Abstract
Late-life depression represents a significant health concern, linked to disruptions in brain connectivity and immune functioning, mood regulation, and cognitive function. This pilot study explores a digital intervention targeting mental health, brain health, and immune functioning in individuals aged 55–60 with subjective cognitive decline, elevated stress and depressive symptoms. Seventeen participants engaged in a two-week intervention comprising spatial cognition, psychological techniques based on mindfulness, attention-training exercises, and cognitive behavioral therapy. Pre-and post-intervention changes in resting-state functional connectivity, inflammation, and psychological health were evaluated. Key findings include: (1) Reduced self-reported depression with a large effect size, (2) Decreased connectivity within the default mode network (DMN), (3) Enhanced anticorrelation between the DMN-Salience networks that was associated with improved depression scores (4) Reduced salivary IL-18 concentration with a medium effect size, correlated with decreased DMN-amygdala connectivity. There was a trend towards reduced anxiety, with no significant changes in quality of life. To our knowledge, this is the first study to investigate the effect of digital intervention on immune markers, clinical behavioral outcomes, and brain function, demonstrating positive synergistic potential across all three levels. These preliminary findings, which need replication in larger, controlled studies, have important implications for basic science and scalable digital interventions.
Similar content being viewed by others
Introduction
Depression is one of the most frequent mental health disorders among older adults, and is the leading cause of disability worldwide, affecting over 300 million people1. In the aging population, depression poses a complex challenge that requires a comprehensive, integrated strategy for diagnosis, treatment, and support2. According to the World Health Organization (WHO), depression rates differ across age groups, with the highest prevalence observed in older adults (above 7.5% among females aged 55–74 years, and above 5.5% among males)3. Older adults face an increased risk, given the many age-related health and social changes that increase vulnerability to clinical depression and depressive symptoms4. Although numerous effective treatment modalities for depression exist, such as psychotherapy and antidepressant medications (e.g., selective serotonin reuptake inhibitors)5, only one-third of individuals reportedly reach full remission after their first treatment6. Moreover, a significant proportion show resistance to all standard existing treatments7. Depression often coexists with aging-related diseases like Mild Cognitive Impairment (MCI) and Alzheimer’s Disease (AD). The relationship between depression and these cognitive disorders is complex and bidirectional. Individuals with a history of depression were found to have a higher risk of developing MCI and dementia AD in later years8,9. In parallel, cognitive decline can result in frustration and a loss of independence, which may eventually lead to late-life depression (LLD)10.
Subjective Cognitive Decline (SCD) refers to the individual’s self-reported experiences of increasing confusion or memory lapses, indicating a form of cognitive impairment11. According to international cohort studies, the prevalence of SCD among adults over the age of 60 is about 25%12. LLD and SCD are crucial early markers for potential cognitive impairment and the development of dementia, including MCI and AD13, therefore, identification and exploring early intervention strategies for LLD are essential.
Given the interplay between depression and cognitive decline, it is unsurprising that these conditions exhibit some similarities in their characteristic patterns of brain resting state functional connectivity (rsFC) imbalance. Although the specific neural mechanisms underlying each condition differ, understanding their dynamics may provide valuable insights into their shared pathways and potential targets for interventions. In both conditions, alterations in rsFC have been reported in the default mode network (DMN), the salience network (SN), and in the interactions between the two. The primary brain areas included in the DMN are the medial prefrontal cortex (mPFC), the posterior cingulate cortex (PCC) and precuneus, and the lateral parietal (LP) regions14. The SN is defined by the functional coupling of the anterior insula (aINS), the dorsal anterior cingulate cortex (dACC), and the rostral prefrontal cortex (rPFC) regions15. While the DMN is primarily involved with self-referential processes14, the SN is associated with awareness to environmental stimuli and emotional processing control15. Since these networks are functionally distinct with respect to attention and information processing, healthy individuals exhibit anticorrelated DMN-SN connectivity16. The balance between the DMN and the SN is known to be crucial for well-being and for the regulation of mood and emotions17.
Aberrant activity both within and between the DMN and SN has been identified in patients with a variety of psychiatric and cognitive disorders compared to controls, such as in pathological anxiety, major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), schizophrenia, obsessive-compulsive disorder (OCD), and dementia18. For example, MDD is often characterized by increased rsFC between the two networks19,20, and increased connectivity within the DMN and SN has been associated with negative psychological states and psychopathology19,20. Network organization of the brain connectome in MDD is reported to vary with symptom onset, age and medication status21. In LLD, increased rsFC within the DMN was found to distinguish depressed elderly individuals from their non-depressed control subjects22. Certain abnormalities in DMN rsFC improved following antidepressant or TMS treatment23,24, and some alterations were suggested as predictors of treatment response24. Recently, a prediction model using rsFC in a large cohort demonstrated that age-related changes in rsFC are accelerated in MDD and are selectively associated with greater impulsivity and increased depression severity, where both conditions share increased internetwork connectivity between the dACC node of the SN and posterior DMN nodes25. Thus, exploring connectivity alterations in DMN-SN areas may be a sensitive approach for detecting associations with psychological changes in SCD following short interventions.
Normal aging is associated with a gradual deterioration of the immune system, immunosenescence, which is characterized by chronic inflammatory processes that affect both the innate and adaptive immune responses26,27,28. Numerous adverse outcomes in the elderly, including chronic inflammatory diseases and geriatric syndromes, have been linked to dysregulation in cytokine networks, while pro-inflammatory cytokines such as IL-18, IL-6, IL-1β and TNF-α have been identified as potential mediators of age-related inflammation29,30. Crucially, negative phenotypes of aging (e.g., susceptibility to chronic diseases, functional and cognitive impairments) have been attributed at least in part to the molecular and cellular damage that occurs when the body’s resilience mechanisms for coping with stressors decline in efficacy over time31,32. This disruption is especially prominent in the central nervous system (CNS), where alterations are seen in brain cells as well as in the immune cells of the peripheral nervous system33. Recent findings have implicated both neuroinflammation and chronic, low-grade, systemic inflammation as underlying mechanisms in the progression of numerous neurodegenerative and age-related diseases26,30, and in the mediation of age-related cognitive impairments34. Notably, neuroinflammation has been implicated in relevant neurobiological correlates of chronic stress35, depression and depressive symptoms36 that often manifest early in the pathogenesis of neurodegenerative disorders.
Recent studies have identified a relationship between depression and increased inflammatory markers such as IL-18, CRP, TNF-α, and IL-637,38, and an improvement in depressive symptoms associated with the use of anti-inflammatory agents (e.g., NSAIDs)39. These findings support the potential role of the immune system in the pathophysiology of psychiatric conditions, including depression36,40. Given the heightened risk for depression in older adults41, the effects of natural aging on immune functioning26, and the association between increased inflammation and psychiatric disorders42, understanding the mechanisms that underpin the effects of increased inflammation on psychological health in older adults is crucial. Consequently, targeting the immune system in SCD may influence both the progression of age-related diseases and depression.
Against the backdrop of growing interest in the interactions between the brain and the immune system, and the notable alterations in synaptic plasticity and cellular function, which contribute to impaired cognitive function in aging, researchers have begun to investigate the interplay between inflammation and neural plasticity43. It has been posited that the deleterious effects of inflammation on brain function may be due to excessive levels of inflammatory mediators known to impair neurogenesis44. Similarly, pro-inflammatory cytokines (e.g., IL-1β and TGF-β) have been implicated in mechanisms of cognitive functioning and synaptic plasticity, as well as AD pathology and impaired age-related neurogenesis30,45. Thus, synaptic plasticity is increasingly being regarded as a potential therapeutic target for treating age-related cognitive decline46. We therefore used the rsFC technique to gain a deeper understanding of the brain-immune relationship, with the aim of developing effective and detectable interventions.
Diagnosis, monitoring, and treatment through comprehensive psychological and cognitive assessments may reduce the risk of cognitive decline47. A specific domain of such therapeutic settings uses digital therapeutics; software-based interventions designed to manage, monitor, or treat psychological and medical disorders or diseases48. Digital therapeutics can function independently, providing standalone treatments through apps or platforms, or be used as an adjunct to traditional medications and medical devices to address specific patient needs49. Studies evaluating digital interventions in mental health conditions such as depression, anxiety, psychological distress, OCD, and others, have demonstrated that engagement with such programs is associated with therapeutic gains49. In the context of depression, existing digital interventions offer various forms of cognitive behavioral therapy (CBT), mood and symptom tracking, mindfulness exercises, and activity scheduling to help individuals manage symptoms of depression48. A recent meta-analysis revealed no significant difference between human-guided digital interventions and face-to-face psychotherapy efficacy for treating depression50. In a recent study in aging individuals experiencing SCD, we evaluated the effect of a digital mobile application training program on spatial cognition and rsFC51. The intervention combined psychological exercises with multisensory spatial navigation tasks, based on the principles of visual masking and sensory visuo-audio substitution52,53. This study demonstrated enhanced perceptual learning and spatial cognition by engaging brain networks involved in visio-motor processing, spatial memory, and emotion control51. In the current study we investigated the psychological aspects of this research and a possible synergistic effect with alterations in rsFC and immune markers, to provide a more comprehensive understanding of the underlying mechanisms of the intervention.
Studies have shown that psychosocial interventions (e.g., mindfulness meditation, cognitive behavioral therapy) have a significant impact on brain connectivity among depressed patients, specifically by upregulating cognitive control and downregulating DMN connectivity54. In addition, such interventions also positively influence immune system function. For example, in a meta-analysis that included 48 RCTs (N = 4683) comparing mindfulness-based interventions to control conditions, Dunn & Dimolareva55 quantified the potential of these interventions for improving immune function and thus impacting somatic disorders. This meta-analysis indicates a reduction in IL-6 and an increase in T cells in both post-intervention and follow-up. In a different meta-analysis, Shields et al.56 included 56 RCTs (N = 4060) comparing psychosocial interventions to control conditions. This meta-analysis indicates that psychosocial interventions were associated with a 14.7% improvement in beneficial immune system function and an 18.0% decrease in harmful immune system function. Intervention-related decrease in levels of proinflammatory cytokines or markers (e.g., IL-6) and an increase in immune cell counts (e.g., T cells) over time, were also shown. These associations persisted for at least 6 months following treatment. Taken together, in this study, we aimed to investigate the potential for a similar effect using a mobile app.
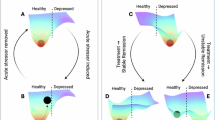
In summary, LLD and SCD are linked through disrupted functional connectivity within and between the DMN and SN brain networks, as well as through increased systemic inflammation. Both conditions are considered potential early markers of MCI and dementia, possibly driven by underlying mechanisms of neural degeneration, making them an important treatment target. We hypothesized that a digital intervention integrating psychological exercises and spatial navigation tasks may produce synergistic improvements for both psychological well-being and immune function (Fig. 1). The psychological interventions aim to enhance well-being and resilience while reducing chronic stress and inflammation through top-down brain regulation. The spatial navigation tasks are designed to strengthen connectivity in brain regions, such as the DMN, which are particularly vulnerable to aging and degenerative diseases52,53. By targeting both of these complementary pathways, this intervention may lead to measurable improvements in psychological indices and immune markers. We further hypothesized that the alterations in brain connectivity will correlate with psychological outcomes and immunological markers, reflecting the top-down modulation influence of the brain.
Results
Participants’ demographics and recruitment
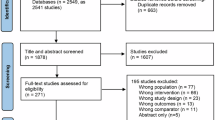
We assessed twenty individuals for eligibility, however, two were excluded due to claustrophobia, and one opted out by withdrawing consent. Consequently, the study proceeded with seventeen participants. The baseline characteristics of these participants are comprehensively detailed in Supplementary Table S1. The mean age of participants at inclusion was 57.2 ± 1.5, the mean PSS-10 score was 15.1 ± 4.5, the mean MoCA score was 27.6 ± 1.9, and 47% were males. Participants’ adherence to the self-guided psychological interventions was 94.1% [78.6− 100%, min-max].
Decreased self-reported depression
Questionnaire analysis is summarized in Table 1. Following the intervention, the CES-D (depression scale) score was reduced by 26.6% with a large effect size (d=-0.829, p = 0.004) (Fig. 2). A trend toward improvement in the anxiety score (GAD7) was shown (p = 0.063, d = -0.484). No significant changes were observed in quality of life and well-being measures.
Based on depression severity criteria categories in older population57 (clinical depression: CES-D ≥ 16, subclinical depression: CES-D 8–15 and no depression CES-D < 8), the average score pre- and post-intervention (12.8 ± 7.7 and 9.5 ± 6.6 respectively) are considered to correspond with subclinical depression. However, the percentage of participants meeting the clinical cut-off score for clinical depression was reduced by 18%, and the percentage of participants classified as “no depression” according to the clinical cut-off scores increased by 18%.
CES-D score changes. (A) The intervention significantly decreased self-reported depression CES-D questionnaire. Following the intervention, CES-D (depression scale) score was reduced by 26.6% with a large effect size (d = − 0.829, p = 0.004). Data shown in boxplot, the central mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The square mark indicates the mean level. (B) Pre- and post-intervention depression severity categories57. The percentage of participants meeting the clinical cut-off for depression decreased by 18%, and the percentage of participants classified as “no depression” according to the clinical cut-off scores increased by 18%.
Improvement in the interplay between the DMN and SN networks
All participants were included in the rsFC analysis. No significant differences in head motion were found between the groups (mean translation, 1.01 ± 0.48 and 0.97 ± 0.57, mean rotation, 1.02 ± 0.52 and 0.95 ± 0.57, p, NS). Inter and intra-network connectivity was assessed for DNM and SN according to ALE based large-scale brain networks parcellation of the Human Connectome Project-Multi Modal Parcellation atlas (HCP-MMP)58,59. The ROI-to-ROI analysis revealed a significant reduction in post-intervention connectivity within the DMN (Fig. 3). Specifically, this reduction was significant between the mPFC and the PCC with a large effect size (p32-p31pd, T = − 4.89, d = − 1.19, pFDR < 0.05), and between the PCC and the right and left LP (31a-PGsL and PGiR, T = − 4.61, − 4.42, d = − 1.12, − 1.07, pFDR < 0.05 respectively) as shown in Fig. 3 and Supplementary Table S2. Conversely, no significant changes were observed intra-network in SN connectivity.
DMN-SN ROI-to-ROI inter and intra-network connectivity analysis. (A) Brain network representation of significant post-intervention differences within the DMN. Image was generated using CONN (RRID: SCR_009550) https://web.conn-toolbox.org, v22a. (B) a connectivity matrix of significant post-intervention alterations within and between the DNM and SN networks. Significant reductions were demonstrated between the mPFC and PCC (T = − 4.89), and between the ACC and the right LP (T = − 5.32). Additional details are provided in Supplementary Table S2. LP, lateral parietal, PCC, posterior cingulate cortex, mPFC, medial prefrontal cortex, aINS, anterior insula, ACC, anterior cingulate cortex, R, right, L, left, POST > PRE-Intervention, n = 17, pFDR < 0.05, White, NS.
Internetwork connectivity between the DMN and SN: The DMN exhibited both increased and decreased connectivity with the SN in different regions as shown in Fig. 3. Specifically, significant alterations were found between the ACC and the right LP with a large effect size (a32pr-PFm, T = − 5.32, d = − 1.29, pFDR < 0.05). Importantly, a significant correlation was found between changes in overall inter-network rsFC and changes in CES-D and MHC-SF scores (r = 0.49, p < 0.05, r = − 0.63, p < 0.01 respectively) following treatment, demonstrating that improvement in depression and mental health scores is correlated with increased internetwork anticorrelation. This suggests a correlation between the extent to which individuals improved in their psychological state and the extent in which their connectivity pattern became healthier (Fig. 4).
DMN-SN intra-network connectivity correlations with psychological state. The improved CES-D depression score (negative change marks improvement in the depression score for the post vs. pre assessment) was correlated with increased negative internetwork DMN-SN rsFC. Additionally, the improved MHC_SF, general mental Health score (positive change marks improvement), was correlated with increased negative internetwork DMN-SN rsFC. This suggests a correlation between the extent to which individuals improved in their psychological state and the extent in which their connectivity pattern became healthier.
Association between salivary IL-18 and large-scale brain networks
Cytokines’ salivary levels were analyzed, pre- and post-intervention, to determine a possible correlation between saliva concentrations and alterations in brain connectivity. The cytokines IFN-α2, TNF-α, IL-10, IL-12p70, IL-17, IL-33 were below the lower limit of detection for more than 50% of the samples. Insignificant post-intervention change was detected in IL-1β, IFN-γ, IL-6, IL-8, IL-23, MCP-1.
Post-intervention salivary IL-18 concentration was significantly reduced by 25%, with a medium effect size (317.9 ± 233.6 pg/mL to 218.4 ± 128.3 pg/mL, p = 0.026, d = − 0.641) as shown in Fig. 5A. Voxel-wise regression analysis was used to test for associations between IL-18 and whole brain connectivity across individuals. The analysis revealed a correlation between the decrease in the rsFC between the right amygdala and right and left precuneus, a node of the posterior DMN, (r = 0.88, p < 0.001) and the change in Salivary il-18 levels (Fig. 5B, C).
IL-18 levels change. (A) The intervention significantly decreased interleukin-18 levels by 25% with a medium effect size (d = − 0.64, p < 0.05, n = 15). Data shown in boxplot, the central mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The square mark indicates the mean level. (B) Association between salivary IL-18 post-intervention reductions and alterations in rsFC: voxel-wise regression analysis maps demonstrating significant positive correlation between decreased rsFC between the right amygdala seed and the left and right precuneus within the posterior DMN and decreased in IL-18 levels. DMN mask, green, overlap, yellow. (C) Significant correlation between decreased rsFC between the right amygdala and the right precuneus. R, right, POST > PRE-Intervention, n = 15, pFDR < 0.05.
Discussion
In this proof-of-concept study, we explored the potential of a multisensory digital psychological intervention to demonstrate a triple-effect relationship between mental health, brain health, and immune function in SCD individuals with elevated levels of stress and depressive symptoms. Testing this triple-effect model, participants engaged in a two-week protocol of daily self-training psychological program featuring short stress regulation techniques based on mindfulness, attention-focusing exercises, and cognitive behavioral therapy (CBT), and were evaluated before and after the session using rsFC, saliva tests and questionnaires. Key results showed: (1) Decreased self-reported depression (Fig. 2), (2) Decreased connectivity within the DMN (Fig. 3). (3) Enhanced anticorrelation between the DMN and SN networks was associated with improved depression scores (Figs. 3 and 4), and (4) Reduced salivary IL-18 concentration correlated with decreased connectivity between the amygdala and the DMN (Fig. 5). These results demonstrate a synergistic relationship between psychological intervention, neuroplasticity, and the enhancement of immune biomarkers. To our knowledge, this is the first study to investigate the effects of digital intervention on immune system markers, clinical behavioral outcomes, and brain imaging, and show correlations to the effect of the intervention in all three levels. Thus, it has important implications both for basic science and the potential use in such innervations that can be scaled for massive use.
The DMN, depression and the immune system
The DMN is a global brain network, which shows higher activity during rest, and is involved with self-referential processes, recalling memories and daydreaming14. Increased rsFC within the DMN is associated with psychological processes and may reflect several clinical aspects of depression, such as rumination, repetitive negative thinking, and unforgiveness60,61. In our study, we demonstrated post-intervention reduction in the intrinsic connectivity within the DMN, specifically, a reduced connectivity between the PCC node and mPFC and LP nodes. This observation aligns with previous studies in LLD that demonstrated increased connectivity, compared to non-depressed subjects, between the PCC and the anterior DMN20,22,62, mPFC and sgACC. Although the sgACC is not typically considered as a part of the DMN, it was found to be a part of the DMN in MDD63. This reduced connectivity may explain the improvement in depression score, as the connectivity between the anterior and posterior DMN regions has been suggested to reflect positive treatment effects64. On the other hand, there are generally consistent findings indicating that aging and cognitive decline are often linked to decreased rsFC within the DMN65, where one of the prominent changes observed is the anterior-posterior disconnection66. However, the loss of intra-network connections is attributed to disease progression and cognitive impairment severity64,67. The balance between age, cognitive function, and psychological symptoms may explain variability between studies and highlight the need for tailored treatments.
The interplay between the SMN and the SN and their relation to depression
Altered intrinsic connectivity is often accompanied by changes in connectivity with associated networks. In this study we report alterations in DMN-SN interconnectivity, most significantly between the SN-ACC and DMN-LP. The LP area has been shown to have overlapping activity with the dorsal attention network and the SN, specifically the dorsal ACC and the insula areas68. This pivotal connectivity pattern supports the role of the LP in both internally and externally oriented processing, facilitating attention control, action planning, and behavior regulation68,69. We showed that the improved depression score correlated with a decrease in DMN-SN global mean inter-network connectivity, or anticorrelation. Global network hyperconnectivity between the DMN and SN has been reported in MDD patients compared to healthy controls70 and specific increased interconnections reported between the mPFC71, sgmFFC72, and PCC73 and the anterior insula. However, variations in age, seed selection, and medication status could explain the different findings regarding specific connections between the studies.
A gradual increase in DMN-SN connectivity was also demonstrated across the healthy adult lifespan74,75, and in degenerative diseases such as AD, dementia and Parkinson’s disease64,67,76. The proposed treatment demonstrated improvements in mental state and in shared neural pathways affected both psychiatric and degenerative diseases. We anticipate that targeting these pathways and key regions vulnerable to aging withing the DMN will positively impact higher cognitive processes, such as memory, attention, and executive function, making the treatment beneficial for both conditions.
Immune system markers via longitudinal salivary analysis and their link to depression
Growing evidence suggests that neuroinflammation plays a significant role in the pathophysiology of both aging and mental health. We examined an array of cytokines to test the hypothesis that a reduction in anti-inflammatory biomarkers is related to changes in brain connectivity and improvements in depression symptoms. Among the tested cytokines, we found that following the intervention, salivary IL-18 concentration was significantly reduced. IL-18 is a pro-inflammatory cytokine, of the IL-1 family, which is involved in inflammatory processes that increase during aging. Neuroinflammation, characterized by the activation of the brain’s immune cells and increased production of inflammatory cytokines, plays a critical role in the pathogenesis of neurodegenerative diseases and cognitive decline29,77. It has been suggested that IL-18 may help modulate the stress response and be implicated in maintaining the balance between neural inflammation and glucocorticoid signaling78. Indeed, IL-18 has been identified as a potential mediator of age-related inflammation, with increased levels linked to neuroinflammatory processes that contribute to cognitive impairments and neurodegenerative diseases such as Alzheimer’s29,77. IL-18 has also been linked to psychiatric disorders, including schizophrenia and bipolar disorder79, PTSD80, and depression81. This reduction in IL-18 suggests that our interventions can play a vital role in managing mental health and age-related inflammatory and neuroinflammatory conditions. Further research is needed to elucidate the precise mechanisms through which these interventions modulate immune function and to explore their long-term benefits for aging individuals.
Given the lack of findings for the remaining immune marker analytes in the present study, it is important to note that while the use of saliva over blood for biomarker analysis carries numerous advantages82, and many analytes detected in blood are also found in saliva, their concentration in saliva is often much lower83, and is limited by the sensitivity of detection technologies. Future studies might benefit from collecting and analyzing blood biomarkers, and this is a limitation of the current study. However, even in plasma, analytes may be undetectable in non-clinical patient samples. For example, Li et al. determined normative data for plasma cytokines of 18 analytes in a cohort of carefully screened healthy adults aged 18–64 (N = 126) and found normative values for only half of the analytes (these included IFN-γ, TNF-α, and IL-18), while the remaining nine were not detected on the assays84. Crucially, circulating levels of IL-18 are detectable even amongst healthy individuals29,84.
The relation between changes in IL18 inflammatory cytokine and brain connectivity
Our study, a whole-brain regression analysis revealed a significant association between IL-18 response and amygdala-precuneus rsFC changes. IL-18 is expressed through the CNS, and its receptors are expressed throughout corticolimbic circuitry including the amygdala85. Cross-species research supports a linkage between the IL-18 system in the amygdala and susceptibility for depressive symptoms and behaviors86, and a potentially reciprocal association between amygdala reactivity and inflammation87. Depression-like behaviors induced by chronic stress exposure were suppressed via local inhibition of IL-18 and its receptors in the basolateral amygdala (BLA)86. The precuneus is implicated in tasks requiring sustained focus and multitasking. Additionally, as a part of the DMN, it is involved in self-awareness, episodic memory retrieval, visuospatial processing, and consciousness, and plays a key role in directing attentional resources. Increased connectivity between the amygdala and precuneus has been observed in individuals with psychiatric disorders such as subthreshold depression and MDD, suggesting its involvement in emotion regulation through attentional processes88,89. In the current study, decreased connectivity between the amygdala and precuneus was not significant at the group level, however reduced connectivity was correlated with a reduction in IL-18 levels suggesting the relationship between inflammation markers and brain connectivity normalization. Although not much is known about the relationship between IL-18 and brain connectivity in depressed individuals, in a mouse model, chronic stress (chronic restraint or chronic foot-shock) was shown to increase IL-18 expression in the BLA and the exhibition of depression-like behaviors while IL-18 knockout mice displayed resilience to the stressors86. Human imaging data also supports abnormal connectivity of the PCC and higher peripheral serum IL-18 levels in depressed patients, a finding does not present in the healthy control group81.
Study limitations
Some limitations should be considered when interpreting our findings. While it is important to discuss such limitations as we do here, it is important to consider these limitations considering the pioneering nature of this study. The homogeneity in clinical characteristics within our participant group due to a narrow age range and a small sample size might restrict the reliability and validity of the findings, as well as the generalizability of our findings to the broader population. On the other hand, as our study included participants with SCD, we did not use baseline depression scores as part of the screening criteria, leading to significant variability in this measure. However, this is quite reasonable and commonplace in first proof-of-concept studies, and our next study aims to replicate this study with a larger sample size. Secondly, there is considerable variability between studies regarding abnormal connectivity patterns in different populations, including study designs, scanning parameters, analysis strategies, and mostly global-networks ROI definitions, complicating direct comparisons. To partially address this variability in seed selection, we used an ALE-based regional mask of the global networks. Additionally, data was gathered following a brief, two-week intervention period and a short daily digital psychological session. While this provides support for our hypothesis that even a short intervention may have significant results, future studies should include a longer follow up period to assess any prolonged impact of the use of the app, as well as the ideal intervention length and any required booster interactions. Furthermore, the digital intervention combined two components: spatial cognition task, and a digitized version of evidence-based psychological interventions, adapted from protocols such as MBSR, MBCT, and attention training. Research suggests that combining these approaches enhances top-down emotion regulation more effectively than using them individually90,91. Integrating them with bottom-up multisensory modulation was hypothesized to lead to an augmentation effect influencing psychological state, immune function, and brain connectivity. However, the study design does not allow for separate assessments of the individual contributions of each intervention type. Lastly, this was a single-arm study, and it is not possible to determine whether the intervention has benefits beyond a placebo effect. While we focused here on correlation between changes in connectivity patterns, immune response, and mental scores, which may suggest causality, incorporating a control or placebo intervention group in future studies would significantly enhance the robustness of the findings.
Conclusions
In conclusion, the present study demonstrates that short-term daily digital interventions using a self-training protocol can improve depression symptoms and elicit alterations in brain connectivity in DMN and SN regions, which are critical for both well-being and cognition, which are often affected in the early stages of AD. Our findings confirm a synergetic effect between mental health, brain health, and immune function in SCD individuals with elevated levels of stress and depressive symptoms. These results suggest potential strategies for reducing inflammation and slowing the progression of degenerative brain diseases. Furthermore, they highlight the potential of digital interventions in reducing depression, particularly in the aging population, who also often experience cognitive decline. Future research should explore the interplay between brain changes and the immune system in response to these interventions in larger groups and different types of populations susceptible to mental health disorders.
Methods
Trial design
A pilot study, designed as a prospective, open-label trial, was undertaken at the Baruch Ivcher Institute for Brain, Cognition & Technology (BCT) within the School of Psychology at Reichman University, Israel. In this study, we recruited healthy adults aged between 55 and 60 who exhibited signs of SCD. The specific age range was selected for this pilot study to enable focusing on a population that is in an age that is already relevant to SCD on one hand92, but that is less likely to display diverse brain connectivity patterns, which occur naturally in both normal and pathological aging93,94 on the other hand. This allowed for a relatively homogenous group and a smaller sample size. In accordance with the conceptual framework for research in SCD offered by Jessen et al.47,95, participants were determined to have SCD by providing a self-report on decreased cognitive function compared to the past, while obtaining normal function in a standardized test for cognitive disfunction, as demonstrated a Montreal Cognitive Assessment (MoCA) score of 24 or higher96,97. As described in the framework, adults diagnosed with mild cognitive impairment, prodromal AD or dementia were excluded, as well as adults presenting with a psychiatric or neurologic disease, serious medical disorder, medication, or substance abuse that could otherwise explain the subjective decline in cognitive function (see exclusion criteria below). In addition, participants scored 5 or above on the Perceived Stress Scale (PSS-10). We screened participants for at least a minimal level of stress, increasing the likelihood of identifying individuals with dysregulated immune system.
Exclusion criteria included: any history of malignancy, traumatic brain injury, brain surgery, chronic subdural hemorrhages, epilepsy and other neurodegenerative diseases, any psychiatric disorder or pathological cognitive decline, and MRI contraindications. After signing an informed consent, participants were engaged in a two-week digital intervention using a mobile application, with supervised sessions on days 1 and 14, supplemented by daily self-training at home. Participants were assessed for changes in the brain’s functional connectivity, well-being, and psychological state (Fig. 6A).
The study was approved by Reichman University Institutional Review Board (IRB) (No. P_2023138). The neuroimaging study protocol was reviewed and approved by the IRB of Sheba Medical Center (No. 8591-21-SMC). All participants signed an informed consent prior to their inclusion. All research was performed according to the relevant guidelines and regulations.
The digital intervention
The participants utilized a comprehensive training mobile application developed by Remepy (https://www.remepy.com), which incorporates unique methodologies informed, among other things, on by our prior research in blind and blindfold individuals using spatial memory and navigation training52 and digitized psychological interventions. The intervention’s spatial cognitive component features virtual spatial navigation exercises that utilize digital 3D Hebb-Williams mazes and incorporate both allocentric and egocentric navigation techniques. These exercises are implemented through a progressive, three-step vision-deprivation multisensory training process, designed to increase navigation complexity over time. Each maze trial starts with a top-down 2D map view (Supplementary Fig. S1A) for allocentric navigation. Participants then navigate the maze fully sighted, using auditory cues for spatial information, such as distance from walls. After completing this phase, the 2D map is shown again, followed by a more challenging 3D navigation phase where 50% of the maze is masked. In the final phase, participants navigate blindfolded using only auditory feedback, employing a sensory substitution strategy (Supplementary Fig. S1B–D). This approach aims to enhance brain connectivity, spatial learning, and balance across sensory and cognitive networks51,53,98,99.
The psychological program featured short stress regulation techniques based on mindfulness, attention-focusing exercises, and cognitive behavioral therapy (CBT), presented by video, audio, and interactive formats. Each daily self-training session lasted approximately 30 min, comprising about 25 min of engagement with the cognitive program and 5 min dedicated to psychological interventions. In this article, we focus on the psychological aspect of the intervention. Table 2 presents a summary of the psychological interventions in the program. The digital navigation training protocol integrates egocentric and allocentric strategies with multisensory stimulation and visual input masking to enhance spatial cognition and brain connectivity. The spatial cognitive related brain connectivity and behavioral endpoints are to be analyzed and reported in future publication.
Outcome measures
Self-reported questionnaires
Changes in the subject’s psychological state were assessed using the Mental Health Continuum Short Form (MHC-SF), General Anxiety Disorder-7 (GAD-7D), Center for Epidemiologic Studies Depression Scale (CES-D), and the 36-Item Short Form Survey (SF-36) validated questionnaires. A description of the questionnaires is provided in Supplementary Information 1.1.
Brain imaging
Brain imaging MRI scans were performed on MAGNETOM Prisma 3T Scanner, configured with a 64-channel receiver head coils (Siemens Healthcare, Erlangen, Germany), at the Ruth and Meir Rosental Brain Imaging Center (MRI), Reichman University. The MRI protocol included the following sequences: Two runs of 300 volumes (9:28 min) resting state fMRI scans were acquired using a multi-band echo planar imaging sequence, CMRR EPI 2D100,101. Scan parameters: TR: 1,870 ms, TE: 30 ms, flip angle: 75°, voxel size: 3.0 × 3.0 × 2.0 mm, FOV: 192, number of slices: 58 axial slices parallel to the AP-PC plane. During scanning, each participant was asked to remain still and relaxed, with their eyes fixated on a cross, and without thinking of anything deliberate. Foam pads and earplugs were employed to reduce head motion and scanning noise. Structural T1-weighted MRI scans were acquired for co-registration purposes using a T1-weighted 3D magnetization-prepared rapid gradient-echo (MPRAGE) sequence in a sagittal plane with 1 mm isotropic resolution. Sequence parameters: TR: 2,000 ms, TE: 1.9 ms, flip angle: 9°, TI: 920 ms, FOV: 256 × 256, and 176 contiguous slices. The MRI protocol also included T2-Fluid-attenuated inversion recovery (FLAIR), and susceptibility-weighted imaging (SWI) sequences, using standard parameters for clinical brain evaluation.
BOLD data preprocessing
Functional connectivity analysis was carried out using the CONN-fMRI toolbox v22a as implemented using statistical parametric mapping software SPM12 (http://www.fil.ion.ucl.ac.uk/spm). Functional volumes pre-processing pipeline included realignment with correction of susceptibility distortion interactions, slice timing correction, outlier detection, direct segmentation, and MNI-space normalization, with a resolution voxel size of 2.0 × 2.0 × 2.0 mm, and spatial smoothing (8 mm FWHM Gaussian kernel) steps102. The preprocessing steps derived (1) the realignment covariate, containing the six rigid-body parameters characterizing the estimated subject motion, (2) the scrubbing covariate containing potential outlier scans performed with CONNs artifact detection tool (ART), and (3) the quality assurance (QA) covariate based on global signal change (> 3 standard deviations from the mean image intensity) and framewise displacement (FD) scan-to-scan head-motion. Age and sex were also used as group (second level) covariates. A component-based noise correction procedure (CompCor) approach103 was used to identify additional confounding temporal factors controlling for physiological noise, BOLD signal present in white matter, and head motion effects. Finally, residual BOLD time series were then bandpass-filtered at a frequency range of 0.01–0.009 Hz102. Individual connectivity maps were generated using the seed-to-voxel approach. We examined rsFC using a priori seeds derived from the extended HCP-MMP atlas (HCPex), a modified and extended version of the Human Connectome Project-MultiModal Parcellation atlas (HCP-MMP)104, which provides the surface-based of 426 human cortical areas. Bivariate correlation analysis was used to determine the linear association of the BOLD time series between the seed and significant voxel clusters. Fisher’s Z transformation was applied to the correlation coefficients to satisfy normality assumptions. Then, functional connectivity maps were thresholded at p < 0.05 false discovery rate (FDR) corrected for multiple comparisons. To examine possible correlations between saliva concentrations, and alterations in rsFC at a whole-brain level, the amygdala and the hippocampus seeds were used. ROI-to-ROI network analysis was focused on depression-related commonly reported large-scale brain networks, which included: default mode (DMN), and salience (SN)14,15. Spatial masks of the DMN and SN were extracted according to activation likelihood estimation (ALE) based large-scale brain networks parcellation of the HCP-MMP atlas58,59. The DMN includes seventeen cortical regions: 10r, 31a, 31pd, 31pv, a24, d23ab, IP1, p32, POS1, POS2, RSC, PFm, PGi, PGs, s32, TPOJ3, and v23ab. The SN includes nine cortical regions: AVI, MI, FOP4, FOP5, a24pr, a32pr, p32pr, SCEF, and 46 (Fig. 6B, Supplementary Table S3). Finally, participants with head motions of > 2 mm in any direction between volumes, rotations of > 2° in any axis during scanning, or mean FD of > 0.5 in either the pre- or post-treatment maps were excluded from the dataset.
Study design and spatial global networks masks. (A) study design and timeline. (B) Network’s masks extracted according to ALE based large-scale brain networks parcellation of the Human Connectome Project-MultiModal Parcellation atlas (HCP-MMP)58,59. DMN, default mode network, SN, salience network, Images were generated using MRIcroGL software (https://www.nitrc.org/projects/mricrogl).
Saliva samples collection and preparation
Saliva samples were collected on days 1 and 14 upon arrival at the research site. Saliva was collected from participants via passive drooling into a clean 5 ml Eppendorf Vial. If participants experienced difficulty producing saliva, they were instructed to massage their jaw. Participants were notified to refrain from smoking, eating, drinking, or oral hygiene procedures for at least 1 h, and refrain from drinking water at least 20 min prior to sample collection. All samples were immediately stored at − 20 °C. To precipitate the mucus, samples underwent three freeze-thaw cycles: freeze at − 80 °C and thaw at 4 °C. Following the fourth cycle, the tubes were centrifuged twice at 1500 g (4000 rpm) for 30 min each. The supernatant was collected, and the aliquots stored at − 20 °C until assay. Saliva samples were diluted at a 1:2 ratio, and cytokines levels were determined using the LEGENDplex™ Human Inflammation Panel I (BioLegend, 740809) in accordance with the manufacturer’s protocol. Measurements were performed with a Cytek® Aurora flow cytometer, and cytokine concentrations were calculated using BioLegend’s LEGENDplex™ data analysis software.
Statistical analysis
Descriptive statistics
The demographics and continuous data are expressed as means ± standard deviations (SD). Categorical data were expressed in numbers and percentages. To evaluate the intervention’s effect, the normality of data distribution was evaluated using the Kolmogorov-Smirnov test. After confirming normality, a student’s t-test was used to compare post-treatment and pre-treatment data. Effect size was evaluated using Cohen’s d method. Data analysis was performed using MATLAB R2021b (MathWorks, Natick, MA) Statistics and Machine Learning toolbox.
Imaging analysis statistics
A bivariate group-level regression analysis with psychological and salivary data covariate model was used to identify global brain correlations. The analysis was implemented in SPM software (version 12, UCL, London, UK) with a parametric analysis approach across the entire brain volume102. RsFC was considered significant at joint-probability thresholds of 0.001 at the voxel level, and p < 0.05 false discovery rate using the Benjamini-Hochberg FDR procedure corrected for multiple comparisons across the whole brain at the cluster level, with a minimum cluster size of 50 voxels. ROI-to-ROI analysis was performed to identify the relationship between and within the DMN and SN. ROI-to-ROI rsFC was considered significant at connection thresholds of 0.01, and cluster threshold p < 0.05 FDR corrected (MVPA omnibus test) across the global networks mask.
Cytokine analysis statistics
Two (out of n = 17) samples were excluded from analysis due to low volume and insufficient quality. Statistical analyses were performed using GraphPad Prism software version 10 (GraphPad Software Inc.). A paired t-test was used to compare cytokine levels between the two time points. Results were considered statistically significant at a p-value of < 0.05.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Ménard, C., Hodes, G. E. & Russo, S. J. Pathogenesis of depression: insights from human and rodent studies. Neuroscience 321, 138–162 (2016).
Mitina, M., Young, S. & Zhavoronkov, A. Psychological aging, depression, and well-being. Aging (Albany NY). 12, 18765 (2020).
World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates (World Health Organization, 2017).
Fiske, A., Wetherell, J. L. & Gatz, M. Depression in older adults. Ann. Rev. Clin. Psychol. 5, 363–389 (2009).
Al-Harbi, K. S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adher., 369–388 (2012).
Rush, A. J. et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. Am. J. Psychiatry. 163, 1905–1917 (2006).
Nuñez, N. A. et al. Augmentation strategies for treatment resistant major depression: a systematic review and network meta-analysis. J. Affect. Disord. 302, 385–400 (2022).
Ma, L. Depression, anxiety, and apathy in mild cognitive impairment: current perspectives. Front. Aging Neurosci. 12, 9 (2020).
Gao, Y. et al. Retracted: depression as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Int. J. Geriatr. Psychiatry. 28, 441–449 (2013).
Formánek, T. et al. Trajectories of depressive symptoms and associated patterns of cognitive decline. Sci. Rep. 10, 20888 (2020).
Molinuevo, J. L. et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s Dement. 13, 296–311 (2017).
Röhr, S. et al. Estimating prevalence of subjective cognitive decline in and across international cohort studies of aging: a COSMIC study. Alzheimers Res. Ther. 12, 1–14 (2020).
Slot, R. E. et al. Subjective cognitive decline and rates of incident Alzheimer’s disease and non–Alzheimer’s disease dementia. Alzheimer’s Dement. 15, 465–476 (2019).
Davey, C. G., Pujol, J. & Harrison, B. J. Mapping the self in the brain’s default mode network. NeuroImage 132, 390–397 (2016).
Seeley, W. W. The salience network: a neural system for perceiving and responding to homeostatic demands. J. Neurosci. 39, 9878–9882 (2019).
Fox, M. et al. (ed, D.) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. 102 9673–9678 (2005).
Shi, L. et al. Brain networks of happiness: dynamic functional connectivity among the default, cognitive and salience networks relates to subjective well-being. Soc. Cognit. Affect. Neurosci. 13, 851–862 (2018).
Sha, Z., Wager, T. D., Mechelli, A. & He, Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol. Psychiatry. 85, 379–388 (2019).
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D. & Pizzagalli, D. A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 72, 603–611 (2015).
Mulders, P. C., van Eijndhoven, P. F., Schene, A. H., Beckmann, C. F. & Tendolkar, I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci. Biobehavioral Reviews. 56, 330–344 (2015).
Yun, J. Y. & Kim, Y. K. Graph theory approach for the structural-functional brain connectome of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 111, 110401 (2021).
Alexopoulos, G. S. et al. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord. 139, 56–65 (2012).
Posner, J. et al. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry. 70, 373–382 (2013).
Dichter, G. S., Gibbs, D. & Smoski, M. J. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J. Affect. Disord. 172, 8–17 (2015).
Dunlop, K., Victoria, L. W., Downar, J., Gunning, F. M. & Liston, C. Accelerated brain aging predicts impulsivity and symptom severity in depression. Neuropsychopharmacology 46, 911–919 (2021).
Fulop, T. et al. Immunology of aging: The birth of inflammaging. Clin. Rev. Allergy Immunol., 1–14 (2021).
Shaw, A. C., Joshi, S., Greenwood, H., Panda, A. & Lord, J. M. Aging of the innate immune system. Curr. Opin. Immunol. 22, 507–513 (2010).
Alpert, A. et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 25, 487–495 (2019).
Dinarello, C. A. Interleukin 1 and Interleukin 18 as mediators of inflammation and the aging process. Am. J. Clin. Nutr. 83, 447S–455S (2006).
Rea, I. M. et al. Age and age-related diseases: role of inflammation triggers and cytokines. Front. Immunol. 9, 334076 (2018).
Schumacher, B., Pothof, J., Vijg, J. & Hoeijmakers, J. H. The central role of DNA damage in the ageing process. Nature 592, 695–703 (2021).
Walker, K. A., Basisty, N., Wilson, D. M. & Ferrucci, L. Connecting aging biology and inflammation in the omics era. J. Clin. Investig. 132 (2022).
DiSabato, D. J., Quan, N. & Godbout, J. P. Neuroinflammation: the devil is in the details. J. Neurochem. 139, 136–153 (2016).
Allison, D. J. & Ditor, D. S. The common inflammatory etiology of depression and cognitive impairment: a therapeutic target. J. Neuroinflamm. 11, 1–12 (2014).
Lavretsky, H. & Newhouse, P. A. Stress, inflammation and aging. Am. J. Geriatric Psychiatry: Official J. Am. Association Geriatric Psychiatry. 20, 729 (2012).
Troubat, R. et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 53, 151–171 (2021).
Corrigan, M., O’Rourke, A. M., Moran, B., Fletcher, J. M. & Harkin, A. Inflammation in the pathogenesis of depression: A disorder of neuroimmune origin. Neuronal Signal. 7 (2023).
Fan, N., Luo, Y., Ou, Y. & He, H. Altered serum levels of TNF-α, IL‐6, and IL‐18 in depressive disorder patients. Hum. Psychopharmacology: Clin. Experimental. 32, e2588 (2017).
Bai, S. et al. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: a systematic review and meta-analysis of randomised controlled trials. J. Neurol. Neurosurg. Psychiatry. 91, 21–32 (2020).
Spellman, T. & Liston, C. Toward circuit mechanisms of pathophysiology in depression. Am. J. Psychiatry. 177, 381–390 (2020).
http://who.int.news-room/fact-sheets/detail/ageing-and-health (2023).
Bauer, M. E. & Teixeira, A. L. Inflammation in psychiatric disorders: what comes first? Ann. N. Y. Acad. Sci. 1437, 57–67 (2019).
Golia, M. T. et al. Interplay between inflammation and neural plasticity: both immune activation and suppression impair LTP and BDNF expression. Brain. Behav. Immun. 81, 484–494 (2019).
Yirmiya, R. & Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain. Behav. Immun. 25, 181–213 (2011).
Lynch, M. A. Age-related neuroinflammatory changes negatively impact on neuronal function. Front. Aging Neurosci. 1, 1206 (2010).
Kumar, A. Vol. 10, 413 (Frontiers Media SA, 2018).
Jessen, F. et al. The characterisation of subjective cognitive decline. Lancet Neurol. 19, 271–278 (2020).
Harvey, P. D. et al. Technology and mental health: state of the Art for assessment and treatment. Am. J. Psychiatry. 179, 897–914 (2022).
Abbadessa, G. et al. Digital therapeutics in neurology. J. Neurol. 269, 1209–1224 (2022).
Moshe, I. et al. Digital interventions for the treatment of depression: A meta-analytic review. Psychol. Bull. 147, 749 (2021).
Amedi, A., Shelly, S., Saporta, N. & Catalogna, M. Perceptual learning and neural correlates of virtual navigation in subjective cognitive decline: A pilot study. Science (2024).
Aggius-Vella, E., Chebat, D. R., Maidenbaum, S. & Amedi, A. Activation of human visual area V6 during egocentric navigation with and without visual experience. Curr. Biol. 33, 1211–1219 (2023). e1215.
Pascual-Leone, A., Amedi, A., Fregni, F. & Merabet, L. B. The plastic human brain cortex. J. R N. 28, 377–401 (2005).
Bursky, M., Egglefield, D. A., Schiff, S. G., Premnath, P. & Sneed, J. R. Mindfulness-enhanced computerized cognitive training for depression: an integrative review and proposed model targeting the cognitive control and default-mode networks. Brain Sci. 12, 663 (2022).
Dunn, T. J. & Dimolareva, M. The effect of mindfulness-based interventions on immunity-related biomarkers: a comprehensive meta-analysis of randomised controlled trials. Clin. Psychol. Rev. 92, 102124 (2022).
Shields, G. S., Spahr, C. M. & Slavich, G. M. Psychosocial interventions and immune system function: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 77, 1031–1043 (2020).
Cohen, C. I., Magai, C. & Yaffee, R. Walcott-Brown, L. Racial differences in syndromal and subsyndromal depression in an older urban population. Psychiatric Serv. 56, 1556–1563 (2005).
Briggs, R. G. et al. Parcellation-based tractographic modeling of the salience network through meta‐analysis. Brain Behav. 12, e2646 (2022).
Sandhu, Z. et al. Parcellation-based anatomic modeling of the default mode network. Brain Behav. 11, e01976 (2021).
Ingersoll-Dayton, B., Torges, C. & Krause, N. Unforgiveness, rumination, and depressive symptoms among older adults. Aging Ment. Health. 14, 439–449 (2010).
Spinhoven, P., Drost, J., van Hemert, B. & Penninx, B. W. Common rather than unique aspects of repetitive negative thinking are related to depressive and anxiety disorders and symptoms. J. Anxiety Disord. 33, 45–52 (2015).
Wu, M. et al. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Research: Neuroimaging. 194, 39–46 (2011).
Zhou, Y. et al. Increased neural resources recruitment in the intrinsic organization in major depression. J. Affect. Disord. 121, 220–230 (2010).
Kim, J. & Kim, Y. K. Crosstalk between depression and dementia with resting-state fMRI studies and its relationship with cognitive functioning. Biomedicines 9, 82 (2021).
Damoiseaux, J. S. et al. Reduced resting-state brain activity in the default network in normal aging. Cereb. Cortex. 18, 1856–1864 (2008).
De Vogelaere, F., Santens, P., Achten, E., Boon, P. & Vingerhoets, G. Altered default-mode network activation in mild cognitive impairment compared with healthy aging. Neuroradiology 54, 1195–1206 (2012).
Brier, M. R. et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. J. Neurosci. 32, 8890–8899 (2012).
Tumati, S., Martens, S., de Jong, B. & Aleman, A. Lateral parietal cortex in the generation of behavior: implications for apathy. Prog. Neurobiol. 175, 20–34 (2019).
Braga, R. M., Sharp, D. J., Leeson, C., Wise, R. J. & Leech, R. Echoes of the brain within default mode, association, and heteromodal cortices. J. Neurosci. 33, 14031–14039 (2013).
Shao, J. et al. Common and distinct changes of default mode and salience network in schizophrenia and major depression. Brain Imaging Behav. 12, 1708–1719 (2018).
Van Tol, M. J. et al. Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychol. Med. 44, 2053–2065 (2014).
Connolly, C. G. et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol. Psychiatry. 74, 898–907 (2013).
Manoliu, A. et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 7, 930 (2014).
Chan, M. Y., Park, D. C., Savalia, N. K., Petersen, S. E. & Wig, G. S. Decreased segregation of brain systems across the healthy adult lifespan. Proc.Natl. Acad. Sci. 111, E4997–E5006 (2014).
Malagurski, B., Liem, F., Oschwald, J., Mérillat, S. & Jäncke, L. Functional dedifferentiation of associative resting state networks in older adults–a longitudinal study. NeuroImage 214, 116680 (2020).
Putcha, D., Ross, R. S., Cronin-Golomb, A., Janes, A. C. & Stern, C. E. Altered intrinsic functional coupling between core neurocognitive networks in Parkinson’s disease. NeuroImage: Clin. 7, 449–455 (2015).
Bossu, P. et al. Interleukin-18 produced by peripheral blood cells is increased in Alzheimer’s disease and correlates with cognitive impairment. Brain. Behav. Immun. 22, 487–492 (2008).
Yamanishi, K. et al. Acute stress induces severe neural inflammation and overactivation of glucocorticoid signaling in interleukin-18-deficient mice. Translational Psychiatry. 12, 404 (2022).
Szabo, A. et al. Increased Circulating IL-18 levels in severe mental disorders indicate systemic inflammasome activation. Brain. Behav. Immun. 99, 299–306 (2022).
Mehta, D. et al. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression studies. Arch. Gen. Psychiatry. 68, 901–910 (2011).
Du, X. et al. Peripheral Interleukin-18 is negatively correlated with abnormal brain activity in patients with depression: a resting-state fMRI study. BMC Psychiatry. 22, 531 (2022).
Kaufman, E. & Lamster, I. B. The diagnostic applications of saliva—a review. Crit. Reviews Oral Biology Med. 13, 197–212 (2002).
Williamson, S., Munro, C., Pickler, R., Grap, M. J. & Elswick, R. Jr Comparison of biomarkers in blood and saliva in healthy adults. Nurs. Res. Pract. 246178 (2012).
Li, Y. et al. Normative dataset for plasma cytokines in healthy human adults. Data Brief. 35, 106857 (2021).
Conti, B. et al. Cultures of astrocytes and microglia express Interleukin 18. Mol. Brain Res. 67, 46–52 (1999).
Kim, T. K. et al. Local interleukin-18 system in the basolateral amygdala regulates susceptibility to chronic stress. Mol. Neurobiol. 54, 5347–5358 (2017).
Muscatell, K. A. et al. Greater amygdala activity and dorsomedial prefrontal–amygdala coupling are associated with enhanced inflammatory responses to stress. Brain. Behav. Immun. 43, 46–53 (2015).
Peng, X., Lau, W. K., Wang, C., Ning, L. & Zhang, R. Impaired left amygdala resting state functional connectivity in subthreshold depression individuals. Sci. Rep. 10, 17207 (2020).
Cullen, K. R. et al. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 71, 1138–1147 (2014).
Fresco, D. M. & Mennin, D. S. All together now: utilizing common functional change principles to unify cognitive behavioral and mindfulness-based therapies. Curr. Opin. Psychol. 28, 65–70 (2019).
Hallis, L., Cameli, L., Bekkouche, N. S. & Knäuper, B. Combining cognitive therapy with acceptance and commitment therapy for depression: a group therapy feasibility study. J. Cogn. Psychother. 31, 171–190 (2017).
Farras-Permanyer, L. et al. Age-related changes in resting-state functional connectivity in older adults. Neural Regeneration Res. 14, 1544–1555 (2019).
Edde, M., Leroux, G., Altena, E. & Chanraud, S. Functional brain connectivity changes across the human life span: from fetal development to old age. J. Neurosci. Res. 99, 236–262 (2021).
Lee, J. S. et al. Distinct brain regions in physiological and pathological brain aging. Front. Aging Neurosci. 11, 147 (2019).
Jessen, F. et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 10, 844–852 (2014).
Yang, C. et al. Montreal cognitive assessment: Seeking a single cutoff score may not be optimal. Evid.-Based Complement. Altern. Med. 9984419 (2021). (2021).
Thomann, A. E., Berres, M., Goettel, N., Steiner, L. A. & Monsch, A. U. Enhanced diagnostic accuracy for neurocognitive disorders: a revised cut-off approach for the Montreal cognitive assessment. Alzheimers Res. Ther. 12, 1–10 (2020).
Amedi, A., Hofstetter, S., Maidenbaum, S. & Heimler, B. Task selectivity as a comprehensive principle for brain organization. Trends Cogn. Sci. 21, 307–310 (2017).
Amedi, A., Raz, N., Pianka, P., Malach, R. & Zohary, E. Early ‘visual’cortex activation correlates with superior verbal memory performance in the blind. Nat. Neurosci. 6, 758–766 (2003).
Feinberg, D. A. et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PloS One. 5, e15710 (2010).
Moeller, S. et al. Multiband multislice GE-EPI at 7 Tesla, with 16‐fold acceleration using partial parallel imaging with application to high Spatial and Temporal whole‐brain fMRI. Magn. Reson. Med. 63, 1144–1153 (2010).
Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141 (2012).
Behzadi, Y., Restom, K., Liau, J. & Liu, T. T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage 37, 90–101 (2007).
Glasser, M. F. et al. The human connectome project’s neuroimaging approach. 19, 1175–1187 (2016).
Acknowledgements
We would like to thank Dr. Ofer Tur-Sinai for commenting on this paper. We would like to thank Yoad Ben-Adiva for managing the trial, and to Rotem Vekslar, and Sofia Sacal from the Institute for Brain Cognition and Technology, as well as Maya Goldberg and Gal Yogev from Remepy, for study coordination and data collection. We wish to thank Dr. Amber Maimon for helping with the literature review and with commenting on an earlier version of the introduction and discussion of the paper. Our appreciation also extends to Lior Benderski, Johnathan Amit Kanarek, and Shahar Har Nesher from Remepy for their assistance in programming and designing the app, and to Ariel Shahaf for his contributions to the development of psychological intervention protocols. We wish to thank Dr. Shai Erlich for his advice regarding the protocol. Special recognition is due to Dr. Dikla Ender-Fox and Dalit Shlayn from the Ruth and Meir Rosenthal Brain Imaging Center at Reichman University for their support with brain scanning. Finally, we would like to express our deepest gratitude to Dr. Michal Tsur and Or Shoval from Remepy for their visionary leadership and profound insights on the project and their help in executing and coordinating this complex project. This research was supported by an ERC Consolidator Grant (773121 NovelExperiSense) and by European Union’s Horizon 2020 GuestXR grant (101017884) (to A.A.).
Author information
Authors and Affiliations
Contributions
A.A. and M.C. conceived the study; M.C. formally analyzed the data; B.N.L., Y.S., and L.E., analyzed the saliva samples, M.C, B.N.L., Y.S., and A.A investigated and interpreted the data; N.S. designed the clinical intervention, M.C., and Y.S., drafted the manuscript, N.S., B.N.L., S.S., O.Z.S., R.F., and A.A., edited and revised the manuscript; A.A. contributed to the founding acquisition. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.A. is a co-founder of Remepy Health Ltd., A.A., M.C., B.N.L., and N.S. are employees of Remepy Health Ltd., S.S. is a consultant of Remepy Health Ltd. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Catalogna, M., Somerville, Y., Saporta, N. et al. Brain connectivity correlates of the impact of a digital intervention for individuals with subjective cognitive decline on depression and IL-18. Sci Rep 15, 6863 (2025). https://doi.org/10.1038/s41598-025-91457-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91457-3
Keywords
This article is cited by
-
Digital emotional regulation paradox: a cross-sectional study on mindful technology use moderates the relationship between social media emotional content exposure and psychological resilience
BMC Psychology (2025)
-
Mobile application leads to psychological improvement and correlated neuroimmune function change in subjective cognitive decline
npj Digital Medicine (2025)