Abstract
Disseminated intravascular coagulation (DIC) is a thrombo-hemorrhagic disorder that can be life-threatening in critically ill children, and the quest for an accurate and efficient method for early DIC prediction is of paramount importance. Candidate predictors encompassed demographics, comorbidities, laboratory findings, and therapy strategies. A stepwise logistic regression model was employed to select the features included in the final model. Six machine learning algorithms—logistic regression (LR), extreme gradient boosting (XGB), random forest (RF), support vector machine (SVM), decision tree (DT), and k-nearest neighbor (KNN)—were employed to construct predictive models for DIC in critically ill children. Models were then evaluated by using area under the curve (AUC), accuracy, specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV), precision, recall and decision curve analysis (DCA). Interpretation of the optimal model was conducted using shapley additive explanations (SHAP). A total of 6093 critically ill children were encompassed in this study, of whom 681 (11.2%) developed DIC. The RF model exhibited the highest levels of accuracy (0.856), sensitivity (0.866), Kappa (0.472), NPV (0.423), and recall (0.866). However, the XGB model outperformed RF, LR, SVM, DT, and KNN in terms of AUC (0.908), specificity (0.859), PPV (0.978), and precision (0.969). Decision curve analysis (DCA) confirmed the superior clinical utility of the XGB model. Overall, the XGB model demonstrated superior clinical utility compared to RF, LR, SVM, DT, and KNN. We named the final model Alfalfa-PICU-DIC. SHAP analysis identified D-dimer, INR, PT, TT, and PLT count as the top predictors of DIC. Machine learning models can be a reliable tool for predicting DIC in critically ill children, which will facilitate timely intervention, thereby reducing the burden of DIC on patients in the pediatric intensive care unit (PICU).
Similar content being viewed by others
Introduction
Disseminated intravascular coagulation (DIC) is a clinical syndrome associated with various underlying conditions, such as infection, trauma, malignant solid tumors, acute leukemia, and liver diseases1. It is characterized by systemic activation of coagulation pathways, resulting in fibrin clot formation, which may lead to organ failure. Additionally, the condition involves the simultaneous consumption of platelets and coagulation factors, which may lead to clinical bleeding1,2,3. Critically ill children often develop DIC as a complication of sepsis, injury (trauma, burn and drowning), malignancy (acute lymphoblastic leukemia, acute promyelocytic leukemia and solid tumors), or other severe illnesses. A study conducted in a pediatric intensive care unit in Thailand report a 24% prevalence of DIC among critically ill children4. In these cases, DIC often progresses rapidly and presents a high mortality risk. Timely identification and prompt intervention of DIC can significantly improve the prognosis of critically ill children5. The International Society on Thrombosis and Hemostasis (ISTH) criteria for DIC are widely recognized as a standard reference for diagnosing DIC in at-risk children1,6. According to the ISTH criteria, patients must undergo blood tests that include prothrombin time (PT), platelet count (PLT), fibrinogen (FIB) levels, and fibrinogen degradation products (FDPs)/D-dimers. A score of 5 or higher indicates overt DIC (ODIC) and is associated with increased mortality7. Although the ISTH-DIC score demonstrating excellent performance, with high sensitivity (91%) and specificity (97%), its time-consuming nature poses a challenge in the context of life-threatening diseases that require timely diagnosis and management to improve outcomes7,8. Additionally, controversy remains regarding the variation of coagulation parameters across different age groups and the selection of appropriate laboratory tests for accurate DIC diagnosis9. Consequently, the development of an accurate and efficient method for early DIC prediction in critically ill children is of great importance.
Several predictive models have been developed to identify the risk of DIC, primarily focusing on adult populations and often utilizing traditional statistical methods with limited predictive power. For instance, a study introduced a predictive model for DIC detection in sepsis patients, emphasizing the need for clinicians to focus on patients with high model scores10. Another research developed a nomogram incorporating coagulation-related risk factors to predict DIC in patients with heatstroke, which may be useful in clinical decision-making11. Additionally, machine learning models have been applied to predict cancer-associated DIC in critically ill colorectal cancer patients, demonstrating the potential of advanced algorithms in this domain12. While previous studies have explored predictive models for DIC, few studies addressing the unique physiological and clinical characteristics of pediatric patients. Furthermore, existing models often lack interpretability, which is detrimental to gaining the trust of clinicians and promoting integration into routine practice.
In recent years, the utilization of machine learning technology in the medical domain has witnessed a significant surge13,14,15. Machine learning algorithms have the capability to discern potential patterns within vast datasets through learning and analysis, thereby offering crucial support for clinical decision-making16,17. However, the use of machine learning in clinical practice often faces skepticism due to the “black-box” nature of many models that limits their interpretability and clinical applicability. For high-stakes conditions such as DIC, particularly in pediatric patients, it is essential to develop models that not only provide accurate predictions but also offer interpretable outputs that clinicians can trust and act upon.
This study aims to develop an interpretable machine learning model for the early prediction of DIC in critically ill children. By leveraging pediatric-specific clinical data and employing techniques that enhance model interpretability, this work seeks to address current limitations in DIC prediction in critically ill pediatric patients.
Materials and methods
Data source and population
The data utilized in this study were obtained from the Pediatric Intensive Care (PIC) database (http://pic.nbscn.org)—a comprehensive pediatric-specific repository maintained by the Children’s Hospital of Zhejiang University School of Medicine. Covering the period from 2010 to 2018, this unique database provides bilingual documentation for children admitted to critical care units18. It contains a wealth of information, including vital signs, medication records, laboratory results, fluid balance data, diagnostic codes, length of hospital stay, survival rates, and more. Notably, the database ensures anonymity through deidentification processes. Access to this valuable resource is granted through a registration process, which requires individuals to complete a training course on research involving human subjects and sign a data use agreement, thereby committing to responsible data management and collaborative research principles. After successfully completing the required online examination, we have obtained a certification number (Record ID: 12739887).
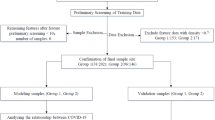
All pediatric patients in the PIC database were eligible for inclusion. For patients with multiple admissions to the pediatric intensive care unit (PICU), only the first admission was considered. The exclusion criteria were as follows: (1) individual data missing more than 30%, (2) PICU stays of less than 24 h, (3) patients younger than 1 month, and (4) patients with congenital coagulopathy. The flowchart detailing the study cohort selection process is presented in Fig. 1.
Feature extraction
A total of 28 variables for the machine learning models were extracted from the PIC database. These included demographic information, such as age and gender, and coexisting disorders, including sepsis, trauma, leukemia, hemophilia, hepatic insufficiency, and renal insufficiency. Treatment administered during PICU admission were also recorded, encompassing surgical procedures, anticoagulant medications (heparin and low molecular weight heparin), vasoactive agents, antibiotics containing N-methylthiotetrazole (NMTT), and nonsteroidal anti-inflammatory drugs (NSAIDs). Laboratory parameters reflecting the highest severity of illness during the first 24 h after ICU admission were extracted. These parameters included hematocrit, platelet count (PLT), international normalized ratio (INR), prothrombin time (PT), partial thromboplastin time (PTT), thrombin time (TT), activated partial thromboplastin time (APTT), D-dimer, fibrinogen (FIB) levels, alanine aminotransferase (ALT), aspartate transaminase (AST), and electrolytes levels, including sodium, potassium, calcium, and chloride.
Outcomes
DIC was defined based on guidelines established by the scientific subcommittee on DIC of the ISTH. The diagnostic criteria for DIC included the following parameters: PLT count: 50–100 × 10^9/L = 1; <50 × 10^9/L = 2; D-dimer: moderate increase (2.5–6 µg/ml) = 2; strong increase (≥ 6 µg/ml) = 3; PT: prolong by ≥ 3 s but < 6 s = 1, prolong by > 6 s = 2; FIB level: <1.0 g/L = 1. The most severe daily values of DIC-related indicators were extracted. A score of ≥ 5 indicated overt DIC, while a score of < 5 suggested non-overt DIC (Supplement Table 1).
Statistical analysis
The distribution of variables was assessed using the Shapiro–Wilk test. Variables with normal distribution were reported as mean ± standard deviation, whereas non-normally distributed variables were presented as median (interquartile range). Categorical variables were presented as counts (percentage). Collinearity among variables was assessed using the variance inflation factor (VIF) method, with a VIF value of less than 5 indicating no significant collinearity. Baseline characteristics between the DIC and non-DIC groups were compared using univariate analyses. Bivariate analyses were performed with a chi-square test for categorical variables. For continuous variables, Student’s t-test was applied to those with normal distributions, while the Mann–Whitney U test was used for non-normally distributed variables. Missing data were addressed through multiple imputation using the “MICE” package.
The dataset underwent random allocation, with 70% designated for the training dataset and the remaining 30% for validation dataset. A stepwise logistic regression model was employed to select the features included in the final model. The Akaike Information Criterion (AIC) was utilized as the selection criterion for feature inclusion. At each step, the AIC was recalculated as features were added through forward selection or removed via backward elimination. The process terminated when no further changes improved the AIC, thus obtaining the model with the lowest AIC. We employed six machine learning methods—logistic regression (LR), extreme gradient boosting (XGB), random forest (RF), support vector machine (SVM), decision tree (DT) and k-nearest neighbor classification (KNN)—to develop models for predicting DIC in critically ill children, utilizing the “tidymodels” package. The models were trained using the training dataset and validated with five-fold cross-validation. This method divides the dataset into five mutually exclusive subsets. In each iteration, four folds are used for training, while the remaining fold serves as the test set. This process is repeated five times, ensuring that each fold is used once as the test set. By systematically rotating the test set, cross-validation reduces overfitting and enhances model robustness. Model performance was assessed using various metrics, including the area under the curve (AUC), accuracy, specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV), precision and recall. Additionally, decision curve analysis (DCA) was employed to evaluate the net clinical benefit. The interpretation of the optimal model was performed using Shapley Additive Explanations (SHAP) with the “fastshap” and “shapviz” packages. Statistical significance was set at an alpha level of 5% (p-value < 0.05). All statistical analyses were performed using R version 4.3.3 (R Foundation for Statistical Computing).
Results
Patient baseline characteristics
Our study included 6,093 critically ill pediatric patients, comprising 2,671 females and 3,422 males, with a median age of 16.08 (4.44–55.20) months. Patients were categorized based on the occurrence of DIC during their PICU stay. The DIC group consisted of 681 patients (11.2%), including 285 females and 396 males, with a median age of 19.92 (4.80-71.04) months. The non-DIC group included 5412 patients (89.8%), with 2,386 females and 3,026 males, and a median age of 15.60 (4.44–53.22) months (Table 1). Pediatric patients with DIC exhibited significantly lower levels of FIB, PLT, calcium, sodium, and chloride compared to those without DIC. Conversely, they showed elevated levels of ALT, AST, creatinine, hematocrit, D-dimer, INR, APTT, PT, TT, and potassium. Moreover, Patients with DIC had a higher rate of surgery, sepsis, hepatic insufficiency, renal insufficiency, and trauma. Additionally, these patients were more likely to receive anticoagulant drugs, antibiotics (containing NMTT), and vasoactive agents compared to those without DIC (Table 1).
Feature selection
Initially, 28 variables were assessed using stepwise logistic regression. Subsequently, 16 variables were selected for inclusion in the final model. These variables including APTT, AST, D-dimer, INR, PLT count, PT, systolic pressure, TT, age, calcium, vasoactive agents, NSAIDs, anticoagulants, sepsis, leukemia, and renal insufficiency. (Supplement Table).
Model development performance
Six distinct machine learning models—LR, XGB, RF, SVM, DT, and KNN— were employed to predict the risk of DIC in critically ill patients using the 16 selected features. The performance of the models was evaluated using multiple metrics, including AUC, accuracy, specificity, sensitivity, PPV, NPV, precision, and recall. The RF model exhibited the highest levels of accuracy (0.856), sensitivity (0.866), Kappa (0.472), NPV (0.423), and recall (0.866). However, the XGB model outperformed RF, LR, SVM, DT, and KNN in terms of AUC (0.908), specificity (0.859), PPV (0.978), and precision (0.969), as illustrated in Table 2; Fig. 2A. DCA was performed on these six models, with results presented in Fig. 2B. Notably, the utilization of the XGB prediction model yielded the greatest net benefit for DIC prediction, surpassing RF, LR, SVM, DT, and KNN. Overall, XGB demonstrated superior clinical utility compared to RF, LR, SVM, DT, and KNN. We named the final model Alfalfa-PICU-DIC (“Alfalfa” is the name of our team, representing happiness and luck).
Assessment of model performance. (A) Receiver operating characteristic curve of machine learning models in the validation cohort; (B) Decision curve of machine learning models in the validation cohort. Abbreviations: DT, decision tree; KNN, k-nearest neighbor classification; RF, random forest; SVM, support vector machine; XGB, extreme gradient boosting.
Model interpretations
To enhance the interpretability of the mode’s output, SHAP analysis was employed to identify the features most strongly associated with DIC in critically ill children. The SHAP summary plot, showing in Fig. 3A, illustrates the importance ranking on the Y-axis and the SHAP value on the X-axis represents each feature’s influence on the prediction. The dot color represented the feature values, with purple denoting low values and yellow representing high values. For instance, higher D-dimer levels typically result in SHAP values above zero, indicating an increased risk of DIC as D-dimer levels rise. Additionally, Fig. 3B shows the feature ranking based on the average absolute SHAP value, with D-dimer, INR, PT, TT, and PLT identified as the top five risk factors. The partial dependence plot illustrates the influence of individual features on the XGB model’s output, where a positive SHAP value indicates an increased risk of DIC in critically ill children (Fig. 4). Our study identified a positive association between APTT, AST, D-dimer, INR, PT, TT, age, receive vasoactive agents and NSAIDs, as well as coexisting of leukemia, and the risk of DIC in critically ill children. Conversely, an elevated PLT count, increased calcium levels, higher systolic pressure and use of anticoagulation were negatively associated with the risk of DIC in critically ill children.
The interpretation of the XGB model. A. Feature importance ranking based on SHAP values. The position on the Y-axis implied the importance ranking, and the X-axis reflected the association between each value of features and the corresponding SHAP value. B The importance ranking of included features according to the mean (|SHAP value|). Abbreviations: INR, international normalized ratio; PT, prothrombin time; TT, thrombin time; PLT, platelet count; APTT, activated partial thromboplastin time; AST, aspartate transaminase; NSAIDs, nonsteroidal anti-inflammatory drugs.
The partial dependence plots of the XGB model based on SHAP. A-P show how the APTT, AST, D-dimer, INR, PLT count, PT, systolic pressure, TT, age, calcium, vasoactive agents, NSAIDs, anticoagulants, sepsis, leukemia, and renal insufficiency affects the output of the XGB prediction model respectively. As the SHAP value exceeds zero, it indicated a promoting effect on the DIC risk. Abbreviations: APTT, activated partial thromboplastin time; AST, aspartate transaminase; INR, international normalized ratio; PLT, platelet count; PT, prothrombin time; TT, thrombin time; NSAIDs, nonsteroidal anti-inflammatory drugs.
Interpretation of individual prediction
To explore the contribution of clinical features to individual patient outcomes, a patient was randomly selected from the validation cohort for analysis. The force plot and waterfall plot depicted the contributions of each feature, with yellow bars indicating positive contributions and purple bars indicating negative contributions to DIC risk. The selected patient, a 3.6-month-old boy, had an XGB-model predicted DIC risk of 14.8%. Analysis revealed that D-dimer (4.17 mg/L), calcium (0.85 mmol/L), and INR (6.34) were the top three contributing factors. Figure 5A and B illustrate the specific impact of these factors on the prediction, showing that the patient did not develop DIC during his PICU stay.
The forceplot (A) and waterfall plot (B) for explaining the contribution of features on a certain patient. The yellow bar and purple bar represented a positive and negative contribution to in-hospital mortality, respectively. Abbreviations: INR, international normalized ratio; PT, prothrombin time; PLT, platelet count; TT, thrombin time; APTT, activated partial thromboplastin time.
Discussion
DIC can lead to hemorrhage or thrombotic vessel occlusion in critically ill children, potentially disrupting the blood and oxygen supply to vital organs. This can result in multiple organ dysfunction, significantly altering the clinical course and leading to adverse outcomes. Therefore, early prediction of DIC in critically ill children is critical. Previous studies have demonstrated the outstanding performance of machine learning models in early prediction of coagulopathy among adult patients, including traumatic brain injury-induced coagulopathy (TBI-IC)19, sepsis-induced coagulopathy (SIC), venous thromboembolism16,20,21, and coagulopathy in spontaneous intracerebral hemorrhage patients in emergency rooms22. However, few studies addressing the unique physiological and clinical characteristics of pediatric patients. In this study, we developed, validated and compared different machine learning models for the early prediction of DIC risk in critically ill children. Specifically, we applied six distinct machine learning models—LR, XGB, RF, SVM, DT, and KNN—to predict DIC, utilizing the 16 features commonly used in clinical practice. In terms of predictive performance, the RF model exhibited the highest levels of accuracy (0.856), sensitivity (0.866), Kappa (0.472), NPV (0.423), and recall (0.866). However, the XGB model outperformed RF, LR, SVM, DT, and KNN in terms of AUC (0.908), specificity (0.859), PPV (0.978), and precision (0.969). Decision curve analysis (DCA) confirmed the superior clinical utility of the XGB model. SHAP analysis identified D-dimer, INR, PT, TT, and PLT count as the top predictors of DIC. To our knowledge, this is the first study to establish a predictive model for DIC in critically ill children.
Several diagnostic criteria for DIC have been established, such as Japanese Ministry of Health and Welfare (JMHW) DIC criteria23, the Japanese Association for Acute Medicine (JAAM) DIC criteria24, the Texas Children’s Hospital (TCH) criteria25, the International Society on Thrombosis and Haemostasis (ISTH) overt DIC criteria1 and the Chinese DIC Scoring System (CDSS)26. However, these criteria typically require multiple patient assessments before a definitive diagnosis can be made. In contrast, our machine learning predictive model utilizes data from the first 24 h of PICU admission to predict DIC at an early stage. Traditional diagnostic criteria rely on global coagulation tests, such as APTT, PT, INR, PLT, FIB, and fibrin/FDP or D-dimer, to evaluate hemostatic abnormalities. In our study, D-dimer, INR, PT, TT, and PLT were identified as the top five predictors of DIC in critically ill children. Notably, our predictive model excludes fibrinogen. Although FIB levels may decrease during the acute phase of DIC, they can also increase in conditions such as acute infection, sepsis, tissue damage, and inflammation, thereby limiting its diagnostic utility. Previous research has highlighted the secondary importance of FIB, with some studies recommending its exclusion from DIC scoring systems7,25,27,28. In our study, although FIB levels were lower in the DIC group compared to the non-DIC group, this reduction did not significantly contribute to the risk of DIC in critically ill children. Therefore, FIB was excluded from our prediction models. Conversely, additional features, such as systolic pressure, AST, age, calcium levels, use of vasoactive agents, NSAIDs, anticoagulants, the presence of sepsis, leukemia, and renal insufficiency, were incorporated into our models.
Our findings revealed a negative correlation between systolic pressure, calcium levels, the use of anticoagulants with the occurrence of DIC in critically ill children. Specifically, patients with DIC exhibited lower systolic pressure compared to those without DIC. The mechanisms underlying this association are multifactorial. First, DIC induces widespread microthrombus formation in capillaries, arterioles, and venules, disrupting hemodynamics and resulting in decreased blood pressure29. Second, the progression of DIC depletes coagulation factors and platelets30, exacerbating microthrombus formation and bleeding, which further compromises circulating blood volume and blood pressure stability. Numerous studies have identified elevated AST levels as a significant risk factor for DIC. Hemostasis is intricately linked to liver function, as most coagulation factors are synthesized by hepatic parenchymal cells. Additionally, the liver’s reticuloendothelial system plays a crucial role in clearing activated coagulation factors. Similarly, our findings demonstrated a significant association between elevated AST levels and an increased risk of DIC. Calcium ions are essential cofactors in the coagulation cascade, and hypocalcemia is linked to abnormal coagulation function. Epstein et al.31 have reported an association between the risk of severe bleeding, while Helsloot D et al.32 identified hypocalcemia as an independent risk factor for trauma-induced coagulopathy (TIC). Consistent with these findings, our results indicated that lower calcium levels were significantly associated with an increased risk of DIC in critically ill children. Additionally, our study demonstrated that the use of anticoagulants including unfractionated heparin (UFH) and low molecular weight heparin (LMWH), significantly reduced the risk of DIC in this population. Liu et al.33 observed that low-dose UFH effectively alleviated the hypercoagulable state associated with sepsis, thereby reducing the incidence of DIC. Similarly, Wen et al.34 reported that both LMWH and UFH therapies were associated with lower mortality rates from DIC compared to the control group in the ICU.
Our findings showed that sepsis, leukemia, and renal insufficiency were significantly associated with DIC in critically ill children, with sepsis being the most common complication related to DIC. During sepsis, widespread inflammation activates the coagulation system, resulting in the consumption of clotting factors and subsequent development of DIC35,36,37,38. Clinically, approximately 50–70% of patients with sepsis experience coagulation dysfunction, and approximately 35% progress to DIC, with mortality rates ranging from 28–43%39,40,41. Leukemia, the most common hematological malignancy in children, is also associated with DIC due to various underlying factors. DIC is most commonly observed in acute promyelocytic leukemia among childhood leukemia cases, with incidence rates ranging from 17 to 100% and mortality rates from 6–73%42. Previous studies have shown that the onset of DIC in acute leukemia patients correlates with elevated levels of circulating tissue factors (TF) or TF-containing particles. These TFs may originate from leukemia cells or be released by vascular endothelial cells43,44. Circulating TFs activate the extrinsic coagulation pathway, resulting in significant consumption of coagulation factors, widespread microthrombus formation, and fibrinolytic system activation. Harada Shirado et al.45 reported that the spontaneous release of nuclear proteins by leukemia cells may contribution to DIC development. Additionally, our findings suggest that renal function could serve as a potential indicator of the risk of DIC. Similarly, various machine learning models have identified renal function as a significant predictor of coagulopathy. For instance, Yang et al.19 developed a machine learning model that utilized renal function indicators, such as blood urea nitrogen (BUN) and creatinine (CRE), to predict the risk of TIC. Likewise, Zhao et al.46 designed a model incorporated urine output and creatinine levels to predict the risk of SIC.
We also found a significant association between the use of vasoactive agents and NSAIDs and the risk of DIC in critically ill children. Previous studies have elucidated that vasoactive agent, such as adrenaline and vasopressin, can enhance activated protein C activity, reduce plasminogen activator inhibitor levels, and exacerbate fibrinolytic abnormalities in TIC47,48. DIC has also been a notable adverse effect associated with NSAIDs49. NSAIDs influence coagulation mechanisms in two primary ways: they impair platelet function by inhibiting thromboxane A₂ synthesis, a key eicosanoid required for platelet activation and vasoconstriction, and they suppress prostacyclin production by endothelial cells, thereby promoting a prothrombotic state50,51,52.
Our study exhibited several limitations, which were consistent with those inherent in many large administrative database studies. Firstly, the study was retrospective and relied on public databases, thus there was a possibility of missing or incomplete data as well as selection bias. Secondly, the study population was from a single center, limiting the generalizability of the findings to other populations. Thirdly, novel coagulation markers, such as thrombin-antithrombin complex (TAT), thrombomodulin (TM), tissue plasminogen activator-inhibitor complex (tPAI-C) and plasmin-α2-antiplasmin complex, are valuable in DIC diagnosis. However, the PIC database does not include records for these indicators. Fourthly, while this study utilized the PIC database to develop and validate the machine learning model, the validation was conducted solely within the same dataset using a 7:3 split for training and validation. Although this approach provides an initial evaluation of the model’s performance, it does not fully address the robustness and generalizability of the machine learning technique. This study is the absence of external validation using independent datasets. Testing the model on datasets from other pediatric intensive care units or similar healthcare domains would provide a more comprehensive assessment of its ability to generalize across diverse patient populations and clinical environments. Without such external validation, the applicability of the model in broader clinical settings remains uncertain.
Conclusion
In summary, among all the models we constructed, the XGB model, named Alfalfa-PICU-DIC, was the most effective model in identifying critically ill children at high risk of DIC early. This early identification facilitates timely intervention, thereby reducing the burden of DIC on patients in the PICU.
Data availability
Data will be available on request from the corresponding author.
References
Taylor, F. B. Jr., Toh, C. H., Hoots, W. K., Wada, H. & Levi, M. Scientific subcommittee on disseminated intravascular coagulation of the international Society on, T., Haemostasis. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 86(5), 1327–1330 (2001).
Levi, M. & van der Poll, T. Disseminated intravascular coagulation: A review for the internist. Intern. Emerg. Med. 8(1), 23–32. https://doi.org/10.1007/s11739-012-0859-9 (2013).
Rajagopal, R., Thachil, J. & Monagle, P. Disseminated intravascular coagulation in paediatrics. Arch. Dis. Child. 102(2), 187–193. https://doi.org/10.1136/archdischild-2016-311053 (2017).
Padungmaneesub, W. et al. Biomarkers of disseminated intravascular coagulation in pediatric intensive care unit in Thailand. Int. J. Lab. Hematol. 41(1), 32–38. https://doi.org/10.1111/ijlh.12917 (2019).
Kunwar, S. et al. Diagnostic scores and treatment options for acute disseminated intravascular coagulation in children. Cureus 13(9), e17682. https://doi.org/10.7759/cureus.17682 (2021).
Soundar, E. P., Jariwala, P., Nguyen, T. C., Eldin, K. W. & Teruya, J. Evaluation of the international society on thrombosis and haemostasis and institutional diagnostic criteria of disseminated intravascular coagulation in pediatric patients. Am. J. Clin. Pathol. 139(6), 812–816. https://doi.org/10.1309/AJCPO64IWNLYCVVB (2013).
Bakhtiari, K., Meijers, J. C., de Jonge, E. & Levi, M. Prospective validation of the international society of thrombosis and haemostasis scoring system for disseminated intravascular coagulation. Crit. Care Med. 32(12), 2416–2421. https://doi.org/10.1097/01.ccm.0000147769.07699.e3 (2004).
Yamakawa, K. et al. Benefit profile of anticoagulant therapy in sepsis: A nationwide multicentre registry in Japan. Crit. Care. 20(1), 229. https://doi.org/10.1186/s13054-016-1415-1 (2016).
Oren, H., Cingoz, I., Duman, M., Yilmaz, S. & Irken, G. Disseminated intravascular coagulation in pediatric patients: Clinical and laboratory features and prognostic factors influencing the survival. Pediatr. Hematol. Oncol. 22(8), 679–688. https://doi.org/10.1080/08880010500278749 (2005).
Fu, Y., He, Y., Zheng, C., Zeng, J. & Ou, H. A predictive model for disseminated intravascular coagulopathy in sepsis: An observational study. Int. J. Gen. Med. 17, 4845–4855. https://doi.org/10.2147/IJGM.S475953 (2024).
Zeng, Q. et al. Nomogram for predicting disseminated intravascular coagulation in heatstroke patients: A 10 years retrospective study. Front. Med. (Lausanne). 10, 1150623. https://doi.org/10.3389/fmed.2023.1150623 (2023).
Qin, L. et al. Machine learning models can predict cancer-associated disseminated intravascular coagulation in critically ill colorectal cancer patients. Front. Pharmacol. 15, 1478342. https://doi.org/10.3389/fphar.2024.1478342 (2024).
Desai, M. Y. Machine learning to predict future disease-specific outcomes: The brave new frontier. JACC Asia. 4(5), 387–388. https://doi.org/10.1016/j.jacasi.2023.12.004 (2024).
Jain, P. et al. Machine learning assisted hepta band THz metamaterial absorber for biomedical applications. Sci. Rep. 13(1), 1792. https://doi.org/10.1038/s41598-023-29024-x (2023).
Jain, P., Yedukondalu, J., Chhabra, H., Chauhan, U. & Sharma, L. D. EEG-based detection of cognitive load using VMD and LightGBM classifier. Int. J. Mach. Learn. Cybernet. 15(9), 4193–4210. https://doi.org/10.1007/s13042-024-02142-2 (2024).
Guan, C., Ma, F., Chang, S. & Zhang, J. Interpretable machine learning models for predicting venous thromboembolism in the intensive care unit: an analysis based on data from 207 centers. Crit. Care. 27(1), 406. https://doi.org/10.1186/s13054-023-04683-4 (2023).
Jain, P. et al. Machine learning techniques for predicting metamaterial microwave absorption performance: A comparison. IEEE Access. 11, 128774–128783. https://doi.org/10.1109/ACCESS.2023.3332731 (2023).
Zeng, X. et al. PIC, a paediatric-specific intensive care database. Sci. Data. 7(1), 14. https://doi.org/10.1038/s41597-020-0355-4 (2020).
Yang, F. et al. Machine learning approach for the prediction of traumatic brain injury induced coagulopathy. Front. Med. (Lausanne). 8, 792689. https://doi.org/10.3389/fmed.2021.792689 (2021).
Xu, Q. et al. Machine learning predicts cancer-associated venous thromboembolism using clinically available variables in gastric cancer patients. Heliyon 9(1), e12681. https://doi.org/10.1016/j.heliyon.2022.e12681 (2023).
Papillon, S. C. et al. Derivation and validation of a machine learning algorithm for predicting venous thromboembolism in injured children. J. Pediatr. Surg. 58(6), 1200–1205. https://doi.org/10.1016/j.jpedsurg.2023.02.040 (2023).
Zhu, F. et al. Machine learning models predict coagulopathy in spontaneous intracerebral hemorrhage patients in ER. CNS Neurosci. Ther. 27(1), 92–100. https://doi.org/10.1111/cns.13509 (2021).
Kobayashi, N., Maekawa, T., Takada, M., Tanaka, H. & Gonmori, H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the research committee on DIC in Japan. Bibl. Haematol.(49), 265–275. https://doi.org/10.1159/000408467 (1983).
Gando, S. et al. Evaluation of new Japanese diagnostic criteria for disseminated intravascular coagulation in critically ill patients. Clin. Appl. Thromb. Hemost. 11(1), 71–76. https://doi.org/10.1177/107602960501100108 (2005).
Jhang, W. K., Ha, E. J. & Park, S. J. Evaluation of disseminated intravascular coagulation scores in critically ill pediatric patients. Pediatr. Crit. Care Med. 17(5), e239–246. https://doi.org/10.1097/PCC.0000000000000705 (2016).
Wu, Y. et al. Evaluation of the new Chinese disseminated intravascular coagulation scoring system in critically ill patients: A multicenter prospective study. Sci. Rep. 7(1), 9057. https://doi.org/10.1038/s41598-017-09190-5 (2017).
Sivula, M., Tallgren, M. & Pettila, V. Modified score for disseminated intravascular coagulation in the critically ill. Intensive Care Med. 31(9), 1209–1214. https://doi.org/10.1007/s00134-005-2685-2 (2005).
Gando, S. et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: Comparing current criteria. Crit. Care Med. 34(3), 625–631. https://doi.org/10.1097/01.ccm.0000202209.42491.38 (2006).
Wada, T. & Gando, S. Phenotypes of disseminated intravascular coagulation. Thromb. Haemost. 124(3), 181–191. https://doi.org/10.1055/a-2165-1142 (2024).
Sharma, R., Cobb, S. & Ramakrishnaiah, R. D. Intravascular coagulation-related microangiopathy. Neurocrit Care. 39(2), 533–538. https://doi.org/10.1007/s12028-023-01733-1 (2023).
Epstein, D. et al. Association between ionised calcium and severity of postpartum haemorrhage: A retrospective cohort study. Br. J. Anaesth. 126(5), 1022–1028. https://doi.org/10.1016/j.bja.2020.11.020 (2021).
Helsloot, D. et al. Trauma-induced disturbances in ionized calcium levels correlate parabolically with coagulopathy, transfusion, and mortality: A multicentre cohort analysis from the traumaregister DGU((R)). Crit. Care. 27(1), 267. https://doi.org/10.1186/s13054-023-04541-3 (2023).
Liu, X. L. et al. Low-dose heparin as treatment for early disseminated intravascular coagulation during sepsis: A prospective clinical study. Exp. Ther. Med. 7(3), 604–608. https://doi.org/10.3892/etm.2013.1466 (2014).
Quenby, S., Mountfield, S., Cartwright, J. E., Whitley, G. S. & Vince, G. Effects of low-molecular-weight and unfractionated heparin on trophoblast function. Obstet. Gynecol. 104(2), 354–361. https://doi.org/10.1097/01.AOG.0000128902.84876.d4 (2004).
Okamoto, K., Tamura, T. & Sawatsubashi, Y. Sepsis and disseminated intravascular coagulation. J. Intensive Care. 4(1), 23. https://doi.org/10.1186/s40560-016-0149-0 (2016).
Levi, M. & van der Poll, T. Inflammation and coagulation. Crit. Care Med. 38(2 Suppl), 26–34. https://doi.org/10.1097/CCM.0b013e3181c98d21 (2010).
Iba, T. et al. Japanese surviving Sepsis C.mpaign guideline working group for disseminated intravascular, C. Diagnosis of sepsis-induced disseminated intravascular C.agulation and C.agulopathy. Acute Med. Surg. 6 (3), 223–232. https://doi.org/10.1002/ams2.411 (2019).
O’Brien, M. The reciprocal relationship between inflammation and coagulation. Top. Companion Anim. Med. 27(2), 46–52. https://doi.org/10.1053/j.tcam.2012.06.003 (2012).
Fei, A. et al. The relationship between coagulation abnormality and mortality in ICU patients: A prospective, observational study. Sci. Rep. 5, 9391. https://doi.org/10.1038/srep09391 (2015).
Singh, B. et al. Trends in the incidence and outcomes of disseminated intravascular coagulation in critically ill patients (2004–2010): A population-based study. Chest 143(5), 1235–1242. https://doi.org/10.1378/chest.12-2112 (2013).
Levi, M. & van der Poll, T. Coagulation and sepsis. Thromb. Res. 149, 38–44. https://doi.org/10.1016/j.thromres.2016.11.007 (2017).
Kongstad, C., Mikkelsen, T. S. & Hvas, A. M. Disseminated intravascular coagulation in children with cancer: A systematic review. Pediatr. Hematol. Oncol. 37(5), 390–411. https://doi.org/10.1080/08880018.2020.1733717 (2020).
Ishihara, T. et al. Hemostatic function in hyperfibrinolytic disseminated intravascular coagulation. Pediatr. Int. 61(9), 872–881. https://doi.org/10.1111/ped.13919 (2019).
Mantha, S., Tallman, M. S. & Soff, G. A. What’s new in the pathogenesis of the coagulopathy in acute promyelocytic leukemia? Curr. Opin. Hematol. 23(2), 121–126. https://doi.org/10.1097/MOH.0000000000000221 (2016).
Harada-Shirado, K. et al. Circulating intranuclear proteins May play a role in development of disseminated intravascular coagulation in individuals with acute leukemia. Int. J. Hematol. 111(3), 378–387. https://doi.org/10.1007/s12185-019-02798-5 (2020).
Zhao, Q. Y. et al. A Machine-Learning approach for dynamic prediction of Sepsis-Induced coagulopathy in critically ill patients with Sepsis. Front. Med. (Lausanne). 7, 637434. https://doi.org/10.3389/fmed.2020.637434 (2020).
Richards, J. E., Harris, T., Dunser, M. W., Bouzat, P. & Gauss, T. Vasopressors in trauma: A never event? Anesth. Analg. 133(1), 68–79. https://doi.org/10.1213/ANE.0000000000005552 (2021).
Berg, D. D., Bohula, E. A. & Morrow, D. A. Epidemiology and causes of cardiogenic shock. Curr. Opin. Crit. Care. 27(4), 401–408. https://doi.org/10.1097/MCC.0000000000000845 (2021).
Martinelli, M. et al. Prescribing patterns, indications and adverse events of ibuprofen in children: Results from a National survey among Italian pediatricians. Ital. J. Pediatr. 47(1), 98. https://doi.org/10.1186/s13052-021-01047-y (2021).
Kenny, G. N. Potential renal, haematological and allergic adverse effects associated with nonsteroidal anti-inflammatory drugs. Drugs 44 Suppl.(5), 31–36. https://doi.org/10.2165/00003495-199200445-00005 (1992). discussion 37.
Schafer, A. I. Effects of nonsteroidal anti-inflammatory therapy on platelets. Am. J. Med. 106(5B), 25S–36S. https://doi.org/10.1016/s0002-9343(99)00114-x (1999).
Auriel, E., Regev, K. & Korczyn, A. D. Nonsteroidal anti-inflammatory drugs exposure and the central nervous system. Handb. Clin. Neurol. 119, 577–584. https://doi.org/10.1016/B978-0-7020-4086-3.00038-2 (2014).
Funding
This project was funded with help from the Natural Science Foundation of Fujian Province of China (2021J01419).
Author information
Authors and Affiliations
Contributions
Conception and design of study: JTZ and JHZ, acquisition of data: YJX, YL and PGN, analysis and interpretation of data: JTZ and YJX, drafting the manuscript: JTZ, YJX and PGN, revising the manuscript critically for important intellectual content: HJC, XPZ and JHZ. All authors approved the version of the manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The Pediatric Intensive Care (PIC) database is a large, freely-available medical database consisting of deidentified data from patients who were admitted to The Children’s Hospital, Zhejiang University School of Medicine. The consent was obtained at the beginning of data collection. Therefore, the ethical approval statement and the need for informed consent were jumped in this manuscript, which were not required for this study in accordance with the national legislation and the institutional requirements.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, J., Xie, Y., Liu, Y. et al. Interpretable machine learning model for early prediction of disseminated intravascular coagulation in critically ill children. Sci Rep 15, 11217 (2025). https://doi.org/10.1038/s41598-025-91434-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91434-w