Abstract
A high purity polysaccharide (UELPP-A1) was isolated from the crude polysaccharide of litchi pericarp (UELPP) by column chromatography, and acetylated polysaccharide (AC-UELPP) was obtained by acetylation modification of the crude polysaccharide of litchi pericarp. The physicochemical properties and in vitro antioxidant activity of UELPP-A1 and AC-UELPP were compared. The C/H on UELPP-A1 was assigned by Congo red test, FTIR, 1D and 2D NMR, and its structural characteristics were characterized. The results showed that the total sugar content of neutral UELPP-A1 was significantly increased to 94.15%, and its structure did not have a triple helix structure. In addition, the in vitro antioxidant activity test showed that both polysaccharides had antioxidant activity in a dose-dependent manner. The enhancement effect of AC-UELPP with the increase of concentration was the most significant (P < 0.05). Among them, the hydroxyl radical scavenging activity was stronger than its reducing ability and superoxide anion radical at the same polysaccharide concentration. Acetylation modification can improve the antioxidant activity of UELPP and has further research value for human health care.
Similar content being viewed by others
Introduction
Litchi was deeply loved by consumers as a fruit with high nutritional value and rich taste, while a large number of uneaten fruit pericarps also have high utilization value. A variety of bioactive compounds such as caffeic acid, flavonoids, phenols, polysaccharides, proanthocyanidins and ferulic acid could be extracted from litchi pericarps1. They had the functions of liver protection, anti-oxidation, anti-depression, anti-diabetes and anti-hypertension2,3, and was widely used in medicine, food, cosmetic products and other fields. Polysaccharides, as one of the active macromolecules beneficial to human health, were increasingly used in biomedicine and materials science, such as wound dressings, drug delivery, health care products and food preservative films4,5. Therefore, extracting polysaccharides from abandoned litchi peel and conducting systematic research on them can not only avoid the waste of resources, but also give full play to their economic benefits.
Most polysaccharides had antibacterial, antioxidant, anti-cancer, immune regulation and other activities6, but some polysaccharides had no significant effect or biological activity. Whether polysaccharides have bioactivity and activities were closely related to their internal structure, relative molecular mass, degree of branched chain and monosaccharide formation7. From this point of view, the structural characteristics of polysaccharides are an important factor affecting their biological activity. Researchers have paid more attention to the relationship between its structure and function. The modification of natural products to explore whether the difference in biological activity is related to its structure and physical and chemical properties is also a problem worthy of study. By means of chemical modification such as phosphorylation, acetylation, carboxymethylation and sulfation, the structural diversity was provided, the physicochemical properties were improved, and the biological activities were greatly improved8,9,10,11. Furthermore, some studies have found that the introduction of new groups into the molecular structure of polysaccharides can enhance their activity, and may also give them new biological functions and ameliorate their dissolubility12. It was a current research hotspot to transform its structure and develop novel polysaccharide compounds. Acetylation changes the internal environment of polysaccharide molecules by grafting acetyl groups to polysaccharides, resulting in acetylation modification also playing an important role in the treatment of tumors, aging, bacterial infection, kidney disease and other diseases13. Because the natural acetylated polysaccharides extracted from plants were extremely limited, acetyl plays a special and important role in the molecules of polysaccharides14. The molecular weight of litchi pericarp polysaccharide is 14 kDa, mainly composed of mannose, galactose, and a small amount of arabinose. At present, there are relatively few studies on the structure and acetylation of litchi pericarp polysaccharides14. Therefore, this paper intends to analyze the physical and chemical properties and structure of litchi pericarp polysaccharides after separation and purification, and introduce acetyl functional groups by modifying litchi pericarp crude polysaccharides to explore whether the difference in antioxidant activity after chemical modification is related to the change of substituent groups. It was very important to comprehensively analyze the active function and structure-activity connection of litchi pericarp polysaccharide, which was of fundamentality to increase the application value of litchi.
Materials and methods
Materials and reagents
The mature litchi was selected as the research object from the Lingnan area of Guangdong Province, China. After crushing, screening and drying. Acetic anhydride was purchased from Chongqing chuandong Chemical Co., Ltd (Chongqing, China). DEAE-cellulose 52 and Sephadex G-100 were bought from Shanghai yuanye Bio-Technology Co., Ltd (Shanghai, China). Other related reagents were analytically pure.
Preparation of Litchi Pericarp Polysaccharide
Ultrasonic assisted enzyme extraction of UELPP
Ultrasonic-assisted pectinase preparation of litchi pericarp polysaccharide15 was as follows. 80 g dried litchi peel powder was put into the water extract at a ratio of 1:30 g/ml, 3.2 g pectinase (BR, 50000 u/g) was added, stirred evenly, preheated for 5 min, ultrasounded for 40 min at 68 °C and 280 W, and filtered. Distill two-thirds of the water from the extract, added four times anhydrous ethanol. The lower substrate was dissolved after standing for 12 h at low temperature (4 ~ 5 °C) and centrifuged. After that, the mixed solution of trichloro-methane: n-butanol = 4 : 1 was used as the protein removal reagent of sevage. According to the ratio of 30% of the volume of the original polysaccharide concentrate, the protein removal reagent was added and oscillated violently for 20 min, stand for 15 min, collect the uppermost extract16, perform two deproteinization operations, merge the upper supernatant, put it into a 3500 Da dialysis bag for dialysis for two days. The unrefined UELPP was gotten by freeze-drying technique.
Purification of UELPP by column chromatography
Firstly, preliminary purification was carried out by cellulose column17. The specific steps are as follows: DEAE-Cellulose 52 filler was immersed for 12 h to achieve the purpose of swelling. Then, the fillers were soaked in 400 mL, 0.5 mol / L sodium hydroxide, hydrochloric acid and sodium hydroxide solution for 60 min to activate for use. After each soaking, the fillers were repeatedly washed and filtered with distilled water until the filtrate was neutral, and then packed into a column (30 * 250 mm). The 50 mg UELPP was dissolved, and the solution was slowly added around the edge of the DEAE-Cellulose 52 column wall after passing through a 0.45 μm strainer for separation. Gradient elution was performed with 300 mL distilled water, 0.1, 0.2, 0.3 and 0.4 mol/L NaCl. And keeping the elution rate between 1.6 and 1.8 min/mL. The recovery liquid of the five components was determined by phenol-sulfuric acid method18. The components collected according to different concentrations of eluent were named UELPP-A, UELPP-B, UELPP-C, UELPP-D and UELPP-E. Enrich the first polysaccharide component and freeze-dried.
Then 15 mg UELPP-A was dissolved in 5 mL distilled water and filtered with 0.45 μm filter membrane. Sephadex G-100 gel column (16 * 350 mm) was used for secondary separation with 400 mL distilled water as eluent19 and determined by phenol-sulfuric acid method. The fraction was enriched and stored by freeze-drying to obtain higher purity UELPP-A1.
Preparation of acetylated UELPP
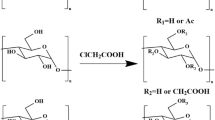
According to the method of acetylation modification of polysaccharides, a slight modification was made20. A mixture of 15 mL of water and 0.2 g of crude litchi pericarp polysaccharide (UELPP) was dissolved, and then a small amount of it was added several times. The acetic anhydride solution (1 mL, 1:5 g/mL) was added while stirring, and then 0.5 mol/L NaOH solution was used to make the pH of mixed solution to 9.5. Stir at 25 °C for 2.5 h. The solution was neutralized with 0.5 mol/L hydrochloric acid and dialyzed with running water for 2 days. After concentration, alcohol precipitation was carried out with 4 times the capacity of anhydrous ethanol, stored at 5 ℃ for 12 h, centrifuged, dissolved, and freeze-dried to obtain AC-UELPP.
Physicochemical property
Determination of total sugar content
The 0.1 mg/mL glucose solution was added in different volumes of 0, 0.1, 0.3, 0.5, 0.7 and 0.9 mL, and the distilled water was supplemented to 1 mL. Then 1 mL 5% phenol solution and 5 mL concentrated sulfuric acid were added respectively, and the reaction was carried out (20 min, 50 ℃). The determination was carried out at 490 nm by UV technique21.
Protein content determination
10 mg Coomassie brilliant blue was dissolved in 5 mL anhydrous ethanol, 10 mL phosphoric acid solution was added, 100 mL volumetric flask was constant volume, and stored in brown bottle (stored at 4 °C). The standard solution of bovine serum albumin (100 µg/mL) was added at 0, 0.2, 0.4, 0.6 and 0.8 mL, respectively, and 1 mL of distilled water was added. Then 5 mL of Coomassie brilliant blue solution was added in turn. The solution was shaken evenly, kept at 30 °C for 10 min, and the absorbance value was measured at λ = 590 nm22.
Determination of uronic acid content
Six dry 10 mL centrifuge tubes were taken, and 0.0, 0.1, 0.3, 0.5, 0.7, 0.9 mL of the prepared galacturonic acid standard solution (0.1 mg/mL) were added, and then the galactose aldehydic acid solution (16 mmol/L) was added to it, and the shaking was fully shaken. They were held at 90 ℃ for 5 min to achieve the purpose of mutual reaction. Then 1.25% carbazole solution (dissolved in 99.99% ethanol) was added and shaken. The response was completed in a water shower(95 °C) for 10 min. Finally, the temperature of the solution was reduced to 25 °C, and the specimens of various concentrations were determined at 520 nm23. The equivalent standard equation of uronic acid content was produced by taking the coefficient of absorption on the Y-axis and the concentration of the galacturonic acid solution on the X-axis.
Determination of acetylation degree of substitution
The degree of acetylation substitution of AC-UELPP was determined by neutralization titration24. According to the determination method of Meng Z et al., the method was modified25. In simple terms, 15 mg acetylated litchi pericarp polysaccharide was mixed with 10 mL NaOH (50 mmol/L), reacted at 50 °C for 150 min, cooled, dropped into 2 drops of phenolphthalein solution, and used 0.05 mol/L hydrochloric acid to end of titration. The volume of hydrochloric acid before and after consumption was recorded, and the substitution value of AC-UELPP can be calculated by the following formula26:
R = (V1C1-V2C2)/M;
DS = 132R/(4300-42R).
In the formula: R was the content of acetyl in polysaccharide (%). V1 and C1 were the volume (L) and concentration (mol/L) of NaOH. V2 and C2 were the volume (L) and concentration (mol/L) of HCl. M was the weight of the specimen (g). The degree of substitution was denoted by DS.
Structure representation
NMR and FT-IR analysis
80 mg polysaccharide and 99.99% heavy water were mixed and shaken to obtain a clear and transparent solution for nuclear magnetic resonance analysis. It includes 1D-NMR (1H NMR, 13C NMR) and 2D-NMR (1H, 1H-COSY, HSQC, HMBC)27,28. The purified sample UELPP-A1 was determined by one-dimensional and two-dimensional spectra, and UELPP and AC-UELPP were determined by 13C NMR.
The three polysaccharides of UELPP, UELPP-A1 and AC-UELPP were subjected to FT-IR analysis (Scanning in the range between 4000 cm−1 and 500 cm−1) by infrared spectrometer29, and the corresponding transmittance was recorded30.
Semi-quantitative adherence assay
Revised slightly with reference to the experimental procedure of Yang W et al.31. 2 milliliter of sodium hydroxide solution with different concentration gradients (0, 0.3, 0.6, 0.9, 1.2, 1.5 mol/L) was added to the six test tubes, and then UELPP-A1 sample solution (3 mg/mL, 1 mL) and rubrum congoensis solution (1 mL, 0.3 mmol/L) were added respectively to the above samples to be tested. The mixture was mixed in a volume ratio of 2:1:1 and stood for 30 min. The UV absorbance was obtained by scanning between 400 ~ 700 nm and the absorption maximum was recorded32. Keeping other conditions unchanged, water was used to replace the equal proportion of UELPP-A1 solution, and the above experimental operation was repeated. The parallel experiment was repeated three times to obtain the mean value. The concentration of sodium hydroxide was used as the abscissa, Draw the correlation plot with the maximum absorption wavelength as the y-axis.
Study on the antioxidant activity
Scavenging capacity of ·OH
Based on Liu M et al.33 method for determining the scavenging activity of polysaccharide·OH, a slight improvement was made. Six groups of different UELPP concentrations were used as variables, namely 0.9, 1.2, 1.5, 1.8, 2.1, 2.4, 2.7, 3.0 mg/mL. The same volume and the same concentration (0.5mL, 1mmol/L) of FeSO4 solution, salicylic acid-ethanol solution and H2O2 solution were added to each group. Stand in a water bath at 40 °C for 35 min, recorded as the experimental group Ab. Water was blank (Aa), and Vc with equal concentration gradient of UELPP-A1 was used as control. The absorbance value was measured at λ = 510 nm. The ability of AC-UELPP and UELPP-A1 to scavenge hydroxyl radicals was determined by the formula, as follows:
The proportion of Hydroxyl radical (·OH)·elimination (%) = [(Aa-Ab)/Aa] × 100%.
In the formula, Aa was the blank control group, and Ab was the absorbance value of polysaccharide solution with different concentration gradients.
Reducing capacity
Based on Hashemifesharaki et al.34, the reduction ability test of UELPP-A1 was adjusted to a certain extent. 2 mL K3Fe(CN)6 and 2 mL phosphate buffer were added to the sample solution with different concentrations in turn, 45 °C reaction for half an hour, cooling, adding 1 mL trichloroacetic acid (12%) to terminate the reaction. After centrifugation, 2 mL clear solution was added with 1% ferric chloride solution and 2 mL deionized water, respectively, and placed at 20 ℃ for 15 min. The value was recorded at a wavelength of 700 nm. The equal concentration gradient of Vc was used as the positive control group, and the above experiments were repeated. The reduction capacity was calculated as follows:
Reduction capacity (%) = [1–(A2-A1)/A3] × 100%.
In the formula, the absorbance of phosphate buffer at 700 nm was taken as A1. Polysaccharide samples as A2. The absorbance of aqua destillata was taken as A3.
Superoxide anion scavenging capacity
It was modified according to the method of Yuan et al.35. Litchi pericarp polysaccharide solution with different mass concentrations of 1 mL was taken, and 2 mL Tris-HCl buffer solution (0.05 mol/L, pH = 8.2) and pyrogallic acid solution (0.05 mL, 8 mmol/L) were added to it in turn. The solution was incubated at 25 °C for 15 min. Then 1 mL concentrated hydrochloric acid was added to terminate the reaction. The absorbance was measured at 420 nm. The superoxide anion scavenging capacity was calculated as follows:
Superoxide anion scavenging capacity (%) =[(A1-A2)/(A1-A3)] × 100%.
In the formula, A1 took distilled water as the blank group; A2 was the experimental group; A3 with buffer solution as the control group corresponding to the absorbance value.
Statistical analysis
All experimental data were recorded as mean ± SD and analyzed using SPSS Statistics 27 for Windows. P < 0.05 was considered statistically significant.
Result and discussion
Physicochemical property
The linear regression equation of glucose standard curve was y = 8.51836x + 0.09507 (R2 = 0.99995). The BSA method was determined by the method of 2.3.2, and the BSA curve was y = 4.1x + 0.8268 (R2 = 0.99990). The linear regression equation of galacturonic acid was y = 4.4711x + 0.18458 (R2 = 0.99987). The UELPP extracted by ultrasonic-assisted enzymatic method was reddish brown powder, with sugar content of 71.82%, uronic acid content of 14.90%, and protein content of 0.22%. The UELPP-A1 purified by column chromatography was a white powder with a sugar content of 94.15% and almost no uronic acid. The polysaccharide was a neutral polysaccharide. And if it was exposed to the air for a long time, it was easy to absorb water, and the surface becomes damp, which had certain water absorption. This may be due to the high purity of UELPP-A1. And the structure of UELPP-A1 contains a large number of -OH. These hydroxyl groups were hydrophilic and can combine with water molecules to form hydrogen bonds, so that the polysaccharide had good hydrophilicity. Therefore, UELPP-A1 should be sealed and preserved. AC-UELPP was brown powder, the yield was 68.20%, and the sugar content was 59.23%. The three kinds of polysaccharides of UELPP, UELPP-A1 and AC-UELPP were shown in Fig. 1.
Extraction and preliminary purification of UELPP
The UELPP obtained by extracting the crude polysaccharide from litchi pericarp without protein removal by sevage method contained 69.45% sugar, 16.53% uronic acid, 4.31% protein and other impurities. The sevage method was used to remove the protein twice. The results showed that the protein content in the crude polysaccharide was 0.22%, and the sevage method effectively removed most of the proteins contained in the crude polysaccharide of litchi pericarp. However, there were still a small amount of proteins and other impurities and pigments after preliminary purification. In order to further improve the content of UELPP, further purification was needed.
Separation and purification
DEAE-Cellulose 52 was used to separate the crude polysaccharide from litchi pericarp. Five elution peaks were obtained by different concentrations of eluent, as shown in Fig. 2 (1). The crude polysaccharides of litchi pericarp were different types of mixtures composed of neutral polysaccharides and acidic polysaccharides. It can be concluded from the comparison of the five components that the higher the elution peak of the UELPP-A and UELPP-B components, the larger the peak area, and the higher the content of the isolated litchi pericarp polysaccharides, which were 82.06% and 75.77%, respectively. However, the yield and sugar content of UELPP-C, UELPP-D and UELPP-E were very low, which was not conducive to collection. Meanwhile, during the separation process using cellulose columns, it was found that when appropriate amount of lychee husk polysaccharide solution was taken for up-sampling, the pigment was mainly adsorbed in the uppermost layer of the packing material, and the enriched lyophilized UELPP-A polysaccharide was in the form of a white powder, which effectively removed the pigment molecules. And no protein was detected in the UELPP-A fraction, indicating that the method was effective in removing proteins from lychee husk crude polysaccharide.
However, when the concentration of the UELPP solution for up-sampling is too large, not only will the pigment be eluted in the early stage, but also it will be difficult to separate each component of the polysaccharide from each other, and there will be overlapping between peaks. Therefore, for polysaccharides, the amount of sample was controlled between 40 and 60 mg. Secondly, the flow rate of the eluent is fast or slow, which also affects the effect of separation. Too fast a flow rate will lead to mixing of polysaccharide fractions with each other and poor separation, and too slow flow rate led to too low an efficiency for large volume collection.
From the comprehensive consideration of separation efficiency and yield, the UELPP-A fraction was selected for the second Sephadex G-100 gel column separation and purification and collection of fractions. A symmetrical and uniform peak was obtained as shown in Fig. 2 (2), and the results indicated that UELPP-A1 obtained by column chromatography purification was a homogeneous polysaccharide.
Acetylation analysis
AC-UELPP was prepared by acetylation modification of crude polysaccharide from lychee pericarp using acetic anhydride. The phosphorus substitution degree DS = 0.47 was calculated by acid-base titration, indicating that the acetylation modification of lychee pericarp polysaccharide was successful. For the reduced yield and polysaccharide content of the chemically modified lychee pericarp polysaccharides, there may be several reasons for the above situation. First of all, it may be because the amount of acetic anhydride would affect the acidity and alkalinity of the whole reaction process. The higher the concentration of acetic anhydride, the full reaction with the original polysaccharide solution and the increase of the probability of acetylation. However, too much dosage would enhance the acidity of the whole reaction, resulting in the breakage of the long chain of polysaccharides and the decrease of the yield. Secondly, it may be because the alkalization time was too long, UELPP was in an alkaline environment for a long time, resulting in polysaccharide degradation, yield and sugar content decreased. Finally, the reaction temperature may also affect the yield, degree of substitution and so on36. With the increase of temperature, the reaction between chloroacetic acid and polysaccharide sodium salt was more sufficient, and the utilization rate of raw materials was improved. However, too high temperature would destroy the structure of polysaccharide and lead to the decrease of sugar content. Therefore, the experimental conditions for the preparation of acetylated litchi pericarp polysaccharides, such as the amount of acetic anhydride, the degree of alkalization, reaction time and temperature, need to be further optimized.
Structure characterization
Determination of triple helix structure
Polysaccharide structures are varied and intricate, and the existence of triple helix usually has a positive effect on its biological activity37. This is an aspect that cannot be ignored when discussing the structure and activity of polysaccharides. Congo rubrum can be mixed with a substance with a triple helix structure, causing in a red shift in the maximum absorption peak of the solution to be measured. This is one of the main detection methods to determine whether the polysaccharide has this structure by Congo red test38. Under the condition of low alkali environment, the formation of the complex makes its maximum absorption peak increase significantly. With the concentration of sodium hydroxide increases, the triple helix structure of the material is broken by breaking the hydrogen bond, and the maximum absorption wavelength decreases sharply39. Figure 3 depicted the maximum wavelength of the blank group acid dye Congo red and the experimental group UELPP-A1 in different content of NaOH solution. The results showed that with the increase of the concentration of NaOH solution, λmax of Congo red decreased gradually. In the 0.0 ~ 0.3 mol/L sodium hydroxide solution, the λmax of UELPP-A1 showed a red shift compared with the blank group, but it was not obvious. With the concentration of sodium hydroxide solution continued to increase, the λmax of UELPP-A1 did not change much, and there was no sharp decrease compared with Congo red. At a higher concentration of NaOH solution, the λmax of polysaccharides did not increase significantly or decrease significantly. Therefore, structure of UELPP-A1 was not a triple helix. Therefore, according to the change trend of AC-UELPP, it could be inferred that it did not have a triple helix structure.
Analysis of FT-IR
In the structural analysis of organic matter, infrared spectroscopy is usually used to identify its characteristic groups. It was a common method for structural analysis. UELPP, UELPP-A1, and AC-UELPP polysaccharides were scanned in the range of 4000 ~ 500 cm−1. According to the typical characteristic absorption peaks, some possible structures were inferred. As shown in Fig. 4, a broad and strong absorption peak at 3500 –3200 cm−1 appears, which is a typical hydroxyl functional group on the polysaccharide sugar ring. The reason is that the oscillation of O-H collapsing in the structure of the material is caused by40. The weak absorption peaks at 2930, 2941 and 2933 cm−1 showed the stretching vibration of C-H, which were the specified peak position of polysaccharides41. The peak types of the three substances were similar, and the projecting absorption peak of purified UELPP-A1 was stronger. It indicated that UELPP, UELPP-A1 and AC-UELPP were all polysaccharides, and the purity of UELPP-A1 homogeneous polysaccharide was higher.
Figure 4 was a comparative analysis of infrared spectra of crude and purified polysaccharides from litchi pericarp. A peak of purified UELPP-A1 at 1623 cm−1 was significantly weakened. The absorption peak at 1623 cm−1 may come from the C = O stretching vibration42. It may be because the purified UELPP-A1 polysaccharide structure did not contain uronic acid, resulting in a significant reduction in the peak area. The results were consistent with the detected uronic acid content. The absorption peak near 1407 cm−1 may be caused by the C-H deformation vibration of methyl or methylene on the sugar chain, which was greatly enhanced after purification. The absorption peak of the pyranose ring showed wide and obvious absorption intensity near 1024 cm−1, which was due to the stretching of the carbon-oxygen bond on the six-membered ring of the polysaccharide34. In addition, the absorption signal appears at 950 –800 cm−1, which often corresponds to the glycosidic bond configuration. The larger red shift was the β glycosidic bond configuration, and the smaller was the α configuration43. UELPP-A1 showed a new strong and sharp peak at 854 cm−1, indicating that the polysaccharide may be α-configuration. In summary, UELPP-A1 may be α-pyranose.
After the introduction of ethanoyl into the polysaccharide structure, it could be seen that the corresponding C = O stretching vibration peaks at 1732 cm−1, 1616 cm−1 was strengthened, and the absorption peaks at 1305 and 1243 cm−1, which belong to -CH3 symmetric stretching vibration and C-H deformation vibration, were obviously strengthened44. From the above, it shows that the acetylation modification of UELPP was successful. In addition, compared with the infrared spectrum of the original litchi pericarp crude polysaccharide, it was found that the feature signal peaks of -O-H, carbon-hydrogen bond and carbon-oxygen bond in the 3287, 2930 and 1032 cm−1 regions of the chemically modified UELPP did not change much compared with the crude polysaccharide. This indicates that the chemical modification method may change the binding degree and amount of bonding of functional radical, methylene radical and other groups on the six-membered ring of the original polysaccharide, but did not change the main internal structure of the polysaccharide.
Analysis of NMR
The research on the structure of litchi pericarp polysaccharide by FT-IR was not perfect. For acetylated litchi pericarp polysaccharide, other structural units cannot be completely excluded, and there may be problems such as incomplete derivation and lack of structural units, which need to be verified and supplemented by NMR technology. The structure of crude polysaccharide, purified polysaccharide and acetylated polysaccharide were identified and compared by NMR technology, and the structure of polysaccharide was further confirmed.
In Fig. 5 (a) and (b), the 1D-13 C and 1D-1 H spectra of crude polysaccharides from litchi pericarp were shown. In the 13C spectra of crude polysaccharides from litchi pericarp, the anomeric carbon of UELPP mainly existed in the region of 90–108 ppm, indicating that there were both alpha and beta glycosidic bonds in UELPP45. In addition, there were some weak peaks in this region. The C2 ~ C6 signals existed between 60 and 85 ppm, and there were many stacking peaks, which were difficult to distinguish. Other than that, two peaks appeared at δ 175.00 and δ 174.95, which were the peak location of carbon on -COOH in uronic acid structure46. Moreover, it very well may be obviously seen that there is serious area of strength for a pinnacle signal at δ 16.63. Combined with the literature, it was deduced that it was the C6 peak signal on the rhamnose residue47. In the 1D-1 H spectrum corresponding to the crude polysaccharide of litchi pericarp, a single peak with the largest peak area appeared at δ 4.73, which was the peak of D2O solvent. The terminal matrix and H2 ~ H6 signal peaks were located on both sides of the heavy water peak, which are distributed in the 4.5–5.7 ppm region and 3.0-4.4 ppm, respectively. All in all, if the chemical shift of H1 was greater than 5.0 ppm, the glycosidic bond residue was an α-configuration; if less than 5.0 ppm, it was β configuration48. It could be seen from the figure that there were α-glycosidic bonds in the crude polysaccharide of litchi pericarp. In addition, we also found a new peak of δ 1.13 in the polysaccharide macromolecule, that is, the H6 signal of 6-deox-L-mannose, which was consistent with the 13C-NMR data, indicating that the H6 in the monosaccharide was not in the same chemical environment, and its pyran-rhamnose site was likely to be discrepant49. Two NMR signals at δ 1.76, δ 2.20 were attributed to the H5 of arabinose, the proportion of arabinose in lychee pericarp polysaccharide was relatively small.
In the carbon spectrum of acetylated litchi pericarp polysaccharide (Fig. 5c), many signal peaks appeared in the NMR spectrum between 20 ~ 185 ppm, of which the region of 60.0 ~ 80.0 ppm belongs to the typical polysaccharide signal peak, and there was less uronic acid at δ 171.12. By comparing with the 13C NMR of UELPP, two new sites, δ 181.57 and δ 160.39, were detected in the downfield area of the carbon spectrum of AC-UELPP, which were related to C atom on the introduced acetyl group. In the high field region, newly added signals of δ 20.36 and δ 23.34 both involve the methyl group on the acetyl group, indicating that the hydroxyl group in UELPP was acetylated successfully. This conclusion was consistent with the outcome of the above FTIR analysis.
For the isolated and purified litchi pericarp polysaccharide UELPP-A1, in its one-dimensional carbon spectrum (Fig. 5d), the 60–110 ppm range was the characteristic correlation peak of the polysaccharide, these anomeric carbons exist between 101 and 108 ppm, of which the δ 107.54 anomeric carbon signal was the strongest. Compared with 13C of UELPP, the peak signal was more significant, the overlapping peak was less, and the impurity peak was less. It turned out that UELPP-A1 had higher purity and uniform composition. There was no peak signal near 175 ppm and 16.63 ppm, and there was no rhamnose H6 signal in the 1D-1 H spectrum of UELPP-A1 (Fig. 5e), indicating that the polysaccharide chain of the purified UELPP-A1 component did not contain or the proportion of uronic acid was less and rhamnose residues. It indicated that the component UELPP-A1 polysaccharide may be neutral. In the hydrogen spectrum of UELPP-A1, many pinnacles were challenging to recognize because of the serious cross-over of pinnacles. Along these lines, just five clear proton top signs were tracked down around here. The monosaccharide residues represented by δ 5.10, 5.07, 5.05, 4.98 and 4.95 were named as Q1, Q2, Q3, Q4 and Q5, respectively.
The two-dimensional nuclear magnetic resonance (COSY, HSQC, HMBC) of polysaccharides was the necessary information for structural analysis. COSY spectrum can improve the coupling relationship between (H1, H2), (H2, H3), (H3, H4), (H4, H5) and (H5, H6). In this experiment, the hydrogen signal of other sites and the coupling between H and its connected C were found by using the HSQC spectral signal from the head hydrogen on the sugar chain. HMBC can obtain the long-distance C/H coupling signal of C and H, so as to infer the linkage fragments between monosaccharide residues. Next, the two-dimensional NMR spectra of UELPP-A1 were assigned to its carbon and hydrogen-related peaks. All C/H attribution results are shown in Table 1. Firstly, in H-H COSY (Fig. 5f), the cross-peak signals of δ 4.28, δ 3.88, δ 3.55 and δ 4.49 starting from the signal of Q1 anomeric hydrogen δ 5.10 were found, representing the H2 to H5 signals of Q1. Secondly, in the COSY spectrum, according to the signal of δ 5.07, five cross-peak signals were found in turn, which were δ 4.25, 3.85, 4.18, 3.77 and 3.43, respectively, belonging to H1 to H6 signals of Q2. The hydrogen signal of Q3 was not significant in the COSY diagram, so it was not discussed here. Thirdly, the H1 ~ H6 signal values of Q4 were δ 4.98, δ 4.02, δ 3.58, δ 3.37, δ 3.13, δ 4.52, respectively. Combined with the HSQC spectrum (Fig. 5g), the corresponding carbon signals, δ H 5.07/δ C 107.54 and δ H 4.98/δ C 107.05, were found by H1. For Q2, 83.69 ppm, 82.36 ppm, 61.46 ppm were the corresponding C3-C5 signal peaks. For the Q4 monosaccharide residue-related signal, the position of C2 was determined by δ H 4.02/δ C 76.59 and C3 was assigned. Be that as it may, no obvious C4-C6 cues were detected. In HMBC (Fig. 5h), we could find δ H 4.97/δ C 76.63, δ H 4.97/δ C 66.85 and δ H 4.94/δ C 74.15, δ H 4.94/δ C 82.25, indicating that H1 had a long-distance coupling relationship with C2 and C3. According to δ H 3.83/δ C 101.49, we attribute a cue of δ 101.45 to the telomere carbon of Q1, thus determine the C1 signal of Q5 as δ 106.44. Other carbon and hydrogen of Q1 were assigned by 1 H–1 H COSY and HSQC spectra, but the position of C5 was not found.
Study on the antioxidant activity
Figure 6 was the results of comparative study on the active function of two kinds of litchi pericarp polysaccharides UELPP-A1, Ac-UELPP and ascorbic acid (Vc). In this study, the hydroxyl clearance rate, reduction ability and superoxide anion clearance rate were used as indicators to explore the antioxidant activity of litchi pericarp polysaccharides in vitro. Figure 6 (1) showed that UELPP-A1 and Ac-UELPP had certain scavenging activity on hydroxyl radicals. When the sample content was 3 mg/mL, the scavenging rate of isolated and purified litchi pericarp polysaccharides was 38.43% ± 0.86%, and the scavenging rate of acetylated litchi pericarp polysaccharides could reach 82.75% ± 1.42%. Its scavenging activity was slightly lower than that of Vc. However, due to the hydroxyl scavenging ability of Ac-UELPP, the activity increased significantly with the increase of polysaccharide concentration and maintained a continuous upward trend (P < 0.05). Therefore, compared with Vc, this gap will gradually decrease. Similarly, UELPP-A1 and AC-UELPP dose-dependently increased antioxidant activity levels (Tables 2 and 3). Among the three different antioxidant experiments, the acetylation had the best enhancement influence for the scavenging activity of ·OH. The difference of hydroxyl scavenging activity and reducing ability activity of UELPP-A1 was relatively small. The explanation might be that the presentation of acetyl bunches in polysaccharides debilitates the separation energy of O-H bonds, subsequently having more noteworthy hydrogen supply.
The reducing power of UELPP-A1 and Ac-UELPP was shown in Fig. 6 (2). When the concentration of UELPP-A1 increased to 2.7 mg/mL, the growth trend of UELPP-A1 reduction ability tended to be gentle. The reducing ability of Ac-UELPP was in stark contrast to that of Ac-UELPP. With the increment of content, the reducing ability of acetylated litchi pericarp continues to increase in an oblique upward trend, showing good antioxidant capacity. It can be seen from Tables 2 and 3 that with the increase of the concentration of the two polysaccharides, the difference in reducing ability was statistically significant (P < 0.05), and the reducing ability of AC-UELPP was significantly higher than that of UELPP-A1.
Figure 6 (3) shows that UELPP-A1 and AC-UELPP have certain scavenging activity on superoxide anion. The superoxide anion radical scavenging activity of UELPP-A1 was not significantly different from the results of the first two active ability tests, while the superoxide anion radical scavenging activity of AC-UELPP was enhanced to a certain extent, which may be due to the introduction of the acetyl group. The introduction changed the spatial arrangement and molecular weight of the sugar chain molecules of the polysaccharide, thereby changing the biological activity. Compared with the purified UELPP-A1, the antioxidant activity of AC-UELPP was significantly improved at all concentrations (P < 0.05). It could be seen that the acetylation modification could significantly improve the antioxidant activity of UELPP. Moderate acetylation modification can enhance the antioxidant capacity of polysaccharides. In addition, the antioxidant activity of polysaccharides may also be affected by changes in degree of substitution, molecular weight and monosaccharide composition. The effect of different substitution degrees of acetylated polysaccharides on antioxidant activity needs to be further explored.
Statistical analysis
The relationship between the two polysaccharides and antioxidant activity was clarified by correlation analysis, and the results are shown in Table 4.
It can be seen from Table 4 that UELPP-A1 and AC-UELPP were significantly positively correlated with hydroxyl radicals, superoxide anion radicals and reducing power (R > 0.9, P < 0.01). It can be seen that the antioxidant capacity of polysaccharides may be closely related to derivatization modification. Considering the differences in the physicochemical properties of crude litchi pericarp polysaccharides, purified litchi pericarp polysaccharides and acetylated litchi pericarp polysaccharides, it could be concluded that the antioxidant activity of litchi pericarp polysaccharides may be closely related to acetylation modification, molecular weight and degree of substitution.
Conclusion
In this study, UELPP-A1 was isolated and purified from the crude polysaccharide extracted from litchi pericarp, and the sugar content was significantly increased to 94.15%. AC-UELPP (DS = 0.47) was obtained by acetylation modification. Congo red test showed that these two polysaccharides had no triple helix structure. Through FT-IR and NMR, we could find that there were a small amount of uronic acid and some rhamnose residues in the crude polysaccharide of litchi pericarp, while the structure of UELPP-A1 did not contain uronic acid and rhamnose, which could be determined that UELPP-A1 was a neutral polysaccharide. It also showed that the acetylation modification was successful. The C/H on UELPP-A1 was partially assigned by 2D NMR, and H1/C1 ~ H6/C6 signals were found in turn. UELPP-A1 may have five monosaccharide residues. This laid a foundation for the study of the structure of natural litchi pericarp polysaccharide. Through the correlation analysis of antioxidant activity, the concentration was significantly positively correlated with hydroxyl radical scavenging rate, reducing power and superoxide anion radical scavenging rate. The in vitro antioxidant activities of AC-UELPP and UELPP-A1 were significantly different. The antioxidant activity of AC-UELPP increased significantly with the increase of concentration (P < 0.05) and was stronger than that of UELPP-A1. This greatly improved the biological activity of UELPP. In addition, the introduction of acetylated polysaccharides has potential application value in the fields of bioactive carriers and functional foods. Acetylated litchi pericarp polysaccharides can provide new ideas for food health and industrial development.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
de Oliveira, J. P. et al. Diet with different concentrations of lychee peel flour modulates oxidative stress parameters and antioxidant activity in zebrafish. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 272, 110964. https://doi.org/10.1016/j.cbpb.2024.110964 (2024).
Tanaka, M. R. et al. Lychee peel extract obtained by ultrasound-assisted extraction: bioactive compounds and functional properties. Acta Scientiarum Technol. 46(1), e66447. https://doi.org/10.4025/actascitechnol.v46i1.66447 (2024).
Cano-Gómez, C. I., Alonso-Castro, A. J., Carranza-Alvarez, C. & Wong-Paz, J. E. Advancements in Litchi chinensis Peel Processing: a scientific review of drying, extraction, and isolation of its Bioactive compounds. Foods 13(10), 1461. https://doi.org/10.3390/foods13101461 (2024).
Wang, H., Huang, G. & Zhang, X. Analysis and properties of polysaccharides extracted from Brassica oleracea L. var. capitata L. by hot water extraction/ultrasonic-synergistic enzymatic method. Ultrason. Sonochem. 114, 107244 (2025).
Wu, J., Zhang, L. & Fan, K. Recent advances in polysaccharide-based edible coatings for preservation of fruits and vegetables: a review. Crit. Rev. Food Sci. Nutr. 64(12), 3823–3838. https://doi.org/10.1080/10408398.2022.2136136 (2024).
Wang, H. & Huang, G. Extraction, purification, structural modification, activities and application of polysaccharides from different parts of mulberry. Food Funct. 15(8), 3939–3958 (2024).
Kwon, P. S. et al. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell. Discovery. 6(1), 50. https://doi.org/10.1038/s41421-020-00192-8 (2020). Jonathan S. Dordick.
Deng, L. & Huang, G. Preparation, structure and application of polysaccharides from Poria cocos. RSC Adv. 14(42), 31008–31020 (2024).
Xia, S. et al. Phosphorylation of polysaccharides: a review on the synthesis and bioactivities. Int. J. Biol. Macromol. 184, 946–954. https://doi.org/10.1016/j.ijbiomac.2021.06.149 (2021).
Zhang, W. & Huang, G. Preparation, characteristics and antioxidant activity of mung bean peel polysaccharides. Sci. Rep. 14(1), 22161 (2024).
Tang, Z. & Huang, G. Antioxidant activity of polysaccharide from Garcinia mangostana rind and their derivatives. BMC Complement. Med. Ther. 24(1), 283 (2024).
Simsek, M., Asiyanbi-Hammed, T. T., Rasaq, N. & Hammed, A. M. Progress in bioactive polysaccharide-derivatives: a review. Food Reviews Int. 39(3), 1612–1627. https://doi.org/10.1080/87559129.2021.1935998 (2023).
Li, H. et al. Naturally and chemically acetylated polysaccharides: structural characteristics, synthesis, activities, and applications in the delivery system: a review. Carbohydr. Polym. 313, 120746. https://doi.org/10.1016/j.carbpol.2023.120746 (2023).
Liu, Y. & Huang, G. Extraction and derivatisation of active polysaccharides. J. Enzyme Inhib. Med. Chem. 34(1), 1690–1696 (2019).
Song, Z., Huang, G. & Huang, H. The ultrasonic-assisted enzymatic extraction, characteristics and antioxidant activities of lychee nuclear polysaccharide. Ultrason. Sonochem. 110, 107038 (2024).
Qin, H. et al. Purification, characterization, and bioactivity of Liupao tea polysaccharides before and after fermentation. Food Chem. 353, 129419. https://doi.org/10.1016/j.foodchem.2021.129419 (2021).
Shi, H. M. et al. Extraction, purification and antioxidant activity of polysaccharides from different parts of Hibiscus manihot L. J. Mol. Struct. 1295, 136598 (2024).
Yang, D., Lin, F., Huang, Y., Ye, J. & Xiao, M. Separation, purification, structural analysis and immune-enhancing activity of sulfated polysaccharide isolated from sea cucumber viscera. Int. J. Biol. Macromol. 155, 1003–1018. https://doi.org/10.1016/j.ijbiomac.2019.11.064 (2020).
Li, X., Rao, Z., Xie, Z., Qi, H. & Zeng, N. Isolation, structure and bioactivity of polysaccharides from atractylodes macrocephala: a review. J. Ethnopharmacol. 296, 115506. https://doi.org/10.1016/j.jep.2022.115506 (2022).
Wang, X. et al. Acetylated polysaccharides: synthesis, physicochemical properties, bioactivities, and food applications. Crit. Rev. Food Sci. Nutr. 64(15), 4849–4864. https://doi.org/10.1080/10408398.2022.2146046 (2024).
Wang, Y., Huang, G. & Huang, H. Ultrasonic/enzymatic extraction, characteristics and comparison of leechee peel polysaccharide. Ultrason. Sonochem. 108, 106948 (2024).
Huang, Y., Xie, W., Tang, T., Chen, H. & Zhou, X. Structural characteristics, antioxidant and hypoglycemic activities of polysaccharides from Mori Fructus based on different extraction methods. Front. Nutr. 10, 1125831. https://doi.org/10.3389/fnut.2023.1125831 (2023).
Shi, H. et al. Two-step hydrolysis method for monosaccharide composition analysis of natural polysaccharides rich in uronic acids. Food Hydrocoll. 101, 105524. https://doi.org/10.1016/j.foodhyd.2019.105524 (2020).
Li, L. et al. Carboxymethylation modification, characterization of dandelion root polysaccharide and its effects on gel properties and microstructure of whey protein isolate. Int. J. Biol. Macromol. 242, 124781. https://doi.org/10.1016/j.ijbiomac.2023.124781 (2023).
Ye, X., Zhao, Z. & Wang, W. Structural characterization and antioxidant activity of an acetylated Cyclocarya paliurus polysaccharide (Ac-CPP0. 1). Int. J. Biol. Macromol. 171, 112–122. https://doi.org/10.1016/j.ijbiomac.2020.12.201 (2021).
Li, J. et al. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrason. Sonochem. 93, 106295. https://doi.org/10.1016/j.ultsonch.2023.106295 (2023).
Dwivedi, R., Maurya, A. K., Ahmed, H., Farrag, M. & Pomin, V. H. Nuclear magnetic resonance-based structural elucidation of novel marine glycans and derived oligosaccharides. Magn. Reson. Chem. 62(4), 269–285. https://doi.org/10.1002/mrc.5377 (2024).
Zhou, S., Huang, G. & Long, R. Ultrasonic-assisted extraction, structural analysis and antioxidative mechanism of polysaccharide from sunflower disc. J. Mol. Struct. 1321, 140200 (2025).
Lin, B., Huang, G. & Huang, H. Extraction and structural analysis of polysaccharides from Shatian Pomelo peel. J. Mol. Struct. 1318, 139564. https://doi.org/10.1016/j.molstruc.2024.139564 (2024).
Hong, T., Yin, J. Y., Nie, S. P. & Xie, M. Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chemistry: X. 12, 100168. https://doi.org/10.1016/j.fochx.2021.100168 (2021).
Yang, W. & Huang, G. Extraction, structural characterization, and physicochemical properties of polysaccharide from purple sweet potato. Chem. Biol. Drug Des. 98(6), 979–985. https://doi.org/10.1111/cbdd.13952 (2021).
Pan, Q., Sun, Y., Li, X., Zeng, B. & Chen, D. Extraction, structural characterization, and antioxidant and immunomodulatory activities of a polysaccharide from Notarchus leachii freeri eggs. Bioorg. Chem. 116, 105275. https://doi.org/10.1016/j.bioorg.2021.105275 (2021).
Liu, M. et al. Functional properties, structural characteristics, and anti-complementary activities of two degraded polysaccharides from strawberry fruits. Int. J. Biol. Macromol. 269, 132263. https://doi.org/10.1016/j.ijbiomac.2024.132263 (2024).
Hashemifesharaki, R., Xanthakis, E., Altintas, Z., Guo, Y. & Gharibzahedi, S. M. Microwave-assisted extraction of polysaccharides from the marshmallow roots: optimization, purification, structure, and bioactivity. Carbohydr. Polym. 240, 116301. https://doi.org/10.1016/j.carbpol.2020.116301 (2020).
Yuan, S. et al. Extraction of polysaccharides from Codonopsis pilosula by fermentation with response surface methodology. Food Sci. Nutr. 8(12), 6660–6669. https://doi.org/10.1002/fsn3.1958 (2020).
Wang, H., Deng, L. & Huang, G. Ultrasound-assisted extraction and value of active substances in Muxu. Ultrason. Sonochem. 113, 107220 (2025).
Zeng, W. et al. Comparative study on the structural properties and bioactivities of three different molecular weights of Lycium barbarum polysaccharides. Molecules 28(2), 701. https://doi.org/10.3390/molecules28020701 (2023).
Fu, Y. L. & Shi, L. Methods of study on conformation of polysaccharides from natural products: a review. Int. J. Biol. Macromol. 263, 130275. https://doi.org/10.1016/j.ijbiomac.2024.130275 (2024).
Wang, C., He, Y., Tang, X. & Li, N. Sulfation, structural analysis, and anticoagulant bioactivity of ginger polysaccharides. J. Food Sci. 85(8), 2427–2434. https://doi.org/10.1111/1750-3841.15338 (2020).
Liu, G. et al. Extraction, structural characterization, and immunomodulatory activity of a high molecular weight polysaccharide from Ganoderma Lucidum. Front. Nutr. 9, 846080. https://doi.org/10.3389/fnut.2022.846080 (2022).
Liang, J. et al. Preparation and structure-activity relationship of highly active black garlic polysaccharides. Int. J. Biol. Macromol. 220, 601–612. https://doi.org/10.1016/j.ijbiomac.2022.08.115 (2022).
Su, Y. & Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 229, 115407. https://doi.org/10.1016/j.carbpol.2019.115407 (2020).
Zhang, H. et al. Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of Pinus koraiensis. Carbohydr. Polym. 251, 117078. https://doi.org/10.1016/j.carbpol.2020.117078 (2021).
Tang, Z., Huang, G. & Huang, H. Ultrasonic-assisted extraction, analysis and properties of purple mangosteen scarfskin polysaccharide and its acetylated derivative. Ultrason. Sonochem. 109, 107010. https://doi.org/10.1016/j.ultsonch.2024.107010 (2024).
Cheng, Y. et al. Structural characterization and in vitro fermentation properties of polysaccharides from Polygonatum Cyrtonema. Int. J. Biol. Macromol. 258, 128877. https://doi.org/10.1016/j.ijbiomac.2023.128877 (2024).
Fan, Y. & Huang, G. Preparation, structural analysis and antioxidant activity of polysaccharides and their derivatives from Pueraria lobata. Chem. Biodivers. 20(4), e202201253. https://doi.org/10.1002/cbdv.202201253 (2023).
dos Santos Ré, A. C., Cury, J. A., Sassaki, G. L. & Aires, C. P. Structure of rhamnoglucan, an unexpected alkali-stable polysaccharide extracted from Streptococcus mutans cell wall. Int. J. Biol. Macromol. 262, 130121. https://doi.org/10.1016/j.ijbiomac.2024.130121 (2024).
Li, X. G. et al. Structural analysis, in vitro antioxidant and lipid-lowering activities of purified Tremella Fuciformis polysaccharide fractions. Process Biochem. 133, 99–108. https://doi.org/10.1016/j.procbio.2023.06.005 (2023).
Li, J. et al. Extraction and properties of Ginkgo biloba leaf polysaccharide and its phosphorylated derivative. Ind. Crops Prod. 189, 115822. https://doi.org/10.1016/j.indcrop.2022.115822 (2022).
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
Yijie Wang wrote the manuscript. Gangliang Huang reviewed & edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Huang, G. Preparation, structure and properties of litchi pericarp polysaccharide. Sci Rep 15, 6331 (2025). https://doi.org/10.1038/s41598-025-90697-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90697-7
Keywords
This article is cited by
-
Modification, analysis and properties of Piper nigrum polysaccharide
Scientific Reports (2025)
-
The Isolation, Purification, Structure, and Properties of Lizhi Seed Polysaccharide
Applied Biochemistry and Biotechnology (2025)