Abstract
With the rapidly growing world population, climate change uncertainties and depletion of our natural resources, edible insects are seen as sustainable and viable bioresources for food and feed to tackle global food and nutritional security issues, for their nutritional value, taste, and environmental sustainability. In this study, we investigated the effect of geographical distribution on the nutrient composition and sensory attributes of commonly consumed edible insects, namely Acheta domesticus, Apis mellifera, Gnathocera trivittata, Gryllotalpa africana, Imbrasia oyemensis, Locusta migratoria, Macrotermes subhylanus, Nomadacris septemfasciata, and Rhyncophorus phoenicis collected from six different geographical areas namely Fizi, Kabare, Kalehe, Idjwi, Mwenge and Walungu, in the Eastern D. R. Congo. Depending on edible insect species, geographical sourcing area affected significantly macronutrient composition and mineral profile as well as sensory attributes of investigated commonly edible insects. A principal component analysis (PCA-Biplot) indicated that the two axes accounted for up to 97.7% of the observed variability in the nutrient composition and sensory attributes of commonly consumed edible insects sourced from different geographical area. Visualized results after cluster analysis using non-metric multidimensional scaling (NMDS) indicated that the geographical sourcing area has substantial and significant effect on the nutrient composition as well as sensory attributes of the studied commonly edible with a stress value of 0.185.
Similar content being viewed by others

Introduction
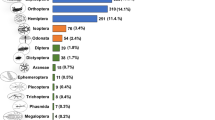
Edible insects are seen as sustainable and viable bioresources for food and feed to address global food and nutrition security issues1,2 linked to rapid global population growth3, climate change uncertainties4 and the depletion of our natural resources5. There are an estimated 5.5 million insect species worldwide, of which only about 1 million have been described6, and over 2,100 are consumed, mainly in tropical countries, and are categorized as beetles (Coleoptera, 31%), caterpillars (Lepidoptera, 18%), bees, wasps and ants (Hymenoptera, 14%), locusts, grasshoppers, crickets and crickets (Orthoptera, 13%), cicadas, leafhoppers, grasshoppers, mealybugs and bedbugs (Hemiptera, 10%), termites (Isoptera, 3%), dragonflies (Odonata, 3%), flies (Diptera, 2%) and 5% other orders being the most widely consumed insect groups7.
Several studies have paid attention to nutritional composition of edible insects reporting protein content ranging from 6.25 to 80.26% on dry matter, fat content from 2.2 to 43%, 1.91–9.2% (moisture content), 1.2-11.38% (ash content) and a rang from 1.01 to 6.8% for fiber content8,9,10,11,12,13. Additionally, as edible insects emerges as a mainstream food source, their acceptance and adoption largely depend on consumer attitudes and perceptions14, of which sensory attributes including taste, texture, aroma, and appearance play a crucial role15. As our understanding of edible insects continues to grow, efforts are being made to develop innovative preparation techniques and recipes that optimize their sensory appeal16. With their diverse flavors, unique textures, intriguing aromas, and visually captivating appearances17.
However, their nutritional content and sensory attributes are subjected to biotic factors including diet, harvesting time and gender, age, and abiotic factors such as temperature, humidity and light18, in addition to processing methods19 and preservation techniques13 as well as applied analytic method20. A number of studies have shown the importance of feeding behavior on nutritional status of edible insects21,22,23, so it is possible to diversify diet in order to meet specific needs.
Although edible insects have gained particular attention, only 10% are currently described, and less so in regard to the effects of geographical sourcing area on their nutritional composition and sensory attributes, despite the fact that the latter are largely depending on biogeochemical conditions. Located in the Eastern of the Democratic Republic of Congo, Fizi, Idjwi, Kabare, Kalehe, Mwenga and Walungu are relatively six territories well known for the widespread of anthropo-entomophagy practices, with diverse agro-ecological conditions24. While the Fizi, Walungu, kabare and Mwenga territories are characterized by acrisols, cambisols, ferralsols and nitisols, the Kalehe and idjwi territories are characterized by haplic acrisols, dystric cambisols and haplic nitisols, humid ferralsols and gleyic solonchaks with different climatic conditions likely to affect not only land/plant cover but also the nutrient composition and sensory attributes of edible insects24. Considering the diversity in term of soil composition, climate, temperature, humidity, rainfall among other biogeochemical conditions, this study therefore aimed at investigating the effect of geographical sourcing area on the nutritional composition and sensory attributes of commonly edible insects in Eastern Democratic Republic of Congo.
Results
Influence of geographical sourcing area on nutrient composition
The geographical sourcing effect on macronutrient composition of commonly consumed edible insects in Eastern D. R. Congo is depicted in Table 1. Although the geographical sourcing area did not affect significantly (p > 0.05) the protein content for Acheta domesticus, Apis mellifera, Gnathocera trivittata, Grillotalpa africana and Nomadacris septemfasciata, a significant effect (p < 0.05) was observed for Imbrasia oyemensis, Locusta migratoria, Macrotermes subhyalinus and Rhynchophorus phoenicis. Fat content varied significantly for all edible insects except for G. trivittata. Ash content varied significantly (p < 0.05) for A. domesticus, A. mellifera and M. subhyalinus, but not for G. trivittata, G. africana, I. oyemensis, L. migratoria, N. septemfasciata and R. phoenicis. A significant variation (p < 0.05) in moisture content was observed for all studied edible insects except for R. phoenicis.
Acheta domesticus macronutrient composition varied significantly (p < 0.05) due to geographical sourcing area, except its protein content (p > 0.05), which ranged between 35.25 and 38.16 g/100 g, with fat content ranging between 20.99 and 24 g/100 g, ash content 4.90 to 11.83 g/100 g and moisture content 57.23 to 66.25 g/100 g. As for A. mellifera, its protein content ranged between 19 and 20.08 g/100 g, fat (22.27–26.29 g/100 g), ash (4.41–8.79 g/100 g) and between 70.06 and 78.84 g/100 g for moisture content.
Gnathocera trivittata had a protein content ranging between 34.40 and 36.13 g/100 g, fat content (16.90 to 17.78 g/100 g), ash (4.94 to 6.57 g/100 g), and moisture content (56.40 to 62.21 g/100 g). The protein, fat, ash as well as moisture content of G. africana ranged between 30.70 and 32.22 g/100 g, 21.23–26.73 g/100 g, 4.45–5.26 g/100 g, and 63.66–79.72 g/100 g respectively. Imbrasia oyemensis was found to be nutritionally potential with 31.18–56.68 g/100 g (protein content), 15.68–27.95 g/100 g (fat), 5.92–6.93 g/100 g (ash), 11.94–28.57 g/100 g (CHO), and 66.58–79.05 g/100 g for moisture. Similarly for L. migratoria, with protein, fat, ash, and moisture contents ranging from 29.63 to 35.25 g/100 g, 16.95–23.10 g/100 g, 4.54–5.67 g/100 g and 59.82–77.34 g/100 g respectively. Finally, R. phoenicis, with a protein content of 31.55–39.19 g/100 g, fat content (25.80–30.60 g/100 g), ash content (6.92–7.02 g/100 g) and moisture content (68.14–68.84 g/100 g).
Geographical sourcing effect on mineral profile of commonly consumed edible insects in Eastern D. R. Congo
Table 2 presents the geographical sourcing area effect on the potassium, sodium, magnesium, iron, calcium and zinc content of A. domesticus, A. mellifera, G. trivittata, G. africana, I. oyemensis, L. migratoria, M. subhyalinus, N. septemfasciata and R. phoenicis, commonly edible insects in Eastern D. R. Congo. Although the geographical sourcing area affected significantly (p < 0.05) the potassium, sodium, iron, calcium and zinc content in studied edible insects, the magnesium content was not significantly (p > 0.05) affected for A. domesticus and A. mellifera. As for A. mellifera, its potassium, sodium, iron and calcium content varied significantly (p < 0.05), except for its magnesium and zinc content. Potassium, sodium, magnesium, iron and calcium content varied significantly (p < 0.05) in G. trivittata, except for its zinc content (p > 0.05).
Geographical sourcing area affected significantly (p < 0.05) potassium, sodium, magnesium, calcium and zinc content for G. africana, except its iron content. In contrast to previous species, geographical sourcing area significantly (p < 0.05) affected some mineral (potassium, sodium and calcium) content in I. oyemensis, while other mineral (magnesium, iron and zinc) content was not significantly (p > 0.05) affected. All assessed mineral varied significantly (p < 0.05) in regards to geographic sourcing area for L. migratoria. Geographical sourcing area affected significantly (p < 0.05) all assessed mineral, with the exception of iron for M. subhyalinus and sodium for R. phoenicis. As for N. septemfasciata, its potassium, magnesium, iron and calcium content varied significantly (p < 0.05) in respect to geographical sourcing area, except for calcium and zinc.
The potassium, sodium, magnesium, iron, calcium and zinc content of A. domesticus ranged between 67.27 and 148.25 mg/100 g, 146 and 161.23 mg/100 g, 40.90 and 59.60 mg/100 g, 4.1 and 6.6 mg/100 g. 87 and 144.27 mg/100 g and 12.2-15.85 mg/100 g respectively. As for A. mellifera, its potassium content varied between 74.79 and 101.7 mg/100 g, sodium (143.5–158.67 mg/100 g), magnesium (45.33–51.72 mg/100 g), iron (4.87–9.24 mg/100 g), calcium (124–147.4 mg/100 g) and zinc (14.27–15 mg/100 g). For G. trivittata, its potassium content varied between 19.54 and 105.38 mg/100 g, 144.23–161.3 mg/100 g (sodium), 32.4–54.77 mg/100 g (magnesium), 6.5–8.8 mg/100 g (iron), 128.5–148.33 mg/100 g (calcium) and 14.4–15.83 mg/100 g (zinc). The G. africana species was found to be rich in potassium (46.91–81.3 mg/100 g), sodium (141.47–168 mg/100 g), magnesium (24.3–42.17 mg/100 g), iron (5.05–5.53 mg/100 g), calcium (129.5–162 mg/100 g) and zinc (15.1–16.3 mg/100 g).
For I. oyemensis, potassium, sodium, magnesium, iron, calcium and zinc contents ranged between 56.43 and 193.16 mg/100 g, 154.44–159.23 mg/100 g, 60.57–66.96 mg/100 g, 7.47–7.57 mg/100 g, 102–107.33 mg/100 g, 12.38–14.36 mg/100 g respectively. With a potassium content of 73.43–108.05 mg/100 g, sodium (154–162.9 mg/100 g), magnesium (44.83–63.43 mg/100 g), iron (4.15–7.17 mg/100 g), calcium (122.67–153 mg/100 g) and zinc (13.1–17.13 mg/100 g), L. migratoria was found to be a good source of minerals. Macrotermes subhyalinus is also a good source of minerals, with 160.43–520.44 mg/100 g (potassium), 157.67–164.33 mg/100 g (sodium), 20.6–67.87 mg/100 g (magnesium), 5.2–5.9 mg/100 g (iron), 97.33–139.1 mg/100 g (calcium) and 15.1–17.8 mg/100 g (zinc). While for N. septemfasciata the potassium, sodium, magnesium, iron, calcium and zinc content varied respectively between 54.37 and 116.17 mg/100 g, 162.07–162.7 mg/100 g, 29.8–56.27 mg/100 g, 6.8–7.79 mg/100 g, 153–160.5 mg/100 g and 13.03–13. 2 mg/100 g, the potassium, sodium, magnesium, iron, calcium and zinc content of R. phoenicis ranged from 27.65 to 28.93 mg/100 g, 169.33–170.67 mg/100 g, 32.3–58.93 mg/100 g, 5.43–8.57 mg/100 g, 98.97–176.05 mg/100 g and 13–18.3 mg/100 g respectively.
Geographical sourcing effect on sensory attributes of commonly consumed edible insects in Eastern D. R. Congo
The geographical sourcing area affected differently the sensory attributes namely appearance, aroma, texture, taste, after taste and overall acceptability of commonly edible insects (A. domesticus, A. mellifera, G. trivittata, G. africana, I. oyemensis, L. migratoria, M. subhyalinus, N. septemfasciata and R. phoenicis) in Eastern D. R. Congo as depicted in Table S3 and Fig. 1. The geographical sourcing area did not significantly (p > 0.05) affect the sensory attributes of A. domesticus except its aroma. On the other hand, it did affect significantly the sensory attributes of A. mellifera. While appearance, aroma and texture of G. trivittata varied significantly (p < 0.05) with geographical sourcing area, taste, after taste and overall acceptability did not. For G. africana and R. phoenicis, the geographical sourcing area significantly (p < 0.05) affected all sensory attributes. As for I. oyemensis, aroma, texture, after taste and overall acceptability varied significantly (p < 0.05) with geographical sourcing area, unlike appearance and taste. While the appearance, aroma, taste and after taste of L. migratoria varied significantly (p < 0.05) with geographical sourcing area, its texture and overall acceptability did not vary significantly (p > 0.05). The appearance, texture and taste of M. subhyalinus were significantly (p < 0.05) affected by geographical sourcing area, but its aroma, after taste and overall acceptability did not vary significantly (p > 0.05). An exception was noted for N. septemfasciata, all its sensory attributes did not vary significantly (p > 0.05) with geographical sourcing area except its taste (p < 0.05).
In Fig. 2, results on Principal component analysis (PCA-Biplot) indicated that the two axes accounted for up to 97.7% of the observed variability in the nutrient composition and sensory attributes of commonly consumed edible insects sourced from different geographical area in Eastern D. R. Congo. The first and second axes accounted for 94.4% and 3.3% of variability, respectively. Visualized results after cluster analysis using non-metric multidimensional scaling (NMDS) indicated that the geographical sourcing area has substantial and significant effect on the nutrient composition as well as sensory attributes of commonly edible in the Eastern D. R. Congo with stress value of 0.185 and p = 0.043 as depicted in Fig. 3D. In the macronutrient composition NMDS plot (Fig. 3A), mineral NMDS plot (Fig. 3B) as well as sensory NMDS plot (Fig. 3C), the edible insects are distant from each other indicating the influence of geographical sourcing area with stress value 0.138 and p = 0.043 (macronutrient composition), stress value 0.107 and p = 0.045 (mineral profile), as well as stress value 0.095 and p = 0.039 (sensory attributes).
PCA-Biplot of nutrient composition and sensory attributes. (1) A. domesticus_Fizi; (2) M. subhyalinus_Fizi ; 3.R. phoenicis_Fizi; 4. A. mellifera_Idjwi; 5. L. migratoria_Idjwi; 6. R. phoenicis_Idjwi; 7. A. domesticus_Kabare 8. A. mellifera_Kabare; 9. G. trivittata_Kabare; 10. G. africana_Kabare; 11. L. migratoria_Kabare; 12. M. subhyalinus_Kabare; 13. N. septemfasciata_Kabare; 14. A. mellifera_Kalehe; 15. I. oyemensis_Kalehe; 16. L. migratoria_Kalehe; 17. A. domesticus_Mwenga; 18. I. oyemensis_Mwenga; 19. A. domesticus_Walungu; 20. A. mellifera_Walungu; 21. G. trivittata_Walungu; 22. G. africana_Walungu; 23. L. migratoria_Walungu; 24. M. subhyalinus_Walungu; 25. N. septemfasciata_Walungu.
Cluster analysis using non-metric multidimensional scaling (NMDS) to determine the extent to which geographical sourcing area influenced edible insects. A: macronutrient composition (stress value 0.138 and p = 0.043), B: mineral profile (stress value 0.107 and p = 0.045), C: sensory attributes (stress value 0.095 and p = 0.039), and D: all parameters combined (stress value 0.185 and p = 0.024), The distance between different points on the plot reflects their similarity level: the more similar the composition of the samples, the smaller the distance between the points. (1) A. domesticus_Fizi; (2) M. subhyalinus_Fizi ; 3.R. phoenicis_Fizi; 4. A. mellifera_Idjwi; 5. L. migratoria_Idjwi; 6. R. phoenicis_Idjwi; 7. A. domesticus_Kabare 8. A. mellifera_Kabare; 9. G. trivittata_Kabare; 10. G. africana_Kabare; 11. L. migratoria_Kabare; 12. M. subhyalinus_Kabare; 13. N. septemfasciata_Kabare; 14. A. mellifera_Kalehe; 15. I. oyemensis_Kalehe; 16. L. migratoria_Kalehe; 17. A. domesticus_Mwenga; 18. I. oyemensis_Mwenga; 19. A. domesticus_Walungu; 20. A. mellifera_Walungu; 21. G. trivittata_Walungu; 22. G. africana_Walungu; 23. L. migratoria_Walungu; 24. M. subhyalinus_Walungu; 25. N. septemfasciata_Walungu.
Discussion
The macronutrient composition and sensory quality of edible insects depend on several aspects, including edible insect species and their development stage25, feeding habit26, processing methods27 and geographical sourcing area28, as well as measurement methods29. The significant geographical sourcing area effect on both nutrient composition and sensory attributes revealed in this study affirms the findings of Romotowska et al.30, Joy et al.31 and Manditsera et al.32 who reported that soil type, geographical sourcing area and season can influence the chemical composition of vegetation, and consequently that of animals such as edible insects that feed on the latter33. It is therefore possible that the difference between soil and vegetation in the different samples sourcing territories influenced the nutrient composition and sensory attributes of the studied edible insects.
This difference could be attributed to the biogeochemistry diversity in the sourcing area as Fizi territory is dominated by acrisols and cambisols with humid wet and dry tropical climate type, Walungu territory is dominated by ferralsols, cambisols and nitisols with humid wet tropical climate type, but Kabare territory is dominated by ferralsols and nitisols with humid wet tropical climate type. While Mwenga territory is dominated by acrisols and cambisols with equatorial climate type, Kalehe territory is dominated by haplic acrisols, dystric cambisols and haplic nitisols and humid ferralsols with humid wet tropical climate type, and Idjwi territory is dominated by gleyic solonchaks, nitisols and humid ferralsols with humid wet tropical climate type24. This corroborates with the findings of Sokol et al.34, who reported that the difference in soil mineral composition is caused by differences in either the type or magnitude of the factors that influence soil nutrient composition, such as parent (rock) material, climate, soil particle size, pH, humus content, aeration, temperature, water content, root surface area and mycorrhizal development among others.
In this study, the geographical sourcing area affected significantly the fat, ash, moisture, potassium, sodium, magnesium, iron, calcium and zinc content of A. domesticus, confirming the findings of Bawa et al.21, who indicated that feeding habits had impacted significantly the protein, fat, ash, moisture, potassium, sodium, iron, calcium and zinc content of A. domesticus. Similarly, a study in Poland on the effects of high-monosaccharide diets on development and biochemical composition of A. domesticus reported a significant variation in terms of fat and moisture content and non-significant variation in protein content22, confirming the findings of this study. Moreover their high iron content would be linked to the soil composition characterized by nitisols a soil known to be rich in iron with species sourced from territories dominated by the latter having the highest iron content24.
The findings is this study are consistent with the findings of Jehlík et al.35, who reported a significant impact of dietary behavior on biological characteristics, such as protein and fat content of A. mellifera. Given that honey bee nutrition is based on the nutritional stored in the hive, with nectar and honeydew as the main source of sugars and pollen, which is the key source of proteins (24%) and fat (5%), variation in pollen availability significantly affects the health and nutritional status of A. mellifera36, so a shortage and poor quality of pollen can lead to nutritional stress with huge impact on the colony37. This study confirms the above findings, showing that geographical source significantly impacts the nutritional and organoleptic quality of A. mellifera. Given the impact of biogeochemical conditions on pollen and nutritional quality, numerous attempts have been made to use artificial supplements, not only to increase bee colony and honey production38. The contribution of other factors such as insect development stage that could possibly explain such variations should be investigated.
Although no significant effect of geographical sourcing area on protein, fat and ash content was observed in this study, a significant effect of the latter on potassium, sodium, magnesium, iron, calcium, zinc content and sensory quality of G. trivittata was observed. As the effect of geographical sourcing area on nutritional and sensory quality of G. trivittata species is still poorly documented, a study in Togo39 reported higher protein values than those presented in this study. This difference could be associated not only to agro-ecological conditions under which the species was harvested28, but also to the conversion factors used to determine protein content. In their study, the general nitrogen-protein conversion factor (GNPCF) of 6.25 was used to calculate protein content, indicating an over-estimation of protein content. However, Boulos et al.20 established a nitrogen-protein conversion factor of 5.33, which was used in this study.
To date, there little to no research assessing the impact of geographical sourcing area on the nutrient and sensory quality of G. africana, nor characterized its nutrient and sensory quality, except a study conducted in Zimbwabwe40 reporting a protein content of 22 g/100 g on dry weight basis (DWB), fat (10.8 g/100 g, DWB) and ash (12.6 g/100 g, DWB). The values are lower in comparison to the ones reported in this study. This difference could be linked to the biogeochemistry differences in both geographical sourcing area. Similarly, a study conducted on I. oyemensis in Kabare territory41 reported a protein content of 56 g/100 g with a GNPCF of 6.25, fat (20.56 g/100 g) and ash of 3.11 g/100 g on DWB. These findings are comparable to those reported in this study (Mwenga territory) and lower than those reported in Kalehe territory, thus underlining the importance of the geographical sourcing area.
In their study, examining the diet effect on the chemical composition of L. migratoria, Oonincx and Van der Poel23 noted that addition of wheat bran decreased the protein content and increased fat content of the latter. The same team reported that addition of carrots to the diet increased fat content of L. migratoria. They also realized that mineral concentrations of Ca, K, Mg and Na were significantly affected by diet. Concentrations of P, K, Cu and Fe were significantly different in penultimate versus adult L. migratoria, showing that chemical composition of the latter can be manipulated by diet. These results corroborate with those reported in this study, highlighting that geographical sourcing area impact on chemical composition and sensory quality of L. migratoria. While a study from Thailand42, reported higher protein content and lower fat content as well as similar ash content in L. migratoria, a study from Spain43 noted higher fat content and comparable ash content in the latter.
In this study, M. subhyalinus presented macronutrient composition similar to that presented by Kinyuru et al.44 in Kenya with a protein content of 39.34 g/100 g and ash (7.78 g/100 g) but with a lower fat content in comparison to the one reported in Kenya. The iron, zinc and magnesium content in M. subhylanus noted in this study is superior, comparable and inferior depending on mineral, to mineral content reported in previous studies, some of which reported mineral contents of 6.2-10.3 mg/100 g (iron), 4.9–13.8 mg/100 g (zinc) and 39.8 mg/100 g (magnesium) for M. subhylanus collected in Benin, and 8.8-9.8 mg/100 g (iron) and 12-12.9 mg/100 g (zinc) for Macrotermes spp collected in South Africa, and 13.9 mg/100 g (iron), 12.9 mg/100 g (zinc) and 95 mg/100 g (magnesium) for Odontotermes spp collected in South East Asia45.
Finally, macronutrient composition in R. phoenicis reported in this study is superior to the one noted by Mba et al.46 in Cameroon, who reported a fat and protein contents of 21.35 g/100 g and 8.18 g/100 g fresh weight (FW), respectively. In this same perspective, Rumpold and Schlüter47 reported protein, fat and ash contents varying between 10.3 and 41.69 g/100 g, 19.50–55.04 g/100 g and 1.43–5.6.06 g/100 g on DWB, respectively. Additionally, Omotoso and Adedire48 reported mineral contents ranging from 13.67 to 17 mg/kg (sodium), 372.5–457.5 mg/kg (potassium), 43.52-60.69 mg/kg (magnesium), 6–22.90 mg/kg (iron), 0.27–2.63 mg/kg (calcium) and 0.31–0.56 mg/kg (zinc) which are inferior or comparable to mineral profile of R. phoenicis observed in this study depending on mineral type.
Materials and methods
Ethics approval
All experimental protocols, as well as methods, were approved and carried out as per relevant guidelines and regulations from the Interdisciplinary Centre for Ethical Research (CIRE) established by the Université Evangélique en Afrique, Bukavu, D.R. Congo, with reference (UEA/SGAC/KM 132/2016). The informed consent describing the study purpose was clearly explained before being signed by all subjects and/or their legal guardian (s).
Geographical sourcing areas
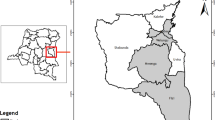
Commonly edible insect samples were obtained from six geographical areas namely Fizi, Idjwi, Kabare, Kalehe, Mwenga and Walungu, in Eastern Democratic Republic of Congo as mapped in Fig. 4.
Map showing the Democratic Republic of the Congo, as well as the South-Kivu Province, and the study area (ArcMap 10.4. https://desktop.arcgis.com/en/arcmap/10.4/).
Agro-ecological conditions of the study area
The agro-ecological conditions of the study area are depicted in Table 3. The Fizi Territory is located between 3°30 and 4°51 32 latitude (South), and 27°45 and 29°14 10 longitude (East), covering an area of ~ 15.789 km2 with estimated population of ~ 1.093.926 in 2019. Its elevation is subdivided into four zones, including the coastline (~ 750 m), the low land valley (~ 1000 m), a highland (~ 1300 m), and the very highland (locally called Haut Plateau with 1,700 m). The climate in Fizi is highly affected by the elevation. The rainfalls are unevenly distributed according to the month and the climatic subdivision. The North, dominated by the coastline and low inland valley, is characterized by humid tropical climate (of Aw3 type according to Köppen-Geiger classification). The greatest rainfall amounts are recorded in March and November, while the smallest amounts are the smallest amounts in February and September. The south part has a dry humid tropical climate. Available climate data mentioned an average annual rainfall of ~ 1,704 mm, the mean temperature ~ 23.54 °C (with the highest observed in April with ~ 25.6 °C and the lowest ~ 21.3 °C in September). The Territory is dominated by forest, comprising two forest reserves and a nature reserve. Acrisols and Cambisols are the dominant soil unities according to the WRB classification.
The Kabare Territory is located between 2°30′ of South latitude and 28°30’ of East longitude. Its altitude varies from ~ 1420 to 3200 m, and the Territory occupies an area of ~ 1690 km2 with an estimated population of ~ 868,616, which makes it among the most populated in the South-Kivu province. The Territory is located in the medium to high altitude AEZ. Available meteorological data mentioned an annual rainfall average of ~ 1572 mm, and a temperature of ~ 22.6 °C. Most of Kabare is savanna with natural vegetation consisting of wild grasses.
The Mwenga Territory is located in the middle of the province and is the only Territory surrounded by the other without any country or province borders. It is located between 28°25’29’’ East longitude and 30°02,16’05’’ South latitude. Its altitude varies between 1500 and 1800 m in the northeast. In the centre and the South, it is more or less 670 m. In the East, it is more or less 200 m and in the West more or less 670 m. It has a humid tropical climate with two seasons: the dry season from June to September and the rainy season from September to May. The temperature varies between 21 and 37 °C in most of the Territory and is low in the Itombwe area because of the high altitude, which goes up to over 2000 m. Rainfall reaches 2000 mm to 3000 mm per year. The vegetation is mainly dense forest and savanna. The forest is home to the Itombwe Nature Reserve (RNI). Relief is dominated by the Itombwe mount uplands and the alluvial valley of the Elila watershed. Soils dominated with clayey (Humic Cambisols) and sandy soil (Acrisols) types.
The Walungu Territory is located between 2º38’ of South latitude and 28º40’ of East longitude. Its altitude varies between 1000 m and 2000 m with a cold tropical climate of low altitude. There are two seasons, the dry season (June to August) and the rainy season from September to March. Available station data presented an annual average of ~ 17–20 ºC for temperature and 900 to 1500 mm rainfall. The vegetation mainly consists of grassland, a few forest reserves of Mugaba and Mushwere and woodlands scattered throughout the Territory.
The agro-ecological conditions of the study area are presented in Table 3. The Idjwi Island is located and surrounded by Lake Kivu. It is located between 1°37’8.85"S and 2°29’5.82"S as well as 29° 5’24.23"E and 28°34’15.91"E of latitude and longitude respectively. With an altitude varying from ~ 1439 m to 2,233 m (average of ~ 1811 m) and a temperature varying from 17 to 30 °C (average ~ 26.1 °C). As a result of its location (surrounded by a lake) and topography, the climate in Idjwi is humid wet tropical and tropical savannah. It is Aw type according to the Köppen-Geiger classification (with an average of 1540 mm of precipitation each year).
There are two seasons, the dry season (May to August) and the rainy season from September to May. The dominant soil unities according to WRB (World Reference Base for Soil) are Gleyic Solonchaks, nitisols and Humid Ferralsols, rich in sand and clay respectively. Threatened vegetation is naturally shrubby and grassy, interspersed with secondary forests. The island is also cover by croplands dominated with coffee, banana and cassava among the others. The Idjwi Territory is among the densely populated Territory in DR Congo and the region leading to high pressure on ecosystems in the island.
Kalehe is a bordering Territory between South-Kivu and North-Kivu. Located in the northern, Kalehe is one of contrasting Territory in South-Kivu based on its topography dominated by mountain (the Mitumba) in East, its altitude varies from 788 to 3035 m dividing the Territory into three AEZ: the high altitude, medium and low altitude (in Western and Northwestern). Lake Kivu borders Kalehe Territory over a distance of ~ 86 km from north to south, opening onto the Bukavu basin. The Kalehe Territory is characterized with a Humid wet tropical climate and in some area temperate with altitude. There are two seasons, the rainy season (from September to May) and the dry season (from June to August), with a precipitation ranging from 1300 to 2000 mm each year, and an annual temperature varying between 18 and 22ºC.
A diversity of soil is observed in the Kalehe Territory, from Haplic Acrisols, Dystric Cambisols, Haplic Nitisols, and Humid Ferralsols. The Dystric Cambisols and Haplic Nitisols are rich in clay very appropriate for agricultural purposes. Its vegetation is dominated by forest, where bamboos and shrubs are unfortunately in the process of disappearing due to an intense deforestation resulting in scarcity of arable land and no appropriate exploitation. Some tea, coffee, banana and cassava exploited lands are also observed. Other men activities such as small-scale mining, sand mining and livestock are dominant activities.
Sampling and sample preparation
About 5 kg of each commonly edible insect namely Apis mellifera larvae, Acheta domesticus, Gnathocera trivittata, Imbrasia oyemensis, Locusta migratoria, Gryllotalpa africana, Nomadacris septemfasciata, Macrotermes subhylanus and Rhyncophorus phoenicis were collected from six geographical sources purposely selected for their familiarity with anthropo-entomophagy practices and unique agroecological conditions. Edible insect samples from each geographical sourcing area were collected using local methods as described by Ishara et al.49,50, then packed in zipping polyethylene bags and delivered to Université Evangelique en Afrique on flaked ice in a cool box before being washed and drained. About half of the samples were frozen at − 20 °C until further analyses and the other half was directly used for sensory assessment purposes.
Macronutrient composition and energy
Macronutrient composition was determined in accordance with Association of Official Analytical Chemists51. Moisture and ash were determined by the hot-air circulating oven (105 °C) and through incineration in a muffle furnace (600 °C) respectively. Crude protein was determined by the Kjeldahl method and its content was obtained by multiplying the corresponding total nitrogen content by a factor of 5.3320. All determinations were carried in triplicate and expressed as mean ± standard error.
Mineral composition
Potassium, Sodium, Magnesium, Iron, Calcium and Zinc was determined in accordance with Association of Official Analytical Chemists51. The mineral content was determined using AA-7000 Atomic Absorption Spectrophotometer (AAS). The residue of ashed samples was dissolved with HCl then filtered using a Whatman filter paper. The absorbance of sample and standard solutions was determined. All the analyses were performed in triplicate and expressed as mean ± standard errors.
Sensory assessment
Insects were cooked using the methods described by Ishara et al.34,54] as shown in Table 4. A. domesticus and N. septemfasciata were deep-fried for 7 min, A. mellifera, I. oyemensis and R. phoenicis were boiled, roasted and deep fried for 10 min, G. trivittata and G. africana were deep-fried for 10 min. Finally, M. subhyalinus was fried for 5 min. Sensory testing of cooked edible insects was carried out using a 7-point hedonic scale. Forty untrained panellist members from the Université Evangélique en Afrique (UEA) took part in the sensory evaluation, and the tests were carried out shortly after cooking. Samples were labelled with a random three-digit number. Between sample tests, panellists used neutral non-carbonated mineral water to rinse the palate. The evaluation was carried out at room temperature and under air circulation. Cooked edible insects were placed on a small plastic plate and a sensory evaluation in relation to appearance, aroma, taste, texture and overall score was carried out with an intensity-based questionnaire using a 7-point hedonic scale (1 = dislike extremely, 2 = dislike moderately, 3 = dislike slightly, 4 = neither like or dislike, 5 = like slightly, 6 = like moderately and 7 = like extremely) according to52. The geographical sourcing area and amount of ingredients used for cooking samples are briefly described in Table 4.
Statistical analysis
Data collected in triplicates were encoded in Microsoft Excel for Mac (Version 16.74). R-Studio Version 4.2.0 and Statistix Version 10 Software were used for statistical analysis, and data were presented as mean ± standard error. Analysis of variance (ANOVA) was used to delineate the effect of geographical sourcing area on the nutritional composition and sensory attributes of commonly edible insects. Means were separated using Tukey’s test at a significance level of 0.05. Cluster analysis using a non-metric multidimensional scale (NMDS) was used to determine the extent to which geographical sourcing area of origin influenced nutritional composition and sensory attributes, as well as all parameters combined. The NMDS was composed using the R package “Vegan”.
Data availability
The data supporting the findings reported herein are available on reasonable request from the corresponding author.
References
Nowakowski, A. C., Miller, A. C., Miller, M. E., Xiao, H. & Wu, X. Potential health benefits of edible insects. Crit. Rev. Food Sci. Nutr. 62, 3499–3508 (2022).
Ishara, J. et al. The contribution of commonly consumed edible insects to nutrition security in the Eastern DR Congo. Sci. Rep. 14, 16186 (2024).
Desa, U. N. World population prospects 2019: highlights. New. York United Nations Dep Econ. Soc. Aff. 11, 125 (2019).
Ishara, J., Ogunyiola, A., Matendo, R., Kiyala, J. C. K. & Karume, K. Climate change and its implications on food security in the great lakes region. In Climate Change and Socio-political Violence in Sub-Saharan Africa in the Anthropocene: Perspectives from Peace Ecology and Sustainable Development 113–140 (Springer, 2024).
Noori, R. et al. Decline in Iran’s groundwater recharge. Nat. Commun. 14, 6674 (2023).
Stork, N. E. How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 63, 31–45 (2018).
Jongema, Y. List of edible insects of the world, Table, Lab. Entomol., Wageningen Univ., Neth. https://www.wur.nl/en/Research-Results/Chair-groups/Plant-Sciences/Laboratory-of-Entomology/Edible-insects/Worldwide-species-list.htm (2019).
Williams, J. P., Williams, J. R., Kirabo, A., Chester, D. & Peterson, M. Nutrient content and health benefits of insects. In Insects as Sustainable Food Ingredients 61–84 (Elsevier, 2016).
Weru, J., Chege, P. & Kinyuru, J. Nutritional potential of edible insects: a systematic review of published data. Int. J. Trop. Insect Sci. 41, 2015–2037 (2021).
Van Huis, A., Rumpold, B., Maya, C. & Roos, N. Nutritional qualities and enhancement of edible insects. Annu. Rev. Nutr. 41, 551–576 (2021).
Lin, X. et al. A review on edible insects in China: nutritional supply, environmental benefits, and potential applications. Curr. Res. Food Sci. 100596 (2023).
Ghosh, S., Lee, S. M., Jung, C. & Meyer-Rochow, V. B. Nutritional composition of five commercial edible insects in South Korea. J. Asia Pac. Entomol. 20, 686–694 (2017).
Baiano, A. Edible insects: an overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 100, 35–50 (2020).
Wendin, K. M. E. & Nyberg, M. E. Factors influencing consumer perception and acceptability of insect-based foods. Curr. Opin. food Sci. 40, 67–71 (2021).
Mishyna, M., Chen, J. & Benjamin, O. Sensory attributes of edible insects and insect-based foods—future outlooks for enhancing consumer appeal. Trends Food Sci. Technol. https://doi.org/10.1016/j.tifs.2019.11.016 (2019).
Melgar-lalanne, G., Hernandez-Alvarez, A. J. & Salinas-Castro, A. Edible insects processing: traditional and innovative technologies. Compr. Rev. Food Sci. Food Saf. 18, (2019).
Ribeiro, J. C., Pintado, M. E. & Cunha, L. M. Consumption of edible insects and insect-based foods: a systematic review of sensory properties and evoked emotional response. Compr. Rev. Food Sci. Food Saf. 23, 1–45 (2023).
Finke, M. D. & Oonincx, D. Insects as food for insectivores. Mass Prod. Benef. Org. Invertebr. Entomopathog. Acad. Press. Waltham, MA 2014, 583–616 (2014).
Mutungi, C. et al. Postharvest processes of edible insects in Africa: a review of processing methods, and the implications for nutrition, safety and new products development. Crit. Rev. Food Sci. Nutr. 59, 276–298 (2019).
Boulos, S., Tännler, A. & Nyström, L. Nitrogen-to-protein Conversion factors for Edible insects on the Swiss Market: T. Molitor, A. Domesticus, and L. Migratoria. Front. Nutr. 7, 89 (2020).
Bawa, M., Songsermpong, S., Kaewtapee, C. & Chanput, W. Effect of diet on the growth performance, feed conversion, and nutrient content of the house cricket. J. Insect Sci. 20, 10 (2020).
Francikowski, J. et al. The effects of high-monosaccharide diets on development and biochemical composition of white-eyed mutant strain of house cricket (Acheta domesticus). Sci. Rep. 11, 21147 (2021).
Oonincx, D. & Van der Poel, A. F. B. Effects of diet on the chemical composition of migratory locusts (Locusta migratoria). Zoo Biol. 30, 9–16 (2011).
Inogwabini, B. I. Land rights land use patterns and soil fertility significantly contribute to the two-decade long regional conflagration in Eastern Congo. In International Yearbook of Soil Law and Policy 2019 127–142 (Springer, 2021).
Kavle, R. R., Carne, A., Bekhit, A. E. D. A., Kebede, B. & Agyei, D. Proximate composition and lipid nutritional indices of larvae and pupae of the edible Huhu beetle (Prionoplus reticularis) endemic to New Zealand. J. Food Compos. Anal. 110, 104578 (2022).
Leonard, A. et al. Host plant-based artificial diets enhance development, survival and fecundity of the edible long-horned grasshopper Ruspolia differens (orthoptera: Tettigoniidae). J. Insect Sci. 22, 8 (2022).
Siddiqui, S. A. et al. Legal situation and consumer acceptance of insects being eaten as human food in different nations across the world—A comprehensive review. Compr. Rev. Food Sci. Food Saf. (2023).
Ssepuuya, G., Smets, R., Nakimbugwe, D., Van Der Borght, M. & Claes, J. Nutrient composition of the long-horned grasshopper Ruspolia differens Serville: Effect of swarming season and sourcing geographical area. Food Chem. 301, 125305 (2019).
Janssen, R. H., Vincken, J. P., van den Broek, L. A. M., Fogliano, V. & Lakemond, C. M. M. Nitrogen-to-Protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 65, 2275–2278 (2017).
Romotowska, P. E., Karlsdóttir, M. G., Gudjónsdóttir, M., Kristinsson, H. G. & Arason, S. Seasonal and geographical variation in chemical composition and lipid stability of Atlantic mackerel (Scomber scombrus) caught in Icelandic waters. J. Food Compos. Anal. 49, 9–18 (2016).
Joy, E. J. M. et al. Soil type influences crop mineral composition in Malawi. Sci. Total Environ. 505, 587–595 (2015).
Manditsera, F. A., Luning, P. A., Fogliano, V. & Lakemond, C. M. M. The contribution of wild harvested edible insects (Eulepida mashona and Henicus Whellani) to nutrition security in Zimbabwe. J. Food Compos. Anal. 75, 17–25 (2019).
Lehtovaara, V. J. et al. The fatty acid contents of the edible grasshopper Ruspolia differens can be manipulated using artificial diets. J. Insects as Food Feed. 3, 253–262 (2017).
Sokol, N. W. et al. Life and death in the soil microbiome: how ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 20, 415–430 (2022).
Jehlík, T. et al. Effects of Chlorella sp. on biological characteristics of the honey bee Apis mellifera. Apidologie 50, 564–577 (2019).
Di Pasquale, G. et al. Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS One. 8, e72016 (2013).
Naug, D. & Gibbs, A. Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie 40, 595–599 (2009).
Eremia, N., Zagareanu, A., Mardari, T. & Modvala, S. Stimulation of resistance of Bee families during Wintering. Sci. Pap Anim. Sci. Biotechnol. Stiint Zooteh si Biotehnol 46, (2013).
Amouzou, K., Tete-Benissan, A. & Badanaro, F. Nutritional potential of two insect species consumed in Togo: Gnathocera trivittata (Swederus, 1787) and Gnathocera impressa (Olivier, 1789). Eur. J. Nutr. Food Saf. 13, 24–32 (2021).
Musundire, R., Zvidzai, C. J., Chidewe, C., Samende, B. K. & Chemura, A. Habitats and nutritional composition of selected edible insects in Zimbabwe. J. Insects as Food Feed. 2, 189–198 (2016).
Ishara, J., Lukausa, S., Mushagalusa, G. & Kinyuru, J. Imbrassia oyemensis and Apis mellifera larvae as a potential source of dietary proteins to alleviate food insecurity in Kabare, Eastern D. R. Congo. Repos Ruforum (2021).
Brogan, E. N., Park, Y. L., Matak, K. E. & Jaczynski, J. Characterization of protein in cricket (Acheta domesticus), Locust (Locusta migratoria), and silk worm pupae (Bombyx mori) insect powders. LWT 152, 112314 (2021).
Barroso, F. G. et al. The potential of various insect species for use as food for fish. Aquaculture 422, 193–201 (2014).
Kinyuru, J. N. et al. Nutrient composition of four species of winged termites consumed in western Kenya. J. Food Compos. Anal. 30, 120–124 (2013).
Verspoor, R. L. et al. Mineral analysis reveals extreme manganese concentrations in wild harvested and commercially available edible termites. Sci. Rep. 10, 6146 (2020).
Mba, A. R. F. et al. Lipid and amino acid profiles support the potential of Rhynchophorus phoenicis larvae for human nutrition. J. Food Compos. Anal. 60, 64–73 (2017).
Rumpold, B. A. & Schlüter, O. K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 57, 802–823 (2013).
Omotoso, O. T. & Adedire, C. O. Nutrient composition, mineral content and the solubility of the proteins of palm weevil, Rhynchophorus Phoenicis f.(Coleoptera: Curculionidae). J. Zhejiang Univ. Sci. B. 8, 318–322 (2007).
Ishara, J. et al. Inventory reveals wide biodiversity of edible insects in the Eastern Democratic Republic of Congo. Sci. Rep. 12, 1576 (2022).
Ishara, J. et al. Edible insect biodiversity and anthropo-entomophagy practices in Kalehe and Idjwi territories, DR Congo. J. Ethnobiol. Ethnomed. 19, 1–17 (2023).
AOAC, B. A. M. Association of official analytical chemists. Off Methods Anal. 12, (2000).
Ihekoronye, A. I. & Ngoddy, P. O. Integrated Food Science and Technology for the Tropics (Macmillan, 1985).
Acknowledgements
The authors would like to thank Amani Kalalizi Benjamin, Badosanya Masirika Augustin, Bajimbe Christophe, Furaha Rubenga Laetitia, Japhet Naluvumbu Jossart and Chiza Birakara Jospin for their contribution to data collection. The assistance of Cirezi Chizungu Nadege in mapping the study area is appreciated. We also extend our acknowledgements to the UEA and the RUFORUM for their support (Grant ID: Grant#RU/2020/GTA/DRG/015).
Author information
Authors and Affiliations
Contributions
J.I., S.N., K.K. and J.K. contributed to the research design, performed the experiments, wrote and revised themanuscript; J.I. processed data, conceptualization and formal analysis; J.I., S.N., K.K., J.K., R.M. and J.N. data curationand investigation; J.I. drafted the manuscript; all authors reviewed the manuscript. All authors contributed tothis work and approved the final text of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ishara, J., Matendo, R., Ng’ang’a, J. et al. Insights into the effects of geographical sourcing area on nutrient composition and sensory attributes of nine edible insects. Sci Rep 15, 11610 (2025). https://doi.org/10.1038/s41598-025-90659-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90659-z
Keywords
This article is cited by
-
Potential health benefits of insect bioactive metabolites and consumer attitudes towards edible insects
npj Science of Food (2025)