Abstract
The aim of this study was to advance post-traumatic stress disorder (PTSD) understanding in older adults (48–77 years) by determining if circulating cytokines (IL-1β, IL-2, IL-4, IL-6, IL-12p70, IL17A and TNFα), brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF-A) and neuroanatomical brain volumes (grey and white matter, hippocampus, and amygdala) significantly differed in those with versus without PTSD. While none of the tested cytokines showed a significant difference, serum BDNF and VEGF-A levels were found to be significantly higher in the PTSD cohort. The assay used for BDNF quantification was important, with differences in general BDNF detected, but not when pro- and mature BDNF were measured specifically. Additionally, BDNF genotyping revealed a significant difference in Val66Met genotype distribution by PTSD diagnosis, with Val66Met carriers generally having lower circulating levels of BDNF compared to their Val66Val counterparts, regardless of PTSD diagnosis. Neuroanatomically, an all-female subset was examined to find total grey and white matter volumes and left and right hippocampal volumes were significantly smaller in those with PTSD. Collectively, these results show that both novel (VEGF-A) and established targets (BDNF and neuroimaging) may serve as useful biomarkers for older adults with PTSD.
Similar content being viewed by others
Introduction
Post-traumatic stress disorder (PTSD) affects 5–10% of people who have experienced a traumatic event, leading to a persistent range of symptoms including distressing and intrusive memories, nightmares, irritable and/or aggressive behaviour, hypervigilance, persistent negative cognitions about oneself/others, concentration difficulties and emotional withdrawal1. A current challenge in the diagnosis and monitoring of PTSD is the reliance on subjective symptoms which need to be recognised as PTSD and evaluated by an appropriately qualified health practitioner. Many PTSD symptoms are shared between with other psychiatric conditions, like major depressive disorder2 and severe anxiety, and specially trained mental health staff are not always available to formalise a diagnosis. Thus, individuals living with PTSD can go undiagnosed and untreated for prolonged periods of time. Hence, the need to better understand the biological markers of PTSD has fostered active research into many molecular, neurochemical and genetic factors associated with this condition1,2,3. Identifying reliable biomarkers that could be used as front-line pre-screening tools and objective monitoring targets could allow a much broader range of health care professionals to be part of the diagnosis and treatment strategy for PTSD. However, while several potential biomarkers have shown significant promise, the consensus across the field has been that no single biomarker is consistently altered across all cases of PTSD examined4. This implies that further research is required to determine which biomarkers are significant for which subgroups of individuals with PTSD and how they should be measured and evaluated most effectively.
Given the increasing age of global populations and the frequently delayed diagnosis of PTSD, the number of studies investigating potential biomarkers for PTSD in older adults is unexpectedly limited. The prevailing association between PTSD and a physiological alteration is the discernible dysregulation of the immune system among individuals diagnosed with PTSD5,6. This dysregulation is often detectable as a systemic, low-grade inflammation7. Overall, immune markers, such as inflammatory cytokines and C-reactive protein (CRP), have been the most studied biomarkers in the older adult PTSD age group8,9,10,11,12,13,14,15,16,17,18,19. More specifically, higher levels of interleukin (IL)−1β19, IL-611, tumour necrosis factor alpha (TNFα)12 and CRP8,10,15,17,18 have been reported in older PTSD cohorts compared to controls. However, reports finding no difference in many investigated immune markers are also common8,9,10,13,14,16,19.
Another important potential biomarker for PTSD is brain-derived neurotrophic factor (BDNF), an essential neurotrophin for learning and memory with crucial functions in neuronal survival, growth, and plasticity20. In the overall PTSD biomarker space, meta-analysis of the BDNF literature suggests generally higher levels of peripheral circulating BDNF in PTSD cohorts compared to controls21. However, there are also reports that peripheral BDNF levels remain unchanged or are lower in PTSD cohorts, including in older PTSD cohorts22. Interpretations of BDNF biomarker findings are complicated by three important factors: (1) this neurotrophin is made as both a precursor (proBDNF) and mature protein, with both forms detectable in peripheral circulation and having important, yet opposite, effects on cellular function23; (2) the majority of peripheral BDNF is stored in platelets24,25 (which is released into serum during coagulation), with only small amounts normally free in circulating plasma, suggesting that serum and plasma may represent two biologically distinct pools of BDNF26; and (3) known single nucleotide polymorphisms (SNPs) within the BDNF gene, such as the Val66Met SNP (rs6265), have generated conflicting outcomes as to whether they contribute to a genetic susceptibility to PTSD or not27,28. As such, the varied outcomes regarding the association (or lack thereof) of BDNF and PTSD likely reflects both technical and biological considerations related to the way this biomarker is investigated.
Vascular endothelial growth factor (VEGF or VEGF-A) has not traditionally been investigated as a PTSD biomarker. In addition to its well-recognised role in angiogenesis, VEGF-A is known to have important neurotrophic activities and effects on blood-brain-barrier permeability29,30,31. Investigations focusing on stress conditions have found that both BDNF and VEGF-A play important roles in the myriad of changes that contribute to stress-induced affective diseases32 and that VEGF-A is either associated with, or required for, BDNF-related antidepressant or anti-psychosis treatment responses33,34,35. Given the parallels and interplay between VEGF-A and BDNF (including platelets also maintaining a reservoir of VEGF-A36), VEGF-A merits investigation as a potential PTSD biomarker.
In addition to biomarker research in the peripheral circulation, the increased availability and impressive technical advances in magnetic resonance imaging (MRI) have allowed for structural brain differences to be investigated as reproducible markers of distinction between PTSD and non-PTSD cohorts. Analysis has suggested that both global brain structures, such as grey and white matter volumes37, and subcortical brain structures, such as hippocampal (for memory) and amygdala (for emotional fear-processing) volumes38,39,40,41, can be different between groups with and without PTSD. Nevertheless, due to the inherent alterations in brain structure that occur with advancing age42, investigating potential correlations between structural brain volumes and PTSD in older adults necessitates a targeted examination of this specific age group.
Collectively, review of the PTSD literature highlights a lack of studies focused on older PTSD cohorts. As such, the aim of this study was to profile two age- and sex-matched cohorts of older adults (48–77 years of age), with and without PTSD, to determine if circulating peripheral biomarkers (cytokines, BDNF and VEGF-A) and neuroanatomical brain volumes (grey and white mater, hippocampus, and amygdala) exhibited significant differences by PTSD diagnosis.
Results
Study cohorts
Two study cohorts, both N = 24, were assembled to represent age- and sex-matched groups of older adults with and without PTSD (Table 1). To confirm the cohorts displayed characteristics typical of PTSD/non-PTSD clinical states, self-reported measures of wellbeing were collected. As anticipated, the PTSD cohort reported significantly lower World Health Organisation Wellbeing Index (WHO-5) quality of life scores and significantly higher levels of anxiety, depression and stress (via the Depression, Anxiety, and Stress Scale (DASS)) compared to their non-PTSD counterparts (Table 1). Additional clinical information about the PTSD cohort is given in Table 1 (trauma type, co-morbid conditions and psychotropic medications used). None of these factors were found to significantly influence the mean biomarker quantifications reported within the PTSD cohort (via Mann Whitney U test).
Cytokines
To investigate potential differences in inflammatory cytokines between these older adult PTSD and non-PTSD cohorts, interleukin (IL)−1β, IL-2, IL-4, IL-6, IL-12p70, IL17A and tumour necrosis factor alpha (TNFα) were quantified from plasma. It was recognized that blood samples were collected at different times between the study cohorts (PTSD cohort bloods were generally collected at midday as non-fasting while the non-PTSD cohort bloods were collected in the early morning as fasting), with the potential to influence cytokines sensitive to circadian rhythms43. As such, Pittsburgh Sleep Quality Index (PSQI) scores were examined in both cohorts (Table 1). Both cohorts reported sleep scores indicative of clinically significant sleep disturbances (average score above 5), suggesting possible chronodisruption43,44,45. Given this matching possible circadian disruption, comparison of cytokine levels between the cohorts proceeded.
Quantification of the cytokine panel determined that a significant number of study samples fell below the lower limit of quantification (LLOQ) for several targets (Table 2). As such, results were analysed as both a binary measure (detectable/not detectable) and as a quantification (continuous) where undetectable samples were assigned a value of half the LLOQ for that target. Regardless of analysis approach, none of the tested immune markers showed a significant difference by PTSD cohort (Table 2).
BDNF
To understand the complex nature of BDNF in peripheral circulation, BDNF was quantified from both plasma and serum using three assays with different target specificities and technologies (BDNF detection via xMAP technology, mature BDNF detection via enzyme-linked immunosorbent assay (ELISA) and proBDNF detection via ELISA). By PTSD diagnosis, only BDNF from serum measured via xMAP technology detected a significant difference between PTSD and non-PTSD cohorts (Table 2; Fig. 1A-C).
Violin plots depicting BDNF levels in plasma and serum between PTSD and non-PTSD cohorts measured as (a) BDNF (via xMAP multiplex technology), (b) Mature BDNF (via ELISA technology), (c) ProBDNF (via ELISA technology) and (d) Mature BDNF and ProBDNF added together (via ELISA technology) (Mann Whitney U test). Coloured circles represent individual sample concentrations (the coloured triangle in non-PTSD plasma plots identifies a participant with an outlier proBDNF value in each test set); Unfilled circles represent samples below the assay detection limit which were assigned values of half the lower limit of quantification (LLOQ) for analysis; Solid faded circles represent samples with concentrations estimated from the analysis software (detectable but below the LLOQ); Line represents the sample set mean; White square represents the sample set median; NS = not significant (p > 0.05). (e) Spearman’s rank correlations of BDNF measurements between testing methods in plasma and serum. Correlations with significance of p ≤ 0.002 are shown in white text with red asterisk; all other non-significant correlations are shown in black text; rs(45) in plasma, rs(46) in serum.
To gain an understanding of the relationship between what forms of BDNF were detected between the test assays, quantified BDNF amounts were compared between tests. In both plasma and serum, mature BDNF values significantly correlated with BDNF values while proBDNF values showed no relationship with either mature BDNF or BDNF values (Fig. 1E). In addition, it was observed that one participant in the non-PTSD cohort had an unusually high proBDNF level in plasma (while having an average mature BDNF level) and that the unusually high level was reproduced in the BDNF assay (Fig. 1A-C, blue triangle marker). This observation suggested that the BDNF assay was measuring both proBDNF and mature BDNF. However, when ELISA assay results from proBDNF and mature BDNF were added together and evaluated by PTSD diagnosis, the significant difference observed in the BDNF xMAP results were not replicated (Fig. 1D). Despite this, a significant correlation between proBDNF + mature BDNF values and BDNF values was detected, with correlation coefficients of 0.499 and 0.429 in plasma and serum, respectively (Fig. 1E). Taken together, this data suggests that the BDNF xMAP assay was able to detect both proBDNF and mature BDNF, but in a way that does not directly equal the sum of measuring each form of BDNF separately by ELISA.
To investigate the contribution of BDNF platelet reserves to the BDNF levels detected in serum compared to plasma, the change in overall quantifiable levels of BDNF was compared between our sample types within each assay. When all the plasma samples were compared to all the serum samples (regardless of PTSD diagnosis), BDNF and mature BDNF showed significantly higher detectable levels in serum over plasma (t(93) = −7.440, p < 0.001 and t(93) = −11.202, p < 0.001, respectively), while matched proBDNF levels revealed no difference by sample type (t(93) = 1.059, p = 0.292) (able to be visualised by comparing the entire serum panel to the entire plasma panels in Fig. 1A, B and C).
To investigate the potential influence of the Val66Met BDNF polymorphism on PTSD diagnosis and BDNF levels, the genotype status of the rs6265 SNP was determined within these cohorts and used to further examine circulating BDNF levels. In agreement with previous reports, a significant difference in genotype distribution between PTSD and non-PTSD cohorts was detected (Fisher-Freeman-Halton exact test = 7.284, p = 0.015), with Val66Val more prevalent in the non-PTSD cohort and Val66Met and Met66Met genotypes more prevalent in the PTSD cohort (Fig. 2A). Separating circulating levels of BDNF, mature BDNF and proBDNF by both genotype and PTSD diagnosis revealed the overall trend for target BDNF levels to be lower in the Val66Met subgroup versus the Val66Val subgroup within both PTSD and non-PTSD cohorts (Fig. 2B-D).
PTSD and non-PTSD cohorts by BDNF Val66Met (rs6265) polymorphism. (a) Genotype distribution by PTSD diagnosis (Fisher-Freeman-Halton Exact test = 7.284, p = 0.015). Violin plots of plasma and serum levels of (b) BDNF (xMAP), (c) Mature BDNF (ELISA) and (d) ProBDNF (ELISA) by both PTSD diagnosis and genotype. Overall group differences were determined using the Kruskal-Wallis H test. Pairwise differences, when significant after Bonferroni correction for multiple tests, are indicated within the graphs by a bar and p-value. Coloured circles represent individual sample concentrations; Unfilled circles represent samples below the assay detection limit which were assigned values of half the lower limit of quantification (LLOQ) for analysis; Solid faded circles represent samples with concentrations estimated from the analysis software (detectable but below the LLOQ); Line represents the sample set mean; White square represents the sample set median; NS = not significant (p > 0.05), *indicates p values still significant after Bonferroni multi-comparison correction (p ≤ 0.013).
VEGF-A
Quantification of VEGF-A circulating levels using xMAP technology resulted in similar results to BDNF levels with the same technology; significantly higher VEGF-A levels were detected in serum from the PTSD cohort compared to the non-PTSD cohort (Table 2; Fig. 3).
Violin plots depicting VEGF-A levels in plasma and serum between PTSD and non-PTSD cohorts measured via xMAP multiplex technology (Mann Whitney U test). Coloured circles represent individual sample concentrations; Line represents the sample set mean; White square represents the sample set median; NS = not significant (p > 0.05).
Neuroanatomical volumes
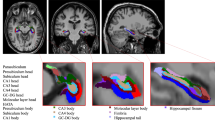
An all-female subset of age-matched participants between the study cohorts (n = 9 per group; PTSD group: age 57.2 ± 4.4 years, range 50–65 years old; non-PTSD group: 57.2 ± 5.2 years, range 49–66 years old) also underwent MRI brain scans, allowing for gross anatomical structures (total grey and white matter volumes) and subcortical segmentations (left and right hippocampus and left and right amygdala volumes) to be evaluated. Two-tailed independent-sample t-tests were performed for each of the structures comparing regional volumes, with results summarised in Table 2. After applying a Bonferroni correction (corrected alpha < 0.0083), total white matter, total grey matter and hippocampal volumes (both left and right) remained significantly different between the cohorts, such that older women with PTSD had smaller volumes compared to their healthy-matched non-PTSD controls (Fig. 4). Amygdala volumes (both left and right) did not show a significant difference by PTSD diagnosis, but the mean trend was also for this brain region to be smaller in the women with PTSD (Fig. 4).
Neuroanatomical volumes by PTSD diagnosis. (a) Overview diagram depicting targeted regions: total white matter (light grey), total grey matter (dark grey), hippocampus (green) and amygdala (red). Violin plots depicting (b) total white matter, (c) total grey matter, (d) hippocampus (left and right) and (e) amygdala (left and right) volumes by PTSD cohort (Independent sample t-test). Circles represent individuals; Line represents the sample set mean; White square represents the sample set median; NS = not significant (p > 0.05).
Discussion
The discovery of clinically informative biomarkers able to support PTSD diagnosis and monitoring would provide tremendous impact for both medical practitioners and their patients. This impact could be particularly meaningful as PTSD can be a lifetime condition and our global populations age. Unfortunately, many potential biomarkers investigated to date have important biological and technical considerations that need to be evaluated before reproducible outcomes can be achieved. This is in addition to the natural population diversity of people living with PTSD. To continue addressing these challenges, this study has examined a selection of established and novel PTSD biomarkers (immune, neurotrophic/vascular and structural) within a focused subgroup of older adults living with PTSD.
In this older adult PTSD/non-PTSD cohort comparison, no significant differences were detected in the collection of cytokine targets tested (IL-1β, IL-2, IL-4, IL-6, IL-12p70, IL17A and TNFα; Table 2). Caution in the interpretation of these results is necessary, as blood collection was not identically timed between cohorts (introducing possible circadian rhythm variations) and both cohorts reported sleep disturbances that could have led to further chronodisruption (Table 1). Cytokine levels are known to be affected by the circadian system and both traumatic stress and ageing have been proven to contribute to circadian distruption43,44,45. As such, consideration of circadian influences that could not be completed controlled for is necessary in the interpretation of these non-significant findings. In this sample set, all the targeted cytokine levels were also low (close to the LLOQ or below detection), which was expected based on previous reports of these targets in plasma from PTSD and non-PTSD individuals46,47. Plasma was specifically chosen for this analysis based on reported evidence that this panel of cytokines showed better stability in plasma over serum48. Additionally, both preliminary study testing (not reported) and published comparisons revealed no significant difference in these cytokines between plasma and serum48,49. So, while some of these inflammatory markers have shown promise as PTSD biomarkers for this age group in other studies11,12,19, this study was unable to support those claims.
Analysis of BDNF as a potential PTSD biomarker in older adults involved multiple comparisons to account for several biological and technical challenges inherent to this neurotrophin. The first set of comparisons analysed BDNF across three different assessments: BDNF by xMAP technology, mature BDNF by ELISA and proBDNF by ELISA. Overall, the only test condition that detected a significant difference between the PTSD and non-PTSD cohorts was the BDNF measurement by xMAP in serum (Fig. 1). Individual participant test results between the three assays suggested that the BDNF assay may represent a total BDNF measurement, as it appeared to detect both proBDNF and mature BDNF (Fig. 1). However, while both BDNF vs. mature BDNF and BDNF vs. proBDNF + mature BDNF results significantly correlated, neither ELISA assay separately nor as a sum together were able to recreate the significant difference observed by the xMAP measurement (Fig. 1). Given the differences in proprietary antibodies used to detect BDNF in each test, the difference in liquid phase (xMAP) versus solid phase (ELISA) of the antibody-BDNF interaction within the assays and the different chemistries used for signal detection (fluorescence for xMAP, colorimetric for ELISA), it was not completely unexpected that each assay showed a unique specificity for BDNF. This difference in BDNF assay specificities has been recognised in the general BDNF literature previously50 and has resulted in calls for greater awareness in regards to reporting BDNF results to account for this difference in assay outcomes23.
Another observation related to the levels of proBDNF/mature BDNF in plasma versus serum. It has been established that the majority of circulating BDNF is stored in platelets and this pool is released into serum during the coagulation process24,26. Interestingly, both proBDNF and mature BDNF have been detected in platelets, but only mature BDNF is reported to be released during platelet activation25. Our assay results support this narrative. Significantly higher BDNF levels were observed in serum over plasma when mature forms of BDNF were assayed for (by the BDNF and mature BDNF assays) while no level changes were seen when only proBDNF was assayed for (by the proBDNF assay) (Fig. 1). This suggests that the significant difference observed in the serum of PTSD participants is being driven specifically by the mature BDNF pools stored in circulating platelets.
Adding a genetic layer to our understanding of circulating BDNF levels, re-evaluating BDNF protein levels by individual Val66Met genotype (which results in either a Val or Met amino acid in position 66 of the pro-domain portion of proBDNF) found that Val66Met individuals trended towards lower levels of circulating BDNF compared to Val66Val individuals within the same cohorts, regardless of PTSD diagnosis. This suggested that some difference in BDNF transcription, translation or protein stability (perhaps related to the known structural change induced by this SNP51) led to lower levels of BDNF circulating in Val66Met individuals versus Val66Val individuals. Whether having less circulating BDNF at the time of trauma contributes to the risk of developing of PTSD, which then leads to an overall more BDNF in circulation (regardless of genotype) during clinically diagnosed PTSD, is an interesting question for future targeted study.
The observation that peripheral BDNF serum levels were significantly higher in individuals diagnosed with PTSD concurs with meta-analysis of BDNF results in the PTSD literature21. These findings may reflect a compensatory mechanism for other alterations that occur during PTSD52 or an over-consolidation of traumatic memories around the time of the trauma53. BDNF has the ability to cross the blood-brain barrier54 and circulating BDNF levels do appear to reflect brain-tissue BDNF levels55. As such, as technical and biological issues are addressed, peripheral BDNF continues to emerge as a strong PTSD candidate biomarker under the right conditions.
Another aim of this study was to characterise peripheral levels of VEGF-A to evaluate its potential as a PTSD biomarker. VEGF-A is emerging as a necessary partner to BDNF in both the development of stress-related disorders and in their treatment32,33,34,35. Despite this, VEGF-A has not previously been investigated as a potential PTSD biomarker. In this study, circulating VEGF-A levels were significantly higher in the serum of the PTSD cohort (Fig. 3). These results paralleled the BDNF findings, also suggesting that the VEGF-A reserves stored in platelets and released during clotting36 is the biological pool that is altered during PTSD. The possible effects of this alteration are not yet known, but these findings suggest that further research in VEGF-A in the context of PTSD is warranted.
Finally, large MRI studies and meta-analyses of many smaller MRI studies indicate brain volume differences at the overall and subregion level have potential to be useful PTSD biomarkers38,39,41. In particular, the important roles of the hippocampus and amygdala in learning, memory and stress regulation have made these regions of particular interest in the PTSD research field. While the neuroanatomical analysis undertaken in this study included only a subset of female older adults with and without PTSD, the results obtained concur with the emerging consensus that smaller brain volumes are observed in PTSD (Fig. 4)37,41. In this study’s comparison, total white matter, total grey matter and both the left and right hippocampus showed significantly smaller volumes in women with PTSD. While it has been postulated that these reduced volumes (particularly in total grey matter and hippocampal regions) could be due to stress exposure41,56, it is generally agreed that more work is needed to understand the underlying causation for these changes. Datasets from groups like the ENIGMA-PGC PTSD consortium also offer an opportunity to extend analysis of older adults with specifically targeted analyses. While neuroimaging researchers continue to expand our understanding of PTSD’s impact on the brain (with investigations into functional changes57 and neural anatomical network alternations58), it is hoped that studies such as this one, which investigated patterns between neuroanatomy, circulating biochemistry and genetic markers in the same patient population, can begin to address the complex interplay between brain, body and mind in PTSD.
This study had limitations that should be taken into consideration when interpreting the outcomes. These included having small cohort sizes (particularly in the imaging group, as only a subset of participants (all female by chance) chose and were able to undergo MRI), different inclusion/exclusion criteria for the PTSD/non-PTSD cohorts, a four-hour processing window from blood collection to storage, different timing schedules for blood collection between cohorts (known to impact some biomarkers sensitive to circadian variation - particularly cytokines), potential confounding personal characteristics which may have affected blood biomarkers, possible biases resulting from recruiting individuals enrolled in clinical trials and the possibility of unreported trauma exposure in the control group. Despite these limitations, statistically significant differences were still detectable between the study cohorts, even after correcting for multiple comparisons, and the findings reported are consistent with previous studies. As such, this study contributes valuable insights into the potential PTSD biomarkers investigated and the potential for future personalised medicine approaches.
Overall, this study has identified that both circulating biomarkers (BDNF and VEGF-A) and structural volumes (of the grey/white matter and hippocampus) have potential as biomarker targets for monitoring PTSD in older individuals. Future research necessitates the analysis of larger and more diverse participant cohorts in order to gain greater understanding of the variability within these targets. PTSD is a diverse, complex condition and many older adults have been living with this condition for extended periods of time. By taking this subgroup-specific, multi-target approach to biomarker investigation, the long-term aim is to recognise relevant clinical subgroups with the PTSD population and increase the biologically informative power of these markers to help facilitate practical outcomes for patients.
Methods
Participants
The present study is a subset analysis of participants enrolled in clinical trials at the Thompson Institute (University of the Sunshine Coast (UniSC), Queensland, Australia) to examine oral ketamine as an augmentation treatment for PTSD (PTSD cohort) or assess a lifestyle intervention for healthy ageing (non-PTSD cohort). All experimental protocols were approved by the Prince Charles Hospital human research ethics committee and the UniSC ethics committee, all methods were carried out in accordance with relevant guidelines and regulations and informed consent was obtained from all participants.
PTSD participant group
Older adults were selected from a participant pool referred by their general practitioner (GP) for participation in either the Oral Ketamine Trial on Post-Traumatic Stress Disorder (OKTOP; Australian New Zealand Clinical Trials Registry (ANZCTR) ID ACTRN12618001965291 (registered 05/12/2018); Prince Charles Hospital HREC Approval: HREC/18/QPCH/288, UniSC Ethics Approval: A181190) or the Transcranial Magnetic Stimulation and Oral Ketamine Combination Treatment for Post Traumatic Stress Disorder (TMS-OK PTSD; ANZCTR ID ACTRN12621000342819; Bellberry Ltd. HREC Approval: 2020-07-653, UniSC Ethics Approval: A211572) to comprise the PTSD cohort. Genetic analysis and reporting are registered under the Genetic Biomarkers of Ketamine on PTSD project (GBOK; Prince Charles Hospital HREC Approval: HREC/18/QPCH/288, UniSC Ethics Approval: S211655). GPs also provided a medical history including diagnosed co-morbidities and medication use for their patients. Individuals’ baseline assessments were included in this analysis (taken prior to anyone receiving treatment), which included blood collection (n = 24, 9 males, 15 females) and MRI scans (n = 9, all females). Blood collection timing and fasting state was not controlled for in this cohort (though most collections occurred around midday). Clinical and demographic information for this cohort is given in Table 1. Eligibility for both oral ketamine trials included males and females over 18 years of age with a current diagnosis of PTSD as assessed using the Clinician Administered PTSD Scale for DSM-5 (CAPS-5)59 with the ability to provide written informed consent and tolerate the clinical trial treatment and monitoring protocol. Exclusion criteria included the presence of psychosis, mania/hypomania, acute suicidality requiring urgent psychiatric intervention, current or history of substance abuse and/or ketamine use disorder, uncontrolled/severe symptomatic cardiovascular disease states (e.g. myocardial infarction within prior 6 months/history of stroke/hypertension (resting blood pressure > 150/100)), body weight of > 150 kg, history of intracranial mass/intracranial haemorrhage or stroke/cerebral trauma or traumatic brain injury/increased intracranial pressure (as assessed by referring general practitioner), liver function test (LFT) results out of normal range, females who were pregnant/currently breastfeeding/planning a pregnancy during the trial, previous adverse reaction to ketamine, presence of MRI contraindications (pacemakers, etc.) or participation in any other clinical intervention trial during study period. Of note, ethics approval for genotyping studies was only available within the OKTOP trial, reducing the PTSD cohort size to n = 17 for genotyping analysis.
Control participant group
Age- and sex-matched individuals were selected as controls from healthy community members who volunteered for the Lifestyle Intervention Study for Dementia Risk Reduction (LEISURE)60 (ANZCTR ID ACTRN12620000054910 (registered 23/01/2020), UniSC Ethics Approval: A191301) to comprise the non-PTSD cohort. Individuals’ baseline assessments were included in this analysis (prior to anyone starting the trial protocol), which including blood collection (n = 24, 8 males, 16 females) and MRI scans (n = 9, all females). Fasting blood was collected from this cohort between 8 and 9 am. Clinical and demographic information for this cohort is given in Table 1. Eligibility for participation in the study included Montreal Cognitive Assessment (MoCA) score of < 26, normal or corrected-to-normal visual acuity and aged between 50 and 85 years. Exclusion criteria for this trial included current or history of the following: prior head injury with loss of consciousness > 60 min, stroke or transient ischemic attack, atherosclerotic cardiovascular disease, myocardial infarction, pulmonary respiratory conditions (e.g. COPD), metabolic disorders (e.g. diabetes type 1 or 2/kidney disease/liver disease), history of schizophrenia or bipolar disorder, major neurological condition (e.g. Parkinson’s disease/multiple sclerosis), epilepsy, current alcohol or other substance misuse, intellectual disability, acute psychosis, insufficient English language skills to complete standardised assessment, test results that indicated study participation was unsafe, participation in conflicting studies, presence of MRI contraindications (pacemakers, etc.) and current usage of medications known to affect central nervous system (e.g. antidepressant medications). Additionally, no history or current diagnosis of PTSD (or any other mental health condition) was confirmed by clinician interview during the pre-screening of this group.
Assessment of clinical characteristics
For the PTSD cohort, PTSD diagnosis was reconfirmed by the study consultant psychiatrist using the CAPS-5 to ensure all participants met the minimum threshold score of ≥ 40 and criterion A-G. Additionally, all study participants self-reported the WHO-561 and the DASS62. A summary of participant clinical characteristics are presented in Table 1.
Sample collection
Blood samples were collected by a certified phlebotomist for both serum and plasma using serum separator tubes (SST) and K2EDTA collection tubes, respectively. After a minimum 30 min at room temperature (to allow for serum clotting), an aliquot of K2EDTA whole blood was removed and stored at −80 °C before all tubes were centrifuged at 2465 x g for 15 min at 4 °C. All samples were processed within four hours of collection. Serum and plasma were separated, aliquoted and stored at −80 °C.
Protein quantification
Circulating peripheral biomarkers were quantified from serum and/or plasma by xMAP microsphere-based assay (Luminex) or ELISA. Before analysis, all samples were thawed on ice and centrifuged at 10,000 x g for 10 min at 4 °C to remove precipitates.
Standard ProcartaPlex simplex assays (ThermoFisher Scientific, Australia) were combined for the detection of BDNF (EPX01A-12116-901, lower limit of quantification (LLOQ) of 2.03 pg/ml) and VEGF-A (EPX01A-10277-901, LLOQ of 4.88 pg/ml). The high-sensitivity 9-plex Human ProcartaPlex panel (EPXS090-12199-901) was used to obtain quantifications for IL-1β (LLOQ of 0.28 pg/ml), IL-2 (LLOQ of 0.88 pg/ml), IL-4 (LLOQ of 1.29 pg/ml), IL-6 (LLOQ of 1.29 pg/ml), IL-12p70 (LLOQ of 0.77 pg/ml), IL-17 A (LLOQ of 0.30 pg/ml) and TNFα (LLOQ of 0.62 pg/ml). This panel also included interferon gamma (IFN-γ) (LLOQ of 1.26 pg/ml) and IL-10 (LLOQ of 0.17 pg/ml), however, 68% and 91% of samples tested fell below the minimum quantification limits for these targets, respectively. As such, these targets were removed from analysis. For the remaining targets, samples that registered below the assay detection limit were assigned a value of half the LLOQ for that target for statistical analysis. Assay were conducted according to the manufacturer’s instruction, read on a Luminex 200 instrument (ThermoFisher) and analysed using the ProcartaPlex Analysis App (ThermoFisher).
Quantifications of mature BDNF and proBDNF separately were performed using the Mature BDNF/proBDNF Combo ELISA assay kit (BEK-2241, Biosensis, Australia). As per the manufacturer’s recommendations, samples were diluted prior to testing (plasma diluted a minimum of 1:20 for both assays, serum diluted a minimum of 1:10 for proBDNF and 1:50 for mature BDNF). This resulted in LLOQs for proBDNF (after accounting for the minimum dilution required) of 0.312 ng/ml in both plasma and serum and LLOQs for mature BDNF of 0.156 ng/ml in plasma and 0.390 pg/ml in serum. Assays were performed according to the manufacturer’s instruction (Biosensis) and analysed using GainData software (Arigo Biolaboratories, https://www.arigobio.com/elisa-analysis). The manufacturer’s information reports the proBDNF assay to be 100% reactive with proBDNF and have no cross-reactivity with mature BDNF while the mature BDNF assay is reported to be 100% reactive with mature BDNF and claims an ~ 5.3% cross-reactivity with proBDNF (Biosensis). By comparison, the ProcartaPlex BDNF assay makes no claims about pro- vs. mature BDNF detection.
BDNF Val66Met genotyping
Genomic DNA was extracted from whole blood using the DNeasy Blood and Tissue kit (Qiagen, Australia) as per the manufacturer’s instructions. Targeted amplification of the genomic region encompassing the Val66Met BDNF polymorphism (rs6265) was performed using 0.5 µM of primers 5’- TGGCCTTTTGATACAGGGACC − 3’ and 5’- GTCTGGTGCAGCTGGAGTTT − 3’ with Platinum SuperFi II Master Mix (Invitrogen, ThermoFisher Scientific) with the manufacturer’s recommended amplification conditions. Products were visualized on a 1% agarose gel before both strands were Sanger sequenced (Macrogen, Korea). The resulting sequences were assembled using Geneious Prime 2023.2 software and manually inspected for the nucleotide identities at the Val66Met position.
Magnetic resonance imaging and analysis
All MRI brain scans were performed at the Nola Thompson Centre for Advanced Imaging (Thompson Institute, UniSC) using a 3T Siemens Skyra (Erlangen, Germany) and a 64-channel head and neck coil. The MRI protocols across studies contained identical anatomical scan parameters: T1-weighted magnetization prepared rapid acquisition gradient echo sequence (MPRAGE: TR = 2200 ms, TE = 1.71 ms, TI = 850 ms, flip angle = 7°, spatial resolution = 1mm3, FOV = 208 × 256 × 256, TA = 3:57). All participants’ T1-weighted scans were visually inspected for image quality, assessing image contrast, field homogeneity, head motion, image artefacts and field of view. No scans were removed due to poor data quality. Structural segmentations were conducted using FMRIB’s Software Library (FSL)63. As part of the FSL anat structural pipeline, whole-brain tissue-classes quantifications were generated with FMRIB’s Automated Segmentation Tool (FAST64). Subcortical segmentation of the amygdala and hippocampus volumes per hemisphere were calculated using FMRIB’s Integrated and Segmentation Tool (FIRST65). Native-space brain extracted images were transformed to standard space (MNI152, 1 mm3) via a skull constrained paired registration approach performed using FMRIB’s Linear Image Registration Tool (FLIRT66). Head-size correction was performed using a proportional method based on the determinant of the registration matrix, generated from the paired registration. All anatomical segmentations and image registrations were manually inspected for accuracy, with all data retained for the subsequent analyses. Six regions of interest were investigated, including whole-brain grey matter, whole-brain white matter, amygdala and hippocampus left and right hemisphere.
Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics software, version 28.0.1.0 (142). Mann Whitney U tests, Kruskal-Wallis H tests, Spearman’s rank correlations and Fisher exact tests were applied as appropriate. To correct for multiple comparisons, Bonferroni correction was applied resulting in a corrected alpha for serum analyses (four targets) of p ≤ 0.013, for plasma analyses (11 targets) of p ≤ 0.005, and for neuroanatomical volume analyses (six targets) of p≤ 0.013. Violin plots were generated in R with ggplots267 and figures were formatted for publication in Inkscape v1.2.1.
Data availability
All molecular biology data generated or analysed during this study are included in this published article (and its Supplementary Information files). Neuroimaging data is available from the corresponding author on reasonable request.
References
Yehuda, R. et al. Post-traumatic stress disorder. Nat. Rev. Dis. Prim. 1, 15057. https://doi.org/10.1038/nrdp.2015.57 (2015).
Almeida, F. B., Barros, H. M. T. & Pinna, G. Neurosteroids and neurotrophic factors: what is their promise as biomarkers for major depression and PTSD? Int. J. Mol. Sci. 22. https://doi.org/10.3390/ijms22041758 (2021).
Aspesi, D. & Pinna, G. Could a blood test for PTSD and depression be on the horizon? Expert Rev. Proteom. 15, 983–1006. https://doi.org/10.1080/14789450.2018.1544894 (2018).
Michopoulos, V., Norrholm, S. D. & Jovanovic, T. Diagnostic biomarkers for posttraumatic stress disorder: promising horizons from translational neuroscience research. Biol. Psychiatry 78, 344–353. https://doi.org/10.1016/j.biopsych.2015.01.005 (2015).
Hori, H. & Kim, Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 73, 143–153. https://doi.org/10.1111/pcn.12820 (2019).
Baker, D. G., Nievergelt, C. M. & O’Connor, D. T. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology 62, 663–673. https://doi.org/10.1016/j.neuropharm.2011.02.027 (2012).
Speer, K., Upton, D., Semple, S. & McKune, A. Systemic low-grade inflammation in post-traumatic stress disorder: a systematic review. J. Inflamm. Res. 11, 111–121. https://doi.org/10.2147/JIR.S155903 (2018).
Eswarappa, M., Neylan, T. C., Whooley, M. A., Metzler, T. J. & Cohen, B. E. Inflammation as a predictor of disease course in posttraumatic stress disorder and depression: A prospective analysis from the Mind your heart study. Brain Behav. Immun. 75, 220–227. https://doi.org/10.1016/j.bbi.2018.10.012 (2019).
Newton, T. L., Fernandez-Botran, R., Miller, J. J. & Burns, V. E. Interleukin-6 and soluble interleukin-6 receptor levels in posttraumatic stress disorder: associations with lifetime diagnostic status and psychological context. Biol. Psychol. 99, 150–159. https://doi.org/10.1016/j.biopsycho.2014.03.009 (2014).
Plantinga, L. et al. Association between posttraumatic stress disorder and inflammation: a twin study. Brain Behav. Immun. 30, 125–132. https://doi.org/10.1016/j.bbi.2013.01.081 (2013).
von Kanel, R. et al. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation 17, 39–46. https://doi.org/10.1159/000243084 (2010).
Bruenig, D. et al. Genetic and serum biomarker evidence for a relationship between TNFalpha and PTSD in Vietnam war combat veterans. Compr. Psychiatry 74, 125–133. https://doi.org/10.1016/j.comppsych.2017.01.015 (2017).
Lima, B. B. et al. Posttraumatic stress disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain Behav. Immun. 75, 26–33. https://doi.org/10.1016/j.bbi.2018.08.015 (2019).
Young, M. D. Investigation of C-reactive protein and AIM2 methylation as a marker for PTSD in Australian Vietnam veterans. Gene 803, 145898. https://doi.org/10.1016/j.gene.2021.145898 (2021).
Miller, K., Driscoll, D., Smith, L. M. & Ramaswamy, S. The role of inflammation in late-life post-traumatic stress disorder. Mil Med. 182, e1815–e1818. https://doi.org/10.7205/MILMED-D-17-00073 (2017).
O’Donovan, A. et al. Current posttraumatic stress disorder and exaggerated threat sensitivity associated with elevated inflammation in the mind your heart study. Brain Behav. Immun. 60, 198–205. https://doi.org/10.1016/j.bbi.2016.10.014 (2017).
Powers, A. et al. The differential effects of PTSD, MDD, and dissociation on CRP in trauma-exposed women. Compr. Psychiatry 93, 33–40. https://doi.org/10.1016/j.comppsych.2019.06.007 (2019).
Spitzer, C. et al. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J. Psychiatr Res. 44, 15–21. https://doi.org/10.1016/j.jpsychires.2009.06.002 (2010).
Wang, W. et al. Characteristics of pro- and anti-inflammatory cytokines alteration in PTSD patients exposed to a deadly earthquake. J. Affect. Disord. 248, 52–58. https://doi.org/10.1016/j.jad.2019.01.029 (2019).
Bathina, S. & Das, U. N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 11, 1164–1178. https://doi.org/10.5114/aoms.2015.56342 (2015).
Mojtabavi, H., Saghazadeh, A., van den Heuvel, L., Bucker, J. & Rezaei, N. Peripheral blood levels of brain-derived neurotrophic factor in patients with post-traumatic stress disorder (PTSD): A systematic review and meta-analysis. PLoS One. 15, e0241928. https://doi.org/10.1371/journal.pone.0241928 (2020).
Domitrovic Spudic, S. et al. Reduced plasma BDNF concentration and cognitive decline in veterans with PTSD. Psychiatry Res. 316, 114772. https://doi.org/10.1016/j.psychres.2022.114772 (2022).
Miranda, M., Morici, J. F., Zanoni, M. B. & Bekinschtein, P. Brain-Derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 13, 363. https://doi.org/10.3389/fncel.2019.00363 (2019).
Fujimura, H. et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 87, 728–734 (2002).
Le Blanc, J. et al. Platelets selectively regulate the release of BDNF, but not that of its precursor protein, ProBDNF. Front. Immunol. 11, 575607. https://doi.org/10.3389/fimmu.2020.575607 (2020).
Gejl, A. K. et al. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci. Rep. 9, 9655. https://doi.org/10.1038/s41598-019-45976-5 (2019).
Bruenig, D. et al. A case-control study and meta-analysis reveal BDNF Val66Met is a possible risk factor for PTSD. Neural Plast. 2016, 6979435. https://doi.org/10.1155/2016/6979435 (2016).
Wang, T. Does BDNF Val66Met polymorphism confer risk for posttraumatic stress disorder? Neuropsychobiology 71, 149–153. https://doi.org/10.1159/000381352 (2015).
Storkebaum, E., Lambrechts, D. & Carmeliet, P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays 26, 943–954. https://doi.org/10.1002/bies.20092 (2004).
Sondell, M., Lundborg, G. & Kanje, M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J. Neurosci. 19, 5731–5740. https://doi.org/10.1523/JNEUROSCI.19-14-05731.1999 (1999).
Davis, B. et al. Role of vasodilator stimulated phosphoprotein in VEGF induced blood-brain barrier permeability in endothelial cell monolayers. Int. J. Dev. Neurosci. 28, 423–428. https://doi.org/10.1016/j.ijdevneu.2010.06.010 (2010).
Nowacka, M. & Obuchowicz, E. BDNF and VEGF in the pathogenesis of stress-induced affective diseases: an insight from experimental studies. Pharmacol. Rep. 65, 535–546. https://doi.org/10.1016/s1734-1140(13)71031-4 (2013).
Kao, C. F. et al. Gene-based analysis of genes related to neurotrophic pathway suggests association of BDNF and VEGFA with antidepressant treatment-response in depressed patients. Sci. Rep. 8, 6983. https://doi.org/10.1038/s41598-018-25529-y (2018).
Deyama, S., Bang, E., Kato, T., Li, X. Y. & Duman, R. S. Neurotrophic and antidepressant actions of brain-derived neurotrophic factor require vascular endothelial growth factor. Biol. Psychiatry 86, 143–152. https://doi.org/10.1016/j.biopsych.2018.12.014 (2019).
Murphy, B. P. et al. Vascular endothelial growth factor and brain-derived neurotrophic factor in quetiapine treated first-episode psychosis. Schizophr Res. Treat. 2014, 719395. https://doi.org/10.1155/2014/719395 (2014).
Webb, N. J., Bottomley, M. J., Watson, C. J. & Brenchley, P. E. Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin. Sci. 94, 395–404. https://doi.org/10.1042/cs0940395 (1998).
Siehl, S. et al. Structural white and gray matter differences in a large sample of patients with posttraumatic stress disorder and a healthy and trauma-exposed control group: diffusion tensor imaging and region-based morphometry. Neuroimage Clin. 28, 102424. https://doi.org/10.1016/j.nicl.2020.102424 (2020).
Karl, A. et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci. Biobehav. Rev. 30, 1004–1031. https://doi.org/10.1016/j.neubiorev.2006.03.004 (2006).
Bromis, K., Calem, M., Reinders, A., Williams, S. C. R. & Kempton, M. J. Meta-analysis of 89 structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. Am. J. Psychiatry 175, 989–998. https://doi.org/10.1176/appi.ajp.2018.17111199 (2018).
Bremner, J. D. Traumatic stress: effects on the brain. Dialogues Clin. Neurosci. 8, 445–461. https://doi.org/10.31887/DCNS.2006.8.4/jbremner (2006).
Logue, M. W. et al. Smaller hippocampal volume in posttraumatic stress disorder: A multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol. Psychiatry. 83, 244–253. https://doi.org/10.1016/j.biopsych.2017.09.006 (2018).
Fjell, A. M. & Walhovd, K. B. Structural brain changes in aging: courses, causes and cognitive consequences. Rev. Neurosci. 21, 187–221. https://doi.org/10.1515/revneuro.2010.21.3.187 (2010).
Comas, M. et al. A circadian based inflammatory response – implications for respiratory disease and treatment. Sleep. Sci. Pract. 1. https://doi.org/10.1186/s41606-017-0019-2 (2017).
Agorastos, A. & Olff, M. Traumatic stress and the circadian system: neurobiology, timing and treatment of posttraumatic chronodisruption. Eur. J. Psychotraumatol. 11, 1833644. https://doi.org/10.1080/20008198.2020.1833644 (2020).
Verma, A. K., Singh, S. & Rizvi, S. I. Aging, circadian disruption and neurodegeneration: interesting interplay. Exp. Gerontol. 172, 112076. https://doi.org/10.1016/j.exger.2022.112076 (2023).
von Kanel, R. et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J. Psychiatr Res. 41, 744–752. https://doi.org/10.1016/j.jpsychires.2006.06.009 (2007).
Dalgard, C. et al. The MCP-4/MCP-1 ratio in plasma is a candidate circadian biomarker for chronic post-traumatic stress disorder. Transl Psychiatry. 7, e1025. https://doi.org/10.1038/tp.2016.285 (2017).
Guo, G. H., Dong, J., Yuan, X. H., Dong, Z. N. & Tian, Y. P. Clinical evaluation of the levels of 12 cytokines in serum/plasma under various storage conditions using evidence biochip arrays. Mol. Med. Rep. 7, 775–780. https://doi.org/10.3892/mmr.2013.1263 (2013).
Aziz, N., Nishanian, P., Mitsuyasu, R., Detels, R. & Fahey, J. L. Variables that affect assays for plasma cytokines and soluble activation markers. Clin. Diagn. Lab. Immunol. 6, 89–95. https://doi.org/10.1128/CDLI.6.1.89-95.1999 (1999).
Polacchini, A. et al. A method for reproducible measurements of serum BDNF: comparison of the performance of six commercial assays. Sci. Rep. 5, 17989. https://doi.org/10.1038/srep17989 (2015).
Anastasia, A. et al. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone Retraction. Nat. Commun. 4, 2490. https://doi.org/10.1038/ncomms3490 (2013).
Zhang, L. et al. PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Mol. Psychiatry. 19, 8–10. https://doi.org/10.1038/mp.2012.180 (2014).
Wu, G. W. Y. et al. Serum brain-derived neurotrophic factor remains elevated after long term follow-up of combat veterans with chronic post-traumatic stress disorder. Psychoneuroendocrinology 134, 105360. https://doi.org/10.1016/j.psyneuen.2021.105360 (2021).
Pan, W., Banks, W. A., Fasold, M. B., Bluth, J. & Kastin, A. J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 37, 1553–1561. https://doi.org/10.1016/s0028-3908(98)00141-5 (1998).
Klein, A. B. et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int. J. Neuropsychopharmacol. 14, 347–353. https://doi.org/10.1017/S1461145710000738 (2011).
Kunimatsu, A., Yasaka, K., Akai, H., Kunimatsu, N. & Abe, O. MRI findings in posttraumatic stress disorder. J. Magn. Reson. Imaging 52, 380–396. https://doi.org/10.1002/jmri.26929 (2020).
Invernizzi, A. et al. Functional changes in neural mechanisms underlying post-traumatic stress disorder in world trade center responders. Transl Psychiatry. 13, 239. https://doi.org/10.1038/s41398-023-02526-y (2023).
Huang, C. et al. Graph theory-based analysis reveals neural anatomical network alterations in chronic post-traumatic stress disorder. Imaging Neurosci. 2, 1–11. https://doi.org/10.1162/imag_a_00141 (2024).
Weathers, F. W. et al. The clinician-administered PTSD scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol. Assess. 30, 383–395. https://doi.org/10.1037/pas0000486 (2018).
Treacy, C. et al. The LEISURE study: A longitudinal randomized controlled trial protocol for a multi-modal lifestyle intervention study to reduce dementia risk in healthy older adults. J. Alzheimers Dis. 94, 841–856. https://doi.org/10.3233/JAD-230193 (2023).
Topp, C. W., Ostergaard, S. D., Sondergaard, S. & Bech, P. The WHO-5 well-being index: a systematic review of the literature. Psychother. Psychosom. 84, 167–176. https://doi.org/10.1159/000376585 (2015).
Lovibond, S. H. & Lovibond, P. F. Manual for the Depression Anxiety Stress Scales 2nd edn. (Psychology Foundation, 1995).
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–790, doi:https://doi.org/10.1016/j.neuroimage.2011.09.015 (2012).
Zhang, Y., Brady, M. & Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 20, 45–57. https://doi.org/10.1109/42.906424 (2001).
Patenaude, B., Smith, S. M., Kennedy, D. N. & Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56, 907–922. https://doi.org/10.1016/j.neuroimage.2011.02.046 (2011).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. https://doi.org/10.1016/s1053-8119(02)91132-8 (2002).
Wickham, H. Ggplot2: Elegant Graphics for Data Analysis 2nd edn (Springer International Publishing, 2016).
Acknowledgements
The study team would like to gratefully acknowledge and thank all the participants who volunteered their time to be part of this work, the Clinical Research Unit and Healthy Brain Ageing teams at the Thompson Institute, UniSC, for their support of the clinical programs underlying this study. We acknowledge the Wilson Foundation, who provided generous funding support for the LEISURE study. This work also wishes to acknowledge the Australian Commonwealth Government’s ‘Prioritizing Mental Health Initiative’ for funding support.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: Underlying clinical trial conception and design: MD, APB, GF, CCG, CT, JL, SCA, ATC, DH This study conception and design: BLQ, NW, JM, DH Data collection: BLQ, NW, JML, MD, APB, GF, CCG, MH, CT, ATCAnalysis and interpretation of results: BLQ, NW, JML Draft manuscript preparation: BLQ, NW, JMLAll authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Quigley, B.L., Wellington, N., Levenstein, J.M. et al. Circulating biomarkers and neuroanatomical brain structures differ in older adults with and without post-traumatic stress disorder. Sci Rep 15, 7176 (2025). https://doi.org/10.1038/s41598-025-91840-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91840-0