Abstract
Glaucoma is a leading cause of irreversible blindness worldwide, with its pathogenesis incompletely understood. Inflammation, as an important aspect of glaucoma, has attracted increasing attention. In this study, we performed a Mendelian randomization (MR) analysis to investigate the association between 91 circulating inflammatory proteins and glaucoma. First, a bidirectional MR was employed to screen for inflammatory proteins that potentially influence glaucoma risk, with the findings further confirmed by a replication sample MR. Then, a mediation analysis was employed to assess the mediating effects of glaucoma endophenotypes on glaucoma. Finally, we performed a subgroup MR to investigate the association between circulating proteins and glaucoma subtypes, including primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG). The bidirectional MR suggested 7 out of the 91 proteins were possibly related with glaucoma risk, with T-cell surface glycoprotein CD5 (CD5) (odds ratio (OR) = 0.87; 95% confidence interval (CI): 0.81–0.94; P = 2.46 × 10−4) passing false discovery rate correction. This result was verified by the replication sample MR. The mediation analysis revealed that intraocular pressure (IOP) (β=-0.05; 95% CI: −0.02-−0.09; P = 1.56 × 10−3) was a mediator of CD5’s protective effect on glaucoma. The subgroup MR indicated that CD5 conferred a protective causal effect specifically on POAG, not PACG. Moreover, IOP served as a mediator in the association between CD5 and POAG, explaining a proportion of 38.29% of CD5’s protective effect against POAG. Our findings suggest a negative causal association between circulating CD5 and POAG risk, which is partially mediated by IOP. This indicates that targeted CD5 therapy may be beneficial to POAG eyes.
Similar content being viewed by others
Introduction
Glaucoma is a heterogeneous spectrum of ocular conditions characterized by optic nerve degeneration and progressive retinal ganglion cell loss due to multiple pathogenic factors1. At present, it stands as the foremost cause of irreversible blindness on a global scale, impacting nearly 95 million people worldwide, with approximately 10 million suffering from blindness1. For long, elevated intraocular pressure (IOP) has been recognized as the primary risk factor for glaucoma, and lowering IOP (whether through medication or surgery) is currently the only valid intervention for glaucoma2. However, nearly half of glaucoma cases occur with a normal IOP, and lowering IOP cannot always effectively preserve patients’ visual function, suggesting that factors other than IOP may be involved in the pathogenesis of glaucoma1,3.
It is now increasingly recognized that the mechanisms underlying glaucomatous optic neuropathy involve not only the classical “mechanical” and “vascular” theories but also immunoregulatory biological processes such as inflammation4,5,6. For instance, previous studies showed that certain systemic inflammatory indices are elevated in patients with primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG) compared to healthy controls, suggesting a potential role of systemic inflammation in the pathogenesis of POAG and PACG7,8. However, establishing these causal relationships is challenging because these studies fall under cross-sectional research, which highlights the necessity for comprehensive assessments to better understand relationship between inflammation and this vision-threatening condition.
Mendelian randomization (MR) offers a cost-effective solution to this challenge. MR is a widely embraced approach in epidemiology that utilizes genetic variants as instrumental variables (IVs) to infer causal effects of specific exposures on health outcomes9. MR exploits the random allocation of genetic variants during meiosis, providing a “natural” randomized controlled trial to infer causality. Compared to traditional observational studies, MR studies are less susceptible to confounding factors and reverse causality, providing strong evidence for causal associations10.
In this study, we performed a comprehensive MR analysis to explore the potential causal association between 91 circulating inflammatory proteins and glaucoma (including both POAG and PACG).
Methods
Study design
The procedural steps of this study are presented in Fig. 1. First of all, a bidirectional MR analysis was conducted to identify circulating inflammatory proteins which are genetically predicted related to glaucoma risk and to detect if any reverse causality exists. Subsequently, for proteins showing causal effects on glaucoma in bidirectional MR analysis, a replication sample MR analysis was used to further validate these detected causal effects. Following this, a two-step MR analysis was employed to assess whether glaucoma endophenotypes, including retinal nerve fiber layer (RNFL) thickness, IOP, and central corneal thickness (CCT) have mediated the causal relationships between circulating inflammatory proteins and glaucoma. Lastly, a subgroup MR analysis was executed to delve deeper into the causal relationships between circulating inflammatory proteins surviving the preceding MR analyses and glaucoma. The subgroup analysis also aimed to elucidate the influence of glaucoma endophenotypes on these relationships. Concurrently, the MR analyses adhered to three fundamental premises: (I) IVs are associated with the exposure; (II) IVs remain unaffected by confounders; (III) Exposure is the only mediator of IV-outcome associations11.
An overview of the study design. MR, mendelian randomization; IVW, inverse variance weighted; WM, weighted median; RNFL, retinal nerve fiber layer thickness; IOP, intraocular pressure; CCT, central corneal thickness; POAG, primary open-angle glaucoma; PACG, primary angle-closure glaucoma; IVs, instrumental variables.
Data source
Summary data for circulating protein levels were obtained from the recently published genome-wide association studies (GWAS) dataset, encompassing up to 14,824 participants of European descent12. Details regarding this GWAS data can be found in Supplementary Table S1. Glaucoma summary data were derived from the 10th round FinnGen GWAS database (https://r10.finngen.fi/; GWAS ID: finngen_R10_H7_GLAUCOMA), comprising 412,181 European participants13. For the replication MR analysis, glaucoma summary data were retrieved from the IEU OPEN GWAS project (https://gwas.mrcieu.ac.uk/; GWAS ID: ebi-a-GCST009722), involving 351,696 European participants14. Additionally, summary data for glaucoma subtypes, namely POAG and PACG, were extracted from recent GWAS datasets (GWAS ID: GCST90011766 and GCST90043782), encompassing 216,257 and 456,348 individuals of European descent, respectively15,16. Finally, GWAS summary data for glaucoma endophenotypes, including RNFL, IOP, and CCT, were obtained from different consortiums where there was no sample overlap with the exposures (circulating inflammatory proteins) and outcome (glaucoma), to ensure the reliability of the analysis17,18. Detailed information about GWAS summary datasets were shown in Supplementary Table S2.
Instrumental variable selection
To ensure the credibility and accuracy of the results concerning the causal effects of circulating inflammatory proteins on glaucoma and its endophenotypes, a series of quality control methods were implemented to select suitable IVs for MR analyzes.
Single nucleotide polymorphisms (SNPs) adhering to the following criteria were chosen as IVs. First, SNPs should surpass the genome-wide significance thresholds of 5 × 10^−6 for circulating inflammatory proteins and 5 × 10^−8 for glaucoma and its endophenotypes, so that these SNPs were significantly associated with these phenotypes19. Second, SNPs demonstrate no linkage disequilibrium (LD) (with a threshold of r^2 = 0.001 and a clumping distance of 10000 KB). Third, SNPs possessing an F statistic ≥ 10 (where F = β_exposure^2/SE_exposure^2) were included to ensure the absence of weak instrument bias20. Lastly, all palindromic SNPs were dropped and MR pleiotropy residual sum and outlier (MR-PRESSO) test was applied to detect horizontal pleiotropy and to eliminate the pleiotropy by removing outlier SNPs.
MR analyses
Several methodologies were employed in the MR analyses: random effects inverse variance weighting (RE-IVW)/fixed effects inverse variance weighting (FE-IVW), weighted median (WM), and MR-Egger regression. In certain scenarios, the IVW method is renowned for its superior effectiveness when compared with the other two methods21. Thus, the IVW method served as the primary approach for the analyses, complemented by the WM and MR-Egger regression methods. For circulating inflammatory proteins, odds ratio (OR) and corresponding 95% confidence interval (CI) were used to quantify the effect size on glaucoma (POAG and PACG). For glaucoma, β coefficients with 95% CI quantified the effects on circulating inflammatory protein levels. Moreover, in the mediation MR analysis, the effects sizes were expressed as β with 95% CI.
In addition, we performed a series of sensitivity analyses to assess the robustness of the observed causal relationships, including Steiger filtering, Cochran’s Q test, and MR-Egger intercept test. Steiger filtering was employed to verify the directionality of the causal associations22. Cochran’s Q test was used to assess heterogeneity, and the RE-IVW method and FE-IVW method was applied when P < 0.05 and P> 0.05, respectively23. Furthermore, potential horizontal pleiotropy was evaluated using MR-Egger intercept test24.
Statistics analysis
All MR analyses were performed using R statistical software (version 4.3.0) with the following packages: “TwoSampleMR” (version 0.5.6), “MendelianRandomization” (version 0.7.0), “MRPRESSO” (version 1.0), and “fdrtool” (version 1.2.17). False discovery rate (FDR) correction was used for multiple testing correction. Results with P value < 0.05 and q-value < 0.1 were considered statistically significant, whereas results with P value < 0.05 but q-value ≥ 0.1 were considered suggestively significant25.
Results
Bidirectional MR analysis
A total of 1,558 IVs meeting the screening criteria were included, with all F-statistics surpassing 10 (range: 20.84 to 3,549.83), suggesting no evidence for weak instrument bias. The details of these IVs are shown in Supplementary Table S3.
Causal effects of Circulating inflammatory proteins on Glaucoma
Among the 91 circulating inflammatory proteins, 7 were identified as causal exposures to glaucoma in the forward bidirectional MR (Fig. 2). Specifically, higher levels of 4 proteins were associated with a decreased risk of glaucoma, including T-cell surface glycoprotein CD5 (CD5) (OR = 0.87; 95% CI: 0.81–0.94; P = 2.46 × 10−4), fibroblast growth factor 19 (FGF-19) (OR = 0.93; 95% CI: 0.86–0.99; P = 4.84 × 10−2), interleukin-1 alpha (IL-1 alpha) (OR = 0.90; 95% CI: 0.81–0.99; P = 2.89 × 10−2), and programmed cell death 1 ligand 1 (PD-L1) (OR = 0.90; 95% CI: 0.82–0.99; P = 4.83 × 10−2). In contrast, higher levels of 3 proteins were associated with an increased risk of glaucoma, including fibroblast growth factor 23 (FGF-23) (OR = 1.17; 95% CI: 1.04–1.31; P = 7.60 × 10−3), leukemia inhibitory factor (LIF) (OR = 1.14; 95% CI: 1.04–1.25; P = 6.60 × 10−3), and tumor necrosis factor (TNF) (OR = 1.14; 95% CI: 1.02–1.27; P = 2.33 × 10−2).
Results of sensitivity analyses were shown in Supplementary Table S4. The Steiger filtering passed the causal direction of the levels of circulating inflammatory proteins on glaucoma. The Cochran’s Q test showed heterogeneity in IVs for IL-1 alpha, hence, RE-IVW was used. No pleiotropy was observed in MR-Egger intercept test.
Of note, among the 7 circulating inflammatory proteins, circulating CD5 remained significantly associated with glaucoma risk in the FDR correction (FE-IVW; q = 0.02). This suggested that of these 7 proteins, CD5 had the strongest association with glaucoma, whereas the other six were suggestively associated with glaucoma.
Results of the forward MR analysis. CD5, T-cell surface glycoprotein CD5; FGF-19, fibroblast growth factor 19; FGF-23, fibroblast growth factor 23; IL-1 alpha, interleukin-1 alpha; LIF, leukemia inhibitory factor; PD-L1, programmed cell death 1 ligand 1; TNF, tumor necrosis factor; N. IVs, the number of IVs; FE-IVW, fixed effects inverse variance weighted; RE-IVW, random effects inverse variance weighted; WM, weighted median.*: P < 0.05 but q > 0.1; **: P < 0.05 and q < 0.1.
Causal effects of Glaucoma on Circulating inflammatory proteins
The reverse MR revealed no causal effect of glaucoma on the 7 circulating inflammatory proteins identified in the forward analysis (Fig. 3). In addition, no evidence of heterogeneity or horizontal pleiotropy was observed in the sensitivity analyses (Supplementary Table S5).
Results of the reverse MR analysis. CD5, T-cell surface glycoprotein CD5; FGF-19, fibroblast growth factor 19; FGF-23, fibroblast growth factor 23; IL-1 alpha, interleukin-1 alpha; LIF, leukemia inhibitory factor; PD-L1, programmed cell death 1 ligand 1; TNF, tumor necrosis factor; N. IVs, the number of IVs; FE-IVW, fixed effects inverse variance weighted; WM, weighted median.
Replication sample MR analysis
For the 7 proteins that were significantly or suggestively associated with glaucoma in the bidirectional MR analysis, we conducted a replication sample MR analysis to further valid the suggestive causality. Totally, 86 IVs were included, and the F-statistics ranged from 20.87 to 198.14, indicating no evidence of weak instrument bias. The information of the included IVs was listed in Supplementary Table S6.
The results of the replication MR analysis, consistent with the forward MR, showed that CD5 maintained a significantly protective effect against glaucoma (OR = 0.87; 95% CI: 0.78–0.97; P = 6.72 × 10−3; q = 0.03) and the 6 suggestive proteins had no significant causal effect on glaucoma (Fig. 4).
Results of sensitivity analyses were listed in Supplementary Table S7. The Steiger filtering verified the direction of causality from circulating inflammatory proteins to glaucoma. The Cochran’s Q test and MR-Egger intercept test confirmed the absence of heterogeneity and pleiotropy, respectively.
Results of the replication MR analysis. CD5, T-cell surface glycoprotein CD5; FGF-19, fibroblast growth factor 19; FGF-23, fibroblast growth factor 23; IL-1 alpha, interleukin-1 alpha; LIF, leukemia inhibitory factor; PD-L1, programmed cell death 1 ligand 1; TNF, tumor necrosis factor; N. IVs, the number of IVs; FE-IVW, fixed effects inverse variance weighted; WM, weighted median. *: P < 0.05 but q > 0.1; **: P < 0.05 and q < 0.1.
Glaucoma endophenotypes mediated the causal effects of Circulating CD5 on Glaucoma
According to above MR analyses, circulating CD5 was associated with a lower risk of glaucoma. To further investigate the potential mediating role of glaucoma endophenotypes in the association between CD5 and glaucoma, we conducted an additional two-step mediation MR analysis.
In the first step, 51 IVs were selected, with F-statistics ranging from 21.10 to 124.29 (Supplementary Table S8). It showed that circulating CD5 was causally negatively associated to IOP (β=−0.33; P = 6.93 × 10−4) (Supplementary Table S9). In addition, no heterogeneity and pleiotropy were observed (Supplementary Table S9). In the second step, a total of 39 IVs were selected, with F-statistics ranging between 29.90 and 256.63 (Supplementary Table S10). It revealed that IOP (β = 0.16; P = 1.88 × 10−18) and CCT (β = 0.04; P = 5.34 × 10−3) were causally positively related to glaucoma risk. Mild heterogeneity was found in IVs for IOP, and CCT. No pleiotropy was observed in this step (Supplementary Table S11).
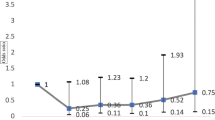
Taken together, IOP (β=−0.05; 95% CI: −0.02–−0.09; P = 1.56 × 10−3) acted as a mediator imposing significant mediation effects on circulating CD5 and glaucoma, with a proportion of 38.39% (95% CI: 14.61–62.17%) (Fig. 5).
Subgroup MR analysis
To delve deeper into the causal association between circulating CD5 and glaucoma subtypes (i.e., POAG and PACG), we performed a subgroup MR analysis. For causal effects of CD5 on POAG and PACG, a total of 34 IVs met the screening criteria, with F-statistics ranging from 20.10 to 124.29 (Supplementary Table S12). Results exhibited that genetically predicted circulating CD5 had a protective causal effect only on POAG (OR = 0.85; 95% CI: 0.76–0.95; P = 3.50 × 10−3) (Fig. 6). No heterogeneity or pleiotropy was observed. Besides, Steiger filtering confirmed that the direction of the causal estimate was correctly orientated from CD5 to POAG and PACG (Supplementary Table S13).
To elucidate mediating role of glaucoma endophenotypes in the association between CD5 and POAG, we conducted a mediation MR analysis. 40 IVs were included, with F-statistics ranging between 29.90 and 256.63 (Supplementary Table S14). The mediation MR analysis indicated that higher levels of circulating CD5 were significantly associated with decreased IOP risk (β=−0.33; P = 6.93 × 10−4) (Supplementary Table S9), and IOP (β = 0.19 P = 1.33 × 10−3) was a contributory factor to the risk of POAG (Supplementary Table S15). Thus, IOP (β=−0.06; P = 1.97 × 10−2) was confirmed as a significant mediator in mediating the causal effect of circulating CD5 on POAG, with a contribution proportion of 38.29% (95% CI: 6.10–70.48%) (Fig. 7). Additionally, no heterogeneity or pleiotropy was observed (Supplementary Table S15).
The mediating role of glaucoma endophenotypes in the relationship between circulating CD5 and POAG. CD5, T-cell surface glycoprotein CD5; IOP, intraocular pressure; POAG, primary open-angle glaucoma. Total effect, the effect of CD5 on glaucoma; Mediation effect, the indirect effect of CD5 on POAG via endophenotypes.
Discussion
This study, to our knowledge, is the first to systematically explore the potential causal relationship between 91 circulating inflammatory proteins and glaucoma using MR. Among the 91 inflammatory proteins, we found that circulating CD5 was associated with a lower risk of POAG. This finding not only enhances our understanding of glaucoma pathogenesis but also suggested a potential therapeutic target for POAG.
Initially, we used bidirectional MR to screen for inflammatory proteins potentially influencing glaucoma. Among the 91 proteins analyzed, only CD5 passed the FDR correction, indicating its prominence. This finding was further confirmed through the replication sample MR with FDR correction, which revealed CD5 was the only protein causally linked to glaucoma. Subsequently, a mediation MR was conducted to explore the role of glaucoma endophenotypes in mediating the association between CD5 and glaucoma. Results highlighted that IOP served as a significant mediator of CD5’s effect on glaucoma. Additionally, considering POAG and PACG were distinct conditions, a subgroup MR analysis was performed. It indicated that CD5 conferred a protective causal effect specifically on POAG, not PACG. Moreover, IOP remained as a mediator in the association between CD5 and POAG, explaining about 38.3% of CD5’s protective effect against POAG.
CD5 is a macromolecular glycoprotein mainly distributed on the surface of T lymphocytes and B-1a lymphocytes26. As an important immune molecule, CD5 not only participates in the innate immune response but also regulates the specific immune response mediated by T and B lymphocytes27. Actually, CD5 is widely recognized as a negative regulator of T cell receptor (TCR) and B cell receptor (BCR) signaling, playing an important role in immune homeostasis28. Previous investigations have shown that upregulation CD5 on T or B cells can provide protection against autoimmunity, possibly due to a higher threshold for TCR- or BCR-mediated activation upon antigen recognition28. In addition, it has been discovered that CD5 can activate the MAPK/Erk pathway, promoting the production of interleukin-10 (IL-10), an anti-inflammatory cytokine that plays a crucial role in regulating immune responses and suppressing inflammation29,30,31. Furthermore, IL-10 can promote the survival of retinal ganglion cells through the STAT-3 pathway, exerting a protective effect against glaucoma32. From this perspective, it makes sense that CD5 is a protective factor for POAG, as it is already well known that inflammation can promote glaucoma, although the underlying mechanisms have not been fully understood4. In a recent study, Okruszko et al. found that glaucoma patients have lower levels of serum CD5 compared with healthy controls33. Their finding is, to some extent, consistent with our observation that lower levels of circulating CD5 may pose a higher risk of glaucoma, particularly POAG. However, Okruszko et al. did not subdivide their glaucoma patients into subgroups, so further research is needed to verify whether there are differences in circulating CD5 levels between POAG and PACG patients.
Notably, we found that higher circulating CD5 levels are associated with lower IOP, and that IOP mediates 38.3% of the negative causal relationship between CD5 and POAG. On the one hand, this suggests that the protective effect of CD5 on POAG is, to a considerable extent, still achieved by optimizing the IOP, highlighting the importance of IOP-lowering treatment for POAG patients. On the other hand, it also indicates that the relationship between CD5 and POAG is complex and involves other pathways, which deserves further experimental research.
This study holds several strengths of note. First of all, the use of MR enabled this study to mimic the effect of a well-designed randomized controlled trial in a time- and labor-saving manner. Moreover, the combined application of bidirectional MR, mediation MR, and subgroup MR analyses enabled us to more accurately assess the causality involved. Last but not least, MR can effectively avoid interference of reverse causation, which is hard to achieve in observational studies.
Despite these merits, this study has some limitations. First, we did not group the glaucoma patients by sex, thus may have overlooked the effect of gender on the association between circulating inflammatory proteins and glaucoma. Second, although we have found the relationship between CD5 and POAG, the specific mechanism of CD5’s role in the pathogenesis of glaucoma still requires further research. Third, despite several rigorous measures taken, it is impossible to completely eliminate the potential impact of heterogeneity on this study.
Conclusions
In summary, we have provided strong genetic evidence that circulating CD5 exerts a protective causal effect on POAG. This may imply that targeted CD5 therapy could potentially offer therapeutic benefits to POAG patients. Furthermore, we also showed that the effect of circulating CD5 on POAG is partially mediated by IOP, indicating that IOP-lowering remains an important treatment option for POAG. Also, we look forward to future studies to further unveiling the mechanisms by which circulating CD5 influences the risk of POAG.
Data availability
Summary statistics used in current study are publicly available from the FinnGen GWAS database (https://r10.finngen.fi/), the IEU OPEN GWAS project (https://gwas.mrcieu.ac.uk/), and references 12 and 15-18.
Abbreviations
- MR:
-
Mendelian randomization
- POAG:
-
Primary open-angle glaucoma
- PACG:
-
Primary angle-closure glaucoma
- CD5:
-
T-cell surface glycoprotein CD5
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- IOP:
-
Intraocular pressure
- IVs:
-
Instrumental variables
- RNFL:
-
Retinal nerve fiber layer
- CCT:
-
Central corneal thickness
- GWAS:
-
Genome-wide association studies
- SNPs:
-
Single nucleotide polymorphisms
- LD:
-
Linkage disequilibrium
- MR-PRESSO:
-
MR pleiotropy residual sum and outlier
- RE-IVW:
-
Random effects inverse variance weighting
- FE-IVW:
-
Fixed effects inverse variance weighting
- WM:
-
Weighted median
- FDR:
-
False discovery rate
- FGF-19:
-
Fibroblast growth factor 19
- IL-1 alpha:
-
Interleukin-1 alpha
- PD-L1:
-
Programmed cell death 1 ligand 1
- FGF-23:
-
Fibroblast growth factor 23
- LIF:
-
Leukemia inhibitory factor
- TNF:
-
Tumor necrosis factor
- TCR:
-
T cell receptor
- BCR:
-
B cell receptor
- IL-10:
-
Interleukin-10
References
Jayaram, H., Kolko, M., Friedman, D. S. & Gazzard, G. Glaucoma: now and beyond. Lancet (London England). 402, 1788–1801. https://doi.org/10.1016/s0140-6736(23)01289-8 (2023).
Stein, J. D., Khawaja, A. P. & Weizer, J. S. Glaucoma in Adults-Screening, diagnosis, and management: A review. JAMA325, 164–174. https://doi.org/10.1001/jama.2020.21899 (2021).
Wang, S. Y. & Singh, K. Management of the glaucoma patient progressing at low normal intraocular pressure. Curr. Opin. Ophthalmol.31, 107–113. https://doi.org/10.1097/icu.0000000000000640 (2020).
Baudouin, C., Kolko, M., Melik-Parsadaniantz, S. & Messmer, E. M. Inflammation in glaucoma: from the back to the front of the eye, and beyond. Prog Retin Eye Res.83, 100916. https://doi.org/10.1016/j.preteyeres.2020.100916 (2021).
McDermott, C. E., Salowe, R. J., Di Rosa, I., O’Brien, J. M. & Stress Allostatic load, and neuroinflammation: implications for Racial and socioeconomic health disparities in Glaucoma. Int. J. Mol. Sci.25https://doi.org/10.3390/ijms25031653 (2024).
Wang, L., Wei, X. T. & Cell-Mediated Autoimmunity in Glaucoma neurodegeneration. Front. Immunol.12, 803485. https://doi.org/10.3389/fimmu.2021.803485 (2021).
Karahan, M., Kilic, D. & Guven, S. Systemic inflammation in both open-angle and angle-closure glaucoma: role of platelet-to-lymphocyte ratio. Bratisl Lek Listy. 122, 45–48. https://doi.org/10.4149/bll_2021_005 (2021).
Li, S. et al. Association of systemic inflammation indices with visual field loss progression in patients with primary angle-closure glaucoma: potential biomarkers for 3P medical approaches. Epma J.12, 659–675. https://doi.org/10.1007/s13167-021-00260-3 (2021).
Emdin, C. A., Khera, A. V., Kathiresan, S. & Mendelian Randomization JAMA318, 1925–1926 https://doi.org/10.1001/jama.2017.17219 (2017).
Gala, H. & Tomlinson, I. The use of Mendelian randomisation to identify causal cancer risk factors: promise and limitations. J. Pathol.250, 541–554. https://doi.org/10.1002/path.5421 (2020).
Boef, A. G., Dekkers, O. M. & le Cessie, S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int. J. Epidemiol.44, 496–511. https://doi.org/10.1093/ije/dyv071 (2015).
Zhao, J. H. et al. Genetics of Circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat. Immunol.24, 1540–1551. https://doi.org/10.1038/s41590-023-01588-w (2023).
Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature613, 508–518. https://doi.org/10.1038/s41586-022-05473-8 (2023).
Elsworth, B. et al. The MRC IEU OpenGWAS data infrastructure. BioRxiv2020.2008.2010.244293https://doi.org/10.1101/2020.08.10.244293 (2020).
Gharahkhani, P. et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat. Commun.12, 1258. https://doi.org/10.1038/s41467-020-20851-4 (2021).
Jiang, L., Zheng, Z., Fang, H. & Yang, J. A generalized linear mixed model association tool for biobank-scale data. Nat. Genet.53, 1616–1621. https://doi.org/10.1038/s41588-021-00954-4 (2021).
Currant, H. et al. Genetic variation affects morphological retinal phenotypes extracted from UK biobank optical coherence tomography images. PLoS Genet.17, e1009497. https://doi.org/10.1371/journal.pgen.1009497 (2021).
Bonnemaijer, P. W. M. et al. Multi-trait genome-wide association study identifies new loci associated with optic disc parameters. Commun. Biology. 2, 435. https://doi.org/10.1038/s42003-019-0634-9 (2019).
Chen, G. et al. Causal association of cytokines and growth factors with stroke and its subtypes: a Mendelian randomization study. Mol. Neurobiol.https://doi.org/10.1007/s12035-023-03752-7 (2023).
Chen, L. et al. Insights into modifiable risk factors of cholelithiasis: A Mendelian randomization study. Hepatol. (Baltimore Md). 75, 785–796. https://doi.org/10.1002/hep.32183 (2022).
Zhou, X., Xu, J., Zhang, X., Zhao, Y. & Duan, X. Causal relationships between gut microbiota and primary open-angle glaucoma: A Mendelian randomization and mediation analysis of Glaucoma endophenotypes. Exp. Eye Res.240, 109788. https://doi.org/10.1016/j.exer.2024.109788 (2024).
Liu, C. et al. Iron status and NAFLD among European populations: A bidirectional Two-Sample Mendelian randomization study. Nutrients14https://doi.org/10.3390/nu14245237 (2022).
Hemani, G., Bowden, J. & Davey Smith, G. Evaluating the potential role of Pleiotropy in Mendelian randomization studies. Hum. Mol. Genet.27, R195–r208. https://doi.org/10.1093/hmg/ddy163 (2018).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: effect Estimation and bias detection through Egger regression. Int. J. Epidemiol.44, 512–525. https://doi.org/10.1093/ije/dyv080 (2015).
Li, P. et al. Association between gut microbiota and preeclampsia-eclampsia: a two-sample Mendelian randomization study. BMC Med.20, 443. https://doi.org/10.1186/s12916-022-02657-x (2022).
Voisinne, G., Gonzalez de Peredo, A. & Roncagalli, R. CD5, an undercover regulator of TCR signaling. Front. Immunol.9, 2900. https://doi.org/10.3389/fimmu.2018.02900 (2018).
Burgueño-Bucio, E., Mier-Aguilar, C. A. & Soldevila, G. The multiple faces of CD5. J. Leukoc. Biol.105, 891–904. https://doi.org/10.1002/jlb.Mr0618-226r (2019).
Dalloul, A. CD5: a safeguard against autoimmunity and a shield for cancer cells. Autoimmun. Rev.8, 349–353. https://doi.org/10.1016/j.autrev.2008.11.007 (2009).
Ip, W. K. E., Hoshi, N., Shouval, D. S., Snapper, S. & Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Sci. (New York N Y). 356, 513–519. https://doi.org/10.1126/science.aal3535 (2017).
Garaud, S. et al. CD5 expression promotes IL-10 production through activation of the MAPK/Erk pathway and upregulation of TRPC1 channels in B lymphocytes. Cell Mol. Immunol.15, 158–170. https://doi.org/10.1038/cmi.2016.42 (2018).
Cevey, Á., Penas, C., Soto, F. N. A., Mirkin, C. D., Goren, N. B. & G. A. & IL-10/STAT3/SOCS3 Axis is involved in the Anti-inflammatory effect of benznidazole. Front. Immunol.10, 1267. https://doi.org/10.3389/fimmu.2019.01267 (2019).
Boyd, Z. S. et al. Interleukin-10 receptor signaling through STAT-3 regulates the apoptosis of retinal ganglion cells in response to stress. Invest. Ophthalmol. Vis. Sci.44, 5206–5211. https://doi.org/10.1167/iovs.03-0534 (2003).
Okruszko, M. A. et al. Inflammation and neurodegeneration in glaucoma: isolated eye disease or a part of a systemic Disorder? - Serum proteomic analysis. J. Inflamm. Res.17, 1021–1037. https://doi.org/10.2147/jir.S434989 (2024).
Acknowledgements
We would like to thank the IEU Open GWAS database and authors of references 12 and 15-18 for providing the data. We also want to acknowledge the participants and investigators of the FinnGen study.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, W.X., Q.F., Y.M.; Methodology: W.X., Q.F., Y.M., S.X., C.C.; Visualization: W.X., Q.F., Y.M., Z.N., A.S., S.X., C.C.; Resources: Z.N., A.S., S.X., C.C.; Supervision: S.X., C.C.; Writing—original draft preparation, W.X., Q.F., Y.M.; Writing—review and editing: Z.N., A.S., S.X., C.C. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable, as all data used in this study were from publicly available database.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, W., Fan, Q., Meng, Y. et al. Association between 91 circulating inflammatory proteins and the risk of glaucoma: A Mendelian randomization study. Sci Rep 15, 8876 (2025). https://doi.org/10.1038/s41598-025-92153-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92153-y