Abstract
Occupational exposure to engineered nanomaterials (ENMs) is increasing in the workplace and can impact human health. Amorphous silicon dioxide nanoparticles (SiO2 NPs) are widely produced respirable ENMs used in commercial products. We have investigated their impact on lung inflammation resolution and bacterial defense. Mice exposed to SiO2 NPs, followed by bacteria, exhibited increased lung inflammation, bacterial proliferation, and lung damage compared to mice not exposed to NPs. SiO2 NPs increased human macrophage production of pro-inflammatory mediators and disrupted phagocytosis of bacteria and efferocytosis of apoptotic neutrophils – pivotal responses for host defense and inflammation resolution. A pro-resolving mediator, resolvin D5 (RvD5), restored macrophage phagocytosis of bacteria and partially controlled excess lung inflammation after SiO2 NPs. These findings demonstrate that SiO2 NPs disrupt endogenous resolution processes to give rise to heightened lung inflammation and infection. RvD5 reduced inflammation and partially restored endogenous resolution cellular processes, suggesting that RvD5 can reduce ENP disruption of resolution.
Similar content being viewed by others
Introduction
Engineered nanomaterials (ENMs) have unique physical and chemical properties, enabling their broad application in fields including agriculture, food, electronics, textiles, and nanomedicine1,2. With the rapid expansion of the nanotechnology industry in recent decades, occupational exposure to ENMs has become an increasing concern, currently affecting an estimated workforce of over six million individuals3. Both historical and recent evidence underscore the potential for ENMs to elicit adverse biological effects4,5,6. Of note, ENMs can translocate across biological barriers, reaching critical organs7. In the lungs, ENM exposure, including amorphous silicon dioxide nanoparticles (SiO2 NPs), is linked to induction of inflammation and tissue damage8,9,10,11. Of note, inhaled crystalline silica particles trigger the production of proinflammatory eicosanoids by tissue-resident alveolar macrophages leading to chronic inflammation in the lung12. Yet, mechanisms and implications for lung disease remain to be determined.
The lungs serve as a mucosal barrier against particles and invading microorganisms13. Upon infection, resident alveolar macrophages initiate inflammatory responses to eliminate harmful inhaled agents and promote the later resolution of inflammation, leading to restoration of tissue homeostasis14. Resolution of inflammation is an active process directed in part by potent molecular effectors known as specialized pro-resolving mediators (SPMs), including resolvins, protectins, and maresins15. Inhaled particulates trigger lung inflammation and the temporal production of specific SPMs16,17. Disruption of pro-resolving pathways is associated with persistent inflammation and exacerbation of pulmonary diseases, including pneumonia18. Specific SPMs such as Maresin 1 and resolvin D1 exhibit protective actions across a range of pulmonary toxicants19,20,21,22, suggesting that harnessing resolution could mitigate the impact of ENMs exposure on lung physiology. Similar to Maresin 1 and resolvin D1, Resolvin D5 (RvD5) is a potent SPM that enhances neutrophil and macrophage phagocytosis of bacteria while counter-regulating proinflammatory responses—key actions for effectively controlling lung infections23. In March 2019, the National Institute of Environmental Health Sciences held an Inflammation Resolution Biology Workshop, to discuss the mechanisms and mediators in the resolution of inflammation that could be impacted by environmental toxicants, thus contributing to human diseases associated with excessive inflammation24.
Here, we report that exposure to ENMs, specifically SiO2 NPs, increased macrophage production of inflammatory mediators and disrupted key cellular mechanisms of resolution of inflammation. These effects led to exacerbated lung inflammation and bacterial proliferation in a mouse model of pneumonia, which were reversed by the administration of the SPM RvD5.
Results
Synthesis, dispersion preparation, and colloidal characterization of SiO2 NPs
To assess their impact on inflammation resolution mechanisms, we prepared amorphous SiO2 NPs that were synthesized by flame-spray pyrolysis. The synthesized SiO2 NPs were measured by Brunauer–Emmett–Teller (BET) and confirmed by transmission electron microscopy (TEM) to exhibit an average primary particle size of 15 nm. Chemical composition analysis by X-ray diffraction confirmed their amorphous nature. The SiO2 NPs complete physicochemical characterization is presented in Supplementary Table 1 (using methods from our published report25).
Dispersion preparation of SiO2 NPs was performed according to our published protocol26. The colloidal properties of as-dispersed SiO2 NPs in deionized water and cell culture media are summarized in Supplemental Table 2. The hydrodynamic diameter (dH), polydispersity index (PDI), and zeta (ζ) potential of SiO2 NPs in water confirmed a stable dispersion with sub-100 nm and uniformly sized particle agglomerates. In cell culture media, some interparticle agglomeration was noted (Supplementary Fig. 1), likely due to an increased z-potential, causing lighter particle clusters and lower effective density compared to the as-synthesized state of SiO2 NPs. Additional details on the physical and chemical properties of the engineered SiO2 NPs synthesized by our high throughput and precision flam-spray synthesis can be found in our published report25.
Airway exposure to SiO2 NPs induces lung neutrophilic inflammation in mice
To investigate the in vivo effects of SiO2 NPs, freshly prepared suspensions in saline were administered intratracheally to mice for 5 consecutive days (Fig. 1a). Mice were euthanized at 48 h after the last exposure. SiO2 NPs exposure increased the number of neutrophils, exudative and inflammatory macrophages (exMACs and iMACs, respectively) in bronchoalveolar lavage (BAL), while reducing resident alveolar macrophages (rAMs) compared to vehicle-treated (H2O) control mice (Fig. 1b-f). In addition, SiO2 NPs increased the BAL levels of CXCL-1 at 48 h post-exposure (Fig. 1g). These results indicate that SiO2 NPs induce neutrophilic and monocyte-derived inflammatory macrophage responses in vivo in a mouse model of exposure.
Airway exposure to SiO2 NPs induces lung inflammation in mice. (a) C57BL/6 mice were exposed intratracheally to either SiO2 NPs (5ug/ day) or vehicle over five consecutive days. Mice were then euthanized 24 h after the last exposure and bronchoalveolar lavage (BAL) was performed. (b) Immunophenotyping of BAL with flow cytometry to evaluate (c) neutrophils, (d) exudative macrophages (CD45+ F4/80+ CD11b+ CD11c+, exMACs), (e) infiltrating macrophages (CD45+ F4/80+ CD11b+ CD11c−, iMACs), and (f) resident alveolar macrophages (CD45+ F4/80+ CD11b− CD11c+, rAMs). (g) BAL fluid (BALF) was assessed for CXCL-1 levels by ELISA. Data expressed as mean ± SEM, n = 3 mice in each group. Statistical significance was determined by Unpaired t-test, with *P < 0.05; **P < 0.01.
SiO2 NPs are cytotoxic to M2-like human macrophages
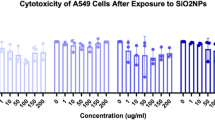
Macrophages play a critical role in the resolution of inflammation27. To determine the direct cellular effects of SiO2 NPs, human monocyte-derived macrophages were produced and then polarized to an M1- or M2-like phenotype prior to exposure to 1, 10, or 100 ug/mL of SiO2 NPs for 24 h (n = 3–5, see Methods). Both M1 and M2 phenotypes were chosen for their distinctly contrasting roles in inflammation and resolution. M2-like macrophages are widely appreciated for their pro-resolving and pro-repair properties28, including efferocytosis of apoptotic neutrophils and production of the lion’s share of SPMs29. Mitochondrial enzymatic activity assays (Supplementary Fig. 2a and 2b) revealed suppressed enzymatic activity in M2, but not M1, macrophages at 10 and 100 ug/mL of SiO2 NPs. M2 macrophages showed a significant loss of enzymatic mitochondrial activity (-10%) at 100 ug/mL, which was not observed with M1 macrophages. Given the changes in mitochondrial activity at high concentrations of SiO2 NPs, levels of mitochondrial membrane potential (ΔΨm) were also investigated since loss of ΔΨm can be an early apoptotic event. M2 macrophages exhibited ~ 40% loss of ΔΨm at the highest SiO2 NP concentration (100 µg/mL) (Supplementary Fig. 2c and 2d), although results did not reach statistical significance because of donor variability. Loss of ΔΨm was less pronounced for M1 macrophages when compared to untreated control. Together these results indicate that M2-like macrophages may be more susceptible to NP-induced dysfunction than M1 cells.
Exposure to SiO2 NPs promotes pro-inflammatory mediator release and impairs neutrophil efferocytosis in human macrophages
Given the observed cytotoxicity of SiO2 NPs in M2-like macrophages, we further examined the impact of SiO2 NPs on M2 release of pro-inflammatory and pro-resolving mediators and efferocytosis of apoptotic neutrophils. Upon exposure to SiO2 NPs (10 µg/mL, 30 min, 37o C; see Methods), human M2-differentiated macrophages showed increased production of the pro-inflammatory bioactive lipid mediators leukotriene B4 (LTB4) and prostaglandin E2 (PGE2) (Fig. 2), as confirmed by targeted LC-MS/MS (see Methods). In addition, small amounts of the pro-resolving mediator RvD5 were produced upon exposure of M2-like macrophages to SiO2 NPs (Fig. 3a). Notably, bacterial stimulation with E. coli in M2-like macrophages exposed to SiO2 NPs enhanced the production of pro-inflammatory mediators but not RvD5, which had a lower mean value (Table 1 and Supplementary Fig. 3). Besides RvD5, no additional SPMs were positively identifiable in the incubations with SiO2 NPs. The retention times and MS/MS fragmentation patterns were consistent with synthetic reference standards of LTB4 and PGE2, and RvD5 (Supplementary Fig. 4).
Exposure to SiO2 NPs promotes pro-inflammatory mediator release in human M2 macrophages. (a) LTB4 was quantified via liquid chromatography-tandem mass spectrometry (LC-MS/MS). Top panel, Multiple reaction monitoring (MRM) chromatogram of LTB4 (m/z 335 > 195) with a retention time (TR) = 13.42 min and signal to noise (S/N) ratio of 5488.1. Inset, quantitation of LTB4 in macrophages stimulated with SiO2 (10 µg/mL) or vehicle. Bottom panel, associated ESI-MS/MS spectrum with a molecular ion at m/z 335 = M-H and accompanying daughter ions at m/z 317 = M-H-H2O, m/z 299 = M-H-2H2O, m/z 273 = M-H-H2O-CO2, m/z 255 = M-H-2H2O-CO2, m/z 205 = 223-H2O, m/z 195, m/z 161 = 223-H2O-CO2, m/z 151 = 195-CO2, and m/z 129. (b) PGE2 was similarly quantified. Top panel, MRM chromatogram of PGE2 (m/z 351 > 189) with a retention time (TR) = 9.29 min and S/N ratio of 309.4. Inset, quantitation of PGE2 in macrophages stimulated with SiO2 or vehicle. Bottom panel, accompanying ESI-MS/MS spectrum with a molecular ion at m/z 351 = M-H and fragment daughter ions at m/z 333 = M-H-H2O, m/z 315 = M-H-2H2O, m/z 289 = M-H-H2O-CO2, m/z 271 = M-H-2H2O-CO2, m/z 235 = 279-CO2, and m/z 189 = 251-H2O-CO2. The retention times and fragmentation spectra of both LTB4 and PGE2 were essentially identical to those of their respective synthetic reference standards.
Exposure to SiO2 NPs promotes RvD5 release and impairs neutrophil efferocytosis in human macrophages. (a) RvD5 was identified via liquid chromatography-tandem mass spectrometry (LC-MS/MS). Top panel, Multiple reaction monitoring (MRM) chromatogram of RvD5 (m/z 359 > 199) with a retention time (TR) = 13.18 min and signal to noise (S/N) ratio of 455.5. Inset, quantitation of RvD5 in M2 macrophages stimulated with SiO2 (10 µg/mL) or vehicle. Bottom panel, Corresponding MS/MS spectrum with a parent ion at m/z 359 = M-H and daughter ions including m/z 341 = M-H-H2O, m/z 323 = M-H-2H2O, m/z 315 = M-H-CO2, m/z 297 = M-H-H2O-CO2, m/z 227 = 289-H2O-CO2, m/z 199 = 217-H2O, and m/z 141. These data were essentially identical to those of synthetic RvD5 reference standard. (b) To assess efferocytosis, human macrophages (Mφ) plated on a 6-well plate (2 × 106 cells/ well) were incubated with vehicle or SiO2 NPs (10–100 µg) for 15 min at 37 °C followed by the addition of CFSE-labeled apoptotic PMN (1:3 ratio; Mφ: neutrophils). Efferocytosis is expressed as the percentage above vehicle control. Data presented as mean ± SEM, n = 3 healthy human donors. Statistical significance was determined using Wilcoxon matched pairs signed rank test (a, b) and RM one-way ANOVA (c) with *p < 0.05.
Next, to assess the cardinal pro-resolving function of macrophage efferocytosis, the human monocyte derived M2 differentiated macrophages were exposed in vitro to either SiO2 NPs (10 µg/mL, 100 µg/mL), or vehicle, followed by the addition of apoptotic neutrophils. SiO2 NP exposure reduced efferocytosis of apoptotic neutrophils, even at the lower dose tested (Fig. 3b).
SiO2 NP exposure impairs human macrophage phagocytosis of bacteria
Phagocytosis of bacteria is another key function of macrophages in both host defense and the resolution of inflammation. Exposure of human macrophages to SiO2 NPs impaired their ability to phagocytose live E. coli (Fig. 4). Specialized pro-resolving mediators such as RvD5 are potent agonists known to enhance macrophage protective mechanisms, including phagocytosis of bacteria18,30. Consequently, the effects of RvD5 on SiO2 NP-exposed macrophages were investigated. Of note, RvD5 (10 nM), validated for authenticity prior to use in the experiments (Supplementary Fig. 5), partially restored the macrophages’ capacity for phagocytosis of E. coli (Fig. 4b and c).
SiO2 NP exposure disrupts human macrophage phagocytosis of E. coli. Human monocytes were isolated from healthy donors and differentiated into macrophages with 20 ng/mL of human recombinant GM-CSF for seven days. Human macrophages (Mφ), plated on a 6-well plate (2 × 106 cells/ well), were incubated with vehicle or SiO2 (10 µg) for 180 min. Macrophages were then incubated with 10 nM of RvD5, or vehicle alone (0.01% vol/vol) for 15 min at 37oC prior to the addition of BacLight Green-labeled Escherichia coli (ratio of 1:50, M2 macrophages: E. coli). Fluorescence-associated Phagocytosis was monitored using flow cytometry. (a) Representative flow cytometry gating strategy for M2 macrophages. (b) Histogram of intracellular BacLight FITC-labeled E. coli in M2 macrophages. (c) Geometric Mean Fluoresce Intensity (MFI) of phagocytosis. (d) Percentage increase in M2 phagocytosis relative. E. coli + vehicle control. Data presented as means ± SEM, n = 6 donors. Statistical significance was analyzed using one-way ANOVA, with *p < 0.05, **p < 0.01, compared to E. coli + vehicle (Panel c-d).
SiO2 NP exposure exacerbates Streptococcus pneumoniae infection in mice
Given the impact of SiO2 NP exposure on macrophage phagocytosis of bacteria, we investigated the impact of these ENMs on Streptococcus pneumoniae lung infection in vivo. As S. pneumoniae is a leading cause of community-acquired pneumonia worldwide and conveys excess morbidity and mortality31, its host-pathogen dynamics were of interest. To evaluate the impact of SiO2 NP-evoked inflammation on host responses to S. pneumoniae infection in the lungs, mice were infected with S. pneumoniae 24 h after the last SiO2 NP exposure and immunophenotyped 48 h later (Fig. 5a). SiO2 NP exposure significantly increased the number of BAL neutrophils, exMACs and iMACs compared to control mice (Fig. 5b-e). In contrast, BAL numbers of rAMs were decreased (Fig. 5f). This overall rise in infiltrated leukocytes in SiO2 NP exposed mice was associated with increased levels of pro-inflammatory cytokines including CXCL-1 and TNFα (Fig. 5g and h). SiO2 NP exposure also augmented infection-induced barrier breach and lung injury, as seen by the increased levels of total protein in the BAL fluid (Fig. 5i), bacterial proliferation in BAL and lung tissues at 48 h post-infection (Fig. 5j and k) and histological changes (Fig. 5l).
SiO2 NP exposure exacerbates severity of S. pneumoniae pneumonia in mice. (a) C57BL/6 mice were exposed intratracheally to SiO2 NPs (5ug/day) or vehicle (H2O) for five days. 24 h after the last exposure, mice were infected intranasally with a mild inoculum of Streptococcus pneumoniae (Serotype 3, 1 × 105 CFU). 48 h post-infection, mice were euthanized, and (b) BAL collected was immunophenotyped by flow cytometry to evaluate numbers of (c) neutrophils, (d) exudative macrophages (CD45+ F4/80+ CD11b+ CD11c+ - exMACs), (e) infiltrating macrophages (CD45+ F4/80+ CD11b+ CD11c− - iMACs), and (f) resident alveolar macrophages (CD45+ F4/80+ CD11b− CD11c+ - rAMs). (g-i) BAL fluid (BALF) was analyzed for (g) total protein content, (h) CXCL-1 levels, and (i) TNF-α levels. Bacterial counts were determined in the (j) bronchoalveolar lavage (BAL) and (k) the left lung after BAL. (l) In separate experiments, lung histology was evaluated using H&E staining. Images were captured at both 10x (bars = 100 μm) and 40x magnifications (bars = 50 μm). Arrows indicate areas of inflammatory cell infiltration and stars (*) mark damaged epithelium. Data is shown as mean ± SEM, n = 6–7 mice in each group. Statistical analysis was performed by (c-i) or unpaired t-test (j-k) Mann Whitney test, with *p < 0.05; **p < 0.01; ***p < 0.001.
RvD5 decreases lung inflammation and enhances S. pneumoniae clearance in mice
RvD5 was given intravenously to mice exposed to both SiO2 NPs and S. pneumoniae to determine its impact on SiO2 NP-exacerbated infectious inflammation (Fig. 6a). RvD5 decreased lung inflammation and damage in both SiO2 NP exposed and unexposed mice as shown by improved lung histology (Fig. 6b). RvD5 also significantly reduced bacterial counts in BAL and lung tissues of vehicle-exposed but not SiO2 NP-exposed infected mice (Fig. 6c and d). RvD5 significantly reduced the number of BAL neutrophils in both vehicle and SiO2 NP-exposed mice infected with S. pneumoniae (Fig. 6e and f). Similar trends for decreased numbers with RvD5 were observed for exMACS and iMACs in vehicle and SiO2 NP-exposed infected mice, but these differences did not reach statistical significance for exMACs (Fig. 6g and h). RvD5 significantly increased numbers of BAL rAMs vehicle-exposed mice but not in the SiO2 NP exposed group (Fig. 6i).
RvD5 decreases lung inflammation and enhances S. pneumoniae clearance in mice. (a) C57BL/6 mice were exposed intratracheally to SiO2 NPs (5µg/day) or vehicle for five consecutive days. 24 h after the final exposure, mice were infected intranasally with a mild inoculum of Streptococcus pneumoniae (Serotype 3, 1 × 105 CFU). At the time of infection and 24 h later, mice were administered RvD5 (100ng/mouse, i.v.) or vehicle. 48 h after infection mice were euthanized and (b) lung histology was performed using H&E staining. Photomicrographs were taken at both 10x (scale bars = 100 μm) and 40x magnification (scale bars = 50 μm). Arrows point to areas of significant inflammatory cell infiltrates and stars (*) indicate damage to the epithelium. In a separate experiment, bacterial counts were determined in (c) bronchoalveolar lavage (BAL) and (d) the left lung after BAL. (e) BAL collected was immunophenotyped by flow cytometry to evaluate (f) neutrophils. Macrophages were further phenotyped and counts of (g) exudative macrophages (CD45 + F4/80 + CD11b + CD11c+ - exMACs), (h) infiltrating macrophages (CD45 + F4/80 + CD11b + CD11c- - iMACs), and (i) resident alveolar macrophages (CD45 + F4/80 + CD11b- CD11c+ - rAMs) were obtained. Analysis of BAL fluid (BALF) was conducted to measure the levels of (j) total protein, (k) CXCL-1, and (l) TNF- a. Data presented as mean ± SEM, n = 3–4 mice in each group pooled from 3 independent experiments. Statistical analysis was performed by one-way ANOVA, with *p < 0.05; **p < 0.01; ***p < 0.001.
With these changes in cellular responses to SiO2 NPs in vivo, and partial regulation with RvD5, we next looked at BAL fluid (BALF) total protein and pro-inflammatory cytokine levels 48 h post-infection. There was a significant and broad increase in BALF pro-inflammatory cytokines with SiO2 NPs (Figs. 6j-l and 7). Of note, the increased BALF pro-inflammatory mediators were reduced with RvD5 in vehicle and SiO2 NP-exposed mice, compared to their respective controls. Together, these results indicate that exposure to SiO2 NPs decreased bacterial clearance and enhanced pro-inflammatory responses, which were partially reversed with administration of exogenous RvD5.
RvD5 decreases S. pneumoniae-triggered proinflammatory cytokine secretion. C57BL/6 mice were exposed intratracheally to SiO2 NPs (5µg/day) or vehicle for five days, and 24 h after last exposure infected intranasally with a mild inoculum of Streptococcus pneumoniae (Serotype 3, 1 × 105 CFU). At the time of infection and 24 h later, mice were administered RvD5 (100ng/mouse, i.v.) or vehicle. (A) Cytokine levels from BAL harvested at 48 h after infection were evaluated by LegendPlex and summarized in a heatmap. (B-K) Analysis of BAL fluid was conducted to measure the levels of pro-inflammatory (B) IL-1a, (C) IL-1β, (D) TNF-a, (E) IFN-γ, (F) IL-6, (G) IFN-β, (H) IL-17, (I) Il-27, (J) IL-12p70, and (K) MCP-1. Data are shown as mean ± SEM, n = 3–4 mice in each group pooled from 3 independent experiments in each group. Statistical analysis was performed by one-way ANOVA, with *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
The acute inflammatory response has evolved to rapidly summon leukocytes from circulation to sites of microbial invasion and/or injury via the production of chemokines and classic eicosanoids such as leukotrienes and prostaglandins that are chemoattractants and mediators that regulate leukocyte diapedesis from vessels32. This process is host protective and, ideally, progresses to complete resolution to restore tissue homeostasis32. Two critical cellular events in the inflammation resolution response are the limiting further neutrophil recruitment and clearance of apoptotic cells and microbes, functions mediated and activated by pro-resolving mediators (e.g., SPMs) as novel resolution agonists33,34. When resolution fails or is incomplete the inflammatory response proceeds to excessive or chronic inflammation, organ failure and ultimately death, as can occur with pneumonia33,35.
In the present manuscript, we investigated the impact of exposure to environmental pollutants as potential disruptors of endogenous mechanisms for pathogen defense and inflammation control, key features of the resolution of inflammation in the lungs. We have used a widely produced industrial engineered nanomaterial, namely amorphous SiO2 NPs to investigate the impact of ENMs on lung homeostasis and resilience to bacterial infections. Exposure to amorphous SiO2 NPs led to exacerbated pathogen induced inflammation and decreased bacterial clearance. This SiO2 NP exacerbation of inflammation in vivo was rescued in part by the immunoresolvent RvD5. With human M2-like macrophages, there was substantial donor-to-donor variation; however, the mean values for LTB4, PGE2 and RvD5 were increased with SiO2 NPs. Of note, when the SiO2 NP exposed macrophages were stimulated with bacteria, there was an exaggerated production of pro-inflammatory LTB4 and PGE2 but not pro-resolving RvD5. In concert with these changes in mediator production, the SiO2 NP exposed M2-like macrophages had reduced phagocytosis of bacteria and decreased efferocytosis of apoptotic neutrophils. These SiO2 NP-induced changes to macrophage function would be predicted to disrupt endogenous mechanisms for bacterial host defense and inflammation control.
Nanotechnology has revolutionized industries worldwide, including electronics, medicine, food, and energy. Although the positive impact on development and progress is evident, the potential toxicity of engineered nanomaterials to lung homeostasis is important to understand. Silicon is the second most abundant element in the Earth’s crust after oxygen. Together, silicon and oxygen form silicon dioxide (SiO2) that can be found in amorphous or crystalline structures. Currently, silica ENMs are used in many economic sectors, which requires their increasing production thus fueling the growth of the nano-silica market36. While not classified as harmful as crystalline silica, amorphous SiO2 NPs still present size- and dose-dependent cytotoxicity for macrophages and bronchial epithelial cells in vitro37. SiO2 NPs have been classified as group 3 (lack of adequate data on their carcinogenicity) by the International Agency of Research on Cancer (IARC), and are generally regarded as safe (GRAS) by the FDA for use as a food additive38,39,40,41. Still, their small size allows them to infiltrate and interact with sub-cellular structures and their versatile surface chemistry renders their toxicological profiling highly contingent on their exact physicochemical properties, especially the silanol groups36. Moreover, silica NPs elicit immune responses upon inhalation and have also shown signs of genotoxicity42,43. The mechanistic knowledge gaps associated with these events and the increasing presence of silica NPs at the occupational and end-consumer level, especially in building materials44, raise the importance of elucidation of their impact as potential disruptors of resolution with far-reaching implications for human health. While NPs can disrupt resolution, nano-structures can be engineered and designed to promote endogenous resolution mechanisms45 and deliver SPMs locally to reduce vascular injury46, infectious inflammation47 and prevent pulmonary fibrosis48.
To simulate work-environment exposure, mice were exposed to SiO2 NPs for five consecutive days. SiO2-exposed mice showed increased BAL neutrophil counts and increased BAL fluid CXCL-1 levels. These results indicated that SiO2 NPs increased inflammation and perturbed lung homeostasis. In addition, resident alveolar macrophages, the major cell type in naïve lungs, were decreased by exposure to SiO2 NPs. Alveolar macrophages are key effectors of lung tolerance to pollutants and particles. Disrupted macrophage responses increase pneumonia severity in mouse models of infection49 and SiO2 NPs can increase pro-inflammatory cytokine production in macrophages50. Here, exposure to SiO2 NPs before Streptococcus pneumoniae infection significantly impaired bacteria control, and increased lung inflammation and damage in mice. With human cells, M2-like macrophages exposed to SiO2 NPs had dysfunctional phagocytosis of bacteria and apoptotic neutrophils (i.e. efferocytosis) and enhanced production of pro-inflammatory lipid mediators. PGE2 can signal efferocytosis by promoting SPM production51 and M2-like macrophages are critical effectors for clearing dead apoptotic cells28. Here, SiO2 NPs disrupted the M2 macrophage phenotype by inducing unexpected secretion of pro-inflammatory mediators and impairing their efferocytosis of apoptotic neutrophils. These functions are crucial for the resolution of inflammation, highlighting the potential of SiO2 NPs to interfere with processes essential for tissue homeostasis. M2-like macrophages were more susceptible to SiO2 NPs when compared to classically activated M1 macrophages. M2 macrophages display a pro-resolving lipid mediator profile, preferentially producing SPMs over pro-inflammatory molecules52 and exhibit greater uptake of SiO2 NPs compared to M1 macrophages53.In agreement with our results, silver NPs have also been shown to disrupt specific pro-resolving mediator pathways54. Thus, these findings indicate that ENMs disrupt cellular effectors of resolution of inflammation.
RvD5 is a potent SPM produced during the resolution of infection by regulatory M2-like macrophages52, and RvD5 reduces LPS-triggered inflammation and promotes bacteria phagocytosis29,55. At an RvD5 dose that significantly decreased S. pneumoniae-elicited lung inflammation and enhanced bacterial clearance, these protective actions of RvD5 were partially disrupted by exposure to SiO2 NPs. RvD5 reduced BAL neutrophil counts and inflammatory mediator levels after S. pneumoniae pneumonia, leading to an overall reduction in lung edema. While RvD5 has not been shown to decrease inflammation related to other respirable particles in indoor or outdoor air pollution, other members of the D-series resolvins have been shown to be lung-protective during airway exposure to larger particulate matter such as cigarette smoke22,56,57. Together, our in vitro and in vivo data indicate that exposure to SiO2 NPs reduced macrophage phagocytosis of bacteria, compromised BAL and lung bacterial clearance and exacerbated pathogen-elicited inflammation as well as partially impaired the RvD5-mediated protective actions.
In summary, our findings indicate that ENMs can adversely impact endogenous resolution of inflammation mechanisms and are previously unappreciated disruptors of vital processes of resolution, implying that resolution pathways must be considered at potential risk when evaluating ENMs in the future of our industrial world.
Materials and methods
SiO2 NP synthesis and physicochemical characterization
The amorphous silica nanoparticles (SiO2 NPs) used in this study were obtained from the engineered nanomaterials (ENMs) repository at Harvard University, part of the Nanotechnology Health Implications Research (NHIR) Consortium and the National Institute of Environmental Health Sciences (NIEHS). SiO2 NPs were synthesized by flame-spray pyrolysis using the Harvard Versatile Engineered Nanomaterials Generation system (VENGES)36,58,59,60. This platform enables the synthesis of property-controlled engineered metal, metal oxide, and metalloid NPs. The detailed physicochemical properties of these SiO2 NPs have been previously reported by Beltran-Huarac et al.25.
SiO2 NP dispersion preparation and colloidal characterization
Dispersion preparation and colloidal characterization of SiO2 NPs in either water or cell culture medium were performed according to previously published Nature protocol26. Briefly, 1.0 mL of SiO2 NP suspension at 0.5 mg/ mL in deionized (DI) water was sonicated using Branson Sonifier S-450D (400 W, with Branson 3-in. cup horn) until 161 J/mL sonication energy was delivered to the suspension, which is the critical energy required for dispersion preparation of this ENM25. The colloidal properties of SiO2 NPs in DI water, including hydrodynamic diameter (z-average, dH), polydispersity index (PDI), and zeta potential were measured by dynamic light scattering (DLS, Zetasizer Nano ZS, Malvern UK). After sonication, SiO2 NPs were diluted in cell culture medium (complete RPMI) to a final concentration of 0.1 mg/mL. The colloidal properties of SiO2 NPs in cell culture medium, including effective density, were then measured according to a protocol developed by the authors61.
Human polymorphonuclear neutrophil (PMN) isolation
Fresh human blood was collected with heparin (10 U/mL) from healthy volunteers, as approved by the Mass General Brigham Institutional Review Board (protocol 1999P001297) and after obtaining informed consent from the subjects. For all experiments involving human subjects, experiments were carried out in accordance with the Declaration of Helsinki. PMNs were isolated by density gradient with Ficoll-histopaque (Sigma-Aldrich, 10771)62,63. Apoptotic neutrophils were prepared by plating 1 × 107 cells/mL in 5 mL DPBS (ThermoFisher Scientific, MA) for 24 h in a 100 mm × 20 mm Petri dish (Corning, AZ). After 24 h incubation, cells were collected with EDTA (5 mM) (Millipore Sigma, MO)64 and apoptotic neutrophils stained with Cell Trace™ Carboxyfluorescein succinimidyl ester (CFSE) at a concentration of 5 µM for 30 min at 37 °C (ThermoFisher Scientific, MA).
Human M1 and M2-like macrophage polarization and differentiation
Human peripheral blood mononuclear cells (PBMCs) were obtained from deidentified leukopacks from Boston Children’s Hospital Blood Bank (Boston, MA) under protocol #1999-P-001279 approved by the Mass General Brigham Institutional Review Board. The experiments were carried out in accordance with the Declaration of Helsinki. PBMCs were isolated by Ficoll-Histopaque-1077 density-gradient, followed by monocyte purification. Monocytes were differentiated for 7 days in RPMI 1640 (Lonza) containing 10% fetal calf serum (ThermoFisher Scientific, 16000-044), 2 mM L-glutamine (Lonza, 17-605E), 2 mM penicillin– streptomycin (Lonza, 17-602E), and 20 (ng/mL) of either with human recombinant granulocyte-macrophage colony-stimulating factor (hr-GMCSF, for M1 differentiation), or human recombinant macrophage colony-stimulating factor (hr-MCSF, for M2 differentiation) (PeproTech, 300 − 25) at 37 °C. Macrophages were then polarized to either M1-like macrophages with LPS (100 ng/ml) plus INF-γ (20 ng/ml) for 48 h or to M2-like macrophages with 20 ng/mL of IL-4 (PeproTech, 200-04) for 48 h at 37 °C65,66.
SiO2 NP impact on human macrophages
M1 and M2 macrophages (105 cells/well) were exposed to 1, 10, or 100 µg/mL SiO2 NPs for 24 h, at 37 °C 5% CO2. Mitochondrial activity on macrophages from five donors was assessed using the Invitrogen™ PrestoBlue® assay (ThermoFisher), a classical resazurin-based assay, following the manufacturer’s instructions. For each donor, signals were normalized along a scale from − 100 (no mitochondrial activity in medium-only wells) to 0% (baseline from unexposed cells). Cells viability was confirmed using a lysis buffer for 45 min as positive control. Next, mitochondrial membrane potential (ΔΨm) was measured using the Biotium JC-1 Kit as per the manufacturer’s instructions on macrophages from three donors. For each donor, signals were normalized along a scale from − 100 100 (no mitochondrial membrane potential in medium-only wells) to 0% (baseline from unexposed cells).
Human macrophage phagocytosis of E. Coli
E. coli serotype O6: K2:H1 was cultured in Luria-Bertani broth, collected at mid-log phase (OD600nm = 0.5; 0.5 × 109 colony-forming units/mL), and labeled with BacLight Green (Molecular Probes, B35000). Human M2 macrophages were plated onto 6 well plates (1 × 106 cells per well) 24 h before the experiments. Macrophages were pre-incubated with RvD5, SiO2 NP (10 µg), or vehicle control (containing 0.01% ethanol vol/vol) in DPBS containing Ca2+ and Mg2+ for 15 min at 37 °C, then E. coli was added at a ratio of 50:1 for 1.5 h. The incubation of M2 macrophages with SiO2 NPs was limited to a maximum of 180 min, because at this timepoint, viability remained greater than 90% when assessed by Annexin V/PI staining and trypan blue exclusion. Cells were then washed with DPBS containing 5 mM ethylenediaminetetraacetic acid (EDTA) to remove undigested and membrane-bound E. coli M2 macrophages were detached using 5 mM of EDTA for 20 min and fixed in FACS buffer consisting of DPBS with 10% FBS also containing 2% paraformaldehyde (Electron Microscopy Sciences). M2-like macrophages were then taken to flow cytometry to assess phagocytosis (BacLight Green). All flow cytometric samples were assessed using BD LSR Fortessa (BD Biosciences, CA) and analyzed using FlowJoX.
Human macrophage efferocytosis of apoptotic human PMNs
Human M2-like macrophages were plated into 6-well plates (2 × 106 cells per well) in DPBS containing Ca2+ and Mg2+. CFSE-labeled apoptotic neutrophils were then added to M2 macrophages at a ratio of 5:1 (PMNs/macrophage) for 120 h at 37 °C. Cells were washed with DPBS containing 5 mM ethylenediaminetetraacetic acid (EDTA) to remove undigested and membrane-bound neutrophils. M2 macrophages were detached using 5 mM of EDTA for 20 min and fixed in FACS buffer consisting of DPBS with 10% FBS also containing 2% paraformaldehyde (Electron Microscopy Sciences). M2-like macrophages were then taken to flow cytometry to assess efferocytosis (CFSE intensity). All flow cytometric samples were assessed using BD LSR Fortessa (BD Biosciences, CA).
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) and UV spectrophotometry
Human M2-like macrophages (10 × 106 cells) were incubated with SiO2 NPs (10 µg/mL), E. coli, or vehicle for 30 min, pH 7.45, 37 °C in DPBS+/+ containing both Ca2+ and Mg2+. Incubations were stopped using two volumes of ice-cold LC-MS-grade methanol (Thermo Fisher Scientific, Waltham, MA) containing deuterated internal standards d4-LTB4 and d4-PGE2 purchased from Cayman Chemical Company (Ann Arbor, Ml). Samples were placed at -80 °C for at least 30 min to allow protein precipitation, followed by centrifugation (3000 rpm, 10 min, 4 °C). Supernatants were collected and concentrated to ~ 1 mL using a gentle stream of nitrogen in a Turbo Vap LV, (Biotage, Charlotte, NC). Each sample was then taken to solid phase extraction (SPE) using an automated system on an Extrahera™ (Biotage, Charlotte, NC) as detailed in67. During extraction, samples were brought to an apparent pH 3.5 using 9 mL of acidified water and were rapidly loaded onto C18 ISOLUTE 100-mg SPE cartridges (Biotage, Charlotte, NC). These cartridges were pre-conditioned with 3 mL of methanol followed by 3 mL of double-distilled water. The samples were then neutralized with 4 mL of double-distilled water, followed by a 4 mL hexane (Supelco, Bellefonte, PA) wash. Lipid mediators were eluted in 4 mL of methyl formate (Sigma-Aldrich, St. Louis, MO). Methyl formate fractions were concentrated to dryness under a gentle stream of nitrogen (Turbo Vap LV, Biotage) and immediately resuspended in 50 µL of LC-MS grade methanol-water mixture (1:1, v/v) for liquid chromatography-tandem mass spectrometric (LC-MS/MS) data acquisition.
The LC-MS-MS data were acquired in negative polarity on a Triple Quadrupole 7500 mass spectrometer (SCIEX, Framingham, MA) coupled with a SCIEX ExionLC system and a Kinetex PS C18 100Å column 100 mm x 3.0 mm x 2.6 μm (Phenomenex, Torrance, CA) maintained at 50 °C as described in68. The mobile phase consisted of Solvent A (Water, 0.1% formic acid) and Solvent B (Methanol, 0.1% formic acid). Mediators were eluted using a 0.5 mL/min flow rate in a gradient of Solvent A/Solvent B (55/45, v, v) from 0 to 2 min, and then changed to Solvent A/Solvent B (20/80, v, v) from 2 min to 16.5 min, and the third segment was increased to Solvent A/Solvent B (2/98, v, v) from 16.6 min to 18.5 min. The final segment consisted of Solvent A/ Solvent B (90/10, v, v) from 18.6 min to 20.9 min. Source and gas parameters were set as indicated: collision gas (CAD) = 12, curtain gas (CUR) = 40, ion source gas 1 (GS1, psi) = 45, ion source gas 2 (GS2, psi) = 70, ion spray voltage (IS, V) = -2000, and temperature (TEM, °C) = 500. Data were obtained using SCIEX OS 3.1.5.3945 and analyzed with SCIEX OS 3.1.5.3945.
Lipid Mediator (LM) authentication and identification were accomplished by matching their chromatographic retention times (TR) and tandem mass spectral (MS/MS) data to those of synthetic and authentic materials67,68. A customized MS/MS library containing spectra of the synthetic standards was utilized for spectral matching. Spectral parameters were set as follows: precursor mass tolerance ± 0.8 Da; collision energy ± 5 eV; use polarity, intensity threshold = 0.05; minimal purity = 5.0%; and intensity factor = 100. Note that the accuracy for this mass spectral data acquisition with this SCIEX 7500 is ± 0.1 atomic mass units (a.m.u.) and that the additional digits presented in spectral data are due to default manufacturer settings. LC/ESI-MS-MS data are presented as screen captures taken from SCIEX software. UV spectral data were acquired using a Cary 3500 Compact Peltier UV-Vis Spectrophotometer with an Agilent Cary UV Workstation 1.3.4 (Agilent Technologies, Santa Clara, CA).
Mice
C57BL/6 male mice (8–12 weeks old – The Jackson Laboratory, Farmington, CT) were maintained in with free access to commercial chow and water. Animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Brigham and Women’s Hospital (2016N000356; AAALAC 1729) and were conducted in accordance with NIH Guidelines for the Care and Use of Laboratory Animals and the ARRIVE guidelines.
In vivo oropharyngeal instillation of SiO2 NPs
Mice were anesthetized with 2% isoflurane in oxygen. Oropharyngeal intratracheal administration of SiO2 NPs was performed by suspending the anesthetized mice by their cranial incisors using a delicate rubber band attached to a board stand. The nostrils were gently clamped, and the tongue was gently maneuvered out of the oral cavity using forceps. 50 µL of either SiO2 NPs (5 µg, diluted in sterile H2O) or H2O was placed at the posterior pharynx (tongue base) and the complete aspiration of the suspension was ensured through continuous monitoring of respiration before releasing the tongue and nostrils. Daily instillations were performed for 5 consecutive days.
Streptococcus pneumoniae infection
Mice were infected intranasally with 1 × 105 CFU of Streptococcus pneumoniae (ATCC 6303, serotype 3) 24 h after the last oropharyngeal intratracheal instillation. For that, the bacterial inoculum was prepared as previously described49 and mice were anesthetized with ketamine/xylazine (80 mg/kg and 10 mg/kg, respectively). Inoculum was confirmed by serial plating in blood agar.
In separate experiments, SiO2 NP-exposed mice or those exposed to vehicle were intravenously treated with RvD5 (100 ng/ mouse) at the time of infection and again 24 h later. At 48 h post-infection, mice were euthanized with an overdose of ketamine/xylazine anesthetic (250 mg/kg ketamine and 20 mg/kg xylazine, intraperitoneally) for immunophenotyping and bacterial counts.
Bronchoalveolar lavage
At 48 h post-infection, mice were euthanized as mentioned above, and trachea were exposed for the bronchoalveolar lavage (BAL). Briefly, an 18-gauge angiocatheter was inserted into the mouse trachea and the lungs were washed three times with 1 mL of ice-cold PBS with 0.6 mM EDTA (2 times). An aliquot of BAL was used for bacteria assessment by plating on blood agar. Next, BAL cell pellet was used for total and differential cell counts by Trypan blue and flow cytometry, respectively. Supernatant of BAL was used for total protein level analysis by the Bradford assay (BioRad®), ELISA (as per manufacturer’s instructions) and LEGENDplex™ (Biolegend Inc.®).
Histology
Another cohort of mice were euthanized, and lungs were fixed with zinc fixative under a transpulmonary pressure of 20 cm H2O. After embedding the tissue in paraffin, lung sections of five-micrometers were prepared and stained with hematoxylin for 10 s followed by a washing step and eosin staining (H&E).
Statistical analysis
All data were expressed as mean ± S.E.M. Statistical significance was defined as p < 0.05. Two-group comparisons were made using a two-tailed unpaired Student’s t-test or Mann–Whitney U test, for non-normal distribution. One-way ANOVA, followed by a post-hoc test, was used for multiple group comparisons. Statistical analyses were performed using GraphPad Prism version 10.0 for Windows (GraphPad Software, San Diego, CA).
Data availability
The data that supports the findings of this study are available in the supplementary material of this article. Raw LC-MS/MS datasets will be available in the BioStudies (https://www.ebi.ac.uk/biostudies/studies/S-BSST1666) database upon publication.
References
Pirela, S. V., Martin, J., Bello, D. & Demokritou, P. Nanoparticle exposures from nano-enabled toner-based printing equipment and human health: State of science and future research needs. Crit. Rev. Toxicol. 47, 678–704. https://doi.org/10.1080/10408444.2017.1318354 (2017).
Eleftheriadou, M., Pyrgiotakis, G. & Demokritou, P. Nanotechnology to the rescue: Using nano-enabled approaches in microbiological food safety and quality. Curr. Opin. Biotechnol. 44, 87–93. https://doi.org/10.1016/j.copbio.2016.11.012 (2017).
Health, N. I., f., O. S. & a. DHHS Publication; no. (NIOSH) 2013 – 101 (Department of Health and Human Services - Centers for Disease Control and Prevention, 2012).
Nel, A., Xia, T., Madler, L. & Li, N. Toxic potential of materials at the nanolevel. Science 311, 622–627. https://doi.org/10.1126/science.1114397 (2006).
Demokritou, P. et al. An in vivo and in vitro toxicological characterisation of realistic nanoscale CeO2 inhalation exposures. Nanotoxicology 7, 1338–1350 (2013).
Wiesner, M. R. Responsible development of nanotechnologies for water and wastewater treatment. Water Sci. Technol. 53, 45–51. https://doi.org/10.2166/wst.2006.105 (2006).
Cohen, J. M. et al. Tracking translocation of industrially relevant engineered nanomaterials (ENMs) across alveolar epithelial monolayers in vitro. Nanotoxicol. Press. https://doi.org/10.3109/17435390.17432013.17879612 (2014).
Lu, X. et al. In vivo epigenetic effects induced by engineered nanomaterials: A case study of copper oxide and laser printer-emitted engineered nanoparticles. Nanotoxicology 10, 629–639. https://doi.org/10.3109/17435390.2015.1108473 (2016).
Bitounis, D. et al. Printer center nanoparticles alter the DNA repair capacity of human bronchial airway epithelial cells. NanoImpact 25, 100379. https://doi.org/10.1016/j.impact.2022.100379 (2022).
Lizonova, D., Nagarkar, A., Demokritou, P. & Kelesidis, G. A. Effective density of inhaled environmental and engineered nanoparticles and its impact on the lung deposition and dosimetry. Part. Fibre Toxicol. 21, 7. https://doi.org/10.1186/s12989-024-00567-9 (2024).
Wang, M. et al. Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction. Part. Fibre Toxicol. 17, 23. https://doi.org/10.1186/s12989-020-00353-3 (2020).
Favor, O. K. et al. Crystalline silica-induced proinflammatory eicosanoid storm in novel alveolar macrophage model quelled by docosahexaenoic acid supplementation. Front. Immunol. 14, 1274147. https://doi.org/10.3389/fimmu.2023.1274147 (2023).
Kageyama, T., Ito, T., Tanaka, S. & Nakajima, H. Physiological and immunological barriers in the lung. Semin Immunopathol. 45, 533–547. https://doi.org/10.1007/s00281-024-01003-y (2024).
Malainou, C., Abdin, S. M., Lachmann, N., Matt, U. & Herold, S. Alveolar macrophages in tissue homeostasis, inflammation, and infection: Evolving concepts of therapeutic targeting. J. Clin. Invest. 133 https://doi.org/10.1172/JCI170501 (2023).
Serhan, C. N. & Levy, B. D. Proresolving lipid mediators in the respiratory system. Annu. Rev. Physiol. https://doi.org/10.1146/annurev-physiol-020924-033209 (2024).
Ma, Q. Polarization of Immune cells in the pathologic response to Inhaled particulates. Front. Immunol. 11, 1060. https://doi.org/10.3389/fimmu.2020.01060 (2020).
Lim, C. S. et al. Resolution of pulmonary inflammation Induced by Carbon Nanotubes and fullerenes in mice: Role of macrophage polarization. Front. Immunol. 11, 1186. https://doi.org/10.3389/fimmu.2020.01186 (2020).
Basil, M. C. & Levy, B. D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 16, 51–67. https://doi.org/10.1038/nri.2015.4 (2016).
Hsiao, H. M. et al. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One. 8, e58258. https://doi.org/10.1371/journal.pone.0058258 (2013). [doi] PONE-D-12-29581 [pii].
Dominguez, E. C. et al. Aspirin-triggered resolvin D1 reduces chronic dust-induced lung pathology without altering susceptibility to dust-enhanced carcinogenesis. Cancers (Basel) 14, (1900). (2022) https://doi.org/10.3390/cancers14081900
Heires, A. J., Samuelson, D., Villageliu, D., Nordgren, T. M. & Romberger, D. J. Agricultural dust derived bacterial extracellular vesicle mediated inflammation is attenuated by DHA. Sci. Rep. 13, 2767. https://doi.org/10.1038/s41598-023-29781-9 (2023).
Hsiao, H. M. et al. Resolvin D1 reduces Emphysema and chronic inflammation. Am. J. Pathol. 185, 3189–3201. https://doi.org/10.1016/j.ajpath.2015.08.008 (2015).
Chiang, N. et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528. https://doi.org/10.1038/nature11042 (2012).
Scruggs, S. Inflammation resolution gets top billing at NIH workshop. Environ. Factor. https://factor.niehs.nih.gov/2019/5; (2019). accessed 2023/09/25.
Beltran-Huarac, J. et al. Development of reference metal and metal oxide engineered nanomaterials for nanotoxicology research using high throughput and precision flame spray synthesis approaches. NanoImpact 10, 26–37. https://doi.org/10.1016/j.impact.2017.11.007 (2018).
Cohen, J. M., Beltran-Huarac, J., Pyrgiotakis, G. & Demokritou, P. Effective delivery of sonication energy to fast settling and agglomerating nanomaterial suspensions for cellular studies: Implications for stability, particle kinetics, dosimetry and toxicity. NanoImpact 10, 81–86. https://doi.org/10.1016/j.impact.2017.12.002 (2018).
Serhan, C. N. & Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 6, 1191–1197. https://doi.org/10.1038/ni1276 (2005).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Invest. 122, 787–795. https://doi.org/10.1172/JCI59643 (2012).
Werz, O. et al. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat. Commun. 9, 59. https://doi.org/10.1038/s41467-017-02538-5 (2018).
Chiang, N. et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 (2012).
Collaborators, G. B. D. L., R. & I Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the global burden of Disease Study 2016. Lancet Infect. Dis. 18, 1191–1210. https://doi.org/10.1016/S1473-3099(18)30310-4 (2018).
Vinay Kumar, A. K. A. & Aster, J. C. Robbins & Cotran Pathologic Basis of Disease10 edn, (Elsevier, 2020).
Serhan, C. N. & Levy, B. D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128, 2657–2669. https://doi.org/10.1172/JCI97943 (2018).
Perretti, M. & Subramanian, M. Resolution pharmacology - A fresh approach to the clinical management of human inflammatory diseases. Semin Immunol. 65, 101669. https://doi.org/10.1016/j.smim.2022.101669 (2023).
Musher, D. M. & Thorner, A. R. Community-acquired pneumonia. N Engl. J. Med. 371, 1619–1628. https://doi.org/10.1056/NEJMra1312885 (2014).
Rubio, L. et al. Safer-by-design flame-sprayed silicon dioxide nanoparticles: The role of silanol content on ROS generation, surface activity and cytotoxicity. Part. Fibre Toxicol. 16, 40. https://doi.org/10.1186/s12989-019-0325-1 (2019).
Zhang, H. et al. Processing pathway dependence of amorphous silica nanoparticle toxicity: Colloidal vs pyrolytic. J. Am. Chem. Soc. 134, 15790–15804. https://doi.org/10.1021/ja304907c (2012).
Konduru, N. V. et al. Surface modification of zinc oxide nanoparticles with amorphous silica alters their fate in the circulation. Nanotoxicology 10, 720–727. https://doi.org/10.3109/17435390.2015.1113322 (2016).
Ma, J. et al. Effects of amorphous silica coating on cerium oxide nanoparticles induced pulmonary responses. Toxicol. Appl. Pharmacol. 288, 63–73. https://doi.org/10.1016/j.taap.2015.07.012 (2015).
Konduru, N. V. et al. Silica coating influences the corona and biokinetics of cerium oxide nanoparticles. Part. Fibre Toxicol. 12, 31. https://doi.org/10.1186/s12989-015-0106-4 (2015).
Camaioni, A. et al. Silica encapsulation of ZnO nanoparticles reduces their toxicity for cumulus cell-oocyte-complex expansion. Part. Fibre Toxicol. 18, 33. https://doi.org/10.1186/s12989-021-00424-z (2021).
Johnston, C. J. et al. Pulmonary chemokine and mutagenic responses in rats after subchronic inhalation of amorphous and crystalline silica. Toxicol. Sci. 56, 405–413. https://doi.org/10.1093/toxsci/56.2.405 (2000).
Yazdimamaghani, M., Moos, P. J., Dobrovolskaia, M. A. & Ghandehari, H. Genotoxicity of amorphous silica nanoparticles: Status and prospects. Nanomedicine 16, 106–125. https://doi.org/10.1016/j.nano.2018.11.013 (2019).
Singh, D. et al. Release of particulate matter from nano-enabled building materials (NEBMs) across their lifecycle: Potential occupational health and safety implications. J. Hazard. Mater. 422, 126771. https://doi.org/10.1016/j.jhazmat.2021.126771 (2022).
Norling, L. V. et al. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J. Immunol. 186, 5543–5547. https://doi.org/10.4049/jimmunol.1003865 (2011).
Levy, E. S. et al. Tissue factor targeting peptide enhances nanoparticle binding and delivery of a synthetic specialized pro-resolving lipid mediator to injured arteries. JVS Vasc. Sci. 4, 100126. https://doi.org/10.1016/j.jvssci.2023.100126 (2023).
Gao, J. et al. Co-delivery of resolvin D1 and antibiotics with nanovesicles to lungs resolves inflammation and clears bacteria in mice. Commun. Biol. 3, 680. https://doi.org/10.1038/s42003-020-01410-5 (2020).
Li, J. et al. Pulmonary delivery of specialized pro-resolving mediators-based Nanotherapeutics attenuates pulmonary fibrosis in Preclinical Animal models. ACS Nano. 17, 15354–15370. https://doi.org/10.1021/acsnano.2c10388 (2023).
L, P. T. et al. Cysteinyl maresins reprogram macrophages to protect mice from Streptococcus pneumoniae after Influenza A Virus Infection. mBio 13, e0126722 (2022). https://doi.org/10.1128/mbio.01267-22
Tsugita, M., Morimoto, N. & Nakayama, M. SiO(2) and TiO(2) nanoparticles synergistically trigger macrophage inflammatory responses. Part. Fibre Toxicol. 14, 11. https://doi.org/10.1186/s12989-017-0192-6 (2017).
Frasch, S. C. et al. Signaling via macrophage G2A enhances efferocytosis of dying neutrophils by augmentation of rac activity. J. Biol. Chem. 286, 12108–12122. https://doi.org/10.1074/jbc.M110.181800 (2011).
Dalli, J. & Serhan, C. N. Specific lipid mediator signatures of human phagocytes: Microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120, e60–72 (2012). https://doi.org/10.1182/blood-2012-04-423525
Hoppstadter, J. et al. M2 polarization enhances silica nanoparticle uptake by macrophages. Front. Pharmacol. 6, 55. https://doi.org/10.3389/fphar.2015.00055 (2015).
Alqahtani, S. et al. Disruption of pulmonary resolution mediators contribute to exacerbated silver nanoparticle-induced acute inflammation in a metabolic syndrome mouse model. Toxicol. Appl. Pharmacol. 431, 115730. https://doi.org/10.1016/j.taap.2021.115730 (2021).
Cardoso, R. D. R. et al. Resolvin D5 (RvD5) reduces renal damage caused by LPS endotoxemia in female mice. Molecules 28 https://doi.org/10.3390/molecules28010121 (2022).
Thatcher, T. H., Woeller, C. F., McCarthy, C. E. & Sime, P. J. Quenching the fires: Pro-resolving mediators, air pollution, and smoking. Pharmacol. Ther. 197, 212–224. https://doi.org/10.1016/j.pharmthera.2019.02.001 (2019).
Wu, W., Jin, Y. & Carlsten, C. Inflammatory health effects of indoor and outdoor particulate matter. J. Allergy Clin. Immunol. 141, 833–844. https://doi.org/10.1016/j.jaci.2017.12.981 (2018).
Gass, S. et al. A safer formulation concept for flame-generated engineered nanomaterials. ACS Sustain. Chem. Eng. 1, 843–857. https://doi.org/10.1021/sc300152f (2013).
Sotiriou, G. A. et al. A novel platform for pulmonary and cardiovascular toxicological characterization of inhaled engineered nanomaterials. Nanotoxicology 6, 680–690. https://doi.org/10.3109/17435390.2011.604439 (2012).
Demokritou, P. et al. Development and characterization of a Versatile Engineered Nanomaterial Generation System (VENGES) suitable for toxicological studies. Inhal Toxicol. 22 Suppl 2, 107–116. https://doi.org/10.3109/08958378.2010.499385 (2010).
DeLoid, G. M., Cohen, J. M., Pyrgiotakis, G. & Demokritou, P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Protoc. 12, 355–371. https://doi.org/10.1038/nprot.2016.172 (2017).
Nauseef, W. M. Isolation of human neutrophils from venous blood. Methods Mol. Biol. 1124, 13–18. https://doi.org/10.1007/978-1-62703-845-4_2 (2014).
Dagur, P. K. & McCoy, J. P. Jr. Collection, storage, and preparation of human blood cells. Curr. Protoc. Cytom. 73, 511–5116. https://doi.org/10.1002/0471142956.cy0501s73 (2015).
Cassatella, M. A. in Chem. Immunol. Allergy Vol. 83, pp. 232 (Karger, Basel, 2003).
Martinez, F. O., Gordon, S., Locati, M. & Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 177, 7303–7311. https://doi.org/10.4049/jimmunol.177.10.7303 (2006).
Locati, M., Curtale, G. & Mantovani, A. Diversity Mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 15, 123–147. https://doi.org/10.1146/annurev-pathmechdis-012418-012718 (2020).
Shay, A. E. et al. Human leukocytes selectively convert 4S,5S-epoxy-resolvin to resolvin D3, resolvin D4, and a cys-resolvin isomer. Proc. Natl. Acad. Sci. U S A. 118 https://doi.org/10.1073/pnas.2116559118 (2021).
Nshimiyimana, R. et al. Biosynthesis of resolvin D1, resolvin D2, and RCTR1 from 7,8(S,S)-epoxytetraene in human neutrophils and macrophages. Proc. Natl. Acad. Sci. U S A. 121, e2405821121. https://doi.org/10.1073/pnas.2405821121 (2024).
Acknowledgements
We thank Julie Nijmeh and Mary H. Small for expert assistance in manuscript preparation. This work was supported by the National Institutes of Health, National Institute EHS R56ES033250 (BDL, CNS), R01 HL168899 (BDL, CNS, PD) and R35GM139430 (CNS). The engineered nanomaterials used in this work were characterized and provided by the Engineered Nanomaterials Resource and Coordination Core established at Harvard T. H. Chan School of Public Health (NIH Grant No. U24ES026946) as part of the NIEHS/Nanotechnology Health Implications Research Consortium. We also thank Dr. Xavier Dela Rosa from the CET&RI for carrying out initial experiments with human macrophages.
Author information
Authors and Affiliations
Contributions
Conceptualization: CNS, BDL & PD. Human cell assays: DB, SL. Mouse experiments: LPT. Lipidomics: RN. Investigation: LPT, SL, CNS, BDL, DB. Statistics: LPT, SL, DB. Funding acquisition: BDL, CNS, PD. Project administration: BDL, CNS. Supervision: BDL, CNS, PD. Writing: LPT, BDL, CNS. Review & editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tavares, L.P., Libreros, S., Bitounis, D. et al. SiO2 nanoparticles as disruptors of endogenous resolution mechanisms of inflammatory responses that exacerbate pneumonia. Sci Rep 15, 6398 (2025). https://doi.org/10.1038/s41598-025-89700-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89700-y