Abstract
Esketamine (EK) has been widely used in the treatment of depression, but the effects of EK prenatal treatment on embryonic heart development have been rarely reported. This study assesses the effects of varying concentrations of EK on embryonic development and cardiogenesis to determine the teratogenic concentration in the zebrafish model, centering on the interaction between the genes nkx2.5 and gata4 to elucidate the mechanisms underlying cardiac morphogenesis. Zebrafish embryos were classified into six distinct groups and exposed to either a vehicle or EK to ascertain the median lethal concentration (LC50) at 48 and 72 h post-fertilization (hpf) analyzing mortality rate data. Embryonic and cardiac morphologies were assessed utilizing live embryo imaging techniques and stereo microscopy. Nkx2.5 and gata4 were identified via whole-mount in situ hybridization (WISH) and reverse transcription quantitative polymerase chain reaction (RT-qPCR). Exposure to EK leads to significant teratogenic effects on zebrafish embryos, which are both concentration- and time-dependent. The 48 h- and 72 h-LC50 of EK for zebrafish embryos were 1.30 (95% CI 0.92, 1.60) millimolar (mM) and 0.71 (95% CI 0.46, 1.01) mM, respectively. A significant reduction in heart rates and body length were observed and the distance between the sinus venosus and bulbar artery (SV-BA) was found expanded, the pericardial edema area showed significant swelling, and the body axis curvature was more pronounced in the EK exposure groups. Both WISH an RT-qPCR analysis showed nkx2.5 staining intensity and expression significantly decreased, while gata4 assay results were in the opposite direction. Our findings indicate that exposure of zebrafish embryos to EK results in embryonic and cardiac malformations, primarily due to the down-regulation of nkx2.5 and the over-expression of gata4. Equilibrium maintenance and compensatory mechanisms are crucial in spatiotemporal gene regulation.
Similar content being viewed by others
Introduction
Perinatal depression (PND) is a public health problem1, which has received much attention in recent years due to its severe social dysfunction. The formation and development of PND have introduced numerous complex challenges to society, frequently resulting in maternal suicidal tendencies. PND refers to severe or mild depressive episodes that occur in a special period: during pregnancy or within one year after delivery, that is, including pregnancy depression and postpartum depression2. Globally, anxiety and depression affect 15–20% of pregnant women today3,4. Pregnancy’s physiological shifts, postpartum recovery, and the psychological toll on new mothers collectively fuel PND. PND, alongside prenatal drug exposure, imperils fetal growth, giving rise to developmental complications and birth defects. These defects, born of inadequate intrauterine progress, stand as the chief culprits behind neonatal disabilities and fatalities. Consequently, they impose substantial economic and psychological burdens on society and families, adversely impacting population health and social progress. However, as the population of pregnant women with PND grows, the challenge of effectively and safely intervening in this condition during pregnancy without adversely affecting fetal development remains a significant and complex issue.
As the classic representative, selective serotonin reuptake inhibitors (SSRIs) have been widely used in the treatment of PND5, but their use in the first trimester may be associated with fetal congenital heart defects (CHD)6,7,8, and even their effectiveness remains unclear9. Being the S-enantiomer of ketamine, EK is an emerging antidepressant drug, which can rapidly and effectively relieve the symptoms of major depressive disorder (MDD) through inhibiting the N-methyl-D-aspatrate (NMDA) receptor10,11. Recently, Wang and his colleagues have reported on BMJ that a single low dose of EK after delivery reduced major depressive episodes by about three-quarters at 42 days postpartum12. However, it remains to be verified whether the use of EK for the treatment of PND, particularly in the first trimester, may adversely affect fetal heart development.
In order to address this issue, the present study will systematically examine the toxicity of EK exposure on the development of embryos and cardiac function in zebrafish embryos. The use of zebrafish as a model organism is advantageous due to its high genetic homology with humans, coupled with the ability to perform in vitro fertilization and observe embryonic development externally, facilitated by the transparency of the zebrafish body13. Therefore, zebrafish embryo has become an excellent model for development biology, basic medicine, neurological and toxicology research during the last decades14,15. The heart, which has a simplified structure consisting of only one atrium and one ventricle16, is the first organ to form and function during zebrafish embryogenesis. The zebrafish heart, despite its anatomical divergence from the human heart, possesses analogous characteristics that encompass atria, ventricles, heart valves, and a conduction system17,18. The critical window for cardiac development in humans spans weeks 2 through 7 of gestation, a phase during which the heart undergoes significant morphogenesis. In contrast, the zebrafish heart achieves full development within approximately 48 h, highlighting species-specific differences in developmental timelines, as documented in Lohr’s study19. Embryonic development represents a highly orchestrated and finely regulated spatiotemporal dynamic process. In the course of development, embryos are required to sustain this dynamic process while simultaneously safeguarding it from disruption by external, non-endogenous factors. Exploring and avoiding the toxic effects of EK exposure so that provide scientific theoretical support for PND women is meaningful treatment protocols, which also be of great significance to improve the health of the population. Therefore, we have conducted a methodical examination of the toxicity of EK exposure on both embryonic and cardiac development by zebrafish model to broaden the scope of EK prescription.
Given the aforementioned considerations, this study aims to elucidate the effects of EK on zebrafish embryonic and cardiac morphology and function, with an initial investigation into the underlying mechanisms by examining the expression patterns of the cardiac development-associated genes nkx2.5 and gata4 during the progression of cardiac dysplasia.
Materials and methods
Ethics
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the South China University of Technology (Guangzhou, Guangdong, P.R China, approval number SCUT-2022-096) and were carried out in accordance with relevant guidelines and regulations. All the methods are performed in accordance with ARRIVE guidelines.
Zebrafish husbandry and embryo collection
Four-month-old healthy wild-type zebrafish Tuebingen (TU) strain in this study are all from the Chinese Academy of Sciences Institute of Aquatic Organisms (Beijing, China). All the adult zebrafish were regularly fed brine shrimp twice a day. Water temperature maintained at 28.5 ± 1°C in the incubator. The salinity of the water was regulated by NaCl and maintained solution conductivity between 450 and 500 μS cm−1. A 14-h light:10-h dark cycle was maintained to mimic natural day-night patterns. The day before the experiment, adult male and female zebrafish were placed in the breeding box at a 1:1 ratio and kept overnight under dark conditions. The next morning at 6:30 am, male and female zebrafish were stimulated to mate and lay eggs. Zebrafish eggs were collected for subsequent experiments.
Esketamine exposure and experimental design
In order to study the EK (Hengrui Medicine, Lianyungang, China) on zebrafish embryonic development and the influence of cardiac toxicity, the egg shells were removed using demembranase (contains chain protease) at a 1:10 dilution on the first day of the experiment, then the collected fertilized eggs at 24 hpf were incubated in six-well plate 24 h with 30 embryos each well, six groups randomly incubated in a range of EK concentrations (0, 0.21, 0.42, 0.84, 1.68 and 3.36 mM, diluted with E3 embryo medium: 5 mM NaCl, 0.67 mM KCl, 2.4 mM NaHCO3 and 0.9 mM CaCl2) through 48 h, exposure solution was changed every 24 h, according to the drug screening protocol20. Each group was set at least three repeated experiments. We utilized a Nikon SMZ18 stereo microscope (Japan) to observe and record the hatching condition and mortalities at 24-h intervals. EK stock solutions were prepared in E3 medium and then diluted to attain the requisite concentrations for the purposes of this study. Zebrafish embryos were treated with the above protocol to determine its toxic effects. Through counting the mortality, heart rate and body length in designed timepoints until 72 hpf, the LC50 values were determined by Graph-pad Prism 10.0 software. The hatching rate of the zebrafish embryos without dechorionating was observed at designed timepoints during the EK exposure in other groups. For calculating the larval hatch rate, successful hatching was defined as a larvae’s head or tail breaking out of the chorion, with the rate expressed as a percentage of living embryos hatched during the designed timepoints. Zebrafish embryos were placed in an incubator at 28.5 ± 1 °C and cultured until the target time.
Determination of the optimal concentration exposure
Based on the mortality of zebrafish embryos that resulted from a series of different concentrations of EK exposure, we have calculated the LC50 of EK in zebrafish embryo model at 48 and 72 hpf. 0.21, 0.42 and 0.84 mM were selected as the low, median and maximum (optimal) concentrations to treat zebrafish embryo for further revealing the teratogenic effects on cardiogenesis and embryonic development. Solution in each group were changed every 24 h. EK or vehicle-treated zebrafish embryos were stored at 4 °C condition in refrigerator for subsequent experiments including observation of zebrafish embryo phenotypes under stereoscopic microscope, WISH test and RT-qPCR analyses. Combined with the mortality, hatching, and malformation phenotypes under a range concentrations of EK, we selected some optimal concentrations to further prove the mechanism of EK developmental cardiotoxicity through WISH and RT-qPCR tests.
Live embryos imaging and heartbeats counting
Selective concentrations of EK exposure were conducted until 72 hpf and solutions were refreshed per 24 h. The embryonic development and morphological changes of zebrafish embryos were recorded under stereo microscope. All survival zebrafish embryo were anesthetized with 0.03% tricaine (MS-222; Sigma-Aldrich, Germany) and mounted in 1% low-melting agarose with 0.03% tricaine, and their phenotypes were observed and measured by stereo microscope equipped with a digital camera (Olympus ZX) at 72 hpf. Quantifying the body length (μm), SV-BA distance (μm), and pericardial area (μm2) of zebrafish three times in each group and the average was analyzed through Image J software. Zebrafish were exposed to 30 g/L methylcellulose, and the heartbeats of 48 and 72 hpf in 1 min were counted under anatomic microscope. The assessment of cardiac morphology and function according to the treatment protocol published by Hoage and Glickman21,22. Any anomaly of cardiac looping may cause disruption of its function. Thirty fish were taken from each group, and each experiment was repeated at least three times.
WISH analysis of the CHD susceptible genes
Congenital heart disease susceptive genes nkx2.5 and gata4 were selected as the indexes for the cardiotoxicity of esketamine23. WISH using digoxigenin (DIG)-labeled antisense RNA probes for nkx2.5, gata4 were performed based on previous protocols24. Subsequently, the corresponding probes (500 ng/mL) were added to the mixture, which was then incubated overnight at 70 °C in a water bath. Combined with the above mortality, hatching, and malformation phenotypes, the optimum concentrations were chosen to further prove the mechanism of EK developmental cardiotoxicity through WISH tests.
Plasmid templates (nkx2.5, gata4) for the preparation of in situ hybridization RNA probes were presented by Wang Qiang Laboratory of South China University of Technology. After that, procedures In Ambion’s MAXIscript In Vitro Transcription Kit were followed. Full RNAase free operation prevents degradation of the probe. After treatment in different concentrations according to experimental requirements embryos were collected for WISH analyses at 72 hpf, with 15 embryos per tube, three tubes for each group. Nkx2.5 and gata4 probes specifically expressed in zebrafish hearts were selected for hybridization. The specific operation process is carried out according to the standard experimental guideline published by Institute of Zoology, Chinese Academy of Sciences. At the end of color development, the embryos were completely immersed in te color development solution and placed in a 28.5 °C incubator for 5 ~ 8 h, washed with buffer solution after color development, and 4% paraformaldehyde was fixed for reserve use. When photographs are needed, the embryos are transferred to 90% glycerol, photographed under a Leica stereo microscope, and the images are taken.
RNA isolation and reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Gene transcription of nkx2.5 and gata4 were detected at 72 hpf in zebrafish embryos exposed to various concentrations of esketamine (0, 0.21, 0.42 and 0.84 mM). Thirty zebrafish embryos were collected in each group, and the total RNA was extracted by Trizol according to the manufacturer’s instructions. The total RNA concentration was detected by a NanoDrop spectrophotometer with a target of OD260: OD280 ≈ 2.0. Then, the cDNA synthesis was conducted employing the PrimeScript RT Reagent Kit of Takara Company. RT-qPCR was performed in triplicateusing tenfold diluted cDNA and a TB Green Premix Ex Taq II kit on a CFX Connect™ Real-Time System (Bio-Rad, Hercules, CA, USA).
The following primers were used for RT-qPCR:
NKX2.5: forward 5’-CGG ATC CTC TCT CTT CAGCG -3',
reverse 5’-TGA CAA CAG CCG ATG TCT TTTT-3';
GATA4: forward 5’CTA CAG GCA CCC CAG CAGA-3 ',
reverse 5’-AGA GCC CGA GAC CCG AAAT-3'.
β-actin: forward 5’- AGC ACG GTA TTG TGA CTA ACTG -3 ',
reverse 5’-TCG AAC ATG ATC TGT GTC ATC-3 '.
The PCR program included an initial denaturation step at 95 ℃ for 5 min, followed by 40 cycles at 95 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s. β-actin was used as the internal reference gene, and all data was analysis using the 2−ΔΔCT method. Each experiment was repeated for at least three times.
Statistical analyses
All experiments were conducted in triplicate to ensure the reliability and validity of the experimental results. Statistically differences were analyzed by one way ANOVA and the Dunnett’s multiple comparison (mean ± SEM). All statistical analysis using GraphPad Prism software (version 10.0) and SPSS software (version 25.0). P < 0.05 was considered statistically significant.
Results
Determination of the optimal concentration
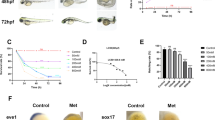
Esketamine exposure was started from 24 to 72 hpf, the medication was changed twice during the period with an interval of 24 h. We observed the mortality at multiple designed timepoints, including 3, 6, 12, 24 and 48 h post-exposure (hpe) to determine its toxicity effect on embryos, respectively. Embryos with no heartbeat were counted and the numbers were marked in each group. As the Fig. 1A shown, consistent with control group, zebrafish embryo survival rate was 100% at the both concentration of 0.21 and 0.42 mM following a long duration of EK exposure from 24 to 72 hpf. In the 0.84, 1.68 and 3.36 mM groups (Fig. 1A), all the survival rate decreased slowly with the exposure duration extended. However, in 0.84 and 1.68 mM groups, when EK exposure time reached to 48 h, the survival rate decreased to 37% and 0%, respectively. In 3.36 mM group, all the zebrafish embryos died at the beginning of the second phase of the study (24hpe). Therefore, concentrations of 1.68 mM and 3.36 mM are unsuitable for further studies on the toxicity effects on zebrafish embryonic development due to the high mortality observed. Based on the results of the above studies, we speculate that the 24 hpe-LC50 value of EK was 1.30 mM, with a 95% confidence interval between 0.92 and 1.60 mM, and the 48 hpe-LC50 value of EK was 0.71 mM, with a 95% confidence interval between 0.46 and 1.01 mM.
Effects of EK exposure on zebrafish embryos during different post-fertilization periods. (A) (Table 1 suppl.), Survival rates in different concentrations and the determination of LC50 at 48 and 72 hpf. (B) (Table 2 suppl.), Hatching rate of zebrafish embryos exposed to vehicle or EK. (C) (Table 3 suppl.), Effects of different exposure concentrations on heart rate in both timepoints. (D) (Table 4 suppl.), Malformation rate of zebrafish embryos exposed to different concentrations and exposure time. The data represent as mean ± SD (P < 0.05).
The number of dead zebrafish embryos were calculated at the designed timepoints in each group. According to the established toxicity grading criteria, EK is identified as having a median toxic effect on zebrafish embryos. Furthermore, a concentration of 0.84 mM has been determined to exhibit both low mortality and a high degree of teratogenicity, representing the optimal concentration for zebrafish embryos. This concentration is notably consistent with the therapeutic range reported for ketamine in zebrafish toxicity studies25,26. A range of three concentrations: 0.21, 0.42 and 0.84 mM were chosen to determine the teratogenicity of EK on zebrafish embryos and the cardiac development defects. Zebrafish embryos were exposed to EK 24 hpf, the mortality, hatching rate and malformation rate showed concentration- and time-effect dependent manner. The above results are based on the pharmacodynamic ratio of EK and ketamine. Each treatment was repeated three times for analysis.
Effects of esketamine on embryonic and cardiac morphology in zebrafish
Assessment of heart phenotype via stereomicroscope (Fig. 2). The pericardial area (μm2) under different concentrations of EK exposure and control group. At 72 hpf, mild pericardial edema was observed in the 0.42 mM group, and the degree of pericardial edema became more severe with the increase of concentration. The phenotype of pericardial edema was in a concentration-dependent manner. Different concentrations of EK exposure result in expanded SV-BA distance (μm). At 72 hpf, mild lengthened SV-BA distance was observed in the 0.42 mM group, and the SV-BA distance became longer with the increase of concentration. The phenotype of SV-BA distance was also in concentration-dependent manner.
EK exposure (0.21, 0.42 and 0.84 mM) induces embryonic dysplasia with pericardial and SV-BA distance (Fig. 2C). (A) (Table 5 suppl.), Pericardial area at 72 hpf of four randomly chosen zebrafish embryos, there was no pericardium swelling in the control and 0.21 mM groups (P > 0.05), the embryos both in 0.42 and 0.84 mM groups were pericardium swelling (rate = 100%, P < 0.01). (B) (Table 6 suppl.), SV-BA distance of four randomly chosen zebrafish embryos at 72 hpf. C, Images of zebrafish at 72 hpf under a stereo microscope; scale bar: 200 μm; magnification: × 6.25. Each group (except 0.84 mM) contains 30 zebrafish embryos and all reperformed for three independent experiments. All data were analyzed using one-way ANOVA with Bonferroni correction. A, bulbar artery; V, sinus venous. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Phenotypes of zebrafish embryo exposed to different concentrations of EK
Exposure to esketamine induced dysplasia within the chevron-shaped somites, where the angle of dashes in the 0.84 mM group was significantly enlarged. The impact of varying concentrations of EK on the body axis length across different groups was examined, a gradual reduction in body axis length was observed, as the concentration increased. These morphogenesis presents a pronounced time- and concentration- dependent manner.
The expression of nkx2.5 and gata4 by WISH analysis
Exposure to EK markedly decreased the expression of nkx2.5 (Fig. 4A), gata4 otherwise was opposite (Fig. 4C). We have analyzed 15 zebrafish embryos in each group against nkx2.5 and gata4 by WISH assay, nkx2.5 staining gradually became lighter with the increase of EK concentration. The ratio of deregulation for nkx2.5 and gata4 is nearly 80% in the 0.42 mM group, but in the 0.84 mM groups the ratio decreased to nearly 40% due to the high mortality.
The zoom images of Fig. 4A showed the cardiac looping defects under 0.21, 0.42 and 0.84 mM exposure. Different types of heart looping at 72 hpf following EK exposure of different concentrations were visualized by nkx2.5 expression (Fig. 4A). The zebrafish embryos showed a “no-looping” heart under the EK exposure, which is demonstrated in the zoom images. Figure 4A,B presents the outcomes derived from the application of WISH.
EK down-regulate nkx2.5 and up-regulate gata4 through Real-Time Quantitative PCR (RT-qPCR) test
Nkx2.5 and gata4 expression levels were quantified using qPCR, which corroborated the trends and findings observed in the WISH assays. (Fig. 5A Table 9 and B Table10 suppl.). The trends identified were consistent with those obtained through WISH results.
Discussion
Previous studies have conclusively demonstrated the neurotoxicity effects of EK exposure on pregnant rats and their offspring. Zhang27 has reported that exposure to EK in the maternal generation results in memory impairments in offspring rats. This phenomenon could potentially result from EK crossing the placental barrier, thereby inducing developmental impairments in the offspring mice. A multitude of epidemiological investigations have examined the potential for first trimester use of SSRIs to cause CHD28. However, whether EK exposure during this special period has any effect on fetal cardiac development remains unascertained and needs in-depth discussion and further study.
In recent years, zebrafish embryo, which is closely parallels mammalian fetal development, has been widely used in various fields as a model for studying drug cardiotoxicity29. In this current research endeavor, we have conducted a thorough investigation into the toxicological implications of EK exposure on zebrafish embryos, meticulously observing alterations in their developmental morphology and functionality. Our study reveals that EK exposure not only affects the molecular mechanisms of zebrafish embryos but also causes damage to cardiac function, such as heat rate. Figures 2 and 3 demonstrate the detrimental and cardiac teratogenic impacts of EK on zebrafish embryos and cardiogenesis, with these effects manifesting in a concentration- and time- dependent fashion.
(A,B) (Table 7 suppl.), Comparison of the zebrafish body length at 72 hpf; length of four embryos were randomly selected to be measured. (C,D) (Table 8 suppl.), chevron-shaped somites angles (small dashes) which induced by malformation of zebrafish at 72 hpf was expressed in (C) and zoomed images, the angles were measured randomly through the survival zebrafish embryos in each group. All data were analyzed using one-way ANOVA with Bonferroni correction. **P < 0.01, ***P < 0.001, ****P < 0.0001.
A specific range concentrations of EK modulates the morphology and functionality of developmental heart, which is consistent with our research hypothesis. The concentrations that we identified as optimal through the 48- and 72-h LC50 assessments exhibit high teratogenic potential with low mortality rates (Fig. 1A,D), which have notably altered the gross morphological development of the organisms, specifically impacting the pericardium (Fig. 2A,C), SV-BA distance (Fig. 2B,C), body length (Fig. 3A,B), and chevron-shaped somites angles of zebrafish (Fig. 3C,D). The exposure to EK also led to a reduction in heart rates (Fig. 1C), and a positive correlation between these two factors was observed, which is in line with the research conducted by Yuan25.
As depicted in Fig. 1A, the exposure to EK exhibits a time- and concentration- dependent effect, the significant differences were observed among the three groups at a same timepoint. When the exposure time reached 24 h, all embryos in the 3.36 mM group succumbed to death, mirroring the fate of embryos in the 1.68 mM group that perished after 48 h of exposure. Therefore, we excluded the aforementioned two concentration groups, 1.68 and 3.36 mM, from further investigation.
In the CTRL and 0.21 mM groups, no instances of malformation were observed. At the identical timepoint, the incidence of deformity in the 0.84 mM group was notably elevated compared to the 0.42 mM group, exhibiting a clear time- dependent trend. In this research endeavor, the teratogenic concentration of EK exposure that was selected aligns precisely with the ketamine concentrations reported by Guo, based on the similarity in their pharmacokinetic profiles30. The 72 h-LC50 of EK was approximately 0.71 mM, as determined through a rigorous analysis of survival rate (Fig. 1A). Consequently, for the teratogenic analysis of zebrafish embryos, three specific concentrations of 0.21, 0.42, and 0.84 mM were carefully selected.
To meticulously investigate the developmental toxicity of drugs on zebrafish embryos, we have selected the hatching rate, heart rate, pericardial area, body length, and SV-BA distance as the key characterized parameters for analysis. Our research concentrated on the initial stages of cardiac development in zebrafish, which serves as a model for the early fetal development process in human. Notably, the aforementioned parameters were most optimally observed during this particular phase. Except for the two elevated concentrations of EK, specifically 1.68 mM and 3.36 mM, which significantly induced pericardial edema and mortality at extremely high levels, we also discerned a consistent trend with Luís M Félix’s reports31, observing a marked slowing of heart rate as the concentration of EK increased within a particular range. Zebrafish embryos exposed to that range of EK concentrations at the early stages of cardiac development showed enlarged pericardial area and longer SV-BA distance, all of these results were consistent with previous studies32,33. Based on the outcomes obtained, concentrations less than 0.84 mM were deemed as the optimal selection for the investigation and analysis of teratological effects, specifically focusing on the examination of two pivotal genes related to CHD formation utilizing the WISH technique (Fig. 4A,C).
Given the high teratogenic rate of cardiac phenotypes in morphant embryos, we utilized nkx2.5 and gata4 markers to confirm disturbances in cardiac development, which have been established as critical factors in CHD during fetal development34. Numerous studies35,36 have conclusively shown that nkx2.5 is localized within heart precursors, serving a crucial regulatory role in cardiac morphogenesis. The mutation or abnormal expression of nkx2.5 leads to impairments in cardiac development, encompassing morphological abnormalities in both the ventricle and atrium. These abnormalities manifest as cardiac looping disorders, characterized by median cardiac morphology, and can ultimately culminate into CHD. The insights derived from our extensive evaluation of cardiac dysplasia are markedly apparent, likely attributable to the diminished expression of nkx2.5 subsequent to EK exposure, which demonstrated that nkx2.5 staining intensity diminished at WISH, and its expression levels were significantly reduced at q-PCR. The influence of EK exposure on nkx2.5 in our research was found to be in alignment with Martin’s, which also has revealed that the exposure to external stimuli, such as drugs, has been observed to elicit a response within 24 h, specifically through affecting the expression of nkx2.537 during embryonic development. Although Harrington38 has discovered that the nkx2.5 loss-of-function zebrafish demonstrates an elevated heart rate, exhibiting a contrasting trend in comparison to ours. This discrepancy may stem from differing drug cardiotoxicities, alternative factors, or genetic compensatory mechanisms.
Gata4 is another factor plays a pivotal role in heart development, and the occurrence of mutations within the gata4 gene also can potentially result in heart dysmorphology, encompassing a range of congenital defects that affect its shape, size, and function39. In the absence of developmental anomalies, the expression of gata4 is gradually increased throughout the whole heart development process40. Vikas Gupta’ study41 has revealed that the zebrafish heart’s responses to various stressors are interconnected through a shared mechanism involving the activation of gata4. The gata4-mediated stress response pathway is consistently employed by zebrafish during growth to confront external stimuli42. Furthermore, gata4 regulation is pivotal for ventricle formation during both zebrafish juvenile morphogenesis and adult heart regeneration43. An additional study44 has likewise demonstrated a correlation between atrial septal defect (ASD) and gata4 mutation. Our study conclusively demonstrated a positive correlation between increasing EK concentrations and heightened gata4 staining intensity via WISH, alongside a statistically significant rise in gata4 gene expression as shown by qPCR analyses.
The aforementioned phenomenon unveils an intriguing pattern: an increase in EK concentration correlates with down-regulation of nkx2.5 and up-regulation of gata4 gene expression. Gata4 has the ability to interact with the C-terminal auto-inhibitory region, thereby releasing the active domain of nkx2.523. This process could potentially serve as a compensatory mechanism in response to the down-regulation of nkx2.5 expression induced by EK exposure45. Tong’s research46 demonstrated that nkx2.5 engages in an interaction with gata4, and any imbalance in this interaction can lead to the development of severe cardiac abnormalities. It is noteworthy that mutations in gata4 may give rise to defective interactions with nkx2.5, ultimately resulting in the development of CHD47. Another investigation has revealed that the nkx2.5 mutation could potentially be linked to Tetralogy of Fallot42, which may also be influenced by gata4 mutations48. Gata4 and nkx2.5 are key synergistic regulators of atrial natriuretic factor and B-type natriuretic peptide gene, which play a critical role in repairing and remodeling cardiogenesis49,50. Carl O. Brown also has disclosed that the incorporation of GATA transcription factors can potentially trigger the early expression of nkx2.5, thereby playing a crucial role in the development of cardiac progenitors43. Daniel Durocher’ research has reported that the nkx2.5 and gata4 provide cooperative crosstalk, which may present a paradigm for transcription factor interaction during cardiogenesis51. Nkx2.5 serves as a pivotal co-factor for gata4, both of which are indispensable for the development of the heart, and their mutations are intricately linked to CHD. Wang and colleages52 speculated that the nkx2.5 lies in the upstream of gata4, and also prior expression to gata4 in the cardiogenic area at embryonic period. The functional cooperation of gata4 and nkx2.5 plays crucial role in the cardiogenetic process53. Nkx2.5, as also a cofactor of gata4, has been shown to be able to recruit gata4 to cardiac gene promoters54, but our findings through WISH (Fig. 4A,C) and RT-qPCR (Fig. 5A,B) analyses indicate that over-expression of gata4 does not produce identical results to those of nkx2.5. Maybe prolonging the EK exposure time under lower concentrations can induce more significant compensatory effect between the two variables.
We furthermore exhibited that as the concentration increased, zebrafish embryos gradually manifested cardiac looping disorder, looks like median cardiac morphology phenotype through WISH from the ventral view. Gradual reduction in atrioventricular overlap and a tendency of distributing along a central axis were observed under the stereo microscope. The initiation of cardiac looping and the process of tube formation may potentially be impeded by exposure to EK, particularly in instances where an imbalance arises between the expression levels of nkx2.5 and gata4 (Fig. 4A, zoom images). This imbalance disrupts the delicate equilibrium necessary for proper heart development, leading to potential CHD.
Conclusions
In conclusion, our research confirms the 48 and 72 h-LC50 of EK and its teratogenic effects on embryonic and cardiac development in time- and concentration- dependent manner. Selective EK exposure leads to compromised cardiac morphology and function in zebrafish embryos, potentially due to an imbalance in the expression of cardiogenesis-susceptible genes such as gata4 and nkx2.5, along with disruptions in their regulatory networks. Our findings suggesting that caution is warranted when considering its use to treat antepartum depression.
Data availability
All of the data presented in this study could be obtained from corresponding author.
References
Dagher, R. K. et al. Perinatal depression: Challenges and opportunities. J. Womens Health (Larchmt) 30(2), 154–159 (2021).
Gaynes, B. N. et al. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evid. Rep. Technol. Assess (Summ) 119, 1–8 (2005).
Cooper, W. O. et al. Increasing use of antidepressants in pregnancy. Am. J. Obstet. Gynecol. 196(6), 544.e1–5 (2007).
Oberlander, T. F. et al. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch. Gen. Psychiatry 63(8), 898–906 (2006).
Daw, J. R. et al. Prescription drug use in pregnancy: A retrospective, population-based study in British Columbia, Canada (2001–2006). Clin. Ther. 34(1), 239-249.e2 (2012).
Bérard, A. et al. First trimester exposure to paroxetine and risk of cardiac malformations in infants: The importance of dosage. Birth Defects Res. B Dev. Reprod. Toxicol. 80(1), 18–27 (2007).
Cole, J. A. et al. Paroxetine in the first trimester and the prevalence of congenital malformations. Pharmacoepidemiol. Drug Saf. 16(10), 1075–1085 (2007).
Allbaugh, L. J. et al. Development of a screening and recruitment registry to facilitate perinatal depression research in obstetrics settings in the USA. Int. J. Gynaecol. Obstet. 128(3), 260–263 (2015).
Qiu, W. et al. Postpartum corticosterone and fluoxetine shift the tryptophan-kynurenine pathway in dams. Psychoneuroendocrinology 130, 105273 (2021).
Gao, S. et al. Process for (S)-ketamine and (S)-Norketamine via resolution combined with racemization. J. Org. Chem. 85(13), 8656–8664 (2020).
Daly, E. J. et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 76(9), 893–903 (2019).
Wang, S. et al. Efficacy of a single low dose of esketamine after childbirth for mothers with symptoms of prenatal depression: Randomised clinical trial. BMJ 385, e078218 (2024).
Howe, K. et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496(7446), 498–503 (2013).
He, J. H. et al. Zebrafish models for assessing developmental and reproductive toxicity. Neurotoxicol. Teratol. 42, 35–42 (2014).
Mi, P. et al. Melatonin protects embryonic development and maintains sleep/wake behaviors from the deleterious effects of fluorene-9-bisphenol in zebrafish (Danio rerio). J. Pineal. Res. 66(1), e12530 (2019).
Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 91(2), 279–288 (2011).
Stainier, D. Y. et al. Cardiovascular development in the zebrafish. I. Myocardial fate map and heart tube formation. Development 119(1), 31–40 (1993).
Beis, D. et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 132(18), 4193–4204 (2005).
Lohr, J. L. et al. Vertebrate model systems in the study of early heart development: Xenopus and zebrafish. Am. J. Med. Genet. 97(4), 248–257 (2000).
Karuppasamy, M. et al. Standardization of zebrafish drug testing parameters for muscle diseases. Dis. Model Mech. 17(1), 50339 (2024).
Glickman, N. S. & Yelon, D. Cardiac development in zebrafish: Coordination of form and function. Semin. Cell Dev. Biol. 13(6), 507–513 (2002).
Hoage, T. et al. Quantifying cardiac functions in embryonic and adult zebrafish. Methods Mol. Biol. 843, 11–20 (2012).
Kinnunen, S. et al. Nuclear receptor-like structure and interaction of congenital heart disease-associated factors GATA4 and NKX2-5. PLoS One 10(12), e0144145 (2015).
Alexander, J. et al. Screening mosaic F1 females for mutations affecting zebrafish heart induction and patterning. Dev. Genet. 22(3), 288–299 (1998).
Yuan, W. et al. Apoptotic mechanism of development inhibition in zebrafish induced by esketamine. Toxicol. Appl. Pharmacol. 482, 116789 (2024).
Félix, L. M. et al. Morphological and behavioral responses of zebrafish after 24h of ketamine embryonic exposure. Toxicol. Appl. Pharmacol. 15(321), 27–36 (2017).
Zhang, L. M. et al. S-ketamine administration in pregnant mice induces ADHD- and depression-like behaviors in offspring mice. Behav. Brain Res. 433, 113996 (2022).
Sadler, T. W. Selective serotonin reuptake inhibitors (SSRIs) and heart defects: Potential mechanisms for the observed associations. Reprod. Toxicol. 32(4), 484–489 (2011).
Sarmah, S. & Marrs, J. A. Zebrafish as a vertebrate model system to evaluate effects of environmental toxicants on cardiac development and function. Int. J. Mol. Sci. 17(12), 2123 (2016).
Matłoka, M. et al. Esketamine inhaled as dry powder: Pharmacokinetic, pharmacodynamic and safety assessment in a preclinical study. Pulm Pharmacol. Ther. 73–74, 102127 (2022).
Félix, L. M., Antunes, L. M. & Coimbra, A. M. Ketamine NMDA receptor-independent toxicity during zebrafish (Danio rerio) embryonic development. Neurotoxicol. Teratol. 41, 27–34 (2014).
Liu, J. & Stainier, D. Y. Zebrafish in the study of early cardiac development. Circ. Res. 110(6), 870–874 (2012).
Liu, X. et al. Protective effects of spermidine and melatonin on deltamethrin-induced cardiotoxicity and neurotoxicity in zebrafish. Cardiovasc. Toxicol. 21(1), 29–41 (2021).
Morgenthau, A. & Frishman, W. H. Genetic origins of tetralogy of fallot. Cardiol. Rev. 26(2), 86–92 (2018).
Jay, P. Y. et al. Cardiac conduction and arrhythmia: insights from Nkx25 mutations in mouse and humans. Novartis Found Symp. 250, 227–238 (2003).
McElhinney, D. B. et al. NKX25 mutations in patients with congenital heart disease. J. Am. Coll. Cardiol. 42(9), 1650–1655 (2003).
Martin, E. D. et al. Plakoglobin has both structural and signalling roles in zebrafish development. Dev. Biol. 327(1), 83–96 (2009).
Harrington, J. K. et al. Nkx2.5 is essential to establish normal heart rate variability in the zebrafish embryo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 313(3), R265–R271 (2017).
Xiang, R. et al. A novel mutation of GATA4 (K319E) is responsible for familial atrial septal defect and pulmonary valve stenosis. Gene 534(2), 320–323 (2014).
Afouda, B. A. Towards understanding the gene-specific roles of GATA factors in heart development: Does GATA4 lead the way?. Int. J. Mol. Sci. 23(9), 1 (2022).
Gupta, V. et al. An injury-responsive gata4 program shapes the zebrafish cardiac ventricle. Curr. Biol. 23(13), 1221–1227 (2013).
Miura, G. I. & Yelon, D. Cardiovascular biology: Play it again, Gata4. Curr. Biol. 23(14), 619–621 (2013).
Brown, C. O. 3rd. et al. The cardiac determination factor, Nkx2-5, is activated by mutual cofactors GATA-4 and Smad1/4 via a novel upstream enhancer. J. Biol. Chem. 279(11), 10659–10669 (2004).
Yang, X. Y. et al. Correlation between GATA4 gene polymorphism and congenital heart disease. Int. J. Clin. Exp. Med. 8(9), 16733–16736 (2015).
Ellesøe, S. G. et al. Familial atrial septal defect and sudden cardiac death: Identification of a novel NKX2-5 mutation and a review of the literature. Congenit. Heart Dis. 11(3), 283–290 (2016).
Tong, Y. F. Mutations of NKX2.5 and GATA4 genes in the development of congenital heart disease. Gene 588(1), 86–94 (2016).
Granados-Riveron, J. T. et al. Combined mutation screening of NKX2-5, GATA4, and TBX5 in congenital heart disease: Multiple heterozygosity and novel mutations. Congenit. Heart Dis. 7(2), 151–159 (2012).
Abhinav, P. et al. Somatic GATA4 mutation contributes to tetralogy of Fallot. Exp. Ther. Med. 27(2), 91 (2024).
Pikkarainen, S. et al. GATA-4 is a nuclear mediator of mechanical stretch-activated hypertrophic program. J. Biol. Chem. 278(26), 23807–23816 (2003).
Kinnunen, S. M. et al. Cardiac actions of a small molecule inhibitor targeting GATA4-NKX2-5 interaction. Sci. Rep. 8(1), 4611 (2018).
Durocher, D. et al. The cardiac transcription factors Nkx2-5 and GATA-4 are mutual cofactors. EMBO J 16(18), 5687–5696 (1997).
Wang, Y. et al. Transcription factors Csx/Nkx25 and GATA4 distinctly regulate expression of Ca2+ channels in neonatal rat heart. J. Mol. Cell Cardiol. 42(6), 1045–1053 (2007).
Shiojima, I. et al. Context-dependent transcriptional cooperation mediated by cardiac transcription factors Csx/Nkx-2.5 and GATA-4. J. Biol. Chem. 274(12), 8231–8239 (1999).
Linhares, V. L. et al. Transcriptional regulation of the murine Connexin40 promoter by cardiac factors Nkx2-5, GATA4 and Tbx5. Cardiovasc. Res. 64(3), 402–411 (2004).
Acknowledgements
We extend our sincere gratitude to the esteemed members of the Q.W. laboratory, affiliated with the prestigious South China University of Technology, located in Guangzhou, China, with the postal code of 510006.
Funding
The funding for this work has been provided by the Guangzhou Municipal Science and Technology Project of China, specifically allocated to J.T. and S.L. under the grant number 2024A03J0195 and 2024A04J5069. Additionally, support has also been received from the Guangzhou Medical University Students Innovation Ability Improvement Plan Project, which has granted to J.T. with the project code 02–408-2304-02122XM. University scientific research project of Guangzhou Education Bureau, which has granted to J.T. with the project code 2024312101.
Author information
Authors and Affiliations
Contributions
J.T., S.L., X.L. and R.Z. collaboratively designed and executed the majority of the experiments, with invaluable assistance from Q.O. contributed to the execution of the zebrafish behavioral experiments. J.T. and S.L. were responsible for devising the experimental protocols, interpreting the data obtained, and drafting the manuscript. Additionally, R.Z. and T.J. dissected the relevant tissues and conducted the WISH procedure. Throughout the process, H.H. offered their insights and provided crucial guidance in discussing the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, S., Li, X., Zhao, R. et al. Esketamine induces embryonic and cardiac malformation through regulating the nkx2.5 and gata4 in zebrafish. Sci Rep 15, 7187 (2025). https://doi.org/10.1038/s41598-025-91315-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91315-2







