Abstract
Religious and spiritual (R/S) struggles, such as questioning of faith, existential and ethical concerns, and interpersonal conflicts, are associated with depressive symptoms. Neuroinflammation is critical in major depressive disorder (MDD) and is linked to stress associated with R/S problems. This study aimed to investigate whether the presence of DSM-5 R/S problems contributes to neuroinflammation. We recruited 93 MDD patients and 93 healthy controls with and without R/S problems. MRI-based restricted fraction (RF) values, an index of neuroinflammation, were measured in the hippocampus, amygdala, and neocortex. Depression and anxiety were assessed using the Hamilton Depression and Anxiety Rating Scales (HAM-D, HAM-A), while R/S problems were quantified using the Religious and Spiritual Struggles Scale (RSS-14). Results revealed elevated RF values in the amygdala and hippocampus of healthy individuals and MDD patients with R/S problems relative to those without R/S problems, with the highest values in MDD patients with R/S problems. Importantly, R/S problems and depressive symptoms were independent predictors of RF values in the amygdala and hippocampus but not in the cortex. Elevated cortical RF values were associated with MDD. These findings indicate that R/S struggles are not secondary manifestations of depression but may independently contribute to neurobiological changes.
Similar content being viewed by others
Introduction
There is a significant link between religious/spiritual (R/S) factors and major depressive disorder (MDD), with both positive and negative influences on mental health outcomes. Negative religious coping, such as blaming God or experiencing a loss of faith, is strongly correlated with higher depressive symptoms1,2,3,4,5. Adolescents who reported a loss of faith showed less improvement in their depressive symptoms over time6. In an international sample of 8,318 volunteers, 10.5% of spiritual participants experienced a depressive episode in the year following the baseline assessment compared to 10.3% of religious participants and 7.0% of secular individuals. However, the findings were heterogeneous, and the difference was significant only in the UK sample7.
However, Kasen et al. (2011) observed that religious service attendance was associated with reduced odds of mood disorders, particularly among high-risk individuals exposed to adverse life events8. A prominent subjective importance of R/S may provide a protective effect against the recurrence of the disorder, especially in individuals with a family history of depression9. The protective effect of R/S has neurobiological underpinnings (a thicker cortex associated with the importance of religion)10. McClintock et al. (2019) identified a multidimensional structure of R/S that remained consistent across familial risk groups and diagnostic histories11. They noted complex interactions between depression, family risk, and R/S. Specifically, a prior diagnosis of MDD was linked to a weaker R/S commitment in high-risk individuals, heightened contemplation in low-risk individuals, and diminished importance of religion in both high- and low-risk groups11. Blazer (2012) reviewed the empirical studies and emphasized the need for longitudinal research to better understand the relationship between R/S and depression12. Overall, the evidence suggests that the impact of R/S on depression risk may vary depending on individual factors, cultural context, and specific aspects of R/S engagement.
Positive religious practices, such as forgiveness, connectedness, and finding existential meaning, can help mitigate depressive symptoms by providing social support and a productive coping framework13,14. Individuals with stronger spiritual beliefs exhibit better existential well-being, which can buffer against depressive symptoms with critical developmental implications. Adolescence is a crucial stage for spiritual growth, and this developmental period also presents an increased risk of depression, substance misuse, and risk-taking behavior. The timing of spiritual development and the onset of depression suggest a shared biological or developmental process, with positive spiritual coping serving as a protective factor against depression and substance-related issues15. However, when spirituality is accompanied by struggles, it can intensify psychological distress, and conversely, stressful life events worsen spiritual struggles16,17,18,19,20,21. Struggles with religious beliefs, such as divine or moral doubts, are linked to lower well-being and higher levels of depression22. As discussed above, the effect of R/S on depression varies by cultural context. For example, in the UK, marked spiritual beliefs were associated with an increased likelihood of depression over time7.
Recently, studies showed that R/S problems are associated with an exaggerated stress response, abnormal brain activation, and subtle neuroinflammatory changes in limbic areas23,24,25. It is especially relevant for understanding the connection between R/S problems and MDD since neuroinflammation has emerged as a potential key factor in psychiatric disorders. Increasing evidence suggests that immune-mediated processes, such as microglial activation and the release of pro-inflammatory cytokines, play a significant role in the emotional, cognitive, and behavioral symptoms of MDD26,27,28,29. Elevated levels of pro-inflammatory cytokines, such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), have been documented in both peripheral blood and cerebrospinal fluid in MDD30. Furthermore, post-mortem studies have demonstrated increased expression of inflammation-related genes in the prefrontal cortex and hippocampus of MDD patients31,32.
Studies using positron emission tomography (PET) imaging have demonstrated increased microglial activation or cell density in brain regions such as the anterior cingulate cortex, prefrontal cortex, and hippocampus in MDD patients33. Translocator protein (18 kDa TSPO) binding, a putative marker of neuroinflammation and microglia density, is higher in MDD in brain regions involved in emotion regulation and cognitive control, including the anterior cingulate cortex, prefrontal cortex, and hippocampus34,35,36. Increased TSPO binding in frontal and cingulate areas is related to attentional dysfunction and suicidal thoughts37,38.
Neuroinflammation interacts with key neurobiological correlates of MDD, including serotonin depletion, hippocampal-pituitary-adrenal axis (HPA) dysregulation (abnormal cortisol secretion and stress response), altered microbiota-gut-brain axis, and perturbed hippocampal neurogenesis39,40. The kynurenine pathway, a metabolic pathway that converts tryptophan into kynurenine, and HPA axis dysregulation contribute to increased glutamate levels, potentially impacting neurogenesis41. Moreover, inflammatory processes may differ among MDD subtypes, with atypical MDD showing distinct inflammatory and neuronal fingerprints42. Large-scale studies indicate that lifestyle, brain structure, immunometabolic changes, and genetics contribute to MDD, particularly highlighting the impact of lifestyle (alcohol consumption, diet, physical activity, sleep, smoking, sedentary behavior, and social connectedness)43.
From a functional neuroanatomical point of view, the involvement of neuroinflammation in emotional dysregulation is particularly relevant in the amygdala and hippocampus44. Studies have found reduced functional connectivity between these regions, the dorsomedial-prefrontal cortex, and the fronto-insular operculum in MDD patients45. Structural changes include decreased gray matter volume in the hippocampus and specific cortical areas46. Functionally, increased glucose metabolism has been observed in the anterior cingulate cortex47. High-resolution connectome analysis revealed decreased connectivity of the hippocampal CA3/4 subregion and reduced clustering of the dentate gyrus in MDD patients. Additionally, dysfunction of a brain network that includes the central nucleus of the amygdala correlates with depression severity48. These alterations may contribute to cognitive deficits, particularly in hippocampus-dependent recollection memory, which can persist even after symptom remission49.
In a large community sample from the UK biobank, Zhang et al. (2023) evaluated the association between depression, peripheral low-grade inflammation, and neuroinflammation. Zhang et al. (2023) measured C-reactive protein (CRP) levels in the blood plasma, an acute-phase protein produced by the liver during inflammation and stress. The authors also used a specific magnetic resonance imaging (MRI) technique to detect neuroinflammation in the brain (diffusion-basis spectral imaging-based restricted fraction (DBSI-RF) MRI)50. The results indicated that elevated peripheral CRP levels were associated with more pronounced depressive symptoms, which were also linked to elevated MRI-restricted fraction (RF) in the amygdala, a putative index of neuroinflammation50.
Using Zhang et al.‘s protocol (2023), we also found elevated MRI RF values in the amygdala and hippocampus of individuals with R/S problems, which were associated with subclinical depressive symptoms and peripheral inflammation25. However, individuals with R/S problems in this study did not meet the clinical criteria of lifetime or current MDD. Therefore, the interaction between R/S problems and MDD regarding MRI neuroinflammatory markers is unclear.
To elucidate this issue, we investigated four groups: healthy individuals with and without R/S problems and MDD patients with and without R/S problems. We had the following hypotheses driven by previous results discussed above25,50: (1) healthy individuals with R/S problems exhibit elevated RF values in the hippocampus and amygdala compared to healthy individuals without R/S problems as a replication of our previous results25; (2) MDD patients with and without R/S problems show elevated hippocampal and amygdala RF values relative to healthy individuals; (3) MDD patients with R/S problems display higher depression scores and RF values than MDD patients without R/S problems. Our hypothesis proposed the following order of neuroinflammation severity in the hippocampus and amygdala: healthy individuals with R/S problems < MDD patients without R/S problems < MDD patients with RS problems.
Methods
Participants
Patients with MDD (n = 93) were recruited from twelve outpatient psychiatric centers and primary care services at the National Psychiatric Center in Budapest and the University of Szeged in Szeged, Hungary. The inclusion criteria were as follows: fulfilling the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria of MDD; age between 18 and 65 years; no significant lifetime psychiatric comorbidities (e.g., evidence of bipolar disorder, psychotic disorders, neurodevelopmental disorders, and substance misuse); never receiving psychotropic medications before the study. The exclusion criteria included neurological disorders or head injury, general medical conditions affecting the central nervous system, substance use within the past six months, pregnancy or breastfeeding, using anti-inflammatory drugs within the past six months, and any general contraindications to MRI.
We also enrolled 93 control participants without a prior history of mental disorders who were matched to the patients with MDD for age, sex, education, and potential confounding variables affecting inflammation (nicotine, caffeine, and alcohol intake, contraception use, body mass index (BMI), chronic diseases, and working night shifts)51 (Table 1). We used social media advertisements to recruit control participants with demographic characteristics similar to those of patients with MDD. All participants were Caucasian and declared themselves cisgender (he/him and she/her).
The study was conducted following the Declaration of Helsinki and approved by the United Ethical Review Committee for Research in Psychology (EPKEB, 2016/032) at the Budapest University of Technology and Economics and the National Medical Research Council (ETT-TUKEB 18814, Budapest, Hungary). Written informed consent was obtained from all subjects.
Clinical evaluation of diagnosis and symptoms
The patients with MDD and the control volunteers received the structured clinical interview for DSM-5, administered by a trained assessor blind to the study’s aim52. Depressive and anxiety symptoms were assessed with the Hamilton Depression Rating Scale (HAM-D) and the Hamilton Anxiety Rating Scale (HAM-A) using semi-structured clinical interviews53,54,55,56,57. We also used a self-report scale to assess quality of life.
The HAM-A consists of 14 items, each rated from 0 (not present) to 4 (severe) (total score: 0–56). The scale evaluates both psychic anxiety (mental and psychological distress) and somatic anxiety (physical symptoms). Scoring is categorized into severity ranges: 0–17 indicates mild anxiety, 18–24 indicates moderate anxiety, and 25–30 indicates severe anxiety53.
The HAM-D consists of 17 items, each evaluating symptoms such as mood, guilt, suicide ideation, insomnia, anxiety, and weight loss. Each item is scored on a scale of 0 to 2 or 0 to 4, depending on the question, leading to a total score that ranges from 0 to 52. The severity is typically categorized as follows: 0–7: normal or no depression; 8–13: mild depression; 14–18: moderate depression; 19–22: severe depression; > 23: very severe depression56.
The Quality of Life in Depression Scale (QLDS) is a patient-reported questionnaire comprising 34 yes-or-no items. Each “Yes” response scores one point, indicating the presence of a problem related to depression, resulting in a total score ranging from 0 to 34; higher scores signify a lower quality of life. The QLDS evaluates critical domains such as emotional well-being, daily activities, social relationships, physical functioning, cognitive functioning, and self-perception. The scale has convincing psychometric properties (Cronbach’s alpha > 0.8)58,59.
Assessing R/S problems
First, to detect R/S problems, we used the structured clinical interview for DSM-552 and the Cultural Formulation Interview60. Forty-three control participants and 37 patients with MDD meet the DSM-5 definition of “Problems related to other psychosocial, personal, and environmental circumstances” (Religious or Spiritual Problem, [code: V62.89]60. The definition of the R/S problem is the following: “This category can be used when the focus of clinical attention is a religious or spiritual problem. Examples include distressing experiences that involve loss or questioning of faith, problems associated with conversion to a new faith, or questioning of spiritual values that may not necessarily be related to an organized church or religious institution.”60.
To quantify R/S problems, we used the Religious and Spiritual Struggles Scale (RSS-14), a 14-item self-report questionnaire61,62. This scale assesses six domains of struggles: divine (feelings of anger or disappointment toward a higher power), demonic (experiences of feeling attacked or oppressed by evil forces), interpersonal (conflicts or tensions with others regarding religious or spiritual matters), moral (internal conflicts about right and wrong or ethical dilemmas), doubt (uncertainty or skepticism about one’s religious or spiritual beliefs), and ultimate meaning (existential concerns about the purpose and meaning of life). Each item is rated on a 5-point Likert scale ranging from 1 (“not at all”) to 5 (“a great deal”), resulting in total scores that range from 14 to 70, with higher scores indicating greater levels of struggle. The Hungarian version of the full scale exhibited high internal consistency, with a Cronbach’s alpha of 0.86. However, confirmatory factor analyses have not uniformly supported the six-factor structure in three independent Hungarian samples, and therefore, we used the total scores in the present study.
Magnetic resonance imaging (MRI)
We acquired diffusion-weighted imaging (DWI) and T1-weighted structural MRI according to the United Kingdom (UK) biobank protocol and our previous study25,50,63,64. We used FreeSurfer v7.4.1 for image processing65. The setup and the technical parameters were as follows: Philips Achieva 3T scanner, MPRAGE (magnetization-prepared rapid acquisition gradient echo), 3D sagittal acquisition, FOV (square field of view) = 5256 mm, 1 × 1 × 1 mm3, TI = 5900 ms, TE (shortest) = 3.16, flip angle: 9 degrees, no fat suppression, full k space, no averages, acquisition time: 6 min and 50 s, acceleration factor: 2. We used a multi-shell approach for DWI (b1 = 1000 s/mm2, b2 = 2000 s/mm2, 2 × 2 × 2 mm3, 50 diffusion encoding directions for each shell). To preprocess the DWI data, we used eddy currents and head motion corrections, outlier slice correction, and gradient distortion correction, as recommended by the UK biobank protocol50,63. Putative neuroinflammatory changes were characterized by restricted fraction (RF) from DWI data (diffusion-basis spectral imaging-based restricted fraction, DBSI-RF)66,67. We investigated two regions of interest (ROIs), the hippocampus and the amygdala, by using FreeSurfer. We extracted DBSI-RF from these ROIs to obtain comparable results across studies50,68,69. The left and right RF values were averaged because they showed high lateralized correlations (left-right correlations: rs > 0.8)50. We also obtained RF for the whole gray matter of the cortex as a control condition for the limbic structures50,70.
Data analysis
We used Spotifre® Data Science Workbench 14.2.0 (Tibco), JASP 0.19.1, and the R-package for statistical analysis. We calculated the sample size with a medium effect size (α = 0.05, power: 0.80).
After checking the normality of data distribution (Kolmogorov-Smirnov test) and homogeneity of variance (Levene’s test) of all variables, we primarily focused on the RF values. We conducted a multivariate analysis of variance (MANOVA) with the group as between-subjects factors (four groups: healthy controls with and without R/S problems and MDD patients with and without R/S problems) and brain regions (amygdala, hippocampus, and cortex). F-tests were used for planned comparisons. The statistical power (η2) was also evaluated in the MANOVA.
To address the relationship between RF values and clinical variables, we conducted hierarchical regression analyses using age, sex, education, BMI, HAM-A, HAM-D, QLDS, and RSS-14 as potential predictors of RF values in the amygdala, hippocampus, and cortex. We also calculated Pearson’s product-moment correlation coefficients with Bonferroni corrections for multiple comparisons. Clinical and demographic variables were compared with one-way ANOVAs, two-tailed t-tests, and chi-square tests for categorical variables. The level of uncorrected statistical significance was α < 0.05.
The critical effects obtained with conventional statistics were re-evaluated using a Bayesian data analytic approach. We calculated Bayes Factors (BF10) to characterize the level of evidence by comparing the alternative hypothesis (H1) and the null hypothesis (H0) (BF10 = 1: no evidence for H1; 1 < BF10 < 3: weak evidence for H1; 3 < BF10 < 10: moderate evidence for H1; BF10 > 10: strong evidence for H1).
Results
Clinical characteristics and demographics
We first compared the clinical and demographic characteristics of MDD patients and control participants with and without R/S problems (Table 1). No significant differences were found between the four groups in education, sex, and BMI (ps > 0.5). Regarding age, MDD patients with R/S problems were older than controls without R/S problems (p < 0.05), but the other groups did not differ significantly (ps > 0.1).
Regarding the degree of R/S problems, both controls and MDD patients with DSM-5 R/S problems scored higher on the RSS14 scale than individuals without R/S problems (one-way ANOVA, main effect of group: F(3,182) = 208.89, p < 0.001, η2 = 0.78; post-hoc comparisons: p < 0.001). However, healthy controls with R/S problems and MDD patients with R/S problems did not differ on the RSS-14 scale (p = 0.08), suggesting a similar level of spiritual distress in the healthy and clinical group.
Contrary to the hypothesis, MDD patients with R/S problems did not exhibit significantly more severe depression and anxiety (HAM-D and HAM-A scores) or a worse quality of life (QLDS scores) relative to MDD patients without R/S problems (ps > 0.2) (Table 1).
RF values in patients with MDD and healthy controls
Next, we compared MRI RF values, a putative index of neuroinflammation, in the amygdala, hippocampus, and cortex of MDD patients with and without R/S problems and healthy control participants with and without R/S problems. The MANOVA conducted on the RF values indicated significant main effects of group (F(3,182) = 44.10, η2 = 0.42, p < 0.001), brain region (F(2,364) = 135.30, η2 = 0.43, p < 0.001), and a two-way interaction between group and brain region (F(6,364) = 3.15, η2 = 0.05, p < 0.05).
Planned comparisons indicated elevated RF values in healthy controls with R/S problems compared to those without R/S problems in the amygdala (F(1,182) = 8.93, p < 0.01, BF10 = 331.3), in the hippocampus (F(1,182) = 8.65, p < 0.01, BF10 = 204.3), but not in the cortex (p = 0.8, BF10 = 0.2).
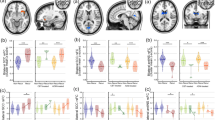
In the MDD group, patients with R/S problems exhibited higher RF values than patients without R/S problems in the amygdala (F(1,182) = 10.76, p < 0.002, BF10 = 4.9), in the hippocampus (F(1,182) = 15.76, p < 0.001, BF10 = 21.1), but not in the cortex (p = 0.7, BF10 = 0.2) (Fig. 1; for a detailed descriptive statistics, see supplementary material).
Violin plots and raw data of restricted fraction (RF) values from patients with major depressive disorder (MDD) and healthy controls with and without religious and spiritual problems (R/S p). The horizontal lines indicate the median, the box plots refer to 25 percentiles, and the vertical lines show 50 percentiles.
Overall, patients with MDD had elevated RF values relative to controls in the amygdala (F(1,182) = 94.80, p < 0.001, BF10 = 3.2 × 1013), in the hippocampus (F(1,182) = 54.46, p < 0.001, BF10 = 2.6 × 107), and in the cortex (F(1,182) = 26.44, p < 0.001, BF10 = 35043.4). Interestingly, healthy controls with R/S problems did not differ from patients with MDD without R/S problems in the hippocampus (p = 0.1, BF10 = 0.9) (Fig. 1).
Clinical and R/S predictors of RF values in MDD
We performed multiple regression analyses to elucidate the clinical predictors of RF values in MDD, controlled for age, education, sex, and BMI.
In the amygdala, there were two significant predictors of RF: HAM-D scores (b* = 0.28, R2 = 0.27, t(84) = 2.56, p < 0.05) and RSS-14 scores (b* = 0.22, R2 = 0.18, t(84) = 2.19, p < 0.05). The Bayesian regression analysis revealed the highest level of evidence (BF10 = 8.7, R2 = 0.27) when HAM-D, RSS-14, age, and education were included in the model.
In the hippocampus, we identified three significant predictors: HAM-D scores (b* = 0.50, R2 = 0.28, t(84) = 5.45, p < 0.001), HAM-A scores (b* = 0.21, R2 = 0.13, t(84) = 2.44, p < 0.05), and RSS-14 scores (b* = 0.19, R2 = 0.18, t(84) = 2.20, p < 0.05). The Bayesian regression analysis revealed the highest level of evidence (BF10 = 30.5, R2 = 0.46) when HAM-D, HAM-A, RSS-14, and age were included in the model.
In the cortex, there was a single significant predictor: education (b* = − 0.33, R2 = 0.10, t(84) = − 3.14, p < 0.05), but the Bayesian approach did not indicate an acceptable level of evidence (BF10 = 1, R2 = 0.10).
Table 2 depicts Pearson’s product-moment correlation coefficients and Bayesian correlations between the RF values and clinical and demographic measures in MDD (the detailed correlation coefficients are shown in the supplementary material). Figures 2 and 3 show the correlations between RF values and their significant clinical predictors in the amygdala and hippocampus in MDD and healthy controls.
Predictors of RF values and correlations in the control group
We also assessed whether R/S problems predict RF values in the control group. In the amygdala, the RSS-14 scores significantly predicted the RF values, controlled for age, education, sex, and BMI (b* = 0.37, R2 = 0.08, t(87) = 3.51, p < 0.01). A similar effect was found in the hippocampus (b* = 0.36, R2 = 0.08, t(87) = 3.56, p < 0.01) but not in the cortex (p > 0.5). However, the Bayesian analysis failed to confirm the results from the conventional statistics, indicating no convincing evidence that the RSS-14 scores significantly predicted RF values in the amygdala and hippocampus (BF10 = 1).
There was a significant positive correlation between amygdala-RF and RSS-14 scores (r = 0.38, p < 0.01; BF10 = 142.4) and between hippocampus-RF and RSS-14 scores (r = 0.37, p < 0.01; BF10 = 104.3) in the whole sample but no such correlations in the cortex (r = − 0.04, BF10 = 0.14). Figure 3 shows the correlations in each group, including MDD.
Discussion
The present study highlights the role that R/S struggles play in neuroinflammatory processes in individuals with MDD. The findings contribute to a growing body of research that demonstrates the complex interplay between psychological, spiritual, and neurobiological factors in depression71,72,73. Specifically, the study’s key findings—that both healthy individuals with R/S struggles and MDD patients with R/S struggles exhibit elevated MRI-RF values in the amygdala and hippocampus—provide new insights into the underlying mechanisms of MDD, particularly as they relate to religious and spiritual distress.
The results suggest that neuroinflammatory processes in the limbic system, indicated by increased RF values, may worsen when religious and spiritual struggles are also present. The hippocampus and amygdala are critical regions involved in emotion regulation and memory processing, often disrupted in MDD74,75,76, and are also associated with R/S problems. The observation that individuals without a clinical diagnosis of MDD but who experience R/S struggles show neuroinflammatory changes similar to those of MDD patients indicates that R/S struggles may represent a significant risk factor for depression or, at the very least, contribute to the same physiological processes that underlie depressive symptoms. However, it is essential to emphasize that depressive symptoms and R/S problems were independent predictors of RF in the amygdala and hippocampus, demonstrating that R/S issues are not secondary manifestations of depression. MDD patients, regardless of R/S problems, did not differ in the severity of depressive symptoms, anxiety, or quality of life. Additionally, some individuals may experience R/S problems and increased amygdala and hippocampus RF without being clinically diagnosed with MDD. This observation also aligns with data suggesting that positive R/S may provide neuroprotective effects10, while negative R/S disrupts brain function similarly to that observed in MDD.
The present findings are consistent with previous research, indicating that inflammation plays a central role in the pathophysiology of MDD74,75,76. Numerous studies investigated fundamental mechanisms of inflammation in MDD, such as elevated levels of pro-inflammatory cytokines and microglial activation. However, developing clinically accessible, non-invasive brain imaging techniques to measure neuroinflammation remains an unresolved issue. Advanced diffusion-weighted MRI approaches used in the present study are among the possible solutions50. Previously, in a large-scale population-based survey including biobank data from 11,512 individuals, it has been demonstrated that higher amygdala-RF was associated with elevated depression, especially in those with a lifetime history of depression50.
However, associations between global-RF or hippocampus-RF and depression were not significant, and no RF values linked depression with peripheral blood markers of low-grade inflammation50. Our results were different because we found increased hippocampus-RF, which was more strongly associated with depressive and even anxiety symptoms than that found in the case of the amygdala. Finally, we also observed increased cortical RF values in MDD, which was the most essential discriminating feature from R/S problems without MDD. However, putative neuroinflammatory changes in the cortex of MDD patients were not linked to depressive or anxiety symptoms, probably indicating a separate cognitive symptom dimension. The differences between the results of the Zhang et al. (2023) data and the present study may be attributed to distinct sample characteristics. In the present study, we included clinically defined MDD patients who never received any treatment before, whereas the Zhang et al. (2023) sample was from an extensive database of heterogeneous individuals50.
These findings have two main implications. First, they underscore the importance of considering R/S struggles as a separate psychological factor that may have measurable effects on brain health. Second, the findings suggest that addressing R/S struggles in therapeutic interventions could be beneficial for patients with MDD. For example, integrating spiritual counseling or interventions that promote positive religious coping could potentially mitigate the neuroinflammatory processes observed in these individuals16.
The strength of the present study is the application of MRI-based techniques to quantify neuroinflammation, allowing for a direct assessment of physiological changes associated with R/S struggles. The methodological rigor, including the application of the DBSI-RF MRI protocol50 and controlling several factors affecting inflammation, ensures that the observed differences in RF values are reliable indicators, adding significant value to the existing neuroimaging literature in MDD. Finally, we included never-treated patients with MDD, which excludes the confounding effects of psychotropic medications.
However, several limitations should be acknowledged. First, the cross-sectional design limits the ability to infer causality and the interaction between MDD, R/S struggles, and neuroinflammation. While the study shows a clear association, it remains unclear whether R/S struggles contribute to increased neuroinflammation or individuals with pre-existing neuroinflammation are more likely to experience R/S struggles. It is also unclear whether neuroinflammatory changes in R/S struggles are reliable predictors of the development of MDD. Longitudinal studies are necessary to clarify this relationship. The available prospective studies examining the relationship between R/S and depression are heterogeneous, and a definitive synthesis is not possible77. Half of the published studies reported that positive R/S predicted a modest decrease in depression, but future investigations into the association between R/S struggles and depressive disorders are indispensable77.
Second, the study sample is confined to individuals who identify themselves as Caucasian in Eastern Europe, limiting the generalizability to more diverse populations. Religious and spiritual experiences are deeply embedded in cultural contexts, and the way R/S struggles manifest and affect mental health may vary across different cultural and ethnic groups. Future studies should include more diverse and larger samples to examine whether the neuroinflammatory effects of R/S struggles are consistent across various demographic groups78. However, convenience sampling and potential biases are difficult to control because of the unique characteristics of the population. Individuals with R/S problems often refuse to participate in psychological and neurobiological investigations because of their religious beliefs, attitudes, and attributions79.
While the study provides evidence that R/S struggles are associated with neuroinflammatory changes in the amygdala and hippocampus, the underlying mechanisms driving these changes remain unclear. The stress associated with religious and spiritual conflicts may activate the hypothalamic-pituitary-adrenal (HPA) axis, leading to the release of cortisol and other stress-related hormones40. Alternatively, chronic R/S struggles may disrupt sleep patterns, which in turn could exacerbate neuroinflammatory processes80. Future research should explore these potential mechanisms to understand how R/S distress translates into neurobiological changes. Finally, we could not separately investigate the dimensions of R/S struggles because of the inconsequent nature of the RSS-14 scale’s factor structure in Hungarian samples.
In conclusion, this study provides evidence that R/S struggles are not only significant psychological stressors but may also contribute to neuroinflammatory processes in the brain, which are associated with more severe alterations in MDD. These findings highlight the importance of addressing spiritual distress in therapeutic settings and pave the way for future research to explore the mechanisms through which this distress influences brain health. Further investigation into the neurobiological underpinnings of R/S struggles, including longitudinal studies and more diverse samples, will be crucial for understanding this complex relationship.
Data availability
The datasets generated and analyzed during the present study are available from the corresponding author upon reasonable request.
References
Exline, J. J. & Rose, E. D. In Handbook of the Psychology of Religion and Spirituality, 2nd edn, 380–398 (The Guilford Press, 2013).
Exline, J. J., Yali, A. M. & Sanderson, W. C. Guilt, discord, and alienation: the role of religious strain in depression and suicidality. J. Clin. Psychol. 56, 1481–1496. https://doi.org/10.1002/1097-4679(200012)56:12<1481::AID-1>3.0.CO;2-A (2000).
Koenig, H. G., King, D. & Carson, V. B. Handbook of Religion and Health, 2nd edn (Oxford University Press, 2012).
Bonelli, R. M. & Koenig, H. G. Mental disorders, religion and spirituality 1990 to 2010: a systematic evidence-based review. J. Relig. Health. 52, 657–673. https://doi.org/10.1007/s10943-013-9691-4 (2013).
Braam, A. W. & Koenig, H. G. Religion, spirituality and depression in prospective studies: a systematic review. J. Affect. Disord. 257, 428–438. https://doi.org/10.1016/j.jad.2019.06.063 (2019).
Dew, R. E. et al. A prospective study of religion/spirituality and depressive symptoms among adolescent psychiatric patients. J. Affect. Disord. 120, 149–157. https://doi.org/10.1016/j.jad.2009.04.029 (2010).
Leurent, B. et al. Spiritual and religious beliefs as risk factors for the onset of major depression: an international cohort study. Psychol. Med. 43, 2109–2120. https://doi.org/10.1017/s0033291712003066 (2013).
Kasen, S., Wickramaratne, P., Gameroff, M. J. & Weissman, M. M. Religiosity and resilience in persons at high risk for major depression. Psychol. Med. 42, 509–519. https://doi.org/10.1017/S0033291711001516 (2012).
Miller, L. et al. Religiosity and major depression in adults at high risk: a ten-year prospective study. Am. J. Psychiatry. 169, 89–94. https://doi.org/10.1176/appi.ajp.2011.10121823 (2012).
Miller, L. et al. Neuroanatomical correlates of religiosity and spirituality: a study in adults at high and low familial risk for depression. JAMA Psychiatry. 71, 128–135. https://doi.org/10.1001/jamapsychiatry.2013.3067 (2014).
McClintock, C. H. et al. Multidimensional understanding of religiosity/spirituality: relationship to major depression and familial risk. Psychol. Med. 49, 2379–2388. https://doi.org/10.1017/S0033291718003276 (2019).
Blazer, D. Religion/spirituality and depression: what can we learn from empirical studies? Am. J. Psychiatry. 169, 10–12. https://doi.org/10.1176/appi.ajp.2011.11091407 (2012).
Bonelli, R., Dew, R. E., Koenig, H. G., Rosmarin, D. H. & Vasegh, S. Religious and spiritual factors in depression: review and integration of the research. Depress. Res. Treat. 2012, 962860. https://doi.org/10.1155/2012/962860 (2012).
Pargament, K. I., Koenig, H. G. & Perez, L. M. The many methods of religious coping: development and initial validation of the RCOPE. J. Clin. Psychol. 56, 519–543. https://doi.org/10.1002/(SICI)1097-4679(200004)56:4<519::AID-JCLP6>3.0.CO;2-1 (2000).
Miller, L. Spiritual awakening and depression in adolescents: a unified pathway or two sides of the same coin. Bull. Menninger Clin. 77, 332–348. https://doi.org/10.1521/bumc.2013.77.4.332 (2013).
Pargament, K. I. & Exline, J. J. In Spirituality and Mental Health Across Cultures. 395–412 (Oxford University Press, 2021).
Horton, E. G., Luna, N. & Malloy, T. Associations between spirituality, meaning in life, and depressive disorders among a sample of individuals in treatment for substance-use disorders. J. Spiritual. Ment Health. 18, 283–299. https://doi.org/10.1080/19349637.2016.1159941 (2016).
Pargament, K. I. The Psychology of Religion and Coping: Theory, Research, Practice (Guilford Press, 1997).
Dein, S. Religion, spirituality and depression: implications for research and treatment. Prim. Care Commun. Psychiatry. 11, 67–72. https://doi.org/10.1185/135525706X121110 (2006).
Koenig, H. G. Religion, spirituality, and health: the research and clinical implications. ISRN Psychiatry 2012, 278730. https://doi.org/10.5402/2012/278730 (2012).
Smith, T. B., McCullough, M. E. & Poll, J. Religiousness and depression: evidence for a main effect and the moderating influence of stressful life events. Psychol. Bull. 129, 614–636. https://doi.org/10.1037/0033-2909.129.4.614 (2003).
Abu-Raiya, H., Pargament, K. I., Krause, N. & Ironson, G. Robust links between religious/spiritual struggles, psychological distress, and well-being in a national sample of American adults. Am. J. Orthopsychiatry. 85, 565–575. https://doi.org/10.1037/ort0000084 (2015).
Kéri, S. Stress responses among individuals with spiritual struggles in Hungary: an experimental study. J. Relig. Health. https://doi.org/10.1007/s10943-023-01819-2 (2023).
Kéri, S. Frontal asymmetry and physiological responses in religious and spiritual problems with and without conversion. Relig. Brain Behav. 1–11. https://doi.org/10.1080/2153599X.2024.2307373 (2024).
Kéri, S. & Kelemen, O. Signatures of neuroinflammation in the hippocampus and amygdala in individuals with religious or spiritual problem. Relig. Brain Behav. 1–13. https://doi.org/10.1080/2153599X.2024.2349784 (2024).
Miller, A. H., Maletic, V. & Raison, C. L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 65, 732–741. https://doi.org/10.1016/j.biopsych.2008.11.029 (2009).
Singhal, G. & Baune, B. T. Microglia: an interface between the loss of neuroplasticity and depression. Front. Cell. Neurosci. 11, 270. https://doi.org/10.3389/fncel.2017.00270 (2017).
Wijaya, M. T., Jin, R. R., Liu, X., Zhang, R. & Lee, T. M. C. Towards a multidimensional model of inflamed depression. Brain Behav. Immun. Health. 26, 100564. https://doi.org/10.1016/j.bbih.2022.100564 (2022).
Yin, Y. et al. Inflamed depression: a review of the interactions between depression and inflammation and current anti-inflammatory strategies for depression. Pharmacol. Res. 207, 107322. https://doi.org/10.1016/j.phrs.2024.107322 (2024).
Enache, D., Pariante, C. M. & Mondelli, V. Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav. Immun. 81, 24–40. https://doi.org/10.1016/j.bbi.2019.06.015 (2019).
Shelton, R. C. et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol. Psychiatry. 16, 751–762. https://doi.org/10.1038/mp.2010.52 (2011).
Dean, B., Tawadros, N., Scarr, E. & Gibbons, A. S. Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. J. Affect. Disord. 120, 245–248. https://doi.org/10.1016/j.jad.2009.04.027 (2010).
Gritti, D., Delvecchio, G., Ferro, A., Bressi, C. & Brambilla, P. Neuroinflammation in major depressive disorder: a review of PET imaging studies examining the 18-kDa translocator protein. J. Affect. Disord. 292, 642–651. https://doi.org/10.1016/j.jad.2021.06.001 (2021).
Setiawan, E. et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 72, 268–275. https://doi.org/10.1001/jamapsychiatry.2014.2427 (2015).
Richards, E. M. et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 8 https://doi.org/10.1186/s13550-018-0401-9 (2018).
Li, H., Sagar, A. P. & Kéri, S. Translocator protein (18 kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Prog. Neuropsychopharmacol. Biol. Psychiatry. 83, 1–7. https://doi.org/10.1016/j.pnpbp.2017.12.011 (2018).
Li, H., Sagar, A. P. & Kéri, S. Microglial markers in the frontal cortex are related to cognitive dysfunctions in major depressive disorder. J. Affect. Disord. 241, 305–310. https://doi.org/10.1016/j.jad.2018.08.021 (2018).
Holmes, S. E. et al. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol. Psychiatry. 83, 61–69. https://doi.org/10.1016/j.biopsych.2017.08.005 (2018).
Du, Y., Gao, X. R., Peng, L. & Ge, J. F. Crosstalk between the microbiota-gut-brain axis and depression. Heliyon 6, e04097. https://doi.org/10.1016/j.heliyon.2020.e04097 (2020).
Hassamal, S. Chronic stress, neuroinflammation, and depression: an overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry. 14, 1130989. https://doi.org/10.3389/fpsyt.2023.1130989 (2023).
Troubat, R. et al. Neuroinflammation and depression: a review. Eur. J. Neurosci. 53, 151–171. https://doi.org/10.1111/ejn.14720 (2021).
Woelfer, M., Kasties, V., Kahlfuss, S. & Walter, M. The role of depressive subtypes within the neuroinflammation hypothesis of major depressive disorder. Neuroscience 403, 93–110. https://doi.org/10.1016/j.neuroscience.2018.03.034 (2019).
Zhao, Y. et al. The brain structure, immunometabolic and genetic mechanisms underlying the association between lifestyle and depression. Nat. Ment. Health. 1, 736–750. https://doi.org/10.1038/s44220-023-00120-1 (2023).
Kim, Y. K. & Won, E. The influence of stress on neuroinflammation and alterations in brain structure and function in major depressive disorder. Behav. Brain Res. 329, 6–11. https://doi.org/10.1016/j.bbr.2017.04.020 (2017).
Tahmasian, M. et al. Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Front. Hum. Neurosci. 7, 639. https://doi.org/10.3389/fnhum.2013.00639 (2013).
Kempton, M. J. et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch. Gen. Psychiatry. 68, 675–690. https://doi.org/10.1001/archgenpsychiatry.2011.60 (2011).
Sacher, J. et al. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J. Affect. Disord. 140, 142–148. https://doi.org/10.1016/j.jad.2011.08.001 (2012).
Jacob, Y. et al. Altered hippocampus and amygdala subregion connectome hierarchy in major depressive disorder. Transl. Psychiatry. 12, 209. https://doi.org/10.1038/s41398-022-01976-0 (2022).
Campbell, S. & Macqueen, G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 29, 417–426 (2004).
Zhang, W. et al. Neuroinflammation in the amygdala is associated with recent depressive symptoms. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. https://doi.org/10.1016/j.bpsc.2023.04.011 (2023).
Narvaez Linares, N. F., Charron, V., Ouimet, A. J., Labelle, P. R. & Plamondon, H. A systematic review of the Trier Social Stress Test methodology: issues in promoting study comparison and replicable research. Neurobiol. Stress. 13, 100235. https://doi.org/10.1016/j.ynstr.2020.100235 (2020).
First, M. B., Williams, J. B. W., Karg, R. S. & Spitzer, R. L. Structured Clinical Interview for DSM-5 Disorders—Clinician Version (SCID-5-CV) (American Psychiatric Association Publishing, 2016).
Maier, W., Buller, R., Philipp, M. & Heuser, I. The Hamilton anxiety scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 14, 61–68. https://doi.org/10.1016/0165-0327(88)90072-9 (1988).
Trajković, G. et al. Reliability of the Hamilton Rating Scale for Depression: a meta-analysis over a period of 49 years. Psychiatry Res. 189, 1–9. https://doi.org/10.1016/j.psychres.2010.12.007 (2011).
Bech, P. Fifty years with the Hamilton scales for anxiety and depression. A tribute to Max Hamilton. Psychother. Psychosom. 78, 202–211. https://doi.org/10.1159/000214441 (2009).
Hamilton, M. Rating depressive patients. J. Clin. Psychiatry. 41, 21–24 (1980).
Perczel-Forintos, D., Ajtay, G., Barna, C., Kiss, Z. & Komlósi, S. Kérdőívek, becslőskálás a klinikai pszichológiában. Medicina (2018).
Hunt, S. M. & McKenna, S. P. The QLDS: a scale for the measurement of quality of life in depression. Health Policy. 22, 307–319. https://doi.org/10.1016/0168-8510(92)90004-u (1992).
McKenna, S. P. et al. International development of the quality of life in Depression Scale (QLDS). J. Affect. Disord. 63, 189–199. https://doi.org/10.1016/S0165-0327(00)00184-1 (2001).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5) 5th edn (American Psychiatric Association, 2013).
Falewicz, A. et al. Polish validation of a 14-Item version of the religious and spiritual struggles scale (RSS-14): factorial structure, psychometric properties, and clinical correlates. J. Relig. Health. 62, 3579–3603. https://doi.org/10.1007/s10943-023-01816-5 (2023).
Exline, J. J., Pargament, K. I., Wilt, J. A., Grubbs, J. B. & Yali, A. M. The RSS-14: development and preliminary validation of a 14-item form of the religious and spiritual struggles Scale. Psychol. Relig. Spiritual. 15, 592–604. https://doi.org/10.1037/rel0000472 (2023).
Alfaro-Almagro, F. et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage 166, 400–424. https://doi.org/10.1016/j.neuroimage.2017.10.034 (2018).
Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 19, 1523–1536. https://doi.org/10.1038/nn.4393 (2016).
Fischl, B. FreeSurfer. NeuroImage 62, 774–781. https://doi.org/10.1016/j.neuroimage.2012.01.021 (2012).
Wang, Y. et al. Quantification of increased cellularity during inflammatory demyelination. Brain 134, 3590–3601. https://doi.org/10.1093/brain/awr307 (2011).
Wang, Q. et al. Quantification of white matter cellularity and damage in preclinical and early symptomatic Alzheimer’s disease. Neuroimage Clin. 22, 101767. https://doi.org/10.1016/j.nicl.2019.101767 (2019).
Fischl, B. et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. https://doi.org/10.1016/s0896-6273(02)00569-x (2002).
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL NeuroImage 62, 782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015 (2012).
Zhang, Y., Brady, M. & Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 20, 45–57. https://doi.org/10.1109/42.906424 (2001).
Köhler, C. A. et al. Mapping risk factors for depression across the lifespan: an umbrella review of evidence from meta-analyses and mendelian randomization studies. J. Psychiatr. Res. 103, 189–207. https://doi.org/10.1016/j.jpsychires.2018.05.020 (2018).
Lima-Ojeda, J. M., Rupprecht, R. & Baghai, T. C. Neurobiology of depression: a neurodevelopmental approach. World J. Biol. Psychiatry. 19, 349–359. https://doi.org/10.1080/15622975.2017.1289240 (2018).
Dean, J. & Keshavan, M. The neurobiology of depression: an integrated view. Asian J. Psychiatr. 27, 101–111. https://doi.org/10.1016/j.ajp.2017.01.025 (2017).
Kiecolt-Glaser, J. K., Derry, H. M. & Fagundes, C. P. Inflammation: depression fans the flames and feasts on the heat. Am. J. Psychiatry. 172, 1075–1091. https://doi.org/10.1176/appi.ajp.2015.15020152 (2015).
Krishnadas, R. & Harrison, N. A. Depression phenotype, inflammation, and the brain: implications for future research. Psychosom. Med. 78, 384–388. https://doi.org/10.1097/psy.0000000000000339 (2016).
Beurel, E., Toups, M. & Nemeroff, C. B. The bidirectional relationship of depression and inflammation: double trouble. Neuron 107, 234–256. https://doi.org/10.1016/j.neuron.2020.06.002 (2020).
Braam, A. W. & Koenig, H. G. Religion, spirituality and depression in prospective studies: a systematic review. J. Affect. Disord. 257, 428–438. https://doi.org/10.1016/j.jad.2019.06.063 (2019).
Moreira-Almeida, A., Mosqueiro, B. P. & Bhugra, D. Spirituality and Mental Health across Cultures (Oxford University Press, 2021).
Beck, J. R. & Banks, J. W. Christian anti-psychology sentiment: hints of an historical analogue. J. Psychol. Theol. 20, 3–10. https://doi.org/10.1177/009164719202000101 (1992).
Dolsen, E. A., Crosswell, A. D. & Prather, A. A. Links between stress, sleep, and inflammation: are there sex differences? Curr. Psychiatry Rep. 21, 8. https://doi.org/10.1007/s11920-019-0993-4 (2019).
Acknowledgements
We thank Ibolya Halász, Katalin Kaza, and Péter Nagy for their assistance in data collection and clinical assessment.
Funding
Open access funding provided by University of Tokaj.
The study was supported by the Thematic Program of Excellence (TKP-2-1/PALY-2020, National Research, Development, and Innovation Fund).
Author information
Authors and Affiliations
Contributions
A.K.: Analyzing and interpreting results and drafting manuscript preparation. O.K.: Study conception and design, draft manuscript preparation. S.K.: Study conception and design, data collection and curation, draft manuscript preparation. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaszás, A., Kelemen, O. & Kéri, S. Magnetic resonance imaging signatures of neuroinflammation in major depressive disorder with religious and spiritual problems. Sci Rep 15, 5407 (2025). https://doi.org/10.1038/s41598-025-89581-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-89581-1
This article is cited by
-
MRI markers of neuroinflammation in untreated patients with subclinical generalized anxiety disorder
Journal of Neural Transmission (2025)