Abstract
We aim to evaluate the myopia control effect of defocus incorporated multiple segments’ (DIMS) spectacle lens in combination with different concentrations of atropine (ATR). A retrospective cohort study was conducted and DIMS users were categorized according to ATR concentration: 55 DIMS alone, 55 DIMS-Low ATR (0.01%) and 50 DIMS-High ATR (0.125%) groups. All three myopia control methods were applied for one year. Primary outcomes measures were changes in spherical equivalent refraction (SER) and axial length (AXL). One-way ANOVA was utilized to compare the outcome differences among the three groups, and multiple linear regression was utilized to analyze the effects of age, sex, baseline SER and baseline AXL on myopia progression among the three groups. The cycloplegic SER progression was-0.30 ± 0.25 D, −0.17 ± 0.49 D and − 0.16 ± 0.14 D in DIMS, DIMS-Low ATR and DIMS-High ATR groups respectively. The DIMS group showed a significant higher cycloplegia SER progression (P = 0.003). The AXL elongation was 0.13 ± 0.08 mm, 0.06 ± 0.20 mm and 0.06 ± 0.14 mm in DIMS, DIMS-Low ATR and DIMS-High ATR groups respectively and AXL elongation was significantly higher in DIMS group (P = 0.011). The young age demonstrated positive correlation to the higher cycloplegia SER progression in all groups (all P < 0.05). The young age is also correlated to higher AXL elongation in the DIMS and DIMS-Low ATR groups (both P < 0.05). The myopia control effects of low- and high-concentration ATRs in DIMS users show no significant difference, while the addition of atropine in combination with DIMS spectacles had a greater effect on myopia control than DIMS spectacles alone.
Similar content being viewed by others
Introduction
Myopia is an ocular disease characterized by defocus anterior to the retinal surface1,2. Myopia has an prevalence above 40% in the United state population3, and the prevalence of myopia in Asian community reach a 2-fold value of above 80%4. The main pathophysiology of myopia includes steep corneal curvature as well as eyeball elongation, the latter of which accounts for the majority of acquired myopia cases5. On the other hand, high myopia, which is regarded as a spherical equivalent refractive error greater than − 6.00 diopters (D), increases the possibility of myopic maculopathy, retinal detachment and optic nerve damage6,7. Thus, the prevention of high myopia cannot be overemphasized.
Due to the need for myopia control, multiple interventions were invented during the late 20th century1,8. The application of high-concentration atropine (ATR) significantly reduces myopia progression2,9. In a previous study, patients treated with high-concentration ATR exhibited significantly less spherical equivalent refraction (SER) progression and axial length (AXL) elongation (up to 60%) than did patients in the control group10. On the other hand, several complications of treatment with high-concentration ATR, such as photophobia, eye allergy reactions and blurry vision at near distances, can develop and influence quality of life2,9. Consequently, a low concentration of atropine has been introduced, which can also significantly retard SER progression and AXL elongation11.
Currently, several new myopia control interventions have been applied and have shown reasonable effectiveness12. The defocus incorporated multiple segments’ (DIMS) spectacle lens is a recently developed myopia control intervention with good myopic control capability concerning AXL elongation13,14, and the DIMS spectacle lens does not harm the ocular surface; thus, its safety is adequate15. However, few studies have evaluated the effectiveness of the combination of DIMS with treatment with different ATR concentrations. Since the combined use of DIMS and treatment with low-concentration ATR better improved myopic control than did DIMS alone16, the effect of DIMS combined with treatment with different ATR concentrations for myopia control may differ and needs to be elucidated.
As a consequence, the objective of this study was to compare the effect of DIMS with that of DIMS combined with treatment with 0.01% ATR with that of DIMS combined with treatment with 0.125% ATR. Changes in the SER and AXL among the three groups were analyzed.
Materials and methods
Ethics declaration
All the interventions in this study were conducted in adherence to the 1964 Declaration of Helsinki and its subsequent amendments. Furthermore, this study was approved by the Institutional Review Board of the National Changhua University of Education (project code: NCUEREC-112-071). The need to obtain written informed consent was waived by the National Changhua University of Education due to the retrospective nature of this study.
Patient selection
A retrospective cohort study was performed at the Nobel Eye Institute, which is a joint clinical bloc located in the central, northern, and southern parts of Taiwan. The inclusion criteria were as follows: (1) aged 6 to 15 years, (2) used a DIMS spectacle lens in any branch of the Nobel Eye Institute from January 1, 2021, to December 31, 2022, and (3) regularly visited any branch of the Nobel Eye Institute for at least one year. Only one type of DIMS spectacle lens (Miyosmart, Hoya, Shinjuku-ku, Tokyo, Japan) was utilized in this study. To standardize the general condition of the population in this study, several exclusion criteria were used: (1) best-corrected visual acuity (BCVA) worse than 20/100 according to the Snellen chart at the first visit, (2) high myopia with a SER higher than − 6.00 D since it is an extreme condition which may increase the likelihood of reduced visual acuity and retinal complications, (3) high astigmatism with cylinder power more than − 3.00 D, (4) use of ATR eye drops with a concentration other than 0.01% and 0.125% at any time point, (5) use of more than one concentration of ATR (i.e. switch the ATR concentration) during the follow-up period, (6) those with prior myopic control interventions including orthokeratology, dual-focus contact lens and myopic control spectacle lenses, and (7) severe ocular defects involving but not limited to microbial keratitis, corneal scarring, infantile glaucoma, major ocular trauma, congenital cataract, retinal detachment, advanced retinopathy of prematurity, and optic nerve degeneration. Then, the patients were categorized into groups based on the concentration of ATR used— the low ATR group comprised patients who used 0.01% ATR and the high ATR group comprised patients who used 0.125% ATR. Notably, only the right eye of each patient was included in this study. A total of 55, 55 and 50 eyes were included in the DIMS group, DIMS-Low ATR group and DIMS-High ATR group, respectively.
Myopia control protocol
With respect to the DIMS protocol, the children who were managed with the DIMS spectacles were advised to wear them for approximately 8–10 h per day, except during exercising and napping, and no break was suggested. On the other hand, the children who used atropine were advised to instill one drop of atropine at approximately 21:00 to 23:00 just before bed. They were also taught to apply atropine eyedrops every day without a break. All the patients/parents used the ATR/DIMS based on our instruction everyday according to the inquiry in the medical records. No severe side effect or adverse effect was noted during the follow-up period.
Ophthalmic examination
The initial data of each child, including age, sex, BCVA, sphere power, cylinder power, simulated keratometry, corneal astigmatism and AXL, were taken from medical documents at the Nobel Eye Institute. Regarding the measurement of myopic progression, the primary outcomes in this study were the extents of SER progression and AXL elongation after one year of treatment. The manifest refraction and AXL were checked by an autorefractor (KR-8900, Topcon, Itabashi-ku, Tokyo, Japan) and a biometry device (IOL Master 500, Carl Zeiss, Göschwitzer Str., Jena, Germany) at the Nobel Eye Institute. The initial simulated keratometry and corneal astigmatism were checked by one topographic device (TMS-5, Tomey Corporation, Nishi-Ku, Nagoya, Japan). The manifest refraction, including the sphere power and cylinder power, was calibrated three times, and the average of the measurements was obtained. The SER was set as the whole sphere power plus half of the cylinder power in this study. Both the SER and AXL before myopic intervention, one month after myopic intervention, three months after myopic intervention, 6 months after myopic intervention, 9 months after myopic intervention and one year after myopic intervention in the study populations were recorded. In addition, the cycloplegia SER was measured before the myopic intervention and one year after the myopic intervention. In more detail, topical agent 0.5% tropicamide (Better Eye Drop, Aseptic Innovative Medicine Co. Ltd., Taoyuan Dist., Taoyuan, Taiwan) was used to induce cycloplegia. Each child received tropciamide instillation for at least one time (the mean times of tropicamide instillations are three) before the optometrists measured the pupil diameter, and cycloplegia refraction was performed if the pupil diameter was larger than 8 mm. The method used for examining and defining cycloplegia SER was the same as that used for manifest refraction.
Statistical analysis
SPSS version 20.0 (SPSS Inc., Chicago, Illinois, USA) was used for all the statistical analyses performed in this study. The statistical power of the current study was 0.90 with a 0.05 alpha value and a medium effect size which was generated using G∗power version 3.1.9.2 (Heinrich Heine Universität at Düsseldorf, Germany). Also, the assumption of equal variances were not violated according to the Levene’s test (all P > 0.05). The Shapiro-Wilk test was used to confirm the normality of the data in all three study populations, and the results showed normal distributions for all baseline data (all P > 0.05). A descriptive analysis was performed to present the initial data among the three groups, and one-way ANOVA and the chi-squared test or Fisher’s exact test were subsequently used to compare the initial data among the three groups depending on the characteristics of the variables. One-way ANOVA was performed again to compare SER progression and AXL elongation after the one-year follow-up period among the three groups, and a post hoc test via the Dunnett T3 method was conducted to identify exactly which groups differed from each other. In the next steps, multiple linear regression was adopted to evaluate the effectiveness of SER and AXL control among the three groups after adjustment for age, sex, initial cycloplegia SER and initial AXL. Then, the beta coefficient and 95% confidence interval (CI) of the intergroup comparisons were calculated. A line chart was drawn to demonstrate the trend of SER progression and AXL elongation among the three groups. Multiple linear regression was used again to evaluate the possible risk factors for SER progression and AXL elongation in the three groups, including young age, sex, initial cycloplegia SER and initial AXL, and to calculate the beta coefficient and 95% CI of each potential risk factor for myopia progression. Statistical significance was defined as a P value < 0.05 in the present study, and a P value less than 0.001 was represented as P < 0.001.
Results
The initial data of the three groups are available in Table 1. The mean initial ages were 9.72 ± 1.24, 9.68 ± 1.11 and 9.69 ± 1.32 years in the DIMS, DIMS-Low ATR and DIMS-High ATR groups, respectively, without significant differences (P = 0.764). The sex distributions (male: female) were 26:29, 28:27 and 23:27 in the DIMS, DIMS-Low ATR and DIMS-High ATR groups, respectively, which revealed similar distributions (P = 0.598). The other initial indices were not significantly different among the three groups (all P > 0.05) (Table 1).
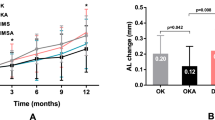
The initial cycloplegia SER was 2.53 ± 0.76 D, -2.57 ± 0.62 D and − 2.56 ± 0.82 D in the DIMS, DIMS-Low ATR and DIMS-High ATR groups, respectively. The difference in the cycloplegia SER among the three groups was not significant (P = 0.273). After one year of DIMS and ATR treatment, cycloplegia SER progression was − 0.30 ± 0.25 D, -0.17 ± 0.49 D and − 0.16 ± 0.14 D in the DIMS, DIMS-Low ATR and DIMS-High ATR groups, respectively, and the DIMS group showed significantly greater cycloplegia SER progression than did the other two groups (P = 0.003) (Table 2). On the other hand, the initial AXL was 23.30 ± 0.64 mm, 23.39 ± 0.92 mm and 23.40 ± 0.82 mm in the DIMS, DIMS-Low ATR and DIMS-High ATR groups, respectively, without significant differences among the three groups (P = 0.492). The changes in the AXL after the intervention were 0.13 ± 0.08 mm, 0.06 ± 0.20 mm and 0.06 ± 0.14 mm in the DIMS, DIMS-Low ATR and DIMS-High ATR groups, respectively, and AXL elongation was significantly greater in the DIMS group than in the other two groups (P = 0.011). About the AXL at 1, 3, 6 and 9-month, there was no significant difference among the three groups (all P > 0.05). Multiple linear regression analysis for the three groups also showed the same results in which both the SER and AXL controls were similar between DIMS-Low ATR and DIMS-High ATR groups and better than the DIMS group (Table 3). The trends in manifest SER progression and AXL elongation are shown in Figs. 1 and 2.
Concerning the risk factors for cycloplegia SER progression, young age was positively correlated with increased progression in all three groups (all P < 0.05) (Table 4). On the other hand, young age was also correlated with greater AXL elongation in the DIMS and DIMS-Low ATR groups (both P < 0.05) but not in the DIMS-High ATR group (P = 0.082) (Table 5). Sex, initial cycloplegia SER and initial AXL were not associated with SER progression or AXL elongation in the three groups (all P < 0.05) (Tables 4 and 5).
Discussion
In this study, the instillation of ATR in the population of patients who were treated with the DIMS spectacles was associated with lower SER progression and AXL elongation than in patients who were treated with only DIMS spectacles. Moreover, the combined used of both high-concentration ATR and low-concentration ATR with DIMS spectacles had similar effects for controlling myopia. On the other hand, except for AXL elongation in patients treated with both DIMS spectacles and high concentrations of ATR, young age was correlated with a greater risk of myopic progression.
The combined use of high-concentration or low-concentration ATR and DIMS spectacles contributes to better myopic control than does DIMS monotherapy in terms of SER progression and AXL elongation. According to previous studies, the DIMS spectacles is an effective intervention for controlling myopia because of its effects on both SER progression and AXL elongation compared to the use of a single-vision spectacle lens for visual correction13. Additionally, the combined use of 0.01% ATR and DIMS spectacles contributed to a greater myopic control rate than did the use of only DIMS spectacles in a previous study16. Nevertheless, few studies have compared the effectiveness of myopia control with DIMS spectacle lenses with different ATR concentrations. To our knowledge, this research may be the first to reveal the similar effectiveness of DIMS spectacle lenses with different ATR concentrations on myopia control in an Asian population. In addition, individuals with a SER higher than − 6.00 D were excluded from this study, thus increasing the homogeneity of our study population. In addition, all three groups in this study were similar in terms of age, sex and initial SER status, which are known confounders in the evaluation of myopia progression17,18. As a consequence, interference from possible confounders may not be prominent in this study. The myopic control efficiency, using AXL elongation as parameter, was similar between the combined treatment groups and DIMS groups until one year after the treatment. We think the possible explanation is that the absolute amount of AXL elongation is small (the largest AXL elongation amount was only 0.13 mm in the DIMS group) after one year. In earlier measurements, the amount of AXL elongation was even lower in all the groups, thus the statistical results tend to present insignificance. Still, the AXL elongation was about 0.03 mm every three months in the DIMS group and about 0.015 months every three months in the two DIMS-ATR groups (as presented in Fig. 2). Accordingly, the additional effect of combined treatments may exist in the early treatment period.
A previous study demonstrated greater myopic control in patients treated with high-concentration ATR19, while later research revealed a similar effect on myopic control between patients treated with high-concentration ATR and those treated with low-concentration ATR with a longer follow-up period20. The mechanism of myopia control in the context of ATR is AXL regulation7, in which the muscarinic receptor on the sclera is stimulated by ATR, and the scleral remodeling would be regulated then AXL elongation would be retarded21. In previous experimental studies, the ATR could contribute to the alteration of gene expression22, and the threshold concentration of ATR to influence muscarinic receptor and inhibit myopia was between 0.01% and 0.1%23. The above evidences may have contributed to the similar myopia control effect of the DIMS-High ATR and DIMS-Low ATR groups in our study. On the other hand, the DIMS spectacle lens controls myopia progression via peripheral myopic defocus effect, which can aggregate peripheral light in front of the retina and reduce the chance of AXL elongation and inhibit myopia13,24. Considering the above evidences and the results of this study, there is an enhanced myopic control effect with the addition of atropine for the children wear DIMS spectacles.
Regarding the possible predisposing factors for myopia progression in the different groups, age was negatively associated with a greater degree of SER progression in all three groups. A young age was a factor that correlated with faster AXL elongation in a previous study25, and children aged 6–10 years presented with more rapid myopia progression than did their older counterparts26. Consequently, it is reasonable that younger individuals had worse myopia control in all three groups in this study. On the other hand, young age was also significantly correlated with greater AXL elongation in the DIMS only and DIMS-Low ATR groups. However, the correlation between young age and AXL elongation was not significant in the DIMS-High ATR group. Few studies have reported this phenomenon. High-concentration ATR demonstrated slightly better myopia control than did low-concentration ATR19. We speculate that the slightly better myopia control effect in the high-concentration ATR group might have a certain effect on the high risk group such as young individuals. Another explanation for the nonsignificant correlation between young age and AXL elongation in the DIMS-High ATR group is that the amplitude of AXL elongation was numerically lower than the amplitude of SER progression; thus, statistical analysis more easily revealed nonsignificant results. Regarding the other factors, sex, initial SER and initial AXL did not accompany worse myopia control in any of the three groups. In a previous study, sex, initial SER and initial AXL were also not significantly correlated with myopia progression in the population treated with the DIMS alone or DIMS-ATR27. The results of this study are in accordance with those of previous studies.
There was a mild difference between the initial SER and AXL among the three groups, in which the DIMS-High ATR group demonstrated a similar SER but a greater AXL. A possible explanation is that some of the patients in the DIMS-High ATR group had previously undergone treatment with ATR; thus, they experienced a longer cycloplegia process than did those in the DIMS only group and the DIMS-Low ATR group. In the 7 patients used high concentration ATR previously in the DIMS-High ATR group, their mean SER and AXL changes were − 0.15 D and 0.06 mm, respectively, which were close to the other patients with DIMS-High ATR treatments. Thus, we did not exclude them. In addition, the simulated keratometry of the DIMS-High ATR group was numerically lower than that of the other two groups, which may indicate that the ratio of patients with axial myopia in the DIMS-High ATR group was greater than that in the other two groups.
There are still some limitations in this study. First, the retrospective nature of the study design reduced the homogeneity of the study population despite the lack of significant differences in the initial parameters among the three groups. Second, the sample size was relatively small in this study, with only 160 eyes enrolled in the study population, which may have contributed to statistical bias. In addition, we did not measure the cycloplegia SER at all time points due to the compliance of both patients and parents in clinical practice; thus, the trend in SER progression was less accurate. Additionally, we wanted to enroll as many patients as possible to reach adequate statistical power; thus, we did not conduct a matching process via certain covariate (i.e. age and sex) and instead enrolled all the patients who met the selection criteria. This may reduce the integrity of our results despite setting an age range and myopia range for all the participants. Besides, we did not measure the amplitude of accommodation which may influence the judgment of fully cycloplegia or not. Finally, the duration of daily DIMS use may have differed among all the participants, which could have significantly influenced myopia control. Nevertheless, all the participants were advised to wear the DIMS spectacles for at least 8 h every day without free days. Accordingly, the influence of DIMS spectacles usage duration might not be prominent.
In conclusion, the overall effect of combined DIMS and ATR interventions on myopia control was not affected by the concentration of ATR. Furthermore, the combined use of DIMS spectacles and high-concentration ATR might improves AXL control in young populations. Consequently, a concurrent intervention with DIMS spectacles and low-concentration ATR may be recommended for the majority of children. Further large-scale prospective studies to evaluate the myopia control effect of combined DIMS and orthokeratology contact lenses compared to single interventions are needed.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- D:
-
Diopter
- ATR:
-
Atropine
- SER:
-
Spherical equivalent refraction
- AXL:
-
Axial length
- DIMS:
-
Defocus incorporated multiple segments
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- N:
-
Number
References
Huang, J. et al. Efficacy comparison of 16 interventions for myopia control in children: A network meta-analysis. Ophthalmology 123, 697–708. https://doi.org/10.1016/j.ophtha.2015.11.010 (2016).
Walline, J. J. Myopia control: A review. Eye Contact Lens 42, 3–8. https://doi.org/10.1097/icl.0000000000000207 (2016).
Vitale, S., Sperduto, R. D. & Ferris, F. L. 3 Increased prevalence of myopia in the united States between 1971–1972 and 1999–2004. Arch. Ophthalmol. 127, 1632–1639. https://doi.org/10.1001/archophthalmol.2009.303 (2009).
Lam, C. S., Goldschmidt, E. & Edwards, M. H. Prevalence of myopia in local and international schools in Hong Kong. Optom. Vis. Sci. 81, 317–322. https://doi.org/10.1097/01.opx.0000134905.98403.18 (2004).
Morgan, I. G., Ohno-Matsui, K., Saw, S. M. & Myopia. Lancet 379, 1739–1748. https://doi.org/10.1016/s0140-6736(12)60272-4 (2012).
Flitcroft, D. I. et al. IMI - Defining and classifying myopia: A proposed set of standards for clinical and epidemiologic studies. Invest. Ophthalmol. Vis. Sci. 60, M20–m30. https://doi.org/10.1167/iovs.18-25957 (2019).
Wu, P. C. et al. Update in myopia and treatment strategy of Atropine use in myopia control. Eye (London) 33, 3–13. https://doi.org/10.1038/s41433-018-0139-7 (2019).
Remón, L., Pérez-Merino, P., Macedo-de-Araújo, R. J., Amorim-de-Sousa, A. I. & González-Méijome, J. M. Bifocal and multifocal contact lenses for presbyopia and myopia control. J. Ophthalmol. 2020 8067657. https://doi.org/10.1155/2020/8067657 (2020).
Tran, H. D. M. et al. A review of myopia control with Atropine. J. Ocul. Pharmacol. Ther. 34, 374–379. https://doi.org/10.1089/jop.2017.0144 (2018).
Bullimore, M. A. & Richdale, K. M. C. : Where are we and where are we heading? Ophthalmic Physiol. Opt. 40, 254–270, https://doi.org/10.1111/opo.12686 (2020).
Yam, J. C. et al. Low-concentration Atropine for myopia progression (LAMP) study: A randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% Atropine eye drops in myopia control. Ophthalmology 126, 113–124. https://doi.org/10.1016/j.ophtha.2018.05.029 (2019).
Chamberlain, P. et al. A 3-year randomized clinical trial of misight lenses for myopia control. Optom. Vis. Sci. 96, 556–567. https://doi.org/10.1097/opx.0000000000001410 (2019).
Lam, C. S. Y. et al. Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br. J. Ophthalmol. 104, 363–368. https://doi.org/10.1136/bjophthalmol-2018-313739 (2020).
Lam, C. S. et al. Myopia control effect of defocus incorporated multiple segments (DIMS) spectacle lens in Chinese children: results of a 3-year follow-up study. Br. J. Ophthalmol. 106, 1110–1114. https://doi.org/10.1136/bjophthalmol-2020-317664 (2022).
Carlà, M. M. et al. Overview on defocus incorporated multiple segments lenses: A novel perspective in myopia progression management. Vision (Basel) 6 https://doi.org/10.3390/vision6020020 (2022).
Nucci, P. et al. A comparison of myopia control in European children and adolescents with defocus incorporated multiple segments (DIMS) spectacles, Atropine, and combined DIMS/atropine. PLoS ONE 18, e0281816. https://doi.org/10.1371/journal.pone.0281816 (2023).
Landreneau, J. R., Hesemann, N. P. & Cardonell, M. A. Review on the myopia pandemic: epidemiology, risk factors, and prevention. Mo Med. 118, 156–163 (2021).
Lanca, C. et al. Three-year change in refractive error and its risk factors: results from the shahroud school children eye cohort study. Eye (London) 37, 1625–1632. https://doi.org/10.1038/s41433-022-02219-8 (2023).
Chia, A. et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the treatment of myopia 2). Ophthalmology 119, 347–354. https://doi.org/10.1016/j.ophtha.2011.07.031 (2012).
Chia, A., Lu, Q. S. & Tan, D. Five-Year clinical trial on Atropine for the treatment of myopia 2: myopia control with Atropine 0.01% eyedrops. Ophthalmology 123, 391–399. https://doi.org/10.1016/j.ophtha.2015.07.004 (2016).
Upadhyay, A. & Beuerman, R. W. Biological mechanisms of Atropine control of myopia. Eye Contact Lens 46, 129–135. https://doi.org/10.1097/icl.0000000000000677 (2020).
Sun, L. et al. Mechanism of myopic defocus or Atropine for myopia control: different or similar ways?? Ophthalmic Res. 65, 698–711. https://doi.org/10.1159/000525744 (2022).
Carr, B. J. et al. Myopia-Inhibiting concentrations of muscarinic receptor antagonists block activation of Alpha2A-Adrenoceptors in vitro. Invest. Ophthalmol. Vis. Sci. 59, 2778–2791. https://doi.org/10.1167/iovs.17-22562 (2018).
Troilo, D. et al. IMI - Report on experimental models of emmetropization and myopia. Invest. Ophthalmol. Vis. Sci. 60, M31–m88. https://doi.org/10.1167/iovs.18-25967 (2019).
Tideman, J. W. L. et al. Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol. 96, 301–309. https://doi.org/10.1111/aos.13603 (2018).
Verkicharla, P. K., Kammari, P. & Das, A. V. Myopia progression varies with age and severity of myopia. PLoS ONE 15, e0241759. https://doi.org/10.1371/journal.pone.0241759 (2020).
Huang, Z., Chen, X. F., He, T., Tang, Y. & Du, C. X. Synergistic effects of defocus-incorporated multiple segments and Atropine in slowing the progression of myopia. Sci. Rep. 12, 22311. https://doi.org/10.1038/s41598-022-25599-z (2022).
Author information
Authors and Affiliations
Contributions
C.-K.C. made conceptualization; S.-F.Y. and C.-K.C. made methodology; C.-K.C. provided software; J.-Y.H. and I.-B.L. made formal analysis; Y.-L.C. and C.-K.C. made data curation; C.-Y.L. wrote original draft; C.-K.C. reviewed and edited the draft; C.-K.C. made validation; C.-K.C. made supervision. All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, CY., Yang, SF., Chang, YL. et al. The effect of defocus incorporated multiple segment spectacles’ lenses combined with different concentrations atropine for myopia control. Sci Rep 15, 12356 (2025). https://doi.org/10.1038/s41598-025-91089-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91089-7