Abstract
Hashimoto’s thyroiditis (HT) is a prevalent autoimmune disorder, yet the metabolic abnormalities associated with HT and their relationship to antibody positivity remain poorly understood. This study aimed to characterize the distinct metabolic profiles associated with thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) positivity in female patients with HT. Serum metabolomic analysis was performed on 14 TPOAb-positive patients, 4 TgAb-positive patients, and 14 sex-matched healthy controls, evaluating a total of 225 metabolites. Partial least squares discriminant analysis (PLS-DA) revealed significant metabolic differences among the groups, identifying 36 key metabolites. Of these, 13 metabolites showed significant differences between the TPOAb-positive group and healthy controls, while 23 metabolites exhibited marked differences between the TgAb-positive group and controls. Further correlation analysis revealed a moderate positive association between TgAb and phenylacetyl-L-glutamine, while TPOAb was strongly correlated with LPC 16:0 sn-1. Additionally, metabolic pathway analysis showed significant activation of glycine, serine, and threonine metabolism in the TPOAb-positive group, whereas the TgAb-positive group exhibited enhanced activity in galactose metabolism. These findings suggest that TPOAb and TgAb positivity are associated with distinct metabolic profiles, reflecting their differential roles in metabolic pathways linked to Hashimoto’s thyroiditis. This study provides valuable exploratory evidence of metabolic abnormalities in HT under different antibody-positive states, laying the foundation for future large-scale investigations to elucidate the underlying mechanisms.
Similar content being viewed by others
Introduction
Hashimoto’s thyroiditis (HT) is the most common form of autoimmune thyroiditis (AIT), characterized by the presence of thyroid autoantibodies in the serum and extensive lymphocytic infiltration of thyroid tissue. Thyroid peroxidase antibodies (TPOAb) and thyroglobulin antibodies (TgAb) are key contributors to the pathogenesis and progression of HT1. Epidemiological studies have shown that HT is significantly more prevalent in women than in men2, with the majority of affected individuals presenting with hypothyroidism3. Notably, approximately 50% of women of reproductive age with HT develop postpartum thyroiditis, which poses significant risks to maternal and fetal health. These include impaired fetal neurodevelopment, increased risk of miscarriage, and preterm delivery during pregnancy2,4,5.
TPOAb is a widely recognized diagnostic marker for autoimmune thyroiditis, detectable in 80–90% of AIT patients and 50–60% of Graves’ disease (GD) patients2. Another antibody relevant to AIT diagnosis is TgAb, although its sensitivity is lower compared to TPOAb. Numerous studies have established a strong association between thyroid autoimmunity and metabolic disorders6. However, most research has predominantly focused on the effects of thyroid hormones on glucose and lipid metabolism, leaving the relationship between thyroid autoantibody positivity and metabolic disturbances largely unexplored.
A cross-sectional study of euthyroid adults revealed a significant positive correlation between TPOAb and/or TgAb levels and HbA1c, obesity, and dyslipidemia, particularly among women7. Moreover, elevated TPOAb titers were identified as a risk factor for metabolic syndrome and were positively associated with the severity of fatty liver disease, a relationship that was also more pronounced in women8. Another large-scale cross-sectional study demonstrated that isolated TgAb positivity could suppress hypertriglyceridemia in women and lower the risk of impaired fasting glucose (IFG) in men. This suggests that TgAb may act as an independent protective factor, potentially mitigating the risk of glucose and lipid metabolic disorders9. Additionally, TPOAb (but not TgAb) has been recognized as a predictive marker for the progression from subclinical hypothyroidism to overt hypothyroidism10,11. These findings highlight the significant association between thyroid autoantibody expression and systemic metabolic disturbances. However, the specific mechanisms underlying the distinct roles of TPOAb and TgAb remain unclear and require further investigation.
Moreover, studies suggest that thyroid antibodies influence the levels of various metabolites, including polyamines. Polyamines, essential polycations present in all living cells, play critical roles in thyroid growth and the regulation of nucleic acid metabolism, making them a key focus of metabolomics research12. Song et al. reported a significant negative correlation between spermine (SPM) levels and serum TgAb and TPOAb levels, independent of thyroid hormone concentrations. Additionally, low arginine levels have been linked to thyroid autoimmunity13. Conversely, a study by Ying Wu et al. demonstrated that, after controlling for thyroid hormone effects, thyroid antibody positivity was positively associated with obesity, elevated LDL-C, and hyperuricemia. Furthermore, increased thyroid antibody levels were found to contribute to distinct metabolic disorders in men and women14. While these findings underscore the relationship between thyroid antibodies and metabolic disturbances, the specific association between isolated TgAb or TPOAb positivity and small-molecule metabolites remains unclear, necessitating further investigation.
Metabolomics is a discipline focused on analyzing dynamic changes in metabolite profiles within biological systems in response to exogenous stimuli. It enables the qualitative and quantitative assessment of small-molecule metabolites and has wide-ranging applications in endocrinology and metabolic diseases, particularly thyroid disorders15. For instance, Liu et al. conducted metabolomic analyses on serum samples from hyperthyroid and hypothyroid patients, identifying abnormal levels of lipids and amino acids, including phosphatidylcholines (PC), lysophosphatidylcholines (LPC), sphingomyelins (SM), and glycodeoxycholic acid (GDCA)16. Similarly, Song et al. reported significant differences in serum polyamine metabolite levels between patients with thyroid diseases—such as Graves’ disease, papillary thyroid carcinoma, Hashimoto’s thyroiditis, and thyroid storm—and healthy controls13. In addition, studies have shown that serum metabolite concentrations are significantly altered by treatment, further highlighting the interplay between metabolite expression and disease states17,18. These findings underscore the potential of metabolite profiling in distinguishing disease states and offer valuable insights for clinical diagnosis and disease management.
However, studies exploring the relationship between small-molecule metabolites and isolated TgAb or TPOAb positivity in female patients with Hashimoto’s thyroiditis remain scarce. To address this gap, our study employs serum metabolomic profiling to preliminarily investigate abnormalities in serum metabolites associated with different antibody states in HT and their correlations with thyroid antibodies. This work aims to provide foundational data for future large-scale longitudinal studies that will further elucidate the underlying mechanisms. Ultimately, we seek to validate and clarify the distinct roles of TPOAb and TgAb in the progression of Hashimoto’s thyroiditis.
Materials and methods
Samples
The study protocol was approved by the Ethics Committee of the Second Hospital of Dalian Medical University (Approval Number: 2021 NO.073). All procedures were performed in accordance with the principles outlined in the Declaration of Helsinki. A total of thirty-two samples, matched for age and sex, were included and divided into three groups for the study. The experimental group consisted of eighteen patients with Hashimoto’s thyroiditis from the Department of Endocrinology at Dalian Medical University. Among them, fourteen patients were assigned to the HT group with positive thyroid peroxidase antibodies (TPOAb (+)), and four were assigned to the HT group with positive thyroglobulin antibodies (TgAb (+)). Fourteen healthy individuals were recruited from the medical examination center of the Second Hospital of Dalian Medical University and assigned to the control group. The inclusion and exclusion criteria were as follows:
Inclusion criteria
The TPOAb (+) group met the following criteria: (a) Female gender. (b) Diffuse swelling of the thyroid gland with surface roughness and nodularity, as determined by ultrasound. (c) Positive thyroid peroxidase antibodies (TPOAb) and normal thyroglobulin antibodies (TgAb). (d) No use of medications or drugs.
The TgAb (+) group met the following criteria: (a) Female gender. (b) Diffuse swelling of the thyroid gland with surface roughness and nodularity, as determined by ultrasound. (c) Positive thyroglobulin antibodies (TgAb) and normal thyroid peroxidase antibodies (TPOAb). (d) No use of medications or drugs.
Exclusion criteria
Any participant who meets any of the following criteria will be excluded: (a) Patients with other autoimmune diseases, such as diabetes mellitus, lupus, or inflammatory bowel disease. (b) P Patients with concurrent acute or chronic infectious diseases, such as acute or chronic hepatitis and pneumonia. (c) Patients taking both non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids. (d) Patients with concurrent malignant tumors or immune deficiency. (e) Pregnant or lactating women.
Thyroid function
Under sterile conditions and in a calm environment, 2 to 3 mL of peripheral blood is drawn from the antecubital fossa of the study participants and promptly sent to the central laboratory for analysis. Thyroid function is assessed using the Siemens ADVIA Centaur XP fully automated chemiluminescent immunoassay system.
Metabolic profiling -LC-MS full-component data acquisition
Metabolites were detected using the LC-MS full-component data system, with chromatographic separations performed in both positive and negative ion modes.
Positive ion mode
Chromatographic column: Waters BEH C8 column (50 mm × 2.1 mm, 1.7 μm) (Waters, Milford, MA). Column temperature: 60 °C. Flow rate: 0.4 mL/min. Mobile phases: water with 0.1% formic acid and acetonitrile with 0.1% formic acid. Gradient: The initial gradient was set to 5% B, maintained for 0.5 min, followed by a linear increase to 40% B within 1.5 min. Subsequently, a further linear increase to 100% B occurred over 6 min, maintained for 2 min, then returned to the initial gradient of 5% B at 10.1 min. Finally, the system was equilibrated for 2 min.
Negative ion mode
Chromatographic column: ACQUITY UPLC HSS T3 (50 mm × 2.1 mm, 1.8 μm) (Waters, Milford, MA). Column temperature: 60 °C. Flow rate: 0.4 mL/min. Mobile phase: Phase A consisted of water with 6.5 mM NH₄HCO₃, while Phase B comprised an aqueous solution containing 95% methanol and 6.5 mM NH₄HCO₃. Gradient: The initial gradient was set to 2% B, maintained for 0.5 min, increased to 40% B within 2 min, followed by a linear increase to 100% B within 6 min, maintained for 2 min, and then returned to the initial gradient of 2% B at 10.1 min. Finally, the system was equilibrated for 1.9 min.
Mass spectrometry data acquisition parameters
The mass spectrometry (MS) full scan range for positive ions was set from m/z 80 to 1200 with a spray voltage of 3.50 kV, while for negative ions, the range was also from 80 to 1200 with a spray voltage of 3.00 kV. The capillary temperature was maintained at 300 °C, and the auxiliary heating gas temperature was set to 350 ℃. The flow rates for sheath gas and auxiliary gas were 45 and 10 (arbitrary units), respectively. The resolution was set to 7e4. The internal standard for serum sample analysis is provided in Table S1.
Statistical analysis
The statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA). Normally distributed data were expressed as mean ± standard deviation (SD), whereas non-normally distributed data were presented as median with interquartile range. For comparisons among three or more groups, analysis of variance (ANOVA) or Kruskal-Wallis tests were employed, followed by appropriate post-hoc tests. To correct for multiple comparisons, the significance threshold was adjusted using the Bonferroni method, with the adjusted p-value threshold set at 0.017 (0.05/3). A p-value of less than 0.017 was considered statistically significant.
Furthermore, Partial Least Squares Discriminant Analysis (PLS-DA) and Orthogonal Projections to Latent Structures Discriminant Analysis (OPLS-DA) were performed using SIMCA version 14.1 (Umetrics AB, Umea, Sweden). Serum metabolites with a Variable Importance in Projection (VIP) score greater than 1.5 in the OPLS-DA model were subjected to statistical significance evaluation using either a t-test or a non-parametric test.
The data were normalized using MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/) to minimize systematic bias and enhance consistency. Features with more than 25% missing values were excluded, and any remaining missing values were imputed with the mean of the original data. The data were normalized by sum, mean-centered, and scaled by dividing each variable by the square root of its standard deviation. Enrichment analysis and heatmap generation of differential metabolites were conducted using MetaboAnalyst. Correlation analysis was performed using Origin 2021. The receiver operating characteristic (ROC) curve was generated using SPSS, and metabolite point plotting was carried out using GraphPad Prism 9.0.
Results
Baseline parameters
The baseline parameters of the control, TPOAb (+), and TgAb (+) groups are presented in Table 1. Significant differences were observed in the levels of thyroid peroxidase antibody (TPOAb) between the control and TPOAb (+) groups. However, no statistically significant differences were found in the levels of thyroglobulin antibody (TgAb), thyroid-stimulating hormone (TSH), free triiodothyronine (FT3), or free thyroxine (FT4). Between the control and TgAb (+) groups, significant differences were observed only in TgAb levels. Both TPOAb and TgAb levels exhibited significant differences between the TPOAb (+) and TgAb (+) groups, with no other significant differences in the remaining parameters.
Metabolomic analysis
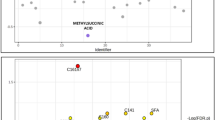
A total of 225 differential metabolites were assessed in each group (Table S2). Partial Least Squares Discriminant Analysis (PLS-DA) was employed to visualize the differences between the Control, TPOAb (+), and TgAb (+) groups (R2X = 0.195, R2Y = 0.44, Q2 = 0.281, Fig. 1a). Orthogonal Projections to Latent Structures Discriminant Analysis (OPLS-DA) revealed distinct separations between the Control (yellow dots) and TPOAb (+) (blue dots) groups (R2X = 0.262, R2Y = 0.756, Q2 = 0.641, Fig. 1b), as well as between the Control (yellow dots) and TgAb (+) (purple dots) groups (R2X = 0.219, R2Y = 0.718, Q2 = 0.570, Fig. 1c).
The PLS-DA score plot comparing the control group to the TPOAb (+) and TgAb (+) groups. (a) PLS-DA score plot of three groups (R2X = 0.195, R2Y = 0.44, Q2 = 0.281). (b) OPLS-DA score plot comparing the Control group to the TPOAb (+) group (R2X = 0.262, R2Y = 0.756, Q2 = 0.641). (c) OPLS-DA score plot comparing the Control group to the TgAb (+) group (R2X = 0.203, R2Y = 0.56, Q2 = 0.233). The t [1] and t [2] values in the figures represent the scores of each sample in principal components 1 and 2, respectively. Each dot on the plot represents a sample in the corresponding group.
Based on the criteria of VIP > 1.5 and P < 0.05, a total of 36 differential metabolites were identified. Thirteen differential metabolites were identified in the comparison between the Control and TPOAb (+) groups, while 23 differential metabolites were identified in the comparison between the Control and TgAb (+) groups (Table 2).
Comparison between the Control and TPOAb (+) groups revealed elevated levels of Glycoursodeoxycholic acid, Glycocholate, LPE 16:1, Glycocholic acid, Indoxyl sulfate, and Phenylacetyl-L-glutamine, as well as reduced levels of LPC 16:0 sn-1, PC 36:3, Hydrocortisone, LPC 18:2 sn-1, SM 36:3, PC 16:0/18:2, and D(-)-Arginine in the TPOAb (+) group compared to the Control (Fig. 2a). The heatmap of differential metabolites is depicted in Fig. 3a. In the TgAb (+) group, concentrations of Dehydroepiandrosterone Sulfate, Androsterone sulfate-4, LPC 16:0 sn-1, Oxypurinol, Androsterone sulfate-3, Androsterone sulfate-1, Decenoylcarnitine, LPC 16:0 sn-2, LPC 15:0 sn-1, FFA 20:5*2, FFA 22:1, 2-Ketohexanoic acid, FFA 18:2, PC 36:3, FFA 18:0, Lactic acid, FFA 16:0, FFA 16:2, and Decadienoylcarnitine were lower than in the Control, while PC 32:2 was elevated, along with Glycoursodeoxycholic acid-pos, Glycochenodeoxycholic acid 3-glucuronide, LPE 20:4 sn-1, and LPE 16:1 (Fig. 2b). The distribution of the measured differential metabolites is depicted in the heatmap (Fig. 3b). These findings suggest distinct metabolic alterations associated with varying antibody profiles, which may provide valuable insights for identifying potential biomarkers for disease monitoring and developing therapeutic strategies.
Correlation analysis
Correlation analysis between clinical data and differential metabolites was conducted using Origin 2021 to explore the relationships between specific metabolites and clinical markers. Pearson correlation coefficients between clinical variables and differential metabolites are depicted in Fig. 3c and d, with different colors representing the strength of correlation. A Pearson correlation coefficient greater than 0.5 and a significance threshold of P < 0.05 were used as criteria to evaluate the correlations. Distinct correlations between differential metabolites and clinical data were observed across the different comparison groups. In the TPOAb (+) group, a moderate correlation was found between TgAb and phenylacetyl-L-glutamine, while a strong correlation was observed between TPOAb and LPC 16:0 sn-1. Conversely, in the TgAb (+) group, a strong correlation was noted between TPOAb and LPE 16:1. The results from each group highlight the specific relationships between metabolites and clinical markers, emphasizing the distinct metabolic profiles associated with different antibody statuses. These findings provide a basis for future research aimed at identifying potential biomarkers for disease monitoring and guiding therapeutic strategies.
(a) Heatmap of differential metabolites comparing the Control group and the TPOAb (+) group. (b) Heatmap of differential metabolites comparing the Control group and the TgAb (+) group. (c,d) Pearson correlation coefficients between clinical variables and differential metabolites displayed by Correlograms.
Enrichment analysis
Enrichment analysis of metabolic pathways, based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, was conducted using MetaboAnalyst to identify significant changes in metabolic pathways. In the comparison between the control and TPOAb (+) groups, notable changes were observed in glycine, serine, and threonine metabolism; glycerophospholipid metabolism; and primary bile acid biosynthesis (Fig. 4a and b). Similarly, the comparison between the control and TgAb (+) groups revealed key roles for galactose metabolism, ascorbate and alternative metabolism, and inositol phosphate metabolism (Fig. 4c and d). These findings suggest that different antibody statuses affect distinct metabolic pathways. Further investigation of these metabolic alterations could enhance our understanding of disease mechanisms and support the development of targeted therapies.
Enrichment analysis was performed to identify the significantly altered metabolic pathways based on the Kyoto Encyclopedia of Genes and Genomes database through Metaboanalyst. (a,b) Comparison between the Control group and the TPOAb (+) group. (c,d) Comparison between the Control group and the TgAb (+) group.
Discussion
Thyroid peroxidase (TPO) is a critical enzyme in thyroid hormone biosynthesis, and thyroid peroxidase antibodies can impair its function, leading to reduced thyroid hormone production. Research has shown a strong association between TPOAb and adverse pregnancy outcomes, including miscarriage, infertility, fetal intellectual impairment, and Hashimoto’s encephalopathy. Anti-thyroglobulin antibodies, which target thyroglobulin, are autoantibodies that serve as biomarkers of thyroid autoimmunity. However, their diagnostic sensitivity for Hashimoto’s thyroiditis is lower than that of TPOAb19.
Wu et al. found significantly higher body mass index (BMI), waist circumference (WC), systolic blood pressure (SBP), diastolic blood pressure (DBP), and 2-hour plasma glucose (OGTT2hPG) in the TPOAb/TgAb-positive group compared to the TPOAb/TgAb-negative group in men. Similarly, in women, BMI, WC, SBP, DBP, total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) were significantly elevated in the TPOAb/TgAb-positive group compared to the TPOAb/TgAb-negative group14. Wang et al. observed that the severity of fatty liver increased with rising titers of TPOAb, particularly in women8. Zhang et al. demonstrated that positive TgAb alone suppressed the development of hypertriglyceridemia in women and reduced the risk of impaired fasting glucose (IFG) in men, suggesting it may serve as an independent protective factor9.
Our study revealed that female patients with HT in the TPOAb-positive group exhibited significantly elevated levels of glycoursodeoxycholic acid, glycocholate, and glycocholic acid compared to the Control group. Enrichment analysis identified the primary bile acid biosynthesis pathway as a key affected pathway, indicating distinct alterations in bile acid metabolism associated with different antibody statuses in HT. Cholic acid, a product of bile acid metabolism, has been implicated in metabolic disorders20. Studies have shown that bile acid metabolism can influence the function and differentiation of Foxp3 + Treg cells21.
Thyroid hormones (TH) have been shown to enhance bile acid metabolism22, while simultaneously suppressing the rate-limiting enzymes (CYP8B1, CYP27A1) involved in bile acid metabolism23,24,25. Kosuge T et al. suggested that deoxycholic acid (DCA or DOCA) levels are elevated in patients with hypothyroidism, while chenodeoxycholic acid (CDA) levels are increased in those with hyperthyroidism26. The ratio of deoxycholic acid to total bile acids and lithocholic acid (LCA) to chenodeoxycholic acid (CDA) has been proposed as a marker of thyroid function. Antibody-dependent cell-mediated cytotoxicity (ADCC) induced by TPOAb accelerates damage to thyroid follicular cells (TFC), resulting in suppressed thyroid hormone synthesis27. Consequently, alterations in serum TPOAb levels in TPOAb-positive patients can impact thyroid hormone levels, modifying bile acid metabolism and its related metabolite products. This, in turn, may affect the ratio of Treg cells, potentially influencing disease progression.
Amino acids are essential components for the body’s vital functions. Amino acid metabolism supports various immune cell functions during immune cell activation and plays a crucial role in autoimmune regulation28. In this study, enrichment analysis revealed dysregulated glycine, serine, and threonine metabolism pathways in the TPOAb (+) group compared to the Control group, indicating that amino acid metabolism is influenced by different thyroid antibody statuses. TPOAb may modulate amino acid metabolism through redox signaling pathways. Oxidative stress has been shown to modulate the function of metabolic enzymes via post-translational modifications. For example, reactive oxygen species (ROS) can alter the activity of key enzymes, thereby affecting both the synthesis and degradation of amino acids. Additionally, thyroid hormones are closely linked to amino acid metabolism. Research indicates that thyroid hormones can enhance glutamine breakdown by facilitating glutamate aminotransferase GPT229, which leads to a decrease in glutamine levels, inversely correlating with THs. When TPOAb reduces TH synthesis by triggering thyroid follicular cell damage via ADCC, glutamine levels are elevated. Additionally, Marta Toral et al. demonstrated that THs promote arginine uptake activation and gene expression of the arginine transporter30. Consequently, as TH levels decrease, the content of arginine also decreases. Thus, the level of TPOAb affects TH levels, subsequently regulating amino acid levels in the body.
Phospholipids are essential for maintaining the structural integrity of cell membranes, facilitating signal transduction, and supporting immune responses in HT. Phospholipids are hydrolyzed by phospholipases into glycerol, fatty acids, phosphoric acid, and a variety of amino alcohols (e.g., choline, ethanolamine, serine)31. Glycerol can be converted to dihydroxyacetone phosphate, which participates in glucose metabolism32. Fatty acids undergo β-oxidation, which influence the development and differentiation of Treg cells33. Phosphoric acid is essential for the metabolism of various substances in the body, while amino alcohols contribute to the resynthesis of phospholipids. The formation and metabolism of phospholipids are closely associated with human physiological functions34. Sphingolipids modulate cellular stress and inflammation35, while lysophospholipids serve as signaling molecules that mediate immunomodulatory functions36,37.
Previous research has demonstrated that thyroid hormones can regulate phospholipid metabolism. In a mouse model of hypothyroidism, phospholipid levels were reduced38. Additionally, Rabkin SW showed a positive correlation between thyroid hormones and phospholipid concentrations in the heart, lung, kidney, and skeletal muscle39. In TPOAb-positive patients, abnormal levels of phospholipid metabolites such as SM, LPC, and PC are found in the serum. Correlation analysis suggests a relationship between TPOAb and LPC. Since TPOAb inhibits thyroid hormone synthesis, the overall phospholipid metabolism decreases, leading to changes in phospholipid metabolites. These changes may lead to the abnormal secretion of inflammatory factors and immune disorders, ultimately resulting in inflammatory abnormalities and immune dysfunction.
TgAb is one of the most common autoantibodies found in the thyroid gland and is often used to diagnose and identify autoimmune thyroiditis, albeit with low sensitivity [8]. Previous studies have suggested that TgAb positivity alone suppresses the development of hypertriglyceridemia in women and reduces the risk of impaired fasting glucose regulation (IFG) in men. In our study, we observed decreased levels of LPC, FFA, and PC in female patients in the TgAb (+) group, indicating suppressed lipid metabolism. Additionally, we found a significant correlation between TgAb levels and serum glutamate. However, due to the lack of literature on the mechanisms underlying the association between serum TgAb levels and suppressed lipid metabolism, further research is needed to elucidate how TgAb influences metabolism.
Conclusion
Our study indicates that female patients with Hashimoto’s thyroiditis exhibit abnormal levels of small molecule metabolites depending on TPOAb or TgAb positivity. TPOAb affects metabolic levels by modulating thyroid hormones, while the mechanisms through which TgAb affects metabolism require further investigation. This research provides insights into potential biomarkers for disease monitoring and treatment. However, longitudinal studies involving larger populations are needed to confirm these results, and further research is needed to elucidate the underlying mechanisms.
Limitiations
There are several limitations to our study. First, the sample size was relatively small. Second, it did not include metabolomic analysis of male patients with Hashimoto’s thyroiditis. Therefore, future research will involve expanding the sample size and adopting more stringent diagnostic criteria, including pathological diagnosis, as inclusion criteria for thyroiditis patients. Additionally, we plan to conduct further studies in larger and more diverse populations to improve statistical power and more robustly validate our findings.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- HT:
-
Hashimoto’s thyroiditis
- AIT:
-
Autoimmune thyroiditis
- TPOAb:
-
Thyroid peroxidase antibody
- TgAb:
-
Thyroglobulin antibody
- GD:
-
Grave’s disease
- SPM:
-
Spermine
- PC:
-
Phosphatidylcholine
- SM:
-
Sphingomyelin
- LPC:
-
Lysophosphatidylcholine
- GDCA:
-
Glucuronide
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- LC-MS:
-
Liquid Chromatograph Mass Spectrometer
- MS:
-
Mass spectrometry
- SD:
-
Standard deviation
- ANOVA:
-
Analysis of variance
- PLS-DA:
-
Partial Least Squares-Discriminant Analysis
- OPLS-DA:
-
Orthogonal projections to latent structures discriminant analysis
- VIP:
-
Variable influences on projection
- ROC:
-
Receiver operating characteristic
- TSH:
-
Thyroid stimulating hormone
- FT3:
-
Free triiodothyronine
- FT4:
-
Free thyroxine
- LPE:
-
Lyso Phosphatidyl Ethanolamine
- FFA:
-
Free fatty acid
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- OGTT2Hpg:
-
2-hour plasma glucose
- IFG:
-
Impaired fasting glucose regulation
- TH:
-
Thyroid hormones
- DOCA:
-
Deoxycholic acid
- CDA:
-
Chenodeoxycholic acid
- LCA:
-
Lithocholic acid
- CDA:
-
Chenodeoxycholic acid
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- ROS:
-
Reactive oxygen species
- TFC:
-
Thyroid follicular cells
References
Stagnaro-Green, A. Detection of At-Risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA 264, 1422 (1990).
Ragusa, F. et al. Hashimotos’ thyroiditis: epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 33, 101367 (2019).
Tunbridge, W. M. G. et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin. Endocrinol. 7, 481–493 (1977).
McLeod, D. S. A. & Cooper, D. S. The incidence and prevalence of thyroid autoimmunity. Endocr 42, 252–265 (2012).
Latina, A., Gullo, D., Trimarchi, F. & Benvenga, S. Hashimoto’s Thyroiditis: similar and dissimilar characteristics in neighboring areas. Possible implications for the epidemiology of thyroid Cancer. PLoS ONE. 8, e55450 (2013).
Chung, G. E. et al. Longitudinal change in thyroid-stimulating hormone and risk of nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 19, 848–849e1 (2020).
Chen, Y. et al. Are thyroid autoimmune diseases associated with cardiometabolic risks in a population with normal thyroid-stimulating hormone? Mediators. Inflamm. 2018, 1–10 (2018).
Wang, C. et al. Elevated TPOAb is a strong predictor of Autoimmune Development in patients of type 2 diabetes Mellitus and non-alcoholic fatty liver disease: a case-control study. DMSO 13, 4369–4378 (2020).
Zhang, J. et al. The Presence of serum TgAb suggests lower risks for glucose and lipid metabolic disorders in Euthyroid General Population from a National Survey. Front. Endocrinol. 11, (2020).
Orgiazzi, J. Thyroid autoimmunity. Presse Med. 41, e611–e625 (2012).
Sanders, P. et al. Crystal structure of the TSH receptor bound to a blocking type TSHR autoantibody. J. Mol. Endocrinol. 46 (2), 81–99 (2011).
Matsuzaki, S. & Suzuki, M. Thyroid function and polyamines II. Thyrotropin stimulation of polyamine biosynthesis in the rat thyroid. Endocrinol. Japon. 22, 339–345 (1975).
Song, J., Shan, Z., Mao, J. & Teng, W. Serum polyamine metabolic profile in autoimmune thyroid disease patients. Clin. Endocrinol. 90, 727–736 (2019).
Wu, Y. et al. The correlation between metabolic disorders and Tpoab/Tgab: A Cross-sectional Population-based study. Endocr. Pract. 26, 869–882 (2020).
Nicholson, J. K. & Lindon, J. C. Systems biology: metabonomics. Nature 455, 1054–1056 (2008).
Liu, J. et al. Serum metabolomic patterns in patients with autoimmune thyroid disease. Endocr. Pract. 26, 82–96 (2020).
Chng, C. L. et al. Physiological and metabolic changes during the transition from hyperthyroidism to Euthyroidism in Graves’ Disease. Thyroid 26, 1422–1430 (2016).
Zhang, X., Chen, D., Xu, K. & Ma, Z. Deciphering the metabolomics-based intervention of Yanghe Decoction on Hashimoto’s thyroiditis. Evid.-based Complement. Altern. Med. 2022, 1–29 (2022).
Dwivedi, S. N., Kalaria, T. & Buch, H. Thyroid autoantibodies. J. Clin. Pathol. 76, 19–28 (2022).
Thomas, C., Pellicciari, R., Pruzanski, M., Auwerx, J. & Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 7, 678–693 (2008).
Campbell, C. et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581, 475–479 (2020).
Mullur, R., Liu, Y. Y. & Brent, G. A. Thyroid hormone regulation of metabolism. Physiol. Rev. 94, 355–382 (2014).
Lammel Lindemann, J. A., Angajala, A., Engler, D. A., Webb, P. & Ayers, S. D. thyroid hormone induction of human cholesterol 7 alpha-hydroxylase (Cyp7a1) in vitro. Mol. Cell. Endocrinol. 388, 32–40 (2014).
Kube, I. et al. Hypothyroidism increases cholesterol gallstone prevalence in mice by elevated hydrophobicity of primary bile acids. Thyroid 31, 973–984 (2021).
Ellis, E. C. S. Suppression of bile acid synthesis by thyroid hormone in primary human hepatocytes. WJG 12, 4640 (2006).
Kosuge, T., Beppu, T., Kodama, T., Hidai, K. & Idezuki, Y. Serum bile acid profile in thyroid dysfunction and effect of medical treatment. Clin. Sci. 73, 425–429 (1987).
Poppe, K., Velkeniers, B. & Glinoer, D. Thyroid disease and female reproduction. Clin. Endocrinol. 66, 309–321 (2007).
Kelly, B. & Pearce, E. L. Amino assets: how amino acids support immunity. Cell. Metab. 32, 154–175 (2020).
Cicatiello, A. G. et al. Thyroid hormone regulates glutamine metabolism and anaplerotic fluxes by inducing mitochondrial glutamate aminotransferase GPT2. Cell. Rep. 38, 110409 (2022).
Toral, M. et al. Thyroid hormones stimulate L-arginine transport in human endothelial cells. J. Endocrinol. 239, 49–62 (2018).
Abe, A., Hiraoka, M., Ohguro, H., Tesmer, J. J. & Shayman, J. A. Preferential hydrolysis of truncated oxidized glycerophospholipids by lysosomal phospholipase A2. J. Lipid Res. 58, 339–349 (2017).
Orozco, J. M. et al. Dihydroxyacetone phosphate signals glucose availability to mTORC1. Nat. Metab. 2, 893–901 (2020).
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4 + T cell subsets. J. I. 186, 3299–3303 (2011).
Patsoukis, N. et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 6, (2015).
Amrein, K., Lasky-Su, J. A., Dobnig, H. & Christopher, K. B. Metabolomic basis for response to high dose vitamin D in critical illness. Clin. Nutr. 40, 2053–2060 (2020).
Peter, C. et al. Migration to apoptotic find-me signals is mediated via the phagocyte receptor G2A. J. Biol. Chem. 283, 5296–5305 (2008).
Mueller, R. B., Sheriff, A., Gaipl, U. S., Wesselborg, S. & Lauber, K. Attraction of phagocytes by apoptotic cells is mediated by lysophosphatidylcholine. Autoimmunity 40, 342–344 (2007).
Dang, G. et al. T lymphocyte-derived extracellular vesicles aggravate abdominal aortic aneurysm by promoting macrophage lipid peroxidation and migration via pyruvate kinase muscle isozyme 2. Redox Biol. 50, 102257 (2022).
Rabkin, S. W. Effect of amiodarone on phospholipid content and composition in heart, lung, kidney and skeletal muscle: relationship to alteration of thyroid function. Pharmacology 76, 129–135 (2006).
Acknowledgements
We gratefully acknowledge the approval from the Second Hospital of Dalian Medical University and the support provided by the Dalian Institute of Chemical Physics, Chinese Academy of Sciences.
Funding
This study was supported by the following grants: the “Xingliao Talent Plan” of Liaoning Province, China (YXMJ-QN-05); the Dalian Science and Technology Innovation Fund, Dalian Science and Technology Bureau (2022JJ12SN048); and the “1 + X” Program for Clinical Competency Enhancement – Clinical Research Incubation Project, The Second Hospital of Dalian Medical University (2022LCYJZD03).
Author information
Authors and Affiliations
Contributions
Conceptualization, Xiao Jiang and Haixia Liu; methodology and validation, Tao Luo and Xinyu Zhao; formal analysis, Tao Luo and Xiao Jiang; data curation, Pengqian Li and Xiaotong Gu; writing—original draf preparation, Tao Luo, Xinyu Zhao and Xiao Jiang; writing—review and editing, Pengqian Li and Xingjie Xie; funding acquisition, Haixia Liu. All authors have reviewed and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval
This study protocol was approved by the Ethics Committee of the Second Hospital of Dalian Medical University (Approval No. 2021NO.073). Informed consent was obtained from all participants. According to national regulations and institutional requirements, written informed consent was not required for this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, T., Zhao, X., Jiang, X. et al. Serum metabolomic analysis in patients with Hashimoto’s thyroiditis positive for TgAb or TPOAb: a preliminary study. Sci Rep 15, 9945 (2025). https://doi.org/10.1038/s41598-025-90467-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90467-5
Keywords
This article is cited by
-
Postmarketing pharmacovigilance study of levothyroxine based on FDA adverse event reporting system (FAERS) and Mendelian randomization
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)