅L−1⋅min−1, \(\:{k}_{2}\) = 29.90 mol⋅L− 1⋅min− 1, and \(\:{k}_{3}\) = 0.04 mol⋅L−1⋅min−1.
Similar content being viewed by others
Introduction
There has been a significant increase in interest in using raw materials from renewable feedstock to produce bio-based products from vegetable oils. Their accessibility, ability to decompose naturally and affordability have all been considered1. Epoxidized vegetable oils have many applications in the chemical industry. Their manufacturing implies the optimization of process parameters to maximize the epoxy yield2. Epoxides serve as highly adaptable chemical intermediates capable of transforming into diverse compounds. Epoxides often undergo ring-opening reactions with various nucleophiles, including water, alcohols, amines, and other substrates3. Palm stearin is commonly used in biodiesel, cosmetics, and food production. Its unsaturated fatty acids, linoleic (C18:2) and oleic acid (C18:1), make it a good candidate for polyol production. Double bonds in these fatty acids make them reactive to epoxidation. Epoxidation involves adding an oxygen atom across the double bond to form an epoxide ring, which is critical for producing polyols. In summary, the unsaturated fatty acids in palm stearin make it a beneficial raw material for epoxidation, which makes it easier to make valuable polyols4. Palm stearin is used only for producing edible food items due to its high melting point. Nevertheless, due to its classification as a by-product and lower cost, it has considerable potential to be used as a fat supply, as conducted in the research3,4,5,6. Epoxides, known as oxiranes in IUPAC nomenclature, are three-membered cyclic ethers. Ethylene oxide, the most basic epoxide, is a vital raw material for producing ethylene glycol, which is applied in antifreeze, coolants, and solvents. Ethylene glycol is crucial for manufacturing polyester and essential for plastic bottles. Epoxides are commonly synthesized through the epoxidation of alkenes with peracids. In situ, epoxidation of vegetable oil converts unsaturated fatty acids directly into epoxides without isolating intermediates. This method provides an eco-friendly alternative to traditional petrochemical-based epoxides. The main contributions of this study are significant improvements in the epoxidation process and the application of epoxidized palm stearin7. Several parameters influence the epoxidation process, including reaction temperature, catalyst type, stirring speed, and reactant ratios. While previous studies have explored these factors, only a few significantly impact epoxide yield. Similar research has been conducted on oils like soybean, canola, castor, sunflower, and jatropha8,9,10. Previous studies have mainly focused on process parameters affecting epoxide formation, with limited research on epoxidation kinetics. A key aspect is the kinetics of epoxide palm stearin formation, which depends on the interfacial reaction between palm stearin and peracids. Accurate quantification of the interfacial area is critical. Although the epoxidation of vegetable oils is a well-known process, this study’s emphasis on palm stearin, a by-product of palm oil fractionation, presents an innovative strategy for converting industrial waste into valuable materials. Furthermore, employing a heterogeneous catalyst (zeolite ZMS-5) in this reaction introduces a more sustainable, reusable, and efficient alternative to conventional homogeneous catalysts. This research seeks to advance epoxidation techniques while promoting using palm-based feedstocks for eco-friendly polymer and material development. The epoxidation mechanism starts with electrophilic species from the lone pair oxygen from peracetic acid reacting with the double bond of an alkene, leading to the formation of an epoxide through a nucleophilic reaction. Palm stearin primarily consists of fatty acids with double bonds, predominantly oleic and linoleic. The lone pair of oxygen atoms in peroxy acid function as nucleophiles by attacking the double bond, forming an epoxide. Hydrogen peroxide is an oxygen donor, while acetic acid and acetic acid function as oxygen transporters; zeolite ZSM-5 acts as a catalyst to speed up the process and lower the activation energy. Multiple nucleophiles can attack the carbon atom in the epoxide ring, resulting in the formation of HPS( polyol), a chemical compound consisting of numerous hydroxyl (OH) hydroxyl groups. Therefore, this paper aims to (1) investigate process parameters for the epoxidation of palm stearin using a zeolite ZSM-5 catalyst and (2) develop kinetic models to determine reaction kinetics using MATLAB.
Materials and methods
Materials
Palm stearin (100 g) is supplied from palm oil industries, glacial acetic acid (75%), hydrogen peroxide (50%), and hydrobromic acid (52%) are supplied by Chemiz, while Thermo Scientific supplies the Zeolite ZSM-5 catalyst.
Experimental procedure for the optimization of palm stearin epoxidation
The epoxidation process for optimizing epoxide palm stearin (EPS) was performed by heating 100 g of liquid palm stearin in a 500-mL beaker using a hot plate. The 100 g of liquid palm stearin was mixed with acetic acid at specified molar ratios (0.5, 1.0, 1.5, or 2.0). The beaker was then immersed in a temperature-controlled water bath. The palm stearin was subjected to various stirring speeds (200, 300, 400, and 500 rpm) and was heated to different temperatures (60, 70, 80, and 90 °C). Hydrogen peroxide was systematically added to the acetic acid-palm stearin mixture in appropriate molar ratios of (0.5, 1.0, 1.5, and 2.0). Subsequently, zeolite ZSM-5 catalyst in varying amounts (0.2, 0.4, 0.6, and 0.8 g) was added to the mixture. Table 1 below lists the parameters for optimizing the epoxidation of palm stearin. The range selection for each variable was primarily informed by a comprehensive literature review, drawing upon previous studies that established parameter ranges for temperature, molar ratios, catalyst concentration, and hydrogen molar ratio11,12. The reaction proceeded for 70 min, after which a 3-gram sample was obtained via a syringe for oxirane concentration analysis by the AOCS Official Method Cd-957.
Relative conversion oxirane (RCO)
The oxirane oxygen content (OOC) is measured by titrating epoxy groups with hydrobromic (HBr) solution in glacial acetic acid, following the AOCS Tentative Method Cd 9–57. This analysis evaluates how process parameters affect the reaction rate and calculates the yield of epoxidized palm stearin. To determine OOC, 10 mL of acetic acid is added to 3 g of the sample in an Erlenmeyer flask and mixed thoroughly. Titration with HBr continues until the colour shifts to green-yellow, as shown in Eqs. (1), (2), (3). The relative conversion to oxirane (RCO) uses theoretical and experimental OOC values, with experiments conducted in triplicate.
Here, \(\:{X}_{0}\) is the initial iodine value, \(\:{\text{A}}_{\text{i}}\) is the molar mass of iodine, \(\:{A}_{o}\) Is the molar mass of oxygen, \(\:N\:\)is the normality of HBr, \(\:V\) is the volume of the HBr solution used for the blank in milimiter (mL), \(\:V\) is the volume of HBr solution used for titration, and \(\:W\) is the weight of the sample.
Scanning electron microscopy (SEM) on zeolite ZSM-5
Scanning Electron Microscopy (SEM, JSM-IT200, JEOL) is a powerful analytical method extensively employed to investigate materials’ surface morphology and microstructural characteristics at high magnification and resolution. SEM employs a concentrated electron beam to produce detailed images showing surface topography and particle dimension. Analysis of SEM is significant in determining the surface roughness, particle uniformity, and crystalline structure of the zeolite ZSM-5 catalyst used in research, as it aids in measuring the catalyst’s performance, stability and recyclability.
Fourier transform infrared (FTIR)
In this investigation, the functional groups of the materials were detected using an FTIR spectrometer (Spectrum One, Perkin Elmer, USA). The FTIR spectra were obtained within a wavenumber range of 400–4000 cm− 1, corresponding to the mid-infrared area associated with the fundamental vibrational modes of the molecules.
Process model of epoxidized palm stearin production procedures
The kinetic model for the epoxidation of palm stearin assumes (1) constant volume for all phases, (2) homogeneous reactions, (3) negligible heat transfer, and (4) reactions that occur away from interfaces. The process is governed by two primary reactions: Eq. 1: in situ formation of peracid (PA) from hydrogen peroxide and carboxylic acid. Equation 2: reaction between peracetic acid and the unsaturated fatty acids in palm stearin, leading to epoxidized product formation. Additionally, Eq. 3 accounts for the formation of polyols, a side product resulting from epoxide ring hydrolysis where AA, HP, PA, PS, and EPS are acetic acid, hydrogen peroxide, peracetic acids, palm stearin and epoxidized palm stearin
The kinetic model for epoxidation and epoxide ring degradation involves the following rate constants. The corresponding rate equations are shown in Eqs. 6–12.
Kinetic modeling involves solving rate equations numerically and minimizing the error between simulation and experimental values. MATLAB’s ode45 solver, using the fourth-order Runge–Kutta method, was employed. Error e is minimized to verify parameter reliability, as shown in Eq. 13.
where \(\:{\text{E}\text{P}\text{O}\text{O}\text{A}}_{i}^{sim}\) and \(\:{\text{E}\text{P}\text{O}\text{O}\text{A}}_{i}^{exp}\) denote the estimated and experimental epoxy concentrations, \(\:i\) is the ith data point, and \(\:n\) is the total number of data points in the simulations and experiments.
Results and discussion
Effect of reaction temperature on epoxidation
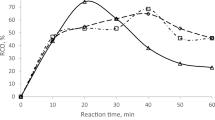
Figure 1 illustrates the relative conversion of oxirane (RCO%) by varying the temperature at 60, 70, 80, and 90 °C. This graph discusses the impact of different temperatures on the (RCO %) by epoxidation at different time intervals from 10 to 70 min. At a temperature of 70 °C, the relative conversion of oxirane is highest at 13.84% in 10 min, while other temperatures result in lower conversion due to reactant activation and side reactions13. A substantial increase in the 70 °C reaction occurs between 30 and 40 min, resulting in the highest percentage of RCO, 43.06%. This increase may be attributed to a critical point when temperature and timing optimize epoxide production14. Meanwhile, other temperatures exhibit minor increases, with 60 °C reaching the second-highest peak 21.82% RCO) at 40 min. At this temperature, oxirane formation continues, but over a long reaction time, the oxirane content decreases significantly due to the depletion of unsaturated bonds.
Specifically, at 70 °C, the oxirane content peaks at 43.06% at 40 min before decreasing sharply at 50 min. These findings indicate that 70 °C is the optimal oxirane content for the measured time intervals. After 40 min at this temperature, a decrease indicates that side reactions outcompete oxirane synthesis, lowering output. At 80 and 90 °C, RCO % at 15.90 and 13.88%, respectively. While the reaction rate initially increases at these temperatures, the higher thermal energy speeds up ring-opening and degradation processes, thereby limiting epoxidation efficiency. The steady decrease in oxirane content after its highest point at 40 min confirms that higher temperatures cause thermal instability, breaking oxirane rings and reducing yield15.
Effect of stirring speed on epoxidation
Figure 2 depicts the epoxidation of palm stearin at different stirrer speeds. This study manipulated the stirring speeds at 200, 300, 400, and 500 rpm while retaining other parameters. The relative conversion of oxirane (RCO %) increases rapidly up to 43.06% at 40 min at 200 rpm due to sufficient mixing to start the reaction. In contrast, the subsequent drop may indicate oxirane ring degradation or side reactions at more extended reaction periods, and long reaction durations can degrade epoxidation products16. At 300 rpm speeds, the graph gradually increases, reaching 13.90% RCO approximately 30 min before stabilizing. The previous study discovered optimal stirring rates for palm oil epoxidation, and this moderate stirring speed appears to balance the reaction rate and product stability17.
RCO% increases gradually to 400 rpm for 30 min, peaking at 15.83% before declining and enhancing mass transfer and reaction rate. From the previous study, dynamic agitation affects epoxide yield and selectivity, which may cause the product to drop due to shear stress. At 20 min, the 500 rpm curve peaks with 11.92% RCO, then drops and stabilizes18. High stirring speeds may speed up the initial conversion but may also speed up reactions or product degradation. These changes in stirring speeds show the relationship between mass transfer, reaction kinetics, and product stability in the epoxidation of palm stearin. Higher stirring speeds increase mixing and initial reaction rates19 found that excessive agitation can cause adverse effects.
Effect of catalyst loading on epoxidation
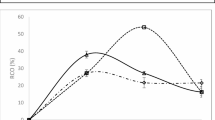
Figure 3 shows the relative conversion of oxirane (RCO%) over time in palm stearin epoxidation using different catalyst weights: 0.2 g, 0.4 g, 0.6 g, and 0.8 g zeolite ZSM-5 catalysts. At 0.2 g, catalyst loading, the relative conversion oxirane rises at 30 min at the percentage of RCO is 15.83 before sharply declining because of initial catalytic activity. Furthermore, a limited catalyst may deactivate rapidly and offer insufficient active sites for persistent conversion17.
At 50 min for 0.4 g and 0.6 g catalyst loading, the % RCO was intermediate, with 9.93% and 13.90%, respectively. The zeolite pores’ diffusion limits and accessible active sites may explain this trend’s slower catalytic process18. The graph of palm stearin’s epoxidation by varying the catalyst loading shows a complex interaction between catalyst concentration and epoxidation efficiency at different catalyst loadings. At 40 min and 0.8 g of catalyst loading, RC0% exhibits a much higher value. This may be because zeolite activates the oxidizing agent and provides enough acidity for the epoxidation reaction19. The increased number of catalytic active sites speeds up epoxidation20.
.
Effect of acetic acid to palm stearin molar ratio on epoxidation
Figure 4 represents the relative oxirane (RCO%) conversion over time for various acetic acid ratios to palm stearin. This study examines the impact of various molar ratios of acetic acid concentrations on the palm stearin epoxidation process over time. At the 10-minute interval, the 1:1 ratio of acetic acid to palm stearin has the greatest RCO% at approximately 13.84%, followed by the 2:1 ratio at around 5.9%. The 0.5:1 and 1.5:1 ratios show a decrease in initial conversion rates. This indicates that the 1:1 ratio yields an ideal starting reaction rate, likely owing to a balanced stoichiometry among the reactants20. As the reaction continues, the 0.5:1 ratio shows a significant increase in RCO%, reaching approximately 17.82% after 30 min. At the same time, the 1:1 ratio maintains the highest overall conversion rate at roughly 23.80%. The highest value for the 0.5:1 ratio may be attributed to using the limiting reagent (acetic acid) during this period10.
The 1:1 molar ratio shows the highest RCO%, which significantly increases between 30 and 40 min, reaching approximately 43.06% at 40 min. The peak indicates that the 1:1 ratio yields the most optimal conversion rate over time, likely attributable to ideal reactant concentrations and minimised side reactions1. Between 40 and 70 min, all ratios consistently decrease RCO %, with the 1:1 ratio sustaining the highest conversion rate overall. The drop may be ascribed to the exhaustion of reactants and the possible reversibility of the epoxidation reaction over prolonged periods21. The 1.5:1 and 2:1 ratios have lower maximum RCO % than the 1:1 ratio, showing delayed peaks at 40 and 30 min, respectively. The delay and diminished efficiency at elevated acetic acid concentrations may result from enhanced side reactions or the generation of by-products that obstruct the epoxidation process1,21.
Effect of hydrogen peroxide/palm stearin molar ratio on epoxidation
Effect of different hydrogen peroxide-to-palm stearin ratios (0.5:1, 1:1, 1.5:1, and 2:1) on the relative conversion of oxirane (RCO %) over time during the epoxidation process of palm stearin as shown in Fig. 5. The RCO % rises quickly at a 0.5:1 ratio, reaching a peak of over 40% at 40 min, and then it starts to drop quickly, most likely due to adverse effects or oxirane ring degradation over time21. The 1:1 ratio shows a minor peak of approximately 25% at 30 min, followed by a slight decrease, indicating enhanced reaction stability but a lower peak conversion relative to the 0.5:1 ratio22. In the 1.5:1 ratio, the RCO% increases steadily, reaching around 15% at 30 min, and remains relatively steady, indicating improved control over the reaction but resulting in a lower overall conversion23,24. The 2:1 ratio, including the highest concentration of hydrogen peroxide, exhibits the lowest peak RCO% (about 12% at 30 min), followed by stabilisation, indicating inefficiencies due to excessive oxidant leading to side reactions or suboptimal peroxide utilisation. A low concentration of molar ratio of hydrogen peroxide (0.5:1 and 1:1) will result in more significant initial conversions but encounter difficulties sustaining oxirane stability over time.
In contrast, higher concentrations (1.5:1 and 2:1) yield more stable but reduced conversion profiles. The findings show how hydrogen peroxide concentration, reaction speed, and product stability all work together in a complex way during the epoxidation of palm stearin. Subsequent research may enhance this process by investigating the impacts of synergistic variables, including stirring velocity, temperature, and catalyst concentration22.
Fourier transform infrared (FTIR) spectroscopy
Fourier Transform Infrared (FTIR) spectroscopy was used to analyze the properties of palm stearin and epoxides palm stearin characteristics. Both samples were analyzed to confirm the presence of a C-O-C ring, indicating an oxirane ring, which supports the existence of epoxidized palm stearin before the epoxidation process. The comparison of the FTIR analysis for both samples is shown in Fig. 6.
FTIR spectra of raw palm stearin (raw PS) and epoxidized palm stearin (epoxide PS) display substantial differences in specific regions and verify the chemical changes during the epoxidation process. In region 3005–3010 cm⁻¹, the raw palm stearin shows a weak or moderate peak corresponding to the = C–H stretching double bonds. However, this peak disappears in the epoxidized sample, indicating the conversion of unsaturated double bonds into epoxide groups.
In the range of 2920 to 2850 cm⁻³, both samples show strong peaks caused by the stretching of aliphatic bonds. This observation corresponds to the saturated hydrocarbon chains remaining unchanged during the epoxidation process. A 1650–1600 cm⁻³ peak is a double bond in raw palm stearin, related to C = C stretching vibrations. In contrast, this peak is either significantly diminished or absent in the epoxidized palm stearin, signifying the successful conversion of double bonds into epoxide groups. The formation of C-O-C oxirane rings in epoxidized palm stearin can be verified at 1242.76 and 1097.42 cm⁻¹25,26.
Catalytic performance and reusability of zeolite ZSM-5
The performance of zeolite ZSM-5 was assessed by evaluating the relative conversion percentage of oxirane (RCO) during the epoxidation of palm stearin across four consecutive cycles of the experiment. The catalyst of each cycle was also analyzed using scanning electron microscope images to observe the surface roughness and porosity performance. This experiment evaluated the catalyst’s performance regarding its selectivity and activity across multiple cycles. After each cycle, the catalyst was rinsed with ethanol and distilled water following every run. The RCO% analysis in Fig. 7 shows that the zeolite ZSM-5 catalyst’s effectiveness goes down with each reuse cycle, with the highest RCO percentage (43.06%) achieved in the first cycle. Subsequent cycles (2nd, 3rd, and 4th) demonstrate a decrease in RCO%, suggesting partial deactivation. Despite the decrease, the catalyst maintains a moderate activity level, showing potential for reusability through further optimization of the regeneration technique27,28.
Zeolites ZSM-5 are microporous aluminosilicate minerals used as catalysts due to their unique characteristics. Their crystalline structure, regular pore diameters, high surface area, and firm acidity make them useful in catalytic reactions27. Figure 7 below shows SEM images: (a) zeolite ZSM-5 1st cycle, (b) zeolite ZSM-5 2nd cycle, (c) zeolite ZSM-5 3rd cycle, (d) zeolite ZSM-5 4th cycle. The structure and porosity of the SEM image zeolite ZSM-5 catalyst significantly degrade with repeated use, as shown by the SEM images in Fig. 8. The SEM image of the first cycle of the zeolite ZSM-5 catalyst reveals a uniform structure with well-defined particles and high porosity, presenting a small crystalline particle that confirms a maximum surface area and substantial active sites. After the second recycle, the SEM image showing slight particle aggregation and reduced uniformity is identified. In the third cycle, increased clustering and reduced porosity indicate blockage of active sites. In the last cycle, the SEM image showed significant structural deterioration with large aggregates and a reduced surface area, which led to a marked decline in catalytic efficiency28,29.
Numerical kinetic modelling of epoxidized palm stearin
Simulated annualizing for kinetic epoxidation involves creating a detailed model to predict the performance and stability of the epoxidation process over a year. This includes developing kinetic models to understand reaction rates and conditions, using simulations to forecast product yield and quality, and optimizing parameters to ensure consistent efficiency. Kinetic studies on epoxidation are crucial for understanding reaction rates and optimizing conditions. These studies use models to estimate residence times and guide reactor design, reducing costs compared to multiple experimental trials.
This study developed a MATLAB-based kinetic model for the catalytic epoxidation of palm stearin, using simulated annulling and Runge-Kutta methods to fit experimental data. The model revealed that performic acid formation is slower than its reverse reaction and epoxidized palm stearin production. High reaction rates for performic acid formation were observed, indicating rapid epoxide formation. This study’s kinetic rates were consistent with previous research but showed variations in degradation times, emphasizing the influence of reaction temperature on the process. The results show that the performic acid formation (k11) reaction rate is lower than its reverse reaction (k12), indicating faster epoxide production, as shown in Table 2. Compared to previous studies, the kinetic values for palm stearin epoxidation are consistent within reported ranges, though oxirane ring degradation occurs earlier.
Figure 9 compares epoxide concentrations from experiments and simulations at 100 °C, showing good agreement with a difference of ~ 0.20. However, deviations were observed in epoxide formation between 30 and 40 min in the simulation. This discrepancy arises because the MATLAB simulation assumes ideal conditions without accounting for heat loss or transfer, while experimental data reflects real, empirical conditions. Besides that, the experimental data and the kinetic model for the epoxidation of palm stearin may result from model simplifications, such as assuming ideal conditions, neglecting side reactions, or using constant rate parameters. Experimental uncertainties, including measurement errors and temperature inconsistencies, may further contribute. Conducting sensitivity analyses and additional experiments under varied conditions could validate the refined model and provide deeper insights into the reaction system.
Conclusion
In this study, epoxidation utilizing non-edible vegetable oils as a sustainable alternative to traditional petroleum-based products significantly advances eco-friendly technologies. These methods will lower fossil fuel dependency and promote sustainability and environmental conservation through worldwide efforts to promote more environmentally friendly industrial processes. The process involves the catalytic epoxidation of unsaturated fatty acids in palm stearin through an in situ peracetic acid mechanism. The highest RCO was achieved, 43.06% at 70 °C, 200 rpm, with 0.8 g catalyst and molar ratios of 1:1 acetic acid and 0.5:1 hydrogen peroxide to palm stearin. The FTIR result showed that the C = C bonds were successfully changed into epoxide of palm stearin. This is shown by the fact that no 1650–1600 cm⁻³ range and epoxide peaks formed in the 1242.76 and 1097.42 cm⁻¹. This result validates the chemical structural change and supports the efficacy of the process.
According to the SEM results, the zeolite ZSM-5 catalysts showed high porosity and surface area, which improved the catalytic performance and facilitated the epoxidation reaction. The findings indicate that the zeolite ZSM-5 catalyst is efficient and recyclable, offering a sustainable alternative for industrial applications. The strong correlation between experimental results and kinetic modelling confirmed the process’s efficiency. Therefore, from this study, the knowledge and data will offer valuable data on the effects of various parameter levels on the epoxidation rate of palm stearin, providing a helpful reference for future researchers. Future research is recommended to emphasise optimizing catalyst properties to enhance the catalyst’s reusability and efficiency and study the long-term performance of epoxidized products in industrial applications. Furthermore, to mitigate the environmental impact by studying green oxidants and solvent-free systems, it is essential to conduct thorough life cycle assessments (LCA) and scale up processes to ensure sustainability and commercial feasibility.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
Ifa, L. et al. Study on the Kinetics of epoxidation reaction of RBD palm olein. In IOP Conference Series: Earth and Environmental Science. https://doi.org/10.1088/1755-1315/175/1/012035 (Institute of Physics Publishing, 2018).
Kanagaratnam, S. et al. Mar., Are characteristics of soft palm stearins similar to soft palm midFractions? J. Oil Palm Res. 32 (1), 103–116. https://doi.org/10.21894/JOPR.2020.0004 (2020).
Rahman, A. A. et al. Vegetable oil based epoxidized oil: A sustainable approach. J. Islamic Soc. Econ. Dev. 7 (46), 17–21. https://doi.org/10.55573/JISED.074603 (2022).
Kaikade, D. S. & Sabnis, A. S. Polyurethane foams from vegetable oil-based polyols: a review. Springer Sci. Bus. Media Deutschland GmbH https://doi.org/10.1007/s00289-022-04155-9 (2023).
Cai, C. et al. Apr., Studies on the kinetics of in situ epoxidation of vegetable oils. Eur. J. Lipid Sci. Technol., 110, (4), 341–346, https://doi.org/10.1002/ejlt.200700104 (2008).
Yang, F., Yu, H., Deng, Y. & Xu, X. Synthesis and characterization of different soybean oil-based polyols with fatty alcohol and aromatic alcohol. E Polym. 21 (1), 491–499. https://doi.org/10.1515/epoly-2021-0052 (2021).
Huey Saw, M., Beng Yeoh, C., Mei Huey, S., Chiew Let, C. & Chee Beng, Y. New developments in palm oil fractionation malaysian palm oil board palm oil developments 62 new developments in palm oil fractionation. https://www.researchgate.net/publication/297170798 (2015).
Rahman, M. A., Mubarak, N. M., Azmi, I. S. & Jalil, M. J. Sustainable approach for catalytic green epoxidation of oleic acid with applied ion exchange resin. Sci. Rep. 13, 1–8. https://doi.org/10.1038/s41598-023-42879-4 (2023).
Mancini, A. et al. Biological and nutritional properties of palm oil and palmitic acid: effects on health. Molecules 20, 17339–17361. https://doi.org/10.3390/molecules200917339 (2015).
Mungroo, R., Pradhan, N. C., Goud, V. V. & Dalai, A. K. Epoxidation of canola oil with hydrogen peroxide catalyzed by acidic ion exchange resin. JAOCS J. Am. Oil Chem. Soc. 85 (9). (2008).
Vanags, E., Kirpluks, M., Cabulis, U. & Walterova, Z. Highly functional polyol synthesis from epoxidized tall oil fatty acids. 1–8. (2018).
Shauqi, A. et al. In situ epoxidation of canola oil via peracetic acid mechanism-optimization and kinetic study. 0 1–15. https://doi.org/10.1177/00952443241243376 (2024).
Bahadi, M., Salih, N. & Salimon, J. D-Optimal design optimization for the separation of oleic acid from Malaysian high free fatty acid crude palm oil fatty acids mixture using urea complex fractionation. Appl. Sci. Eng. Prog. 14, 175–186. https://doi.org/10.14416/J.ASEP.2021.03.004 (2021).
Derawi, D. & Salimon, J. Optimization on epoxidation of palm olein by using performic acid. J. Chem. 7 (4). (2010).
Retno Sawitri, D., Mulyono, P., Rochmadi & Budiman, A. Pseudo-homogeneous kinetic evaluation for in-situ epoxidation of oleic acid. In IOP Conference Series: Materials Science and Engineering. (2020).
Bahadi, M., Salih, N. & Salimon, J. Synthesis and characterization of green biodegradable palm oleic acid based polyester. Biointerface Res. Appl. Chem. 11 (6), 14359–14371. https://doi.org/10.33263/Briac116.1435914371 (2021).
Kongyai, C., Chalermsinsuwan, B. & Hunsom, M. Epoxidation of waste used-oil biodiesel: Effect of reaction factors and its impact on the oxidative stability. Korean J. Chem. Eng., 30, (2) 327–336. https://doi.org/10.1007/s11814-012-0159-0 (2013).
Sinadinović-Fišer, S., Janković, M. & Borota, O. Epoxidation of castor oil with peracetic acid formed in situ in the presence of an ion exchange resin. Chem. Eng. Process. Process Intens. 62, 106–113, https://doi.org/10.1016/j.cep.2012.08.005 (2012).
Abolins, A., Kirpluks, M., Vanags, E., Fridrihsone, A. & Cabulis, U. Tall oil fatty acid epoxidation using homogenous and heterogeneous phase catalysts. J. Polym. Environ. 28 (6). (2020).
Varghese, T. P. & Gopalakrishnan, J. Effect of peroxide concentration on the epoxidation of vegetable seed oil. Mater. Today (2023).
Govedarica, O., Janković, M., Sinadinović-Fišer, S. & Govedarica, D. Optimization of the epoxidation of linseed oil using response surface methodology. Chem. Ind. Chem. Eng. Q 24 (4), 357–368. https://doi.org/10.2298/CICEQ171012008G (2018).
Chen, J. et al. Highly efficient epoxidation of vegetable oils catalyzed by a manganese complex with hydrogen peroxide and acetic acid. Green. Chem. 21, 2436–2447 (2019).
Goud, M., Santacesaria, S. & Varma, P. Investigating hydrogen peroxide utilization in epoxidation reactions of palm oil. J. Am. Oil Chem. Soc. 96 (2), 183–190 (2020).
Li, P., Wang, Z. & Liu, Y. Effects of hydrogen peroxide concentration on the epoxidation of Vegetable oils: a study on reaction kinetics and product stability. Ind. Eng. Chem. Res. 57 (10), 2562–2571 (2019).
Lin, B., Yang, L., Dai, H. & Yi, A. Kinetic studies on oxirane cleavage of epoxidized soybean oil by methanol and characterization of polyols. JAOCS J. Am. Oil Chem. Soc. 85 (2), 113–117. https://doi.org/10.1007/s11746-007-1187-5 (2008).
Tunku Ozir, T. A. Z. et al. Bio-lubricant production based on epoxidized oleic acid derived dated palm oil using in situ peracid mechanism. Int. J. Chem. React. Eng. 21 (6), 793–800. https://doi.org/10.1515/ijcre-2022-0161 (2023).
Bowman, R. S. Applications of surfactant-modified zeolites to environmental remediation. In Microporous and Mesoporous Materials 61, (1–3), 43–56. https://doi.org/10.1016/S1387-1811(03)00354-8. (Elsevier, 2003).
Lin, F. et al. Facile morphology and porosity regulation of zeolite ZSM-5 mesocrystals with synergistically enhanced catalytic activity and shape selectivity. Nanomaterials 12 (9). https://doi.org/10.3390/nano12091601 (2022).
Xue, T. & Yang, L. Zeolite-based materials for the catalytic oxidation of VOCs: A mini review. In Frontiers in Chemistry 9. https://doi.org/10.3389/fchem.2021.751581 (Frontiers Media S.A., 2021).
Acknowledgements
This work was supported by the Fundamental Research Grant Scheme, Ministry of Education Malaysia Ref: FRGS/1/2023/TK09/UITM/03/2.
Author information
Authors and Affiliations
Contributions
Norin Hafizah Rahim: Writing and experiment work; Intan Suhada Azmi: Data curation; Mohd Jumain Jalil: conceptualization and methodology Nabisab Mujawar Mubarak and Gnanasambandam Anbuchezhiyan: Review and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rahim, N.H., Jalil, M.J., Mubarak, N. et al. Catalytic epoxidation of unsaturated fatty acids in palm stearin via in situ peracetic acids mechanism. Sci Rep 15, 4789 (2025). https://doi.org/10.1038/s41598-025-89399-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89399-x
Keywords
This article is cited by
-
Catalytic epoxidation of linoleic acid derived corn oil via in situ performic acid mechanism
Scientific Reports (2025)
-
Enhanced catalytic performance and recycling of sulfate-impregnated ZSM-5 in the epoxidation of castor oil
Biomass Conversion and Biorefinery (2025)
-
Kinetic Analysis and Particle Swarm Optimization Simulation Algorithm-Based Error Minimization in the Epoxidation of Castor Oil Via the Prilezhaev Reaction
Journal of Polymers and the Environment (2025)
-
Ring opening of epoxidized castor oil with applied hybrid kinetic modelling model of particle swarm & simulated annealing
Journal of Polymer Research (2025)