Abstract
Red lionfish, Pterois volitans, a non-native marine species with potential to cause significant damage to Caribbean coral reefs, reached Barbados in late 2011. In 2012, before lionfish became locally established, fish surveys at ten reef sites in Barbados were undertaken every four months over a year to generate baseline data. Fisher catch surveys were also undertaken at two main landing sites twice in a year. A decade later, all surveys were repeated at the same sites. Post-invasion lionfish biomass was low across most sites and increased with site depth, likely due to fishing. A comparison of reef fish biomass of selected key herbivores of high ecological and commercial importance (parrotfishes and surgeonfishes) and forage fish groups (damselfishes and wrasses) pre- and post-invasion indicated no detectable effects of lionfish on the key herbivores and wrasses, although damselfish biomass did decline with lionfish biomass increases. We also found no evidence of a decline in fisher catch rates, suggesting no negative impacts on fisher earnings. Furthermore, catch composition remained virtually unchanged for trap fishers, while lionfish had become an important component of the catch of spearfishers. Overall, our results suggest that control of lionfish through sustained fishing effectively protects key fish herbivores and might indirectly benefit reefs through a release of fishing pressure on native fishes by spearfishers now targeting lionfish.
Similar content being viewed by others
Introduction
The lionfish invasion (comprising two species) is unprecedented with regard to the speed and geographical range over which it has spread1,2, and its potential to become the most damaging marine fish invasion globally to date3. Both species (the red lionfish Pterois volitans and the common lionfish P. miles) are now well established across the western North Atlantic since their first introductions in Florida in the late 1980s and early 1990s4,5. Since then, P. volitans has spread throughout the Gulf of Mexico and Caribbean basin from 2006, reaching the eastern Caribbean islands by 20102, and was first reported in Barbados in late November 20116. The lionfish invasion continues to expand with P. volitans now in the South Atlantic along the coast of Brazil7, and P. miles is spreading rapidly across the Mediterranean8.
This remarkable invasion of the western Atlantic has been aided by the fact that P. volitans has been exceptionally successful at dispersing across locations and colonizing unfamiliar environments, reaching densities of more than 300 individuals per hectare and in some cases several 1000 individuals per hectare on patch reefs in the Bahamas9,10,11,12. As such, the red lionfish is considered a potentially significant ecological threat to the coral-reef ecosystems of the Caribbean through the consumption of native prey and displacement of potential competitors and resultant changes in the species composition and ecosystem services of reefs3,13,14. Of particular concern is lionfish predation on parrotfishes and other key herbivore populations, such as surgeonfishes3,15,16, which provide two main types of services to humans. First, these herbivores graze large amounts of algae on the reefs, playing a key role in maintaining healthy and attractive reefs17,18 that contribute to coastal protection and support high biodiversity and recreational diving, an industry that generates millions of dollars annually across the Caribbean, including Barbados19. Second, they are among the most important target families in the nearshore fisheries of many Caribbean islands20. For example, in Barbados they contribute to food security and provide important income to fishers in small-scale fisheries that contribute several hundred metric tons to the island’s annual fish landings21,22. Thus, the invasion has also been viewed as a substantial socio-economic threat to Caribbean countries14,23,24, especially those whose economies rely heavily on healthy reefs, such as Barbados25. The ultimate impact of this threat being the loss of vital ecosystem services provided by reefs, including provision of food and livelihood security to reef fishers and the wider community and the aesthetic quality and value to the watersports tourism sector. As a result, the lionfish invasion continues to be viewed as a key concern for marine conservation and management23,24.
Considering that management of established non-native species invasions is likely to involve extensive effort and associated costs, there is need to evaluate the actual costs of management action in relation to the true costs of ecosystem consequences (good and bad) of a non-native species invasion26. Proactive management, including early detection and intervention, are key aspects in slowing down the spread of biological invasions27. In this regard, the late arrival of lionfish in Barbados within the context of the Caribbean invasion afforded the country time to learn from others and prepare. In anticipation, the Government drafted a ‘Lionfish Response Plan’28, which included a public education campaign, the promotion of harvesting lionfish for human consumption, and training in handling and preparing lionfish for the market. Today, lionfish are actively targeted by commercial and recreational fishers and have become a valued market fish in Barbados29.
Importantly, the Lionfish Response Plan also involved conducting underwater reef fish surveys at ten sites representative of Barbados’ reefs during the fall, winter, and summer of 2012 to characterize natural spatiotemporal variability in native reef fish assemblages shortly before lionfish became locally established. These surveys focused on key fish herbivores (parrotfishes and surgeonfishes) and highly abundant forage fishes (wrasses and damselfishes). Reef fisher surveys were also conducted twice in the same year (fall and summer) at two of the main landing sites in Barbados to characterize catch yields and composition. Thus, this Plan allowed the provision of critical pre-invasion data that could be used as an ecosystem baseline in this study.
Here, we use these pre-invasion data as a baseline to assess whether the management response in Barbados has helped minimize negative impacts of lionfish on both native reef fishes and fisher yields in Barbados. We do so by (1) quantifying post-invasion lionfish biomass and densities across the selected reef sites and the contribution of lionfish to fishers’ catches; (2) assessing whether the biomass of key fish herbivores and forage fish and/or reef fisher yields have decreased 10 years post-invasion, and (3) testing whether any such potential decreases in reef fish biomass are explained by lionfish biomass.
Results
Post-invasion lionfish biomass and density across reefs
Lionfish were never recorded during the pre-invasion period, but were recorded in 11.8% of the 297 transects, 43.3% of the 60 surveys, and 80.0% of the ten sites during the post-invasion period (Table 1). Mean lionfish biomass estimates ranged between 0.00 and 0.53 kg per 100 m2 across the 30 surveys (3 seasons × 10 sites; Table 1), yielding between 0.00 and 0.38 kg per 100 m2 per survey when survey data were averaged for each site (Fig. 1). Mean lionfish density estimates varied between 0.00 and 1.46 lionfish per 100 m2 across the 30 surveys, yielding between 0.00 and 1.28 lionfish per 100 m2 per survey when survey data were averaged for each site. These data also revealed that, overall, lionfish were more frequently found on the deep bank reefs (32.2% of 297 transects) than on shallow patch (4.4% of 90 transects) and fringing reefs (2.5% of 120 transects). This translated into a significant positive relationship between average lionfish biomass per survey at each site and site depth (p = 0.046; Fig. 1).
Relationship between post-invasion lionfish biomass and depth across 10 reefs (three bank, four fringing, and three patch reefs) on the west and south coasts of Barbados. Biomass values represent the averages of three surveys at each site (winter, summer and fall surveys) (See Table 1 for survey values) during the post-invasion period. The results of a Spearman rank correlation test between site depth and average lionfish biomass per survey are also shown (n = 10).
Spatiotemporal variability in native reef fish
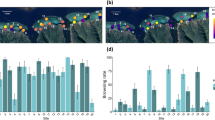
Visual inspection of boxplots of the parrotfish biomass transect data supported overall differences among sites based on median values per seasonal survey (e.g. Accra vs Sixmens; Fig. 2a), but no consistent evidence that post-invasion parrotfish biomass was lower than pre-invasion levels when data were examined for each site across surveys (Fig. 2a). The only instances supporting lower post-invasion parrotfish biomass, based on a lack of overlap in boxplot interquartile ranges, took place during the fall survey (October) at Coconut Court and Southern Palms (2 out of 30 comparisons; Fig. 2a).
Boxplots of fish biomass transect data during the pre-invasion (light blue boxplots) and post-invasion (orange boxplots) period for parrotfishes (a) and surgeonfishes (b) at 10 reefs (one panel per reef) representative of three reef types (B: bank; F: fringing; P: patch) during the fall, winter and summer seasons. All surveys involve ten 60 m2 transects except for Accra during the fall (n = 8) and winter (n = 9) seasons of the post-invasion period and Holetown during the winter of the pre-invasion period (n = 9). Data were standardized to 100 m2. Dashed vertical reference line represents the overall median transect value for the data pooled across all sites and surveys. Biomass values on the y-axis are shown on a fourth root transformed scale.
Similarly, there was evidence of marked differences among sites in overall surgeonfish biomass (e.g. Accra vs Southern Palms; Fig. 2b) but no consistent evidence that post-invasion biomass was lower than pre-invasion levels when data were examined for each site across surveys (Fig. 2b). The only case supporting lower post-invasion surgeonfish biomass was during the winter survey (January and February) at Holetown (1 out of 30 comparisons; Fig. 2b).
For wrasses, there was also evidence of differences among sites in overall biomass based on median values (e.g. Batt’s Rock vs South Bellairs; Fig. 3a), although these differences were not as marked as those observed for parrotfishes and surgeonfishes. That said, there was again no consistent evidence that post-invasion biomass was lower than pre-invasion levels when data were examined for each site across surveys (Fig. 3a). The only instance supporting lower post-invasion surgeonfish biomass took place during the fall survey at Batt’s Rock (1 out of 30 comparisons; Fig. 3a).
Boxplots of fish biomass transect data during the pre-invasion (light blue boxplots) and post-invasion (orange boxplots) period for wrasses (a) and damselfishes (b) at ten reefs (one panel per reef) representative of three reef types (B: bank; F: fringing; P: patch) during the fall, winter, and summer seasons. All surveys involve ten 60 m2 transects except for Accra during the fall (n = 8) and winter (n = 9) seasons of the post-invasion period and Holetown during the winter of the pre-invasion period (n = 9). Data were standardized to 100m2. Dashed vertical reference line represents the overall median transect value for the data pooled across all sites and surveys. Biomass values on the y-axis are shown on a fourth root transformed scale.
Finally, for damselfishes, there was also evidence of differences among sites in overall biomass based on median values (e.g. Coconut Court vs Josef’s; Fig. 3b), although these differences were again not as marked as those observed for parrotfishes and surgeonfishes. There was again no consistent evidence that post-invasion biomass was lower than pre-invasion levels when data were examined for each site across surveys (Fig. 3b). There were, however, more instances to support lower post-invasion damselfish biomass than for the other three fish groups, including the summer (May and June) and fall surveys at Accra, the summer survey at South Bellairs, and the winter survey at both Speightstown and Sixmens (5 out of 30 comparisons; Fig. 3b).
Linking lionfish to variability in reef fish community structure
Changes in fish community structure
A multivariate analysis of variance (MANOVA) of the transect-level data confirmed that the fish community structure (based on parrotfish, surgeonfish, wrasse and damselfish biomass) differed significantly among the ten sites and periods (pre- vs post-invasion) (all p ≤ 0.002; Table 2), with differences between the pre- and post-invasion periods depending on a given site, as evidenced by the significant interaction between these two variables (p = 0.008; Table 2). In contrast, such variability was not significantly linked to season or lionfish biomass, as evidenced by the lack of statistical significance for these two variables (p ≥0.582; Table 2).
Changes in individual fish groups
The mixed-effects linear model analyses focusing separately on parrotfish, surgeonfish, wrasse and damselfish biomass (with data averaged across transects during each survey) as a function of season and period (and their interaction) found that including lionfish biomass as a predictor significantly improved the models only in the case of damselfishes (Table 3). In this case, lionfish biomass had a significant negative effect on damselfish biomass (Fig. 4; Table S1), whereas season and period (or their interaction) did not significantly contribute to explain damselfish biomass variability (Table S1).
Modelled fixed effect of average lionfish biomass per transect on average damselfish biomass per transect at a given reef survey. In the mixed effects model, period (pre- and post-invasion) and season (winter, summer and fall) (and their two-way interaction) and lionfish biomass were treated as fixed effect factors and site was treated as a random effect. Only lionfish biomass was significant (Table 3 and Table S1).
Effects of lionfish on fishing yields and catch composition
Pre- and post-invasion period estimates of fishing yields (after pooling data across seasons and landing sites) were remarkably similar for both trap fishing (Fig. 5a) and spearfishing (Fig. 5b). Consistent with this, the effect of period was not statistically significant (p ≥ 0.236) for either fishing type or season (p ≥ 0.122), although we did find a statistically significant effect of landing site (p ≤ 0.013; Table 4).
Average fishing yields for a trap fishing during the pre-invasion period (n = 22 fishing trips, average yield 3.0 kg/trap) and post-invasion period (n = 34 fishing trips, average yield 3.1 kg/trap) and b spearfishing during the pre-invasion period (n = 11 fishing trips, average yield 1.5 kg/hr) and post-invasion period (n = 27 fishing trips, average yield 1.7 kg/hr).
For trap fishing, the numerical composition of the catch (by family), after pooling data across seasons and landing sites, was broadly similar between the pre- and post-invasion periods with parrotfishes (Scaridae), followed by grunts (Haemulidae), goatfishes (Mullidae), jacks (Carangidae) and surgeonfishes (Acanthuridae) accounting for approximately three quarters (74–77%) of the catch (Fig. 6a). Although lionfish was recorded during the post-invasion period, it represented a negligible proportion of the catch (< 1%) (Fig. 6a). The MANOVA indicated that the effect of period and landing site was significant (p ≤ 0.017), but not that of season (p = 0.240; Table 5). The indicator species analyses identified squirrelfishes (Holocentridae) (statistic: 0.237, p = 0.008) as more specific to the pre-invasion period, whereas no fish group was identified as particularly specific to the post-invasion one.
Average catch relative composition (by fish numbers within families) in a fish trap landings during the pre-invasion (N = 62 fishing trips) and post-invasion period (n = 58 fishing trips) and b spearfishing landings during the pre-invasion (n = 32 fishing trips) and post-invasion period (n = 28 fishing trips).
In contrast, for spearfishing, there was a marked difference in numerical catch composition between the pre- and post-invasion periods (Fig. 6b). Most notably, lionfish (Scorpaenidae) accounted for nearly a quarter of the fish in the catch (23%) during the post-invasion period, whereas it was completely absent in the pre-invasion period (Fig. 6b). Lionfish thus became the second most important fish group caught by spearfishers after parrotfishes (Scaridae; Fig. 6b). The MANOVA indicated that the effects of period and season were significant (p ≤ 0.017), but not that of landing site (p = 0.237; Table 5). The indicator species analyses identified five families, namely grunts (Haemulidae), surgeonfishes (Acanthuridae), large wrasses (Labridae), filefishes (Monacanthidae) and squirrelfishes (Holocentridae) (all statistics: 0.460–0.661, all p ≤ 0.023) as more specific to the pre-invasion period, whereas lionfish was the only fish group identified as more specific to the post-invasion one (statistic: 0.237, p = 0.008).
Discussion
In contrast to the ‘gloom and doom’ scenarios predicted by studies early in the Caribbean lionfish invasion e.g.11,13,30,31, we find no evidence of negative impacts on key reef fish herbivores, i.e. parrotfishes and surgeonfishes, or on reef fisher catch yields for both trap fishers and spearfishers in Barbados. This is likely due to the low lionfish density observed across most of the study sites, which is likely the result of sustained levels of lionfish fishing in Barbados since its arrival. The changes we reveal in both the reef fish communities and spearfisher catch composition indicate that the current level of management is sufficient to mitigate expected negative impacts of the lionfish invasion.
First, we only found evidence of negative effects of lionfish for damselfishes, with a general trend of biomass decrease (relative to the seasonal baseline) at the deepest bank reefs (Accra and Speightstown; Figs. 3b and 4), where lionfish was most abundant (Fig. 1). This is not unexpected given that these damselfishes are one of the most abundant small prey fishes on Barbados’ reefs (representing 51.4% of all small fishes in our surveys). The high abundance of damselfishes (which are not taken by reef fishers) is also likely to be driven by the unnatural lack of competitors and predators32 due to the high levels of fishing pressure to which the reefs of Barbados have been subjected for many years33 preventing recovery of reef fish biomass34. Damselfishes are also known to be highly susceptible to consumption by lionfish35 and are the most frequently reported family in lionfish diet studies across the Caribbean14. Although S. partitus, a generalist feeder, accounted for most (59.4%) of the damselfish biomass recorded during our study, the remaining biomass (40.3%) included ‘algal farming’ Stegastes species such as S. planifrons, S. adustus and S. diencaeus (Microspathodon chrysurus only accounted for 0.3%). A controlling effect of lionfish on the populations of these ‘algal farming’ damselfishes could even be seen as potentially beneficial for live coral in Barbados (which has dramatically decreased since the 1970’s with little evidence of recovery34,36) since farming members of the genus, and most notably S. planifrons, are known to cause significant damage to coral by biting live tissue to extend their turf algae territories37,38.
Second, we found no evidence of a decrease in fishing yields (and hence income) for either trap fishers or spearfishers, with the latter actively targeting lionfish as a commercially valuable species. This contrasts with the only other studies of lionfish impacts on Caribbean fisheries of which we are aware, where the abundance of lobsters in both lobster condos in the Bahamas39,40 and lobster traps in the Florida Keys41,42 significantly decreased in the presence of lionfish, lowering the lobster catch per unit effort in those fisheries. In our study, the fact that fishing yields remained constant while lionfish had become the second most important fish group in the catch of spearfishers during the post-invasion period suggests a release from spearfishing pressure for native fish groups as fishers shift their attention towards lionfish as a viable commercial alternative. That said, we acknowledge that our sample sizes for the fishing yield estimates were small, which warrants some caution in the interpretation of this finding and should be the focus of future research.
Recent studies highlight the ongoing debate regarding negative impacts of lionfish invasions on native reef communities across the Caribbean14,43. The review by Côté and Smith43 noted that not all the early gloomy predictions have come to pass (e.g. strong competition with native predators, shifts in composition of reef benthos, local species extinctions). Interestingly, they also pointed out a potential geographical bias, with the few studies so far conducted in natural reef systems of the southern Caribbean (e.g. Belize: Hackerott et al.44; Venezuela: Elise et al.45) reporting little to no impact of lionfish, thus contrasting with studies reporting impacts on surveyed reefs from more northerly locations (e.g. SE USA: Ballew et al.13 ; Bahamas: Green et al.31, Lesser and Slattery16). However, this variation is likely a result of very different lionfish densities as well as reef characteristics, an issue that remains a topic of active research and contention.
For example, Benkwitt12 determined that the relationship between lionfish density and native prey fish communities is non-linear, with low impacts at low densities, significant impacts at medium densities and leveling off at very high densities. However, the experiments were conducted on small, isolated patch reefs with a range of lionfish densities from 1–12 lionfish m−2. As such her lowest experimental lionfish density (1 m−2) equates to a density of 10,000 lionfish ha-1. Although this was well below the highest natural density observed on her study site reefs (8 lionfish m-2), it nonetheless could be considered an extraordinarily high density of lionfish. Other authors of manipulative experimental studies finding significant impacts of lionfish on native fishes in small Bahamian patch reefs did not state the densities of lionfish used per area, but did give numbers per reef. For example, Albins and Hixon46 placed single lionfish on reefs of approximately 1 m2 and 3 m2, equating to densities of 10,000 and 3,300 lionfish ha-1 respectively. Likewise, Albins47 used densities of one lionfish per 4 m2 reef (2,500 lionfish ha-1). Again, these are at the high end of observed natural densities. Green et al.9 modelled prey biomass production response to variable lionfish densities to predict a critical threshold of lionfish abundance for Bahamian patch reefs below which minimal impacts on native reef communities would be likely (i.e. prey species productivity is able to outstrip lionfish consumption). Their model, intended as a decision support tool for managers, used a metabolic theory of ecology (MTE) framework incorporating ambient temperature, lionfish size and density, and standing biomass of prey to determine this critical threshold and thus an appropriate target abundance for lionfish mitigation efforts. They noted, however, that the high variation in prey abundance among reefs precluded a definitive threshold level of lionfish abundance that could be applied to other locations. Although they did not explicitly state the lionfish densities across their 26 experimental patch reefs, they indicated that reefs were between 100–150 m2 and stated lionfish numbers per reef. Using these values and extrapolating density based on the median reef area (125 m2) to give a ballpark estimate of density, suggests that reef-specific critical threshold density values appear to range between 66–480 lionfish ha-1 for these reefs. This model was subsequently challenged48,49 on the basis of its extreme sensitivity to temperature, although further defended by its authors50.
An alternative model based on mortality-weight coefficients was proposed by Valderrama et al.49, although not yet widely field-tested, and neither model has yet been used as a management tool to the best of our knowledge. As such the critical threshold for lionfish density remains elusive. More recently, Green and Grosholz51 agreed that low densities of lionfish do not elicit a marked decline in prey species and suggest that maintaining lionfish density at or below approximately 25 lionfish ha-1 could prevent declines in native fish and result in low rates of recolonization by lionfish. Similar to the findings of Hackerott et al.44 and Elise et al.45 across natural reef systems at a scale relevant to management, we have demonstrated that lionfish densities in Barbados (between 0 and 128 lionfish ha-1) have remained low enough (8 out of 10 reefs below 25 ha-1 threshold) not to cause detectable negative impacts on native fish communities (except potentially for damselfishes in the two reefs above the threshold) or fisher catches, an important benchmark for ongoing management efforts. The lionfish densities in Barbados are within the range of those reported from Belize and Mexico44 and references therein where, like Barbados, management efforts have encouraged lionfish consumption and targeted spearfishing by recreational and commercial fishers52. These and other studies e.g.53,54,55 provide support for the suggestion by Green and Grosholz51 that lionfish is a good candidate for managing through ‘functional eradication’ (i.e. suppressing invader populations below levels that cause unacceptable ecological effects). Using targeted spearfishing for human consumption is now being promoted or adopted as a cost-effective mitigation tool across many other locations in the Caribbean e.g.52,56,57,58,59, as opposed to attempting full eradication. In line with our own findings, Chapman et al.52 point out that this management strategy not only suppresses lionfish abundance but provides livelihood support to fishers and could alleviate fishing pressure on other reef species. An important role of spearfishing in controlling lionfish in Barbados is further supported by the negative relationship found here between depth and lionfish biomass (Fig. 1), with deeper reefs likely acting as a depth refuge e.g.60,61.
In summary, our study highlights the importance of establishing baseline data to adequately assess the ecological and economic impact of invasive non-native species. This is critical for guiding and prioritizing cost-effective local and regional management actions against invasives when resources are limited, as is generally the case in small Caribbean Island states. Importantly, it shows that functional eradication of lionfish via sustained fishing can be feasible and effective in such a context.
Methods
Assessment of reef fish communities
Underwater fish surveys using SCUBA gear were conducted on 10 coral reef sites in 2012, before the establishment of a local lionfish population; these data represent the pre-invasion baseline period. The same surveys were repeated at the same sites 10 years after, between 2021 and 2022; these data represent the post-invasion period. The 10 sites were selected to represent the three main reef types present in Barbados, namely nearshore shallow fringing reefs (depth: 3–5 m; located on the leeward west coast), nearshore shallow patch reefs (depth: 7–13 m; located on the windward south coast), and offshore deeper bank reefs (depth: 14–26 m; located on both coasts) (Fig. 7).
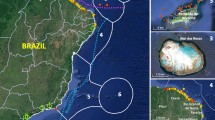
Location of the ten coral reef sites on the west and south coasts of Barbados used for the repeat surveys of native coral reef fish and lionfish (orange, blue and black circles) and main reef fish landing sites where fisher surveys were conducted (blue stars). Map created using ArcGIS Desktop 10.8.2 (https://www.esri.com/en-us/arcgis/products/arcgis-desktop/overview).
In 2012, a total of ten 30 × 2 m belt transects were haphazardly deployed and georeferenced at each site during each survey. All fish belonging to a few key fish families were subsequently counted, identified to species level, and sized along each transect. Because of their size and mobility, we distinguished between large mobile fishes and small sedentary fishes during the surveys. Large mobile fishes here included all species of parrotfishes (Sparisoma spp. and Scarus spp.), surgeonfishes (Acanthurus spp.), and lionfish (Pterois volitans) and one relatively large species of damselfish, M. chrysurus. Small sedentary fishes included all small wrasses (Thalassoma bifasciatum and all Halichoeres spp. except H. garnoti) and all small damselfishes (Stegastes spp.). Our surveys were thus specifically designed to assess potential impacts of lionfish on the key roving herbivores on Caribbean reefs, the parrotfishes and surgeonfishes62, which are also of economic importance for the reef fishery in Barbados21,22. We were also particularly interested in assessing potential effects of lionfish on wrasses and damselfishes because they are the dominant and conspicuous small fish taxa on Barbados’ reefs and thus likely to be commonly preyed upon by lionfish14.
During each fish survey, we first moved along the 2 m-wide belt transect identifying and recording all the large mobile fishes and sizing them to the nearest 1 cm in total length. We then moved back recording the small sedentary fishes along the same transect length, but using a belt transect width of 1 m, and assigning each fish to one of three size classes, namely < 5 cm in total length, 5–10 cm, and > 10 cm. We used FishBase63 length-to-weight parameter values to convert individual fish length estimates to individual biomass estimates. For the large mobile fishes we used their size (recorded to the nearest cm) and for small sedentary fish, we used the mid-points of each size interval (2.5 cm, 7.5 cm and 12.5 cm, respectively) for the length-to-weight conversions. Overall, small sedentary fish in the > 10 cm category were rare (< 10% of all small sedentary fish counts). The large mobile fish and small sedentary fish abundances and biomass estimates were standardized to 100 m2.
In 2012, these underwater fish surveys were first conducted at each site in the winter (January 2012) and then repeated in the summer (May and June 2012) and fall (October 2012) to ensure that any seasonal variation in fish population abundance was captured. Between 2021 and 2022, the post-invasion period, these fish surveys were again repeated at each site using the same georeferenced transects in the summer (June 2021), fall (Oct 2021) and winter (Feb 2022) seasons. In three out of the 60 surveys (2 periods × 3 seasons × 10 sites), 8–9 transects were deployed (instead of ten) due to logistic constraints.
Assessment of reef fishers’ catch composition
Reef fisher surveys were conducted at the two main reef fish landing/marketing sites (Oistins and Pile Bay; Fig. 7) in 2012, before the establishment of a local lionfish population; these data represent the pre-invasion baseline period. Fishers from these two sites are known to fish all reef areas on the west and south coasts where our fish surveys were conducted33. The same surveys were repeated at the same landing sites 10 years later, between 2021 and 2022; these data represent the post-invasion period. For both the pre- and post-invasion periods, two surveys were conducted: one within the winter pelagic fishing season (extending from November–June) and the second within the summer off-season for pelagic fishing (July–October)64. This was to ensure that all reef fishers (those fishing full-time as well as those engaging in the reef fishery only during the pelagic off-season) were sampled, and to ensure that data were not biased by any potential seasonality in the reef fish themselves.
Reef fishers using spears or fish traps were haphazardly surveyed to obtain information on their fishing effort (number of traps hauled, number of hours spearfishing) and the species composition of the catch at the end of a fishing trip. The latter implied either identifying to species level all individual fish landed or identifying the fish from a haphazard sub-sample of the catch of approximately 2.5 kg. When possible, the total fresh weight of landed fish for a fishing trip was also recorded at the time of sale, which allowed calculation of fishing yields for trap fishing (kg caught per trap deployed) and spearfishing (kg per hour of fishing).
Data handling and analysis
Reef fish assemblage data
To assess changes in reef fish assemblage between the pre-invasion and post-invasion period and potential effects of lionfish, we focused on reef fish biomass because this metric incorporates information in both fish size and abundance and because we expected lionfish impacts on the selected fish groups to primarily act through trophic interactions (predation) for which biomass represents the best currency. We then summed species-level biomass data within the parrotfish, surgeonfish, wrasse, and damselfish families and treated these families as our target fish groups.
We expected a decrease in biomass for all the fish groups in the post-invasion period relative to the pre-invasion one and we expected such a decrease to be associated with lionfish biomass. To assess these expectations, for each fish group, we first used boxplots to visually compare (using median values and overlap (or lack thereof) of interquartile ranges) between the pre- and post-invasion period the fish biomass data collected during the three surveyed seasons (winter, summer, and fall) at each site.
We then conducted two sets of analyses. The first analysis operated at the reef fish assemblage level and involved conducting a MANOVA that tested for the effect of site (10 sites), period (pre- and post-invasion), and season (winter, fall and summer) and their three-way and two-way interactions on variability in fish assemblage structure using parrotfish, surgeonfish, wrasse and damselfish biomass as a response matrix. Lionfish biomass (fourth-root transformed) was also included in this analysis as an additional explanatory factor. The transect values were used as replicates (n = 596); thus this analysis also explored links between reef fish biomass composition and lionfish at the transect level. The fish assemblage biomass response matrix was first fourth-root transformed to minimize the influence of extreme values and subsequently Hellinger-transformed65. This analysis was performed using the adonis function in the vegan package66 in R67.
The second analysis explored links between fish biomass and lionfish biomass for each fish group separately and did so at the site level. This involved conducting a mixed-effects linear model using the average biomass per transect of the fish group of interest as response variable and period (pre- and post-invasion) and season (winter, fall and summer) (and their two-way interaction) and corresponding average lionfish biomass per transect estimate as fixed-effect predictors. Sites were treated as a random effects factor. This full model was then compared with a nested one in which lionfish biomass was removed from the model. The statistical significance of including lionfish biomass in the model was assessed via a likelihood ratio test based on maximum likelihood68. Average fish biomass estimates (n = 60) were square-root transformed to ensure meeting conditions for parametric testing. Model fitting and confirmation of meeting parametric conditions for model fitting were conducted in R using the glmmTMB69 and DHARMA70 packages, respectively.
Reef fishers catch data
Fishing yield per unit effort
For trap fishing, we used total fish weight landed per trap during a fishing trip as an estimate of fishing yield per unit effort. For spearfishing, we divided total weight landed during a fishing trip by an individual fisher by the number of hours spent fishing to yield an estimate of weight landed per hour of spearfishing. We then ran a linear model to test for the effect of period (pre- vs post-invasion), season (on- vs off-pelagic season), and landing site (Pile Bay vs Oistins) on fishing yield for trap fishing and spearfishing, separately. The number of fishing yield records across combinations of levels for the three main factors was small and unbalanced, which precluded reliably assessing interaction terms between factors. Thus, only the main effects were assessed. Yield data were log-transformed to ensure meeting conditions for parametric testing.
Fisher catch composition
For both trap fishing and spearfishing, we turned the numbers of individual fish recorded during a given (trap or spear) fishing trip into relative abundance estimates broken down by family. Lionfish was the only member of the Scorpaenidae family recorded in catches, so they are reported here as lionfish. We subsequently used a MANOVA to test for the effect of period (pre- vs post-invasion), season (on- vs off- pelagic season), and landing site (Pile Bay vs Oistins) on the fish family composition of the fishing trip catch. The number of relative abundance records across combinations of levels for the three main factors was small and unbalanced, which precluded reliably assessing interaction terms between factors. Thus, only the main effects were assessed. Relative abundance data were Hellinger-transformed prior to this analysis. If the effect of period on catch composition (after accounting for that of season and landing site) was significant, we identified the fish families that were more strongly associated with the pre- and post-invasion periods, respectively, using the species indicator framework71. This is a powerful approach to identify relationships between species and environmental factors, combining a species relative abundance with its relative frequency of occurrence in the various groups of sites72. This implied using the default settings of the “multipatt” function of the “indicspecies” package71, whereby the raw numerical abundances of the families were used as community data and the pre- and post-invasion periods were treated as two groups of separate sites (with the composition of separate fishing trip landings representing “sites”). The statistical significance of these indicator species tests was assessed via restricted permutations that accounted for the effect of the other potentially significant factors identified by the MANOVA (i.e. season and/or landing site).
All graphs were produced using MS Excel and the ggplot R73 and jtools74 packages in R.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Schofield, P. Update on geographic spread of invasive lionfishes (Pterois volitans [Linnaeus, 1758] and P. miles [Bennett, 1828]) in the Western North Atlantic Ocean, Caribbean Sea and Gulf of Mexico. Aquatic Invasions 5, S117-S122, https://doi.org/10.3391/ai.2010.5.S1.024 (2010).
USGS. Lionfish distribution, geographic spread, biology, and ecology. Nonindigenous Aquatic Species Database, <https://www.usgs.gov/centers/wetland-and-aquatic-research-center/science/lionfish-distribution-geographic-spread-biology> (2016).
Hixon, M. A., Green, S. J., Albins, M. A., Akins, J. L. & Morris, J. A. Lionfish: a major marine invasion. Mar. Ecol. Prog. Ser. 558, 161–165. https://doi.org/10.3354/meps11909 (2016).
Freshwater, D. W. et al. Mitochondrial control region sequence analyses indicate dispersal from the US East Coast as the source of the invasive Indo-Pacific lionfish Pterois volitans in the Bahamas. Mar. Biol. 156, 1213–1221. https://doi.org/10.1007/s00227-009-1163-8 (2009).
Whitfield, P. E. et al. Biological invasion of the Indo-Pacific lionfish Pterois volitans along the Atlantic coast of North America. Mar. Ecol. Prog. Ser. 235, 289–297 (2002).
Walcott, J., Bissada, C. & Oxenford, H. A. Initial sightings and derby data from the red lionfish invasion (Pterois volitans) (Scorpaeniformes: Scorpaenidae) in Barbados. Biodivers. Data J. 7, e38219. https://doi.org/10.3897/BDJ.7.e38219 (2019).
Soares, M. O. et al. Lionfish on the loose: Pterois invade shallow habitats in the tropical southwestern Atlantic. Front. Mar. Sci. 9, 956848 https://doi.org/10.3389/fmars.2022.956848 (2022).
Ulman, A. et al. The lionfish expansion in the Aegean Sea in Turkey: A looming potential ecological disaster. Regional Stud. Mar. Sci. 36, 101271 https://doi.org/10.1016/j.rsma.2020.101271 (2020).
Green, A. J. et al. Linking removal targets to the ecological effects of invaders: a predictive model and field test. Ecol. Appl. 24, 1311–1322 (2014).
Green, S. J. & Cote, I. M. Record densities of Indo-Pacific lionfish on Bahamian coral reefs. Coral Reefs 28, 107–107. https://doi.org/10.1007/s00338-008-0446-8 (2009).
Albins, M. A. & Hixon, M. A. Worst case scenario: Potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ. Biol. Fishes 96, 1151-1157 https://doi.org/10.1007/s10641-011-9795-1 (2013).
Benkwitt, C. E. Non-linear effects of invasive lionfish density on native coral-reef fish communities. Biol. Invasions 17, 1383–1395. https://doi.org/10.1007/s10530-014-0801-3 (2015).
Ballew, N. G., Bacheler, N. M., Kellison, G. T. & Schueller, A. M. Invasive lionfish reduce native fish abundance on a regional scale. Sci. Rep. 6, 32169. https://doi.org/10.1038/srep32169 (2016).
Del Rio, L. et al. Biology and ecology of the lionfish Pterois volitans/Pterois miles as invasive alien species: A review. PeerJ 11, e15728. https://doi.org/10.7717/peerj.15728 (2023).
Kindinger, T. L. & Albins, M. A. Consumptive and non-consumptive effects of an invasive marine predator on native coral-reef herbivores. Biol. Invasions 19, 131–146. https://doi.org/10.1007/s10530-016-1268-1 (2016).
Lesser, M. P. & Slattery, M. Phase shift to algal dominated communities at mesophotic depths associated with lionfish (Pterois volitans) invasion on a Bahamian coral reef. Biol. Invasions 13, 1855–1868. https://doi.org/10.1007/s10530-011-0005-z (2011).
Bonaldo, R. M., Hoey, A. S. & Bellwood, D. R. The ecosystem roles of parrotfishes on tropical reefs. Oceanogr. Mar. Biol. Annu. Rev. 52, 81–132 (2014).
Tebbett, S. B., Siqueira, A. C. & Bellwood, D. R. The functional roles of surgeonfishes on coral reefs: Past, present and future. Rev. Fish Biol. Fish. 32, 387–439. https://doi.org/10.1007/s11160-021-09692-6 (2022).
Burke, L. & Maidens, J. Reefs at Risk in the Caribbean. (World Resource Institute, 2004).
Harms-Tuohy, C. A. Parrotfishes in the Caribbean: a regional review with recommendations for management. (FAO Rome, 2021).
Schuhmann, P. W., Oxenford, H. A., Gill, D. & Staskiewicz, T. Landings, costs, net profit and return on investment in two contrasting fisheries. Part 2: The nearshore trap fishery. (Fisheries Division, Governement of Barbados, Barbados, 2011).
Simpson, N., Oxenford, H. A., Gill, D. & Turner, R. The spear fishery of Barbados. Proc. Gulf. Caribb. Fish. Inst. 66, 136–139 (2014).
Lozano, G. et al. Regional strategy for the control of invasive lionfish in the Wider Caribbean. (International Coral Reef Initiative, 2013).
Morris, J. A. Invasive Lionfish: A Guide to Control and Management. (Gulf and Caribbean Fisheries Institute, 2012).
Oxenford, H. A. & Mahon, R. The state of marine ecosystems that support blue economies in the Wider Caribbean in The Caribbean Blue Economy (eds P. Clegg, R. Mahon, P. McConney, & H. A. Oxenford) 35–47 (Routledge, 2021).
Muthukrishnan, R. et al. Little giants: A rapidly invading seagrass alters ecosystem functioning relative to native foundation species. Mar. Biol. 167, 81. https://doi.org/10.1007/s00227-020-03689-8 (2020).
Vander Zanden, M. J., Hansen, G. J. A., Higgins, S. N. & Kornis, M. S. A pound of prevention, plus a pound of cure: Early detection and eradication of invasive species in the Laurentian Great Lakes. J. Great Lakes Res. 36, 199–205. https://doi.org/10.1016/j.jglr.2009.11.002 (2010).
Biodiversity Working Group. Lionfish invasion response plan for Barbados. (Natural Heritage Department, Government of Barbados, Barbados, 2011).
Vallès, H., Walcott, J. & Oxenford, H. A. Assessment and management of lionfish and status of other marine invasive species of threat to high biodiversity-value reef ecosystems. Final report. (UWI, Cave Hill, Barbados, Department of Biological and Chemical Sciences and Centre for Resource Management and Environmental Studies, 2023).
Arias-Gonzalez, J. E., Gonzalez-Gandara, C., Cabrera, J. L. & Christensen, V. Predicted impact of the invasive lionfish Pterois volitans on the food web of a Caribbean coral reef. Environ. Res. 111, 917–925. https://doi.org/10.1016/j.envres.2011.07.008 (2011).
Green, S. J., Akins, J. L., Maljkovic, A. & Cote, I. M. Invasive lionfish drive Atlantic coral reef fish declines. PLoS ONE 7, e32596. https://doi.org/10.1371/journal.pone.0032596 (2012).
Edwards, C. B. et al. Global assessment of the status of coral reef herbivorous fishes: Evidence for fishing effects. Proc. Biol. Sci. 281, 20131835. https://doi.org/10.1098/rspb.2013.1835 (2014).
Gill, D. A., Oxenford, H. A., Turner, R. A. & Schuhmann, P. W. Making the most of data-poor fisheries: Low cost mapping of small island fisheries to inform policy. Mar. Policy 101, 198–207. https://doi.org/10.1016/j.marpol.2017.10.040 (2019).
CERMES. The Barbados Coral Reef Monitoring Programme: Changes in coral reef communities on the west and south coasts 2012–2022. (The University of the West Indies, Cave Hill, Barbados, 2023).
Kindinger, T. L. Behavioral response of native Atlantic territorial three spot damselfish (Stegastes planifrons) toward invasive Pacific red lionfish (Pterois volitans). Environ. Biol. Fishes 98, 487–498. https://doi.org/10.1007/s10641-014-0279-y (2014).
Bieg, C., Vallès, H., Tewfik, A., Lapointe, B. E. & McCann, K. S. Toward a multi-stressor theory for coral reefs in a changing world. Ecosystems 27, 310–328. https://doi.org/10.1007/s10021-023-00892-8 (2024).
Schopmeyer, S. A. & Lirman, D. Occupation dynamics and impacts of damselfish territoriality on recovering populations of the threatened staghorn coral, Acropora cervicornis. PLoS ONE 10, e0141302. https://doi.org/10.1371/journal.pone.0141302 (2015).
Vermeij, M. J. A. et al. Negative effects of gardening damselfish Stegastes planifrons on coral health depend on predator abundance. Mar. Ecol. Prog. Ser. 528, 289–296. https://doi.org/10.3354/meps11243 (2015).
Henderson, E. B. & Côté, I. Potential effects of the Indo-Pacific lionfish invasion on the Bahamian lobster fishery. Proc. Gulf Caribb. Fish. Inst. 64, 55–56 (2012).
Henderson, E. B. Economic and ecological Implications of interactions between lobsters and invasive lionfish in the Bahamas. MSc thesis, Simon Frazer University (2012).
Lazarre, D., Die, D., Morris, J. & Akins, L. Lionfish bycatch in the Florida Keys commercial spiny lobster fishery. Proc. Gulf Caribb. Fish. Inst. 66, 208–209 (2014).
Akins, L., Lazarre, D., Die, D. & Morris, J. Lionfish bycatch in the Florida lobster fishery: First evidence of occurrence and impacts. Proc. Gulf Caribb. Fish. Inst. 65, 329–330 (2013).
Côté, I. M. & Smith, N. S. The lionfish Pterois sp. invasion: Has the worst-case scenario come to pass? J. Fish Biol. 92, 660–689. https://doi.org/10.1111/jfb.13544 (2018).
Hackerott, S., Valdivia, A., Cox, C. E., Silbiger, N. J. & Bruno, J. F. Invasive lionfish had no measurable effect on prey fish community structure across the Belizean Barrier Reef. PeerJ 5, e3270. https://doi.org/10.7717/peerj.3270 (2017).
Elise, S., Urbina-Barreto, I., Boadas-Gil, H., Galindo-Vivas, M. & Kulbicki, M. No detectable effect of lionfish (Pterois volitans and P. miles) invasion on a healthy reef fish assemblage in Archipelago Los Roques National Park, Venezuela. Mar. Biol. 162, 319–330. https://doi.org/10.1007/s00227-014-2571-y (2014).
Albins, M. A. & Hixon, M. A. Invasive Indo-Pacific lionfish Pterois volitans reduce recruitment of Atlantic coral-reef fishes. Mar. Ecol. Prog. Ser. 367, 233–238. https://doi.org/10.3354/meps07620 (2008).
Albins, M. A. Effects of invasive Pacific red lionfish Pterois volitans versus a native predator on Bahamian coral-reef fish communities. Biol. Invasions 15, 29–43. https://doi.org/10.1007/s10530-012-0266-1 (2013).
Valderrama, D. & Fields, K. H. Linking removal targets to the ecological effects of invaders: a predictive model and field test—Letter to the Editor. Ecol. Appl. 25, 2047–2048. https://doi.org/10.1890/14-2485.1 (2015).
Valderrama, D., Fields, K. H. & Hunsicker, M. Flawed evidence supporting the Metabolic Theory of Ecology may undermine goals of ecosystem-based fishery management: The case of invasive Indo-Pacific lionfish in the western Atlantic. ICES J. Mar. Sci. 74, 1256–1267. https://doi.org/10.1093/icesjms/fsw223 (2017).
Green, S. J. et al. Response to Valderrama and Fields: Effect of temperature on biomass production in models of invasive lionfish control. Ecol. Appl. 25, 2048–2050. https://doi.org/10.1890/14-2485a.1 (2015).
Green, S. J. & Grosholz, E. D. Functional eradication as a framework for invasive species control. Front. Ecol. Environ. 19, 98–107. https://doi.org/10.1002/fee.2277 (2020).
Chapman, J. K., Anderson, L. G., Gough, C. L. A. & Harris, A. R. Working up an appetite for lionfish: A market-based approach to manage the invasion of Pterois volitans in Belize. Mar. Policy 73, 256–262. https://doi.org/10.1016/j.marpol.2016.07.023 (2016).
Côté, I. M., Akins, L., Underwood, E., Curtis-Quick, J. & Green, S. J. Setting the record straight on invasive lionfish control: Culling works. PeerJ Preprints 2, e398v391. https://doi.org/10.7287/peerj.preprints.398v1 (2014).
de León, R. et al. Effectiveness of lionfish removal efforts in the southern Caribbean. Endanger. Species Res. 22, 175–182. https://doi.org/10.3354/esr00542 (2013).
Frazer, T. K., Jacoby, C. A., Edwards, M. A., Barry, S. C. & Manfrino, C. M. Coping with the lionfish invasion: Can targeted removals yield beneficial effects?. Rev. Fish. Sci. 20, 185–191. https://doi.org/10.1080/10641262.2012.700655 (2012).
Bogdanoff, A. K. et al. Optimum lionfish yield: a non-traditional management concept for invasive lionfish (Pterois spp.) fisheries. Biol. Invasions 23, 795–810. https://doi.org/10.1007/s10530-020-02398-z (2020).
Chagaris, D. et al. An ecosystem-based approach to evaluating impacts and management of invasive lionfish. Fisheries 42, 421–431. https://doi.org/10.1080/03632415.2017.1340273 (2017).
Harris, H. E. et al. The bioeconomic paradox of market-based invasive species harvest: A case study of the commercial lionfish fishery. Biol. Invasions 25, 1595–1612. https://doi.org/10.1007/s10530-023-02998-5 (2023).
Ulman, A. et al. Lessons from the Western Atlantic lionfish invasion to inform management in the Mediterranean. Front. Mar. Sci. 9,865162. https://doi.org/10.3389/fmars.2022.865162 (2022).
Lindfield, S. J., McIlwain, J. L. & Harvey, E. S. Depth refuge and the impacts of SCUBA spearfishing on coral reef fishes. PLoS ONE 9, e92628. https://doi.org/10.1371/journal.pone.0092628 (2014).
Tyler, E. H. M., Speight, M. R., Henderson, P. & Manica, A. Evidence for a depth refuge effect in artisanal coral reef fisheries. Biol. Conserv. 142, 652-667. https://doi.org/10.1016/j.biocon.2008.11.017 (2009).
Burkepile, D. E. & Hay, M. E. Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc. Natl. Acad. Sci. USA 105, 16201–16206. https://doi.org/10.1073/pnas.0801946105 (2008).
Froese, R. & Pauly, D. FishBase. World Wide Web electronic publication. www.fishbase.org (2015).
Mahon, R., Hunte, W., Oxenford, H. A., Storey, K. & Hastings, R. Seasonality in the commercial marine fisheries of Barbados. Proc. Gulf. Caribb. Fish. Inst. 34, 28–37 (1982).
Legendre, P. & Gallagher, E. D. Ecologically meaningful transformations for ordination of species data. Oecologia 129, 271–280 (2001).
Oksanen, J. et al. vegan: Community Ecology Package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan (2019).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Version 3.6.1. URL https://www.R-project.org/. (2019).
Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A. & Smith, G. M. Mixed effects models and extensions in ecology with R. (Springer, 2009).
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R J. 9, 378–400. https://doi.org/10.32614/RJ-2017-066 (2017).
Hartig, F. _DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.6. (2022).
De Caceres, M. & Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 90, 3566–3574 (2009).
Dufrene, M. & Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 67, 345–366 (1997).
Wickham, H. ggplot2: Elegant graphics for data analysis. (Springer-Verlag New York, 2016).
Long, J. A. jtools: Analysis and presentation of social scientific data. R package version 2.1.0. (2020).
Acknowledgements
This project was funded by the GEF Trust Fund, through the Ministry of Environment and National Beautification, Government of Barbados, as a component of the regional GEF Project # 9408 ‘Preventing Costs of Invasive Alien Species (IAS) in Barbados and Countries of the OECS’, managed by the Centre for Agriculture and Bioscience International (CABI). We are extremely grateful to those who participated in the data collection, including C. Bissada, H. Trew, A. Cox, R. Bourne, M. Small and J. Weekes. We are also grateful to Barbados Blue and R. Armstrong for boat support and to the trap fishers and spearfishers of Pile Bay and Oistins for their collaboration.
Author information
Authors and Affiliations
Contributions
H.V., H.A.O. and J.W. designed the study and participated in the underwater fish surveys. J.W. led the fisher surveys. H.V. and H.A.O. wrote the main manuscript text. H.V. analyzed the data and produced Figs. 1, 2, 3, 4, 5, 6. H.A.O. produced Fig. 7. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Vallès, H., Walcott, J. & Oxenford, H.A. No change in key reef fish herbivores or reef fisher yields in Barbados a decade after the lionfish invasion. Sci Rep 15, 6253 (2025). https://doi.org/10.1038/s41598-025-90218-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90218-6